The Interaction of Polyphenols and the Gut Microbiota in Neurodegenerative Diseases
Abstract
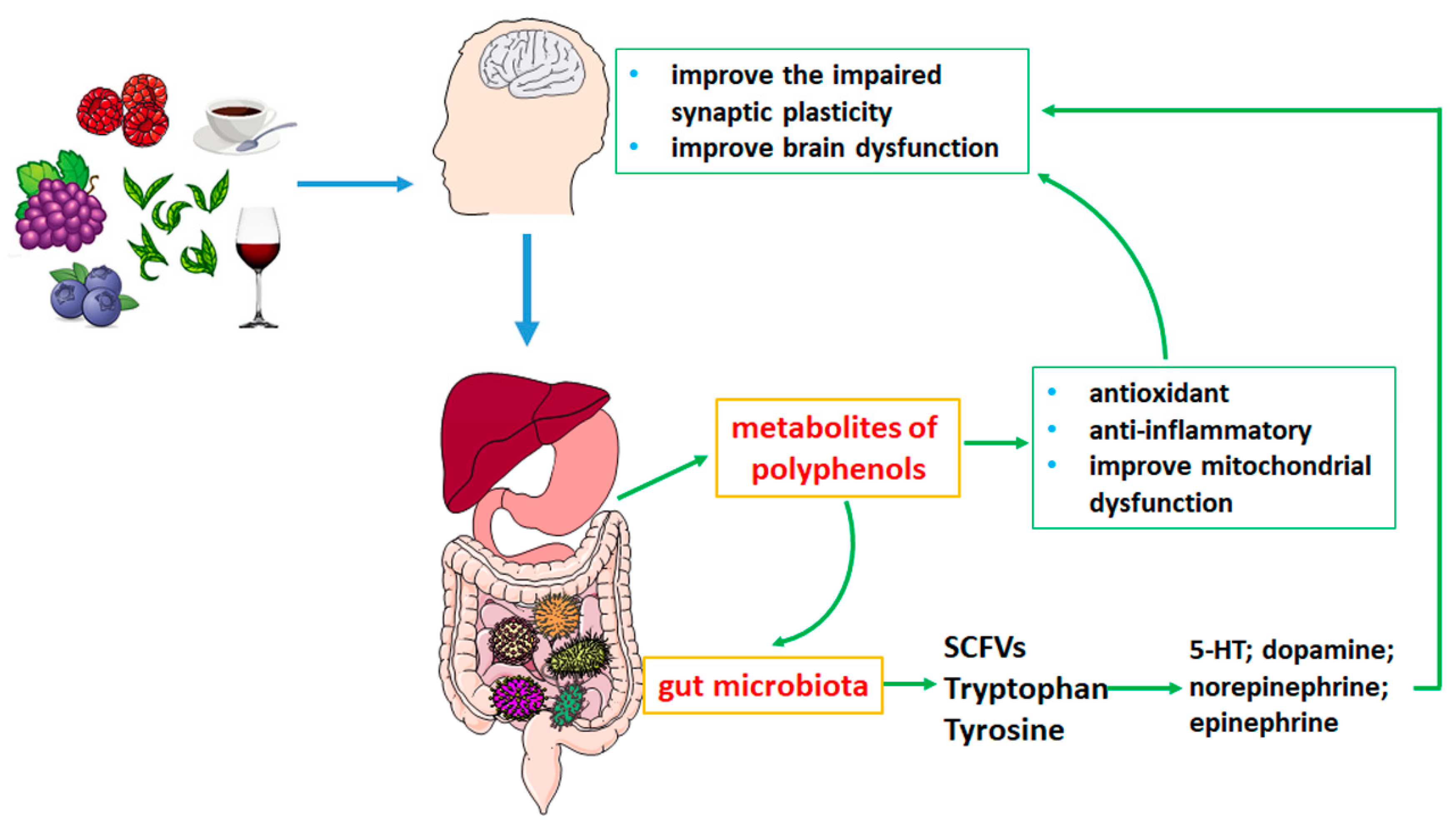
1. Introduction
2. Metabolism of Polyphenols in the Gastrointestinal Tract
3. Effects of Polyphenols on Neurodegenerative Diseases by Gut Microbiota Metabolism
3.1. Polyphenols Affect the Composition of Gut Microbiota
3.1.1. Isoorientin
3.1.2. Quercetin/Quercetin-3-O-Glucuronide
3.1.3. Fisetin
3.1.4. Anthocyanins
3.1.5. Curcumin
3.1.6. Resveratrol
| Polyphenols | Diseases | Models | Composition of Gut Microbiota | Changes of Microbial Metabolites | Functions | Reference |
|---|---|---|---|---|---|---|
| Isoorientin | AD | APP/PS1 mice | in the fecal microbiota: dominated by the class Mollicutes, family Prevotellaceae, and genus Prevotellaceae UCG 001. in the cecal microbiota: dominated by the phylum Proteobacteria (Pasteurellales: Pasteurellaceae). | —— | decreased Aβ plaque deposition in the cortex and hippocampus of AD mice; TNF-α ↓, IL-6 ↓, IL-4 ↑, IL-10 ↑; iNOS ↓, COX-2 ↓, ROS ↓ | [42] |
| Quercetin | sporadic AD | streptozotocin-induced neuropathy rats | increased Actinobacteria at phylum and class level; increased the abundance of p_Actinobacteria and c_Actinobacteria; decreased the abundance of f_Porphyromonadaceae, f_Oxalobacteraceae, g_Oxalobacter and g_Klebsiella, | —— | prevented myelin and axonal damage; ROS ↓ | [43] |
| Quercetin-3-O-Glucuronide | AD | intracerebroventricular injection of Aβ1-42 induced AD mouse model | increased the abundance of g_Barnesiella and g_Lactobacillus; decreased the abundance of g_Alistipes and g_Rikenella | SCFAs ↑ | alleviate spatial memory impairment; Aβ accumulation ↓, tau phosphorylation ↓; | [44] |
| Fisetin | PD | mouse model of PD induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) | increased the abundance of Lachnospiraceae; decreased the abundance of uncultured_bacterium_g_Escherichia-Shigella and uncultured_bacterium_g_Bacillus | —— | improve behavioral impairments, tyrosine hydroxylase ↑ | [46] |
| Anthocyanins | NDD | High-fat diets induced neuroinflammatory in mice | increased the abundance of Pseudoflavonifractor and Sporobacter | Tryptophan and kynurenic acid ↑ | attenuate neuroinflammation | [48] |
| NDD | High-fat diets induced neuroinflammatory in mice | increased the abundance of Bifidobacterium, Lactobacillus, Roseburia, Faecalibaculum, Parabacteroides and Ruminiclostridium, and decreased the abundance of Staphylococcus | SCFAs: butyrate↑ | SOD ↑, GSH-Px ↑; 5-HT ↑, dopamine ↑ | [49] | |
| Curcumin | AD | APP/PS1 mice | increased the abundance of Bacteroidaceae, Prevotellaceae and Lactobacillaceae, and decreased the abundance of Rikenellaceae at family level; increased the abundance of Parabacteroides, and decreased the abundance of Prevotella and Bacteroides decreased at genus level | —— | improved the ability of learning; accumulation of Aβ ↓ | [52,53] |
