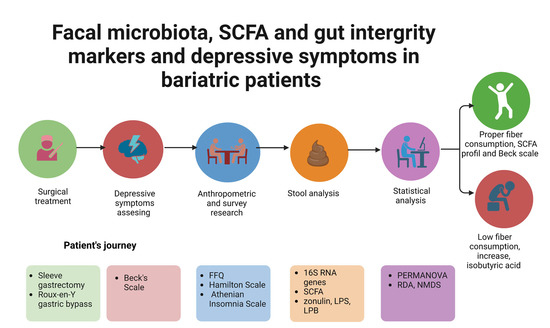
Association between Fecal Microbiota, SCFA, Gut Integrity Markers and Depressive Symptoms in Patients Treated in the Past with Bariatric Surgery—The Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Anthropometric Research
2.2. Survey Research
- Beck’s Scale—a self-assessment questionnaire used to assess the occurrence and intensity of depressive symptoms. The total score ranges from 0 to 63 points, where the higher the score, the greater the severity of depressive symptoms. ≥12 points was considered to suggest the presence of depressive symptoms [16,17,18].
- Athenian Insomnia Scale—a scale consisting of 8 items assessing nighttime sleep patterns (falling asleep and waking up at night) as well as total sleep time, sleep quality, well-being, psychophysical fitness, and daytime sleepiness. Scores were assessed utilizing a total score of between 0 and 24 points, with a higher score representing a poorer quality of sleep. Score ≥6 points indicated insomnia [22].
2.3. Eating Habits
2.4. Laboratory Tests
2.5. The Assessment of Intestinal Barrier Integrity
2.6. Sequencing Analysis of the Bacterial 16S RNA Genes
2.7. The Assessment of SCFA Content in Faecal Samples
2.8. Statistical Analysis
3. Results
3.1. Mental State
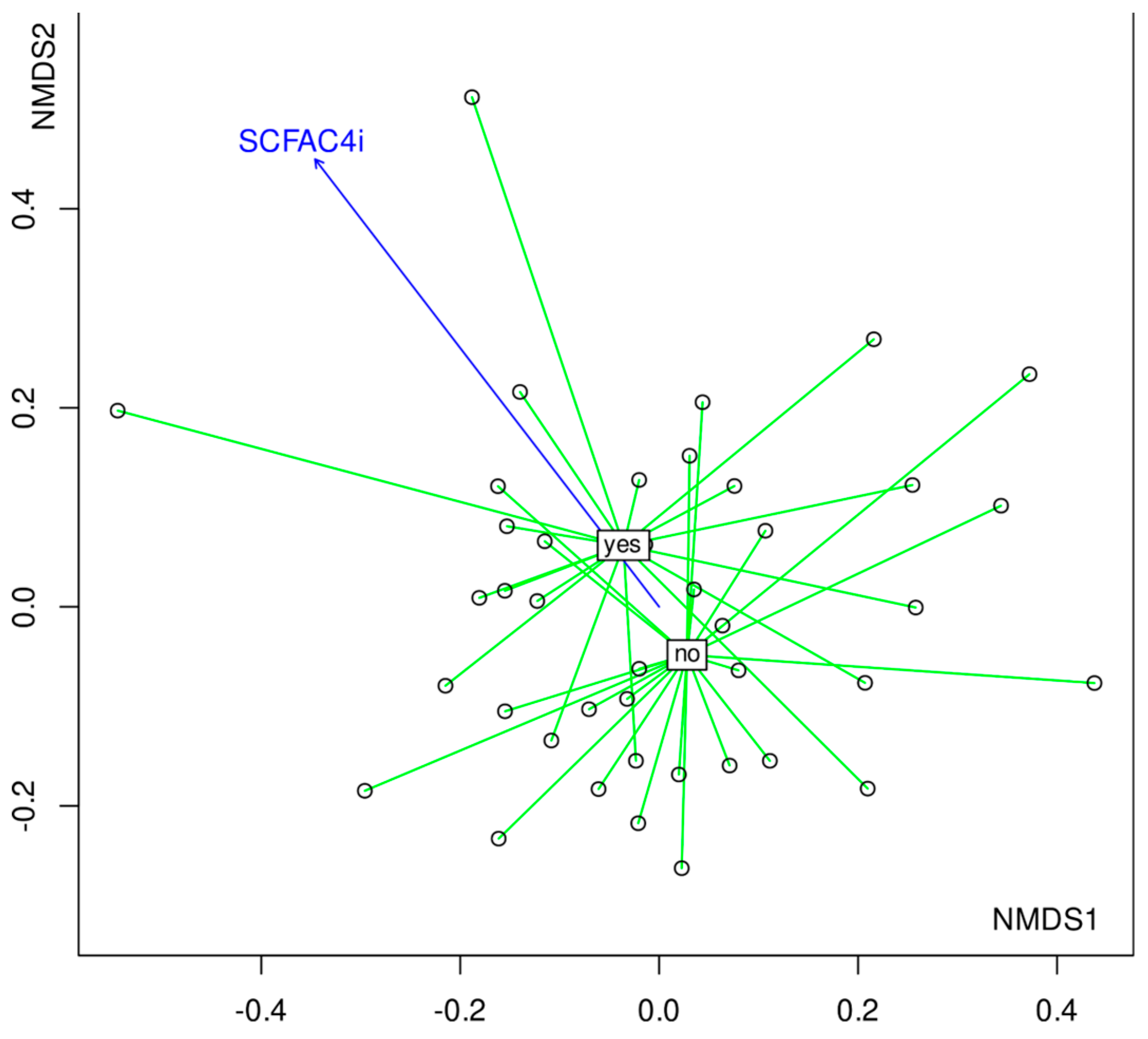
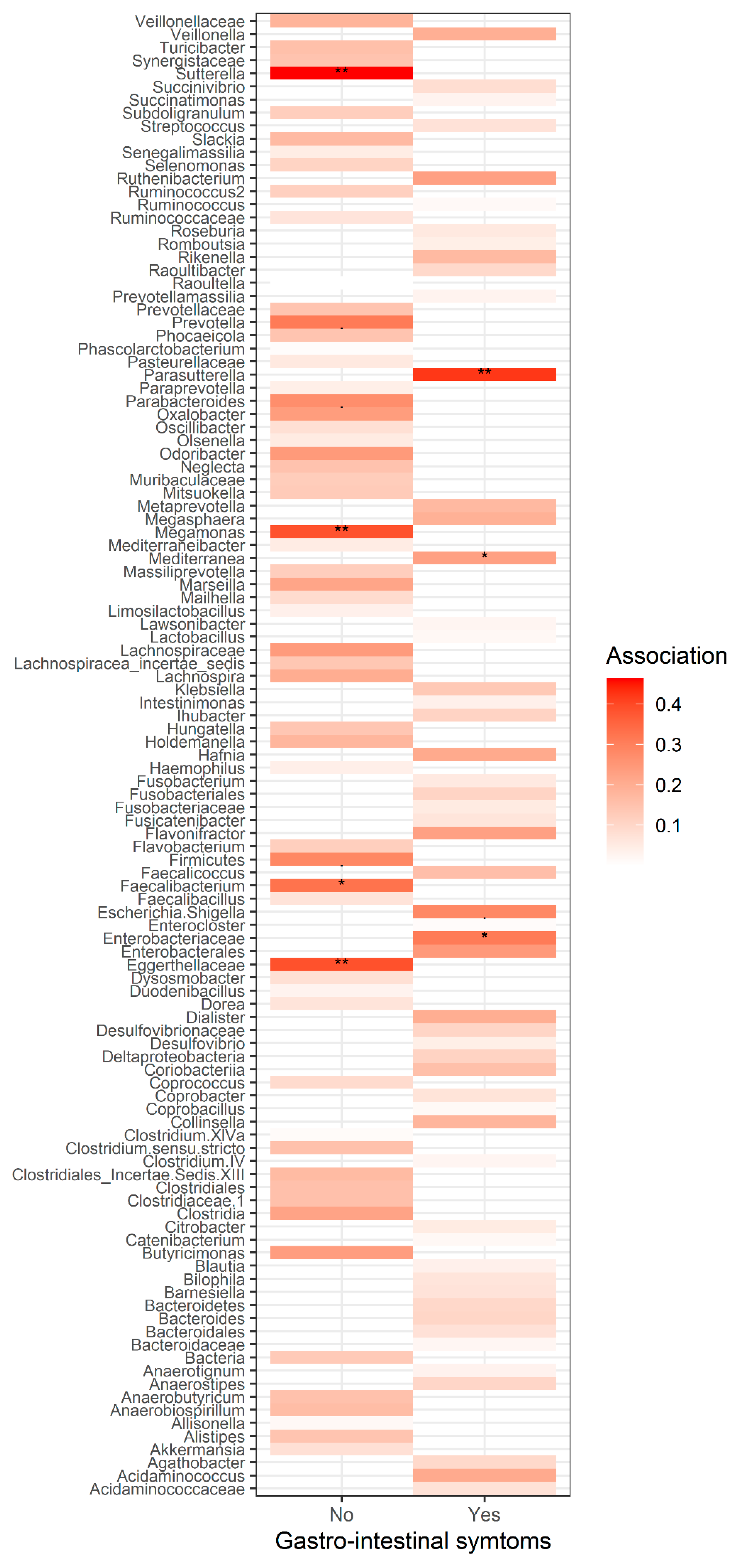
3.2. Gastrointestinal Symptoms, Endotoxaemia Parameters and Other Biochemical Results
3.3. The Analysis of Diet
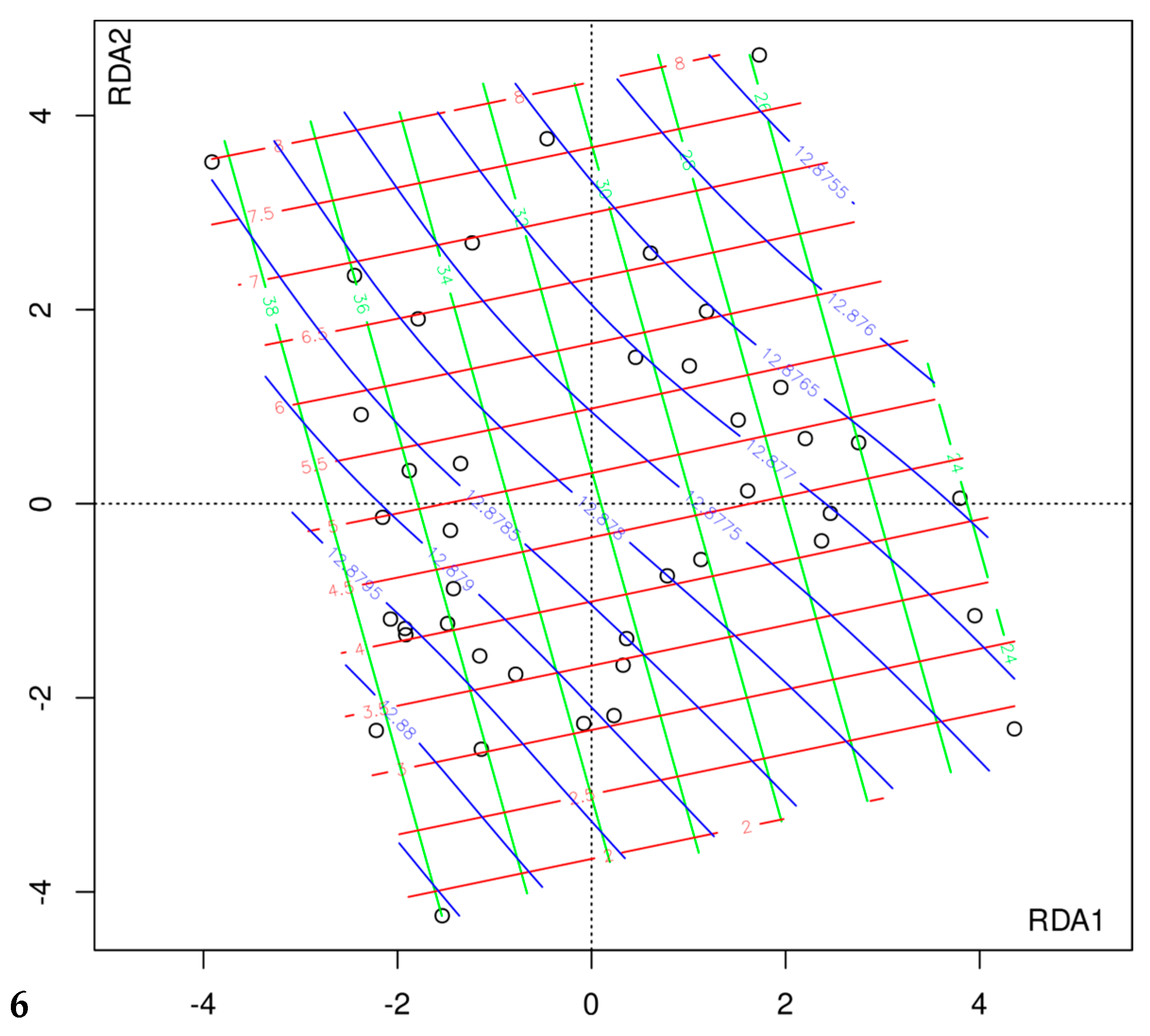
3.4. The Composition of Gut Microbiota
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Lo, T.; Tavakkoli, A. Bariatric surgery and its role in obesity pandemic. Curr. Opin. Physiol. 2019, 12, 51–56. [Google Scholar] [CrossRef]
- English, W.J.; DeMaria, E.J.; Hutter, M.M.; Kothari, S.N.; Mattar, S.G.; Brethauer, S.A.; Morton, J.M. American Society for Metabolic and Bariatric Surgery 2018 estimate of metabolic and bariatric procedures performed in the United States. Surg. Obes. Relat. Dis. 2020, 16, 457–463. [Google Scholar] [CrossRef]
- Steenackers, N.; Vanuytsel, T.; Augustijns, P.; Tack, J.; Mertens, A.; Lannoo, M.; Van der Schueren, B.; Matthys, C. Adaptations in gastrointestinal physiology after sleeve gastrectomy and Roux-en-Y gastric bypass. Lancet Gastroenterol. Hepatol. 2021, 6, 225–237. [Google Scholar] [CrossRef]
- Pucci, A.; Batterham, R.L. Mechanisms underlying the weight loss effects of RYGB and SG: Similar, yet different. J. Endocrinol. Investig. 2019, 42, 117–128. [Google Scholar] [CrossRef]
- Anhê, F.F.; Varin, T.V.; Schertzer, J.D.; Marette, A. The Gut Microbiota as a Mediator of Metabolic Benefits after Bariatric Surgery. Can. J. Diabetes 2017, 41, 439–447. [Google Scholar] [CrossRef]