| Curcumin | PD | mouse model of PD induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) | increased the abundance of Muribaculaceae, Lactobacillaceae, Lachnospiraceae and Eggerthellaceae, and decreased the abundance of Aerococcaceae and Staphylococcaceae | tyrosine, methionine, sarcosine and creatine ↑ | improved motor deficits; glial cell activation ↓; the aggregation of a-synuclein (a-syn) ↓; dopa in the brain ↑ | [55] |
| Resveratrol functional selenium nanoparticles (Res@SeNPs) | AD | mouse model of AD induced by aluminum chloride (AlCl3) and D-galactose(D-gal) | increased the abundance of Faecalibaculum and Desulfovibrio, and reduced the abundance of Alistipes, Helicobacter and Rikenella | —— | improves cognitive dysfunction; Aβ aggregation ↓; ROS ↓, IL-10 ↑ | [62] |
3.2. Polyphenols Influence the Metabolites of Gut Microbiota in Neurodegenerative Diseases
3.2.1. SCFAs
3.2.2. Tryptophan
3.2.3. Tyrosine
4. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Renaud, J.; Martinoli, M.-G. Considerations for the Use of Polyphenols as Therapies in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 1883. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.Z.; Jalili, F.; Farhadian, N.; Joshi, T.; Wang, M.; Zou, L.; Cao, H.; Farzaei, M.H.; Xiao, J. Polyphenols and neurodegenerative diseases: Focus on neuronal regeneration. Crit. Rev. Food Sci. Nutr. 2022, 62, 3421–3436. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Torrisi, S.A.; Mogavero, M.P.; Currenti, W.; Castellano, S.; Godos, J.; Ferri, R.; Galvano, F.; Leggio, G.M.; Grosso, G.; et al. Polyphenols and neuroprotection: Therapeutic implications for cognitive decline. Pharmacol. Ther. 2022, 232, 108013. [Google Scholar] [CrossRef]
- Caracci, F.; Harary, J.; Simkovic, S.J.; Pasinetti, G.M. Grape-Derived Polyphenols Ameliorate Stress-Induced Depression by Regulating Synaptic Plasticity. J. Agric. Food Chem. 2020, 68, 1808–1815. [Google Scholar] [CrossRef]
- Kang, R.-R.; Sun, Q.; Chen, K.-G.; Cao, Q.-T.; Liu, C.; Liu, K.; Ma, Z.; Deng, Y.; Liu, W.; Xu, B. Resveratrol prevents benzo(a)pyrene-induced disruption of mitochondrial homeostasis via the AMPK signaling pathway in primary cultured neurons. Environ. Pollut. 2020, 261, 114207. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Du, C.; Kuang, S.; Zhou, X.; Zhang, J.; Ao, X. Underlying mechanisms of epithelial splicing regulatory proteins in cancer progression. J. Mol. Med. 2022, 100, 1539–1556. [Google Scholar] [CrossRef]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Mithul Aravind, S.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef]
- Sochocka, M.; Donskow-Łysoniewska, K.; Diniz, B.S.; Kurpas, D.; Brzozowska, E.; Leszek, J. The Gut Microbiome Alterations and Inflammation-Driven Pathogenesis of Alzheimer’s Disease—A Critical Review. Mol. Neurobiol. 2019, 56, 1841–1851. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Huang, S.; Li, T.; Li, N.; Han, D.; Zhang, B.; Xu, Z.Z.; Zhang, S.; Pang, J.; Wang, S.; et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome 2021, 9, 184. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, X.; Zhou, X.; Wang, J.; Ao, X. FADD as a key molecular player in cancer progression. Mol. Med. 2022, 28, 132. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ao, X.; Zhou, X.; Du, C.; Kuang, S. The regulation of PBXs and their emerging role in cancer. J. Cell. Mol. Med. 2022, 26, 1363–1379. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zhao, X.; Ma, Y.; Tang, T.; Wang, S.; Wang, L.; Huang, J. Virtual screening analysis of natural flavonoids as trimethylamine (TMA)-lyase inhibitors for coronary heart disease. J. Food Biochem. 2022, e14376. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, C.; Zhang, H.; Qu, G.; Li, C.; Liu, L. Biotransformation of Polyphenols in Apple Pomace Fermented by beta-Glucosidase-Producing Lactobacillus rhamnosus L08. Foods 2021, 10, 1343. [Google Scholar] [CrossRef]
- Sorrenti, V.; Ali, S.; Mancin, L.; Davinelli, S.; Paoli, A.; Scapagnini, G. Cocoa Polyphenols and Gut Microbiota Interplay: Bioavailability, Prebiotic Effect, and Impact on Human Health. Nutrients 2020, 12, 1908. [Google Scholar] [CrossRef] [PubMed]
- Brito Sampaio, K.; Luiz de Brito Alves, J.; Mangueira do Nascimento, Y.; Fechine Tavares, J.; Sobral da Silva, M.; Dos Santos Nascimento, D.; Dos Santos Lima, M.; Priscila de Araujo Rodrigues, N.; Fernandes Garcia, E.; Leite de Souza, E. Nutraceutical formulations combining Limosilactobacillus fermentum, quercetin, and or resveratrol with beneficial impacts on the abundance of intestinal bacterial populations, metabolite production, and antioxidant capacity during colonic fermentation. Food Res. Int. 2022, 161, 111800. [Google Scholar] [CrossRef]
- Ireson, C.R.; Jones, D.J.L.; Orr, S.; Coughtrie, M.W.H.; Boocock, D.J.; Williams, M.L.; Farmer, P.B.; Steward, W.P.; Gescher, A.J. Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2002, 11, 105–111. [Google Scholar]
- Di Meo, F.; Margarucci, S.; Galderisi, U.; Crispi, S.; Peluso, G. Curcumin, Gut Microbiota, and Neuroprotection. Nutrients 2019, 11, 2426. [Google Scholar] [CrossRef]
- Lou, Y.; Zheng, J.; Hu, H.; Lee, J.; Zeng, S. Application of ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry to identify curcumin metabolites produced by human intestinal bacteria. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 985, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Sesink, A.L.A.; Arts, I.C.W.; Faassen-Peters, M.; Hollman, P.C. Intestinal Uptake of Quercetin-3-Glucoside in Rats Involves Hydrolysis by Lactase Phlorizin Hydrolase. J. Nutr. 2003, 133, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, U.; Rubab, M.; Daliri, E.B.-M.; Chelliah, R.; Javed, A.; Oh, D.-H. Curcumin, Quercetin, Catechins and Metabolic Diseases: The Role of Gut Microbiota. Nutrients 2021, 13, 206. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Castaño, G.P.; Dorris, M.R.; Liu, X.; Bolling, B.; Acosta-Gonzalez, A.; Rey, F.E. Bacteroides thetaiotaomicron Starch Utilization Promotes Quercetin Degradation and Butyrate Production by Eubacterium ramulus. Front. Microbiol. 2019, 10, 1145. [Google Scholar] [CrossRef]
- Winter, J.; Popoff, M.R.; Grimont, P.; Bokkenheuser, V.D. Clostridium orbiscindens sp. nov., a Human Intestinal Bacterium Capable of Cleaving the Flavonoid C-Ring. Int. J. Syst. Evol. Microbiol. 1991, 41, 355–357. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Brown, N.M.; Lydeking-Olsen, E. The Clinical Importance of the Metabolite Equol—A Clue to the Effectiveness of Soy and Its Isoflavones. J. Nutr. 2002, 132, 3577–3584. [Google Scholar] [CrossRef]
- Schoefer, L.; Mohan, R.; Braune, A.; Birringer, M.; Blaut, M. Anaerobic C-ring cleavage of genistein and daidzein by Eubacterium ramulus. FEMS Microbiol. Lett. 2002, 208, 197–202. [Google Scholar] [CrossRef]
- Hur, H.G.; Beger, R.D.; Heinze, T.M.; Lay, J.O., Jr.; Freeman, J.P.; Dore, J.; Rafii, F. Isolation of an anaerobic intestinal bacterium capable of cleaving the C-ring of the isoflavonoid daidzein. Arch. Microbiol. 2002, 178, 8–12. [Google Scholar] [CrossRef]
- Wang, P.; Sang, S. Metabolism and pharmacokinetics of resveratrol and pterostilbene. BioFactors 2018, 44, 16–25. [Google Scholar] [CrossRef]
- Chaplin, A.; Carpene, C.; Mercader, J. Resveratrol, Metabolic Syndrome, and Gut Microbiota. Nutrients 2018, 10, 1651. [Google Scholar] [CrossRef]
- Fang, J. Bioavailability of anthocyanins. Drug Metab. Rev. 2014, 46, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Talavéra, S.; Felgines, C.; Texier, O.; Besson, C.; Manach, C.; Lamaison, J.-L.; Rémésy, C. Anthocyanins Are Efficiently Absorbed from the Small Intestine in Rats. J. Nutr. 2004, 134, 2275–2279. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Tan, Y.; Chen, G.; Wang, G.; Sun, J.; Ou, S.; Chen, W.; Bai, W. Metabolism of anthocyanins and consequent effects on the gut microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Jurgoński, A.; Juśkiewicz, J.; Fotschki, B.; Kołodziejczyk, K.; Milala, J.; Kosmala, M.; Grzelak-Błaszczyk, K.; Markiewicz, L. Metabolism of strawberry mono- and dimeric ellagitannins in rats fed a diet containing fructo-oligosaccharides. Eur. J. Nutr. 2017, 56, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Kiss, A.K.; Piwowarski, J.P. Ellagitannins, Gallotannins and their Metabolites—The Contribution to the Anti-Inflammatory Effect of Food Products and Medicinal Plants. Curr. Med. Chem. 2018, 25, 4946–4967. [Google Scholar] [CrossRef] [PubMed]
- García-Villalba, R.; Tomás-Barberán, F.A.; Iglesias-Aguirre, C.E.; Giménez-Bastida, J.A.; González-Sarrías, A.; Selma, M.V.; Espín, J.C. Ellagitannins, urolithins, and neuroprotection: Human evidence and the possible link to the gut microbiota. Mol. Asp. Med. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.M.; Choo, J.J.; Heber, D. Nongallated Compared with Gallated Flavan-3-ols in Green and Black Tea Are More Bioavailable. J. Nutr. 2008, 138, 1529S–1534S. [Google Scholar] [CrossRef]
- Monagas, M.; Urpi-Sarda, M.; Sánchez-Patán, F.; Llorach, R.; Garrido, I.; Gómez-Cordovés, C.; Andres-Lacueva, C.; Bartolomé, B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010, 1, 233–253. [Google Scholar] [CrossRef]
- Tao, W.; Zhang, Y.; Shen, X.; Cao, Y.; Shi, J.; Ye, X.; Chen, S. Rethinking the Mechanism of the Health Benefits of Proanthocyanidins: Absorption, Metabolism, and Interaction with Gut Microbiota. Compr. Rev. Food Sci. Food Saf. 2019, 18, 971–985. [Google Scholar] [CrossRef]