- Palleja, A.; Kashani, A.; Allin, K.H.; Nielsen, T.; Zhang, C.; Li, Y.; Brach, T.; Liang, S.; Feng, Q.; Jørgensen, N.B.; et al. Roux-en-Y gastric bypass surgery of morbidly obese patients induces swift and persistent changes of the individual gut microbiota. Genome Med. 2016, 8, 67. [Google Scholar] [CrossRef]
- Davies, N.K.; O’Sullivan, J.M.; Plank, L.D.; Murphy, R. Altered gut microbiome after bariatric surgery and its association with metabolic benefits: A systematic review. Surg. Obes. Relat. Dis. 2019, 15, 656–665. [Google Scholar] [CrossRef]
- Spirou, D.; Raman, J.; Smith, E. Psychological outcomes following surgical and endoscopic bariatric procedures: A systematic review. Obes. Rev. 2020, 21, e12998. [Google Scholar] [CrossRef]
- Madison, A.; Kiecolt-Glaser, J.K. Stress, depression, diet, and the gut microbiota: Human–bacteria interactions at the core of psychoneuroimmunology and nutrition. Curr. Opin. Behav. Sci. 2019, 28, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Limbana, T.; Khan, F.; Eskander, N. Gut Microbiome and Depression: How Microbes Affect the Way We Think. Cureus 2020, 12, e9966. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.T.; Rowan-Nash, A.D.; Sheehan, A.E.; Walsh, R.F.L.; Sanzari, C.M.; Korry, B.J.; Belenky, P. Reductions in anti-inflammatory gut bacteria are associated with depression in a sample of young adults. Brain Behav. Immun. 2020, 88, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Shi, X.; Li, Z.; Shen, Y.; Shi, X.; Wang, L.; Li, G.; Yuan, Y.; Wang, J.; Zhang, Y.; et al. Possible association of Firmicutes in the gut microbiota of patients with major depressive disorder. Neuropsychiatr. Dis. Treat. 2018, 14, 3329–3337. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-T.; Lai, J.-B.; Du, Y.-L.; Xu, Y.; Ruan, L.-M.; Hu, S.-H. Current Understanding of Gut Microbiota in Mood Disorders: An Update of Human Studies. Front. Genet. 2019, 10, 98. [Google Scholar] [CrossRef]
- Wang, Y.-P.; Gorenstein, C. Assessment of depression in medical patients: A systematic review of the utility of the Beck Depression Inventory-II. Clinics 2013, 68, 1274–1287. [Google Scholar] [CrossRef]
- Kapci, E.G.; Uslu, R.; Turkcapar, H.; Karaoglan, A. Beck Depression Inventory II: Evaluation of the psychometric properties and cut-off points in a Turkish adult population. Depress. Anxiety 2008, 25, E104–E110. [Google Scholar] [CrossRef]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An Inventory for Measuring Depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef]