- Song, Y.; Kim, H.-D.; Lee, M.-K.; Hong, I.-H.; Won, C.-K.; Bai, H.-W.; Lee, S.S.; Lee, S.; Chung, B.Y.; Cho, J.-H. Maysin and Its Flavonoid Derivative from Centipedegrass Attenuates Amyloid Plaques by Inducting Humoral Immune Response with Th2 Skewed Cytokine Response in the Tg (APPswe, PS1dE9) Alzheimer’s Mouse Model. PLoS ONE 2017, 12, e0169509. [Google Scholar] [CrossRef]
- Tan, X.; Liang, Z.; Li, Y.; Zhi, Y.; Yi, L.; Bai, S.; Forest, K.H.; Nichols, R.A.; Dong, Y.; Li, Q.X. Isoorientin, a GSK-3β inhibitor, rescues synaptic dysfunction, spatial memory deficits and attenuates pathological progression in APP/PS1 model mice. Behav. Brain Res. 2020, 398, 112968. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tan, X.; Sun, X.; Wei, J.; Li, Q.X.; Wu, Z. Isoorientin Affects Markers of Alzheimer’s Disease via Effects on the Oral and Gut Microbiota in APP/PS1 Mice. J. Nutr. 2022, 152, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Song, W.; Liang, X.; Zhang, Q.; Shi, Y.; Liu, W.; Shi, X. Protective effect of quercetin on streptozotocin-induced diabetic peripheral neuropathy rats through modulating gut microbiota and reactive oxygen species level. Biomed. Pharmacother. 2020, 127, 110147. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Huang, H.; Mo, X.; Zhu, Y.; Chen, X.; Li, X.; Peng, X.; Xu, Z.; Chen, L.; Rong, S.; et al. Quercetin-3-O-Glucuronide Alleviates Cognitive Deficit and Toxicity in Abeta(1-42) -Induced AD-Like Mice and SH-SY5Y Cells. Mol. Nutr. Food Res. 2021, 65, e2000660. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, S.; Wang, X.; Jiang, H.; Yang, Y.; Wang, Y.; Cheng, J.; Zhang, C.; Liang, W.; Feng, H. Fisetin Exerts Antioxidant and Neuroprotective Effects in Multiple Mutant hSOD1 Models of Amyotrophic Lateral Sclerosis by Activating ERK. Neuroscience 2018, 379, 152–166. [Google Scholar] [CrossRef]
- Chen, T.-J.; Feng, Y.; Liu, T.; Wu, T.-T.; Chen, Y.-J.; Li, X.; Li, Q.; Wu, Y.-C. Fisetin Regulates Gut Microbiota and Exerts Neuroprotective Effect on Mouse Model of Parkinson’s Disease. Front. Neurosci. 2020, 14, 549037. [Google Scholar] [CrossRef]
- Marques, C.; Fernandes, I.; Meireles, M.; Faria, A.; Spencer, J.P.E.; Mateus, N.; Calhau, C. Gut microbiota modulation accounts for the neuroprotective properties of anthocyanins. Sci. Rep. 2018, 8, 11341. [Google Scholar] [CrossRef]
- Wu, H.-Q.; Pereira, E.F.R.; Bruno, J.P.; Pellicciari, R.; Albuquerque, E.X.; Schwarcz, R. The Astrocyte-Derived α7 Nicotinic Receptor Antagonist Kynurenic Acid Controls Extracellular Glutamate Levels in the Prefrontal Cortex. J. Mol. Neurosci. 2010, 40, 204–210. [Google Scholar] [CrossRef]
- Si, X.; Bi, J.; Chen, Q.; Cui, H.; Bao, Y.; Tian, J.; Shu, C.; Wang, Y.; Tan, H.; Zhang, W.; et al. Effect of Blueberry Anthocyanin-Rich Extracts on Peripheral and Hippocampal Antioxidant Defensiveness: The Analysis of the Serum Fatty Acid Species and Gut Microbiota Profile. J. Agric. Food Chem. 2021, 69, 3658–3666. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H.; Manczak, M.; Yin, X.; Grady, M.C.; Mitchell, A.; Tonk, S.; Kuruva, C.S.; Bhatti, J.S.; Kandimalla, R.; Vijayan, M.; et al. Protective Effects of Indian Spice Curcumin Against Amyloid-beta in Alzheimer’s Disease. J. Alzheimer’s Dis. JAD 2018, 61, 843–866. [Google Scholar] [CrossRef]
- Sun, Z.-Z.; Li, X.-Y.; Wang, S.; Shen, L.; Ji, H.-F. Bidirectional interactions between curcumin and gut microbiota in transgenic mice with Alzheimer’s disease. Appl. Microbiol. Biotechnol. 2020, 104, 3507–3515. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Liu, L.; Ji, H.-F. Alzheimer’s Disease Histological and Behavioral Manifestations in Transgenic Mice Correlate with Specific Gut Microbiome State. J. Alzheimer’s Dis. 2017, 56, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C.; et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 2017, 49, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Han, Y.; Li, H.; Yu, H.; Zhang, B.; Li, G. Curcumin-driven reprogramming of the gut microbiota and metabolome ameliorates motor deficits and neuroinflammation in a mouse model of Parkinson’s disease. Front. Cell. Infect. Microbiol. 2022, 12, 887407. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ho, L.; Zhao, Z.; Seror, I.; Humala, N.; Dickstein, D.L.; Thiyagarajan, M.; Percival, S.S.; Talcott, S.T.; Pasinetti, G.M. Moderate consumption of Cabernet Sauvignon attenuates Abeta neuropathology in a mouse model of Alzheimer’s disease. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2006, 20, 2313–2320. [Google Scholar]
- Frozza, R.L.; Bernardi, A.; Hoppe, J.B.; Meneghetti, A.B.; Matte, A.; Battastini, A.M.; Pohlmann, A.R.; Guterres, S.S.; Salbego, C. Neuroprotective effects of resveratrol against Abeta administration in rats are improved by lipid-core nanocapsules. Mol. Neurobiol. 2013, 47, 1066–1080. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Ahmad, A.; Ishrat, T.; Khan, M.B.; Hoda, M.N.; Khuwaja, G.; Raza, S.S.; Khan, A.; Javed, H.; Vaibhav, K.; et al. Resveratrol attenuates 6-hydroxydopamine-induced oxidative damage and dopamine depletion in rat model of Parkinson’s disease. Brain Res. 2010, 1328, 139–151. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Zhu, J.X.; Xie, W.; Le, W.; Fan, Z.; Jankovic, J.; Pan, T. Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson’s disease. Neuro-Signals 2011, 19, 163–174. [Google Scholar] [CrossRef]
- Chung, J.Y.; Jeong, J.-H.; Song, J. Resveratrol Modulates the Gut-Brain Axis: Focus on Glucagon-Like Peptide-1, 5-HT, and Gut Microbiota. Front. Aging Neurosci. 2020, 12, 588044. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Sun, J.; Xia, S.; Tang, X.; Shi, Y.; Le, G. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct. 2014, 5, 1241–1249. [Google Scholar] [CrossRef]
- Li, C.; Wang, N.; Zheng, G.; Yang, L. Oral Administration of Resveratrol-Selenium-Peptide Nanocomposites Alleviates Alzheimer’s Disease-like Pathogenesis by Inhibiting Abeta Aggregation and Regulating Gut Microbiota. ACS Appl. Mater. Interfaces 2021, 13, 46406–46420. [Google Scholar] [CrossRef] [PubMed]