- Fauziana, R.; Jeyagurunathan, A.; Abdin, E.; Vaingankar, J.; Sagayadevan, V.; Shafie, S.; Sambasivam, R.; Chong, S.A.; Subramaniam, M. Body mass index, waist-hip ratio and risk of chronic medical condition in the elderly population: Results from the Well-being of the Singapore Elderly (WiSE) Study. BMC Geriatr. 2016, 16, 125. [Google Scholar] [CrossRef]
- Hamilton, M. A RATING SCALE FOR DEPRESSION. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef]
- Zimmerman, M.; Martinez, J.H.; Young, D.; Chelminski, I.; Dalrymple, K. Severity classification on the Hamilton depression rating scale. J. Affect. Disord. 2013, 150, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Sirajudeen, M.S.; Dilshad Manzar, M.; Alqahtani, M.; Alzhrani, M.; Albougami, A.; Somasekharan Pillai, P.; Spence, D.W.; Pandi-Perumal, S.R. Psychometric Properties of the Athens Insomnia Scale in Occupational Computer Users. Healthcare 2020, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Haines, P.S.; Siega-Riz, A.M.; Popkin, B.M. The Diet Quality Index-International (DQI-I) provides an effective tool for cross-national comparison of diet quality as illustrated by China and the United States. J. Nutr. 2003, 133, 3476–3484. [Google Scholar] [CrossRef]
- Masella, A.P.; Bartram, A.K.; Truszkowski, J.M.; Brown, D.G.; Neufeld, J.D. PANDAseq: Paired-end assembler for illumina sequences. BMC Bioinform. 2012, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.J.; Gross, R.; Bittinger, K.; Sherrill-Mix, S.; Lewis, J.D.; Collman, R.G.; Bushman, F.D.; Li, H. Power and sample-size estimation for microbiome studies using pairwise distances and PERMANOVA. Bioinformatics 2015, 31, 2461–2468. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Wickham, H., Ed.; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- De Caceres, M.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- Sherf Dagan, S.; Goldenshluger, A.; Globus, I.; Schweiger, C.; Kessler, Y.; Kowen Sandbank, G.; Ben-Porat, T.; Sinai, T. Nutritional Recommendations for Adult Bariatric Surgery Patients: Clinical Practice12. Adv. Nutr. 2017, 8, 382–394. [Google Scholar] [CrossRef]
- Major, P.; Matłok, M.; Pędziwiatr, M.; Migaczewski, M.; Budzyński, P.; Stanek, M.; Kisielewski, M.; Natkaniec, M.; Budzyński, A. Quality of Life After Bariatric Surgery. Obes. Surg. 2015, 25, 1703–1710. [Google Scholar] [CrossRef]
- Major, P.; Stefura, T.; Dziurowicz, B.; Radwan, J.; Wysocki, M.; Małczak, P.; Pędziwiatr, M. Quality of Life 10 Years After Bariatric Surgery. Obes. Surg. 2020, 30, 3675–3684. [Google Scholar] [CrossRef]