- Erny, D.; Dokalis, N.; Mezö, C.; Castoldi, A.; Mossad, O.; Staszewski, O.; Frosch, M.; Villa, M.; Fuchs, V.; Mayer, A.; et al. Microbiota-derived acetate enables the metabolic fitness of the brain innate immune system during health and disease. Cell Metab. 2021, 33, 2260–2276. [Google Scholar] [CrossRef] [PubMed]
- Sears, S.M.; Hewett, S.J. Influence of glutamate and GABA transport on brain excitatory/inhibitory balance. Exp. Biol. Med. 2021, 246, 1069–1083. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Nagappan, G.; Lu, Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2014; Volume 220, pp. 223–250. [Google Scholar]
- Li, Q.; Li, L.; Niu, X.; Tang, C.; Wang, H.; Gao, J.; Hu, J. Probiotics alleviate depressive behavior in chronic unpredictable mild stress rat models by remodeling intestinal flora. NeuroReport 2021, 32, 686–693. [Google Scholar] [CrossRef]
- Lyte, M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: Microbial endocrinology in the design and use of probiotics. BioEssays News Rev. Mol. Cell. Dev. Biol. 2011, 33, 574–581. [Google Scholar] [CrossRef]
- Otaru, N.; Ye, K.; Mujezinovic, D.; Berchtold, L.; Constancias, F.; Cornejo, F.A.; Krzystek, A.; de Wouters, T.; Braegger, C.; Lacroix, C.; et al. GABA Production by Human Intestinal Bacteroides spp.: Prevalence, Regulation, and Role in Acid Stress Tolerance. Front. Microbiol. 2021, 12, 656895. [Google Scholar] [CrossRef]
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F., 3rd; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2015, 29, 1395–1403. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Shen, T.; Yue, Y.; He, T.; Huang, C.; Qu, B.; Lv, W.; Lai, H.Y. The Association Between the Gut Microbiota and Parkinson’s Disease, a Meta-Analysis. Front. Aging Neurosci. 2021, 13, 636545. [Google Scholar] [CrossRef]
- Aho, V.T.E.; Houser, M.C.; Pereira, P.A.B.; Chang, J.; Rudi, K.; Paulin, L.; Hertzberg, V.; Auvinen, P.; Tansey, M.G.; Scheperjans, F. Relationships of gut microbiota, short-chain fatty acids, inflammation, and the gut barrier in Parkinson’s disease. Mol. Neurodegener. 2021, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Marizzoni, M.; Cattaneo, A.; Mirabelli, P.; Festari, C.; Lopizzo, N.; Nicolosi, V.; Mombelli, E.; Mazzelli, M.; Luongo, D.; Naviglio, D.; et al. Short-Chain Fatty Acids and Lipopolysaccharide as Mediators Between Gut Dysbiosis and Amyloid Pathology in Alzheimer’s Disease. J. Alzheimer’s Dis. JAD 2020, 78, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 2017, 112 Pt B, 399–412. [Google Scholar] [CrossRef]
- Roth, W.; Zadeh, K.; Vekariya, R.; Ge, Y.; Mohamadzadeh, M. Tryptophan Metabolism and Gut-Brain Homeostasis. Int. J. Mol. Sci. 2021, 22, 2973. [Google Scholar] [CrossRef] [PubMed]
- Bonda, D.J.; Mailankot, M.; Stone, J.G.; Garrett, M.R.; Staniszewska, M.; Castellani, R.J.; Siedlak, S.L.; Zhu, X.; Lee, H.G.; Perry, G.; et al. Indoleamine 2,3-dioxygenase and 3-hydroxykynurenine modifications are found in the neuropathology of Alzheimer’s disease. Redox Rep. Commun. Free. Radic. Res. 2010, 15, 161–168. [Google Scholar] [CrossRef]
- Ogawa, T.; Matson, W.R.; Beal, M.F.; Myers, R.H.; Bird, E.D.; Milbury, P.; Saso, S. Kynurenine pathway abnormalities in Parkinson’s disease. Neurology 1992, 42, 1702–1706. [Google Scholar] [CrossRef]
- Conlay, L.A.; Zeisel, S.H. Neurotransmitter precursors and brain function. Neurosurgery 1982, 10, 524–529. [Google Scholar] [CrossRef]
| Polyphenols | Composition of Gut Microbiota | The Metabolites of Polyphenols | Reference |
|---|---|---|---|
| Curcumin | firmicute Blautia sp. (MRG-PMF1), Escherichia fergusonii (ATCC 35469), and two E. coli strains (ATCC 8739 and DH10B) | Demethylcurcumin, bisdemethylcurcumin, dihydrocurcumin, tetrahydrocurcumin, and ferulic acid | [20] |
| Quercetin and rutin | Eubacterium ramulus, Clostridium orbiscindens, Eubacterium oxidoreducens, Butyrovibrio spp., Bacteroides fragilis, Eubacterium ramulus, Clostridium perfringens, Bacteroides JY-6, Bifidobacterium B-9, Lactobacillus L-2, and Streptococcus S-2 | homo-procatechuic, protocatechuic, 4-hydroxybenzoic, and 3-(3-hydroxyphenyl) propionic acids | [23,24,25] |
| Daidzein and genistein | Lactobacillum, Bifidobacterium and Bacteroides; Lactococcus strains, E. faecium INIA P455 and L. paracasei INIA P461; Eggerthella sp. YY7918, Eubacterium ramulus and Clostridium sp. HGH 136 | S-equol, 2-(4-hydroxyphenyl)-propionic acid, and O-desmethylangolensin (O-DMA) | [8,27,28] |
| Resveratrol | Bifidobacteria infantis and Lactobacillus acidophilus; Slackia equolifaciens and Adlercreutzia equolifaciens | dihydroresveratrol | [30] |
| Anthocyanins | Lactobacilli and Bifidobacteria increased; Staphylococcus aureus and Salmonella typhimurium reduced; Eubacterium ramulus and Clostridium saccbarogumia | protocatechuic acid, gallic, syringic, vanillic, and p-coumaric acids | [33] |
| Ellagitannins | Gordonibacter genus and Clostridium coccoides group | urolithins (Uros) | [36] |
| Proanthocyanidins | Adlercreutzia equolifaciens JCM 14793T, Eubacterium sp. SDG-2, Eggerthella lenta rK3, Eggerthella lenta CAT-1 | (−)-epigallocatechin (EGC), (−)-gallocatechin (GC), (±)-epicatechin (EC), and (±)-catechin (C) | [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yu, W.; Zhang, L.; Wang, M.; Chang, W. The Interaction of Polyphenols and the Gut Microbiota in Neurodegenerative Diseases. Nutrients 2022, 14, 5373. https://doi.org/10.3390/nu14245373
Zhang Y, Yu W, Zhang L, Wang M, Chang W. The Interaction of Polyphenols and the Gut Microbiota in Neurodegenerative Diseases. Nutrients. 2022; 14(24):5373. https://doi.org/10.3390/nu14245373
Chicago/Turabian StyleZhang, Yuan, Wanpeng Yu, Lei Zhang, Man Wang, and Wenguang Chang. 2022. "The Interaction of Polyphenols and the Gut Microbiota in Neurodegenerative Diseases" Nutrients 14, no. 24: 5373. https://doi.org/10.3390/nu14245373
APA StyleZhang, Y., Yu, W., Zhang, L., Wang, M., & Chang, W. (2022). The Interaction of Polyphenols and the Gut Microbiota in Neurodegenerative Diseases. Nutrients, 14(24), 5373. https://doi.org/10.3390/nu14245373