- Felske, A.N.; Williamson, T.M.; Scurrey, S.R.M.; Telfer, J.A.; Campbell, T.S.; Rash, J.A. The Influence of Weight-Related Self-Esteem and Symptoms of Depression on Shape and Weight Concerns and Weight-Loss 12 Months After Bariatric Surgery. Obes. Surg. 2021, 31, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Ivezaj, V.; Grilo, C.M. The Complexity of Body Image Following Bariatric Surgery: A Systematic Review of the Literature. Obes. Rev. 2018, 19, 1116–1140. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.; Kang, S.; Lee, Y.; Rosenblat, J.D.; Brietzke, E.; Zuckerman, H.; McIntyre, R.S. The long-term effect of bariatric surgery on depression and anxiety. J. Affect. Disord. 2019, 246, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.A.; Nathens, A.B.; Thiruchelvam, D.; Grantcharov, T.; Goldstein, B.I.; Redelmeier, D.A. Self-harm Emergencies After Bariatric Surgery: A Population-Based Cohort Study. JAMA Surg. 2016, 151, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Lagerros, Y.T.; Brandt, L.; Hedberg, J.; Sundbom, M.; Bodén, R. Suicide, Self-harm, and Depression After Gastric Bypass Surgery: A Nationwide Cohort Study. Ann. Surg. 2017, 265, 235–243. [Google Scholar] [CrossRef]
- Morgan, D.J.R.; Ho, K.M. Incidence and Risk Factors for Deliberate Self-harm, Mental Illness, and Suicide Following Bariatric Surgery: A State-wide Population-based Linked-data Cohort Study. Ann. Surg. 2017, 265, 244–252. [Google Scholar] [CrossRef]
- Azam, H.; Shahrestani, S.; Phan, K. Alcohol use disorders before and after bariatric surgery: A systematic review and meta-analysis. Ann. Transl. Med. 2018, 6, 148. [Google Scholar] [CrossRef]
- Lu, C.-W.; Chang, Y.-K.; Lee, Y.-H.; Kuo, C.-S.; Chang, H.-H.; Huang, C.-T.; Hsu, C.-C.; Huang, K.-C. Increased risk for major depressive disorder in severely obese patients after bariatric surgery—A 12-year nationwide cohort study. Ann. Med. 2018, 50, 605–612. [Google Scholar] [CrossRef]
- Ribeiro, G.A.N.D.A.; Giapietro, H.B.; Belarmino, L.B.; Salgado-Junior, W. Depression, anxiety, and binge eating before and after bariatric surgery: Problems that remain. Arq. Bras. Cir. Dig. 2018, 31, e1356. [Google Scholar] [CrossRef]
- Schachter, J.; Martel, J.; Lin, C.-S.; Chang, C.-J.; Wu, T.-R.; Lu, C.-C.; Ko, Y.-F.; Lai, H.-C.; Ojcius, D.M.; Young, J.D. Effects of obesity on depression: A role for inflammation and the gut microbiota. Brain Behav. Immun. 2018, 69, 1–8. [Google Scholar] [CrossRef]
- Milano, W.; Ambrosio, P.; Carizzone, F.; De Biasio, V.; Di Munzio, W.; Foia, M.G.; Capasso, A. Depression and Obesity: Analysis of Common Biomarkers. Diseases 2020, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Cheung, S.G.; Goldenthal, A.R.; Uhlemann, A.-C.; Mann, J.J.; Miller, J.M.; Sublette, M.E. Systematic Review of Gut Microbiota and Major Depression. Front. Psychiatry 2019, 10, 34. [Google Scholar] [CrossRef]
- Safadi, J.M.; Quinton, A.M.G.; Lennox, B.R.; Burnet, P.W.J.; Minichino, A. Gut dysbiosis in severe mental illness and chronic fatigue: A novel trans-diagnostic construct? A systematic review and meta-analysis. Mol. Psychiatry 2021, 27, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Skonieczna-Żydecka, K.; Grochans, E.; Maciejewska, D.; Szkup, M.; Schneider-Matyka, D.; Jurczak, A.; Łoniewski, I.; Kaczmarczyk, M.; Marlicz, W.; Czerwińska-Rogowska, M.; et al. Faecal Short Chain Fatty Acids Profile is Changed in Polish Depressive Women. Nutrients 2018, 10, 1939. [Google Scholar] [CrossRef]
- Knight, E.L.; Majd, M.; Graham-Engeland, J.E.; Smyth, J.M.; Sliwinski, M.J.; Engeland, C.G. Gender differences in the link between depressive symptoms and ex vivo inflammatory responses are associated with markers of endotoxemia. Brain Behav. Immun. Health 2020, 2, 100013. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Inohara, N.; Nuñez, G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal. Immunol. 2017, 10, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Wu, H.; Wu, S.-D.; Lu, N.; Wang, Y.-T.; Liu, H.-N.; Dong, L.; Liu, T.-T.; Shen, X.-Z. Parasutterella, in association with irritable bowel syndrome and intestinal chronic inflammation. J. Gastroenterol. Hepatol. 2018, 33, 1844–1852. [Google Scholar] [CrossRef]
- Krawczyk, B.; Wityk, P.; Gałęcka, M.; Michalik, M. The Many Faces of Enterococcus spp.—Commensal, Probiotic and Opportunistic Pathogen. Microorganisms 2021, 9, 1900. [Google Scholar] [CrossRef]
- Muñoz, M.; Guerrero-Araya, E.; Cortés-Tapia, C.; Plaza-Garrido, A.; Lawley, T.D.; Paredes-Sabja, D. Comprehensive genome analyses of Sellimonas intestinalis, a potential biomarker of homeostasis gut recovery. Microb. Genom. 2020, 6, mgen000476. [Google Scholar] [CrossRef]
- Rios-Covian, D.; González, S.; Nogacka, A.M.; Arboleya, S.; Salazar, N.; Gueimonde, M.; de los Reyes-Gavilán, C.G. An Overview on Fecal Branched Short-Chain Fatty Acids Along Human Life and as Related With Body Mass Index: Associated Dietary and Anthropometric Factors. Front. Microbiol. 2020, 11, 973. [Google Scholar] [CrossRef]
- Jaskiewicz, J.; Zhao, Y.; Hawes, J.W.; Shimomura, Y.; Crabb, D.W.; Harris, R.A. Catabolism of Isobutyrate by Colonocytes. Arch. Biochem. Biophys. 1996, 327, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.A.; Macfarlane, G.T. Enumeration of amino acid fermenting bacteria in the human large intestine: Effects of pH and starch on peptide metabolism and dissimilation of amino acids. FEMS Microbiol. Ecol. 1998, 25, 355–368. [Google Scholar] [CrossRef]
- Mahawar, K.K.; Sharples, A.J. Contribution of Malabsorption to Weight Loss After Roux-en-Y Gastric Bypass: A Systematic Review. Obes. Surg. 2017, 27, 2194–2206. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.K.; Muir, J.G.; Gibson, P.R. Review article: Insights into colonic protein fermentation, its modulation and potential health implications. Aliment. Pharmacol. Ther. 2016, 43, 181–196. [Google Scholar] [CrossRef]
- Diether, N.E.; Willing, B.P. Microbial Fermentation of Dietary Protein: An Important Factor in Diet–Microbe–Host Interaction. Microorganisms 2019, 7, 19. [Google Scholar] [CrossRef]
- Szczesniak, O.; Hestad, K.A.; Hanssen, J.F.; Rudi, K. Isovaleric acid in stool correlates with human depression. Nutr. Neurosci. 2016, 19, 279–283. [Google Scholar] [CrossRef]
- Tremaroli, V.; Karlsson, F.; Werling, M.; Ståhlman, M.; Kovatcheva-Datchary, P.; Olbers, T.; Fändriks, L.; le Roux, C.W.; Nielsen, J.; Bäckhed, F. Roux-en-Y Gastric Bypass and Vertical Banded Gastroplasty Induce Long-Term Changes on the Human Gut Microbiome Contributing to Fat Mass Regulation. Cell Metab. 2015, 22, 228–238. [Google Scholar] [CrossRef]
- Hoozemans, J.; de Brauw, M.; Nieuwdorp, M.; Gerdes, V. Gut Microbiome and Metabolites in Patients with NAFLD and after Bariatric Surgery: A Comprehensive Review. Metabolites 2021, 11, 353. [Google Scholar] [CrossRef]
- Farup, P.G.; Valeur, J. Changes in Faecal Short-Chain Fatty Acids after Weight-Loss Interventions in Subjects with Morbid Obesity. Nutrients 2020, 12, 802. [Google Scholar] [CrossRef]
- Pakiet, A.; Wilczynski, M.; Rostkowska, O.; Korczynska, J.; Jabłonska, P.; Kaska, L.; Proczko-Stepaniak, M.; Sobczak, E.; Stepnowski, P.; Magkos, F.; et al. The Effect of One Anastomosis Gastric Bypass on Branched-Chain Fatty Acid and Branched-Chain Amino Acid Metabolism in Subjects with Morbid Obesity. Obes. Surg. 2020, 30, 304–312. [Google Scholar] [CrossRef]
- Afshar, S.; Kelly, S.B.; Seymour, K.; Woodcock, S.; Werner, A.-D.; Mathers, J.C. The Effects of Bariatric Procedures on Bowel Habit. Obes. Surg. 2016, 26, 2348–2354. [Google Scholar] [CrossRef] [PubMed]
- Grosse, C.S.; Cope, V.C. Dietary Fibre Intake and Bowel Habits After Bariatric Surgery: A Structured Literature Review. Obes. Surg. 2019, 29, 2247–2254. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef] [PubMed]
- Barbarroja, N.; López-Pedrera, R.; Mayas, M.D.; García-Fuentes, E.; Garrido-Sánchez, L.; Macías-González, M.; El Bekay, R.; Vidal-Puig, A.; Tinahones, F.J. The obese healthy paradox: Is inflammation the answer? Biochem. J. 2010, 430, 141–149. [Google Scholar] [CrossRef]
- Tuomi, K.; Logomarsino, J.V. Bacterial Lipopolysaccharide, Lipopolysaccharide-Binding Protein, and Other Inflammatory Markers in Obesity and After Bariatric Surgery. Metab. Syndr. Relat. Disord. 2016, 14, 279–288. [Google Scholar] [CrossRef]
- Erridge, C.; Attina, T.; Spickett, C.M.; Webb, D.J. A high-fat meal induces low-grade endotoxemia: Evidence of a novel mechanism of postprandial inflammation. Am. J. Clin. Nutr. 2007, 86, 1286–1292. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Clemente-Postigo, M.; Queipo-Ortuño, M.I.; Murri, M.; Boto-Ordoñez, M.; Perez-Martinez, P.; Andres-Lacueva, C.; Cardona, F.; Tinahones, F.J. Endotoxin increase after fat overload is related to postprandial hypertriglyceridemia in morbidly obese patients. J. Lipid Res. 2012, 53, 973–978. [Google Scholar] [CrossRef]
- Pussinen, P.J.; Havulinna, A.S.; Lehto, M.; Sundvall, J.; Salomaa, V. Endotoxemia Is Associated With an Increased Risk of Incident Diabetes. Diabetes Care 2011, 34, 392–397. [Google Scholar] [CrossRef]
- Cani, P.D.; Possemiers, S.; Van deWiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef]
- Cani, P.D.; Osto, M.; Geurts, L.; Everard, A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes 2012, 3, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Repiso, C.; Moreno-Indias, I.; de Hollanda, A.; Martín-Núñez, G.M.; Vidal, J.; Tinahones, F.J. Gut microbiota specific signatures are related to the successful rate of bariatric surgery. Am. J. Transl. Res. 2019, 11, 942–952. [Google Scholar] [PubMed]
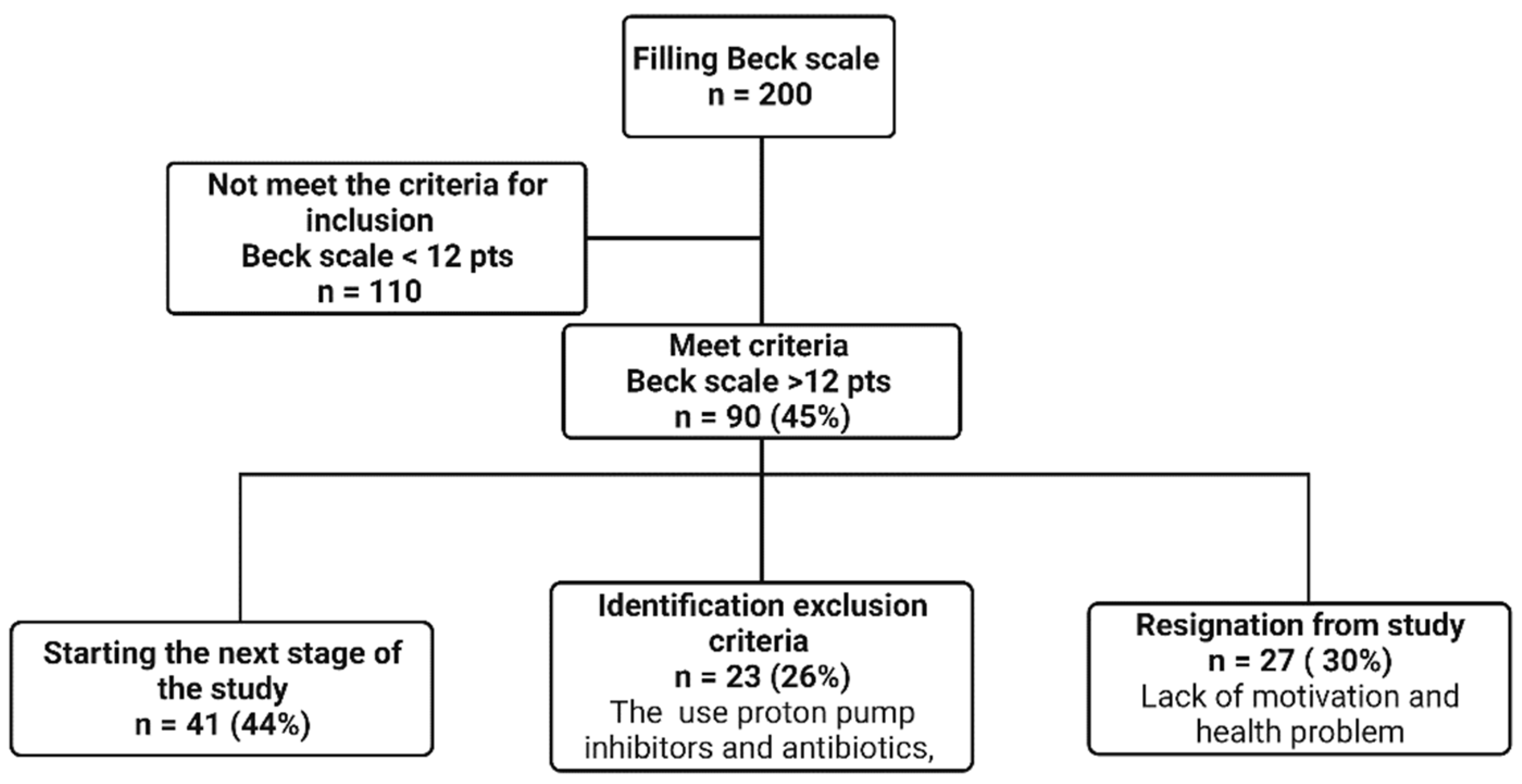
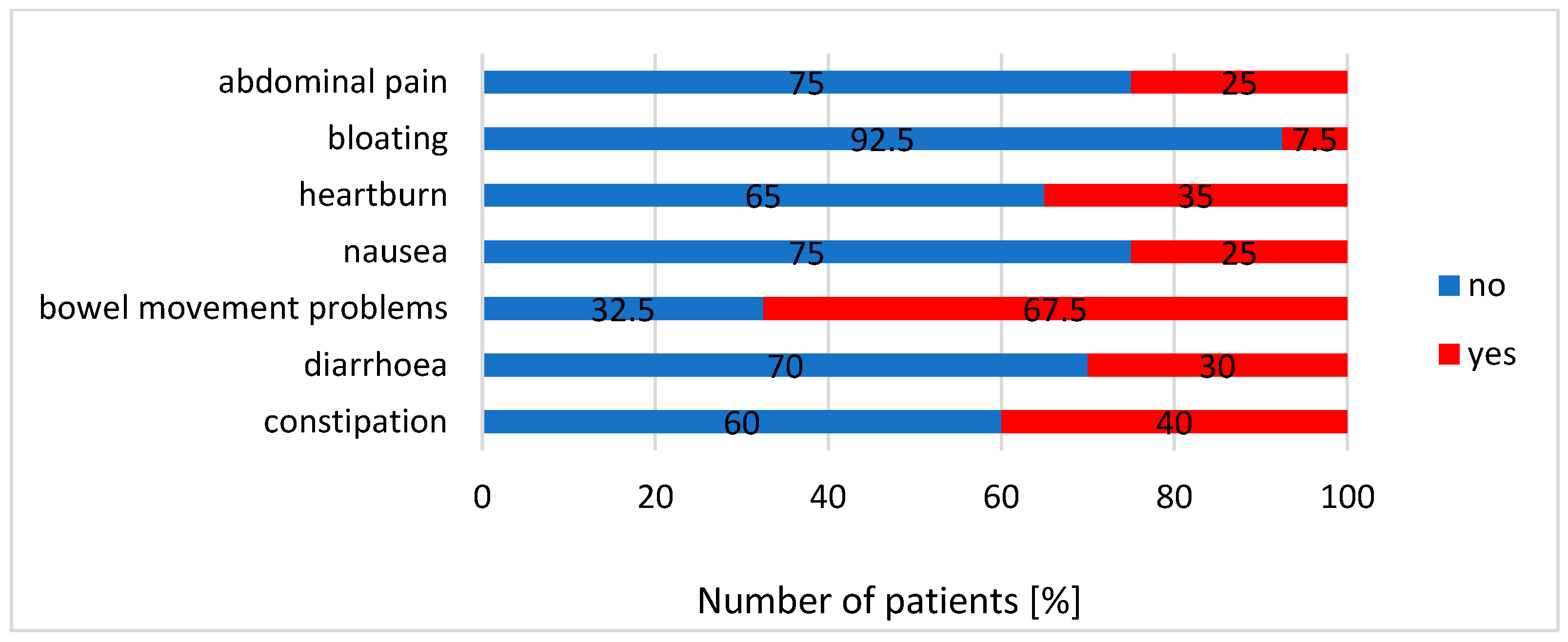
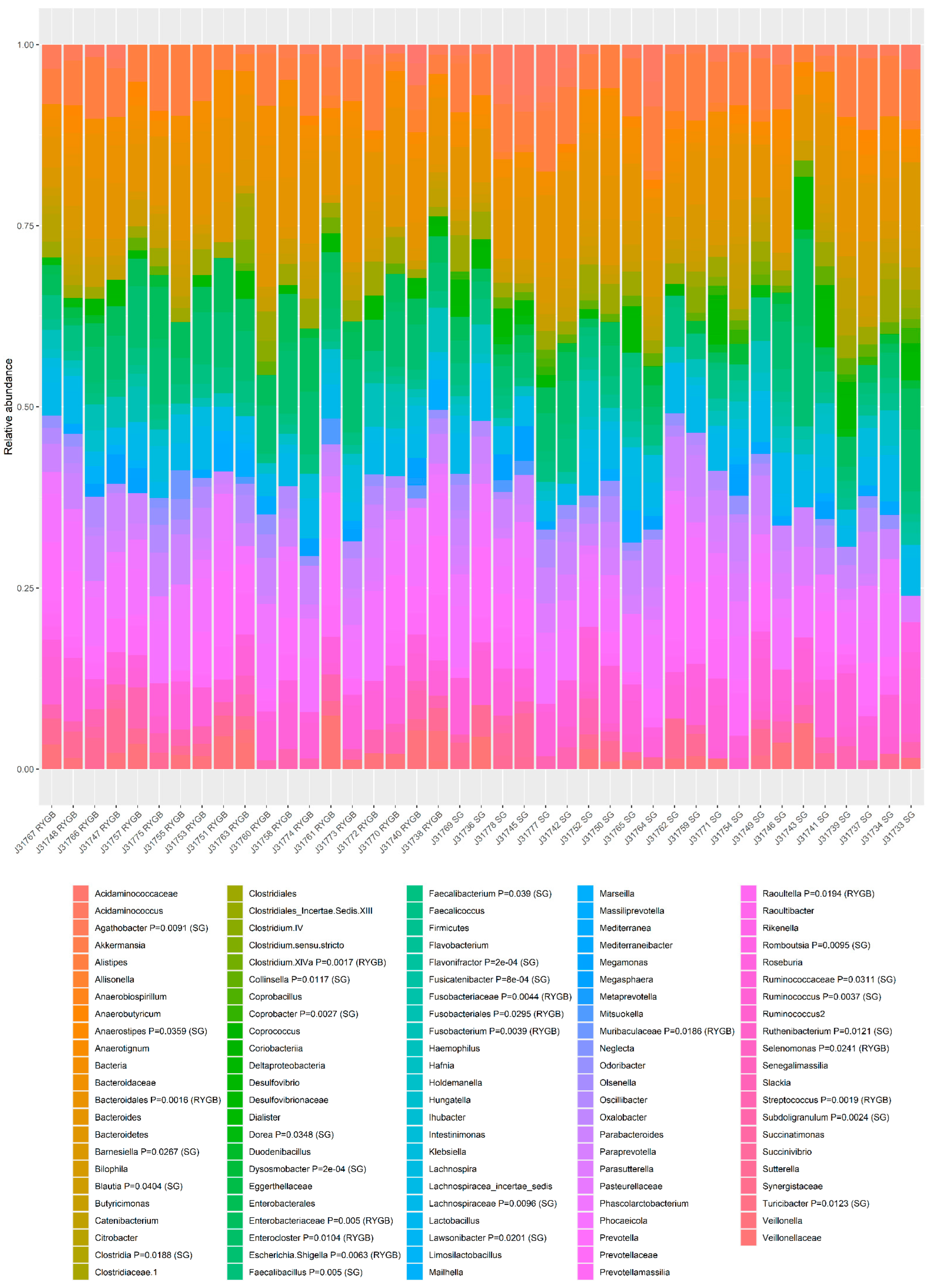
| UPN | Age | Gender | Type of Surgery | Months after Surgery | Beck’s Scale | Hamilton’s Scale | Athenian Insomnia Scale |
|---|---|---|---|---|---|---|---|
| J31767 | 47 | Woman | RYGB | 60 | 16 | 11 | 8 |
| J31748 | 47 | Woman | RYGB | 60 | 12 | 11 | 10 |
| J31766 | 59 | Woman | RYGB | 60 | 2 | 2 | 5 |
| J31747 | 59 | Woman | RYGB | 60 | 12 | 12 | 10 |
| J31757 | 60 | Woman | RYGB | 48 | 14 | NA | NA |
| J31775 | 45 | Woman | RYGB | 6 | 2 | NA | NA |
| J31755 | 45 | Woman | RYGB | 6 | 12 | NA | NA |
| J31753 | 48 | Woman | RYGB | 19 | 18 | 14 | 10 |
| J31751 | 38 | Woman | RYGB | 49 | 3 | 10 | 7 |
| J31763 | 30 | Woman | RYGB | 25 | 6 | NA | NA |
| J31760 | 54 | Woman | RYGB | 145 | 16 | 17 | 11 |
| J31758 | 38 | Woman | RYGB | 20 | 10 | 10 | 12 |
| J31774 | 52 | Woman | RYGB | 8 | 9 | 7 | 11 |
| J31761 | 48 | Woman | RYGB | 13 | 7 | 4 | 3 |
| J31773 | 48 | Woman | RYGB | 25 | 6 | 4 | 4 |
| J31772 | 41 | Woman | RYGB | 109 | 9 | NA | NA |
| J31770 | 64 | Woman | RYGB | 7 | 14 | 19 | 3 |
| J31740 | 51 | Woman | RYGB | 9 | 29 | 17 | 11 |
| J31738 | 38 | Woman | RYGB | 120 | 19 | 19 | 6 |
| J31769 | 45 | Woman | SG | 30 | 9 | 0 | 0 |
| J31736 | 45 | Woman | SG | 30 | 12 | NA | NA |
| J31778 | 36 | Woman | SG | 11 | 5 | NA | NA |
| J31745 | 36 | Woman | SG | 11 | 23 | 15 | 11 |
| J31777 | 34 | Woman | SG | 9 | 4 | 8 | 7 |
| J31742 | 34 | Woman | SG | 9 | 12 | 13 | 6 |
| J31752 | 28 | Woman | SG | 14 | 5 | 9 | 6 |
| J31750 | 55 | Woman | SG | 15 | 2 | 2 | 6 |
| J31765 | 23 | Woman | SG | 18 | 22 | 6 | 14 |
| J31764 | 48 | Woman | SG | 15 | 18 | 7 | 11 |
| J31762 | 49 | Woman | SG | 61 | 11 | 10 | 10 |
| J31759 | 44 | Woman | SG | 61 | 32 | 21 | 11 |
| J31771 | 66 | Woman | SG | 109 | 3 | NA | NA |
| J31754 | 36 | Man | SG | 24 | 12 | NA | NA |
| J31749 | 31 | Man | SG | 18 | 21 | 13 | 10 |
| J31746 | 36 | Woman | SG | 48 | 12 | 9 | 9 |
| J31743 | 28 | Woman | SG | 6 | 21 | 12 | 13 |
| J31741 | 50 | Woman | SG | 48 | 12 | NA | NA |
| J31739 | 47 | Woman | SG | 14 | 34 | 19 | 18 |
| J31737 | 35 | Man | SG | 15 | 18 | 7 | 6 |
| J31734 | 49 | Woman | SG | 12 | 12 | 9 | 13 |
| J31733 | 44 | Woman | SG | 9 | 12 | 7 | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komorniak, N.; Martynova-Van Kley, A.; Nalian, A.; Wroński, M.; Kaseja, K.; Kowalewski, B.; Kaźmierczak-Siedlecka, K.; Łoniewski, I.; Kaczmarczyk, M.; Podsiadło, K.; et al. Association between Fecal Microbiota, SCFA, Gut Integrity Markers and Depressive Symptoms in Patients Treated in the Past with Bariatric Surgery—The Cross-Sectional Study. Nutrients 2022, 14, 5372. https://doi.org/10.3390/nu14245372
Komorniak N, Martynova-Van Kley A, Nalian A, Wroński M, Kaseja K, Kowalewski B, Kaźmierczak-Siedlecka K, Łoniewski I, Kaczmarczyk M, Podsiadło K, et al. Association between Fecal Microbiota, SCFA, Gut Integrity Markers and Depressive Symptoms in Patients Treated in the Past with Bariatric Surgery—The Cross-Sectional Study. Nutrients. 2022; 14(24):5372. https://doi.org/10.3390/nu14245372
Chicago/Turabian StyleKomorniak, Natalia, Alexandra Martynova-Van Kley, Armen Nalian, Michał Wroński, Krzysztof Kaseja, Bartosz Kowalewski, Karolina Kaźmierczak-Siedlecka, Igor Łoniewski, Mariusz Kaczmarczyk, Konrad Podsiadło, and et al. 2022. "Association between Fecal Microbiota, SCFA, Gut Integrity Markers and Depressive Symptoms in Patients Treated in the Past with Bariatric Surgery—The Cross-Sectional Study" Nutrients 14, no. 24: 5372. https://doi.org/10.3390/nu14245372
APA StyleKomorniak, N., Martynova-Van Kley, A., Nalian, A., Wroński, M., Kaseja, K., Kowalewski, B., Kaźmierczak-Siedlecka, K., Łoniewski, I., Kaczmarczyk, M., Podsiadło, K., Bogdański, P., Palma, J., & Stachowska, E. (2022). Association between Fecal Microbiota, SCFA, Gut Integrity Markers and Depressive Symptoms in Patients Treated in the Past with Bariatric Surgery—The Cross-Sectional Study. Nutrients, 14(24), 5372. https://doi.org/10.3390/nu14245372