The Influence of Diet on Tinnitus Severity: Results of a Large-Scale, Online Survey
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Data Preparation
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Future Work
4.2. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Ridder, D.; Schlee, W.; Vanneste, S.; Londero, A.; Weisz, N.; Kleinjung, T.; Shekhawat, G.S.; Elgoyhen, A.B.; Song, J.J.; Andersson, G.; et al. Tinnitus and tinnitus disorder: Theoretical and operational definitions (an international multidisciplinary proposal). Prog. Brain Res. 2021, 260, 1–25. [Google Scholar] [CrossRef] [PubMed]
- McCormack, A.; Edmondson-Jones, M.; Somerset, S.; Hall, D. A systematic review of the reporting of tinnitus prevalence and severity. Hear. Res. 2016, 337, 70–79. [Google Scholar] [CrossRef]
- Biswas, R.; Lugo, A.; Akeroyd, M.A.; Schlee, W.; Gallus, S.; Hall, D.A. Tinnitus prevalence in Europe: A multi-country cross-sectional population study. Lancet Reg. Health Eur. 2022, 12, 100250. [Google Scholar] [CrossRef]
- Jarach, C.M.; Lugo, A.; Scala, M.; van den Brandt, P.A.; Cederroth, C.R.; Odone, A.; Garavello, W.; Schlee, W.; Langguth, B.; Gallus, S. Global Prevalence and Incidence of Tinnitus: A Systematic Review and Meta-analysis. JAMA Neurol. 2022, 79, 888–900. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.; El Rafaie, A. Epidemiology of Tinnitus; Singular, Thomas Learning: San Diego, CA, USA, 2000. [Google Scholar]
- Henry, J.A.; Dennis, K.C.; Schechter, M.A. General review of tinnitus. J. Speech Lang. Hear. Res. 2005, 48, 1204–1235. [Google Scholar] [CrossRef]
- Henry, J.A.; Roberts, L.E.; Caspary, D.M.; Theodoroff, S.M.; Salvi, R.J. Underlying mechanisms of tinnitus: Review and clinical implications. J. Am. Acad. Audiol. 2014, 25, 5–22. [Google Scholar] [CrossRef]
- Mulders, W.H.; Ding, D.; Salvi, R.; Robertson, D. Relationship between auditory thresholds, central spontaneous activity, and hair cell loss after acoustic trauma. J. Comp. Neurol. 2011, 519, 2637–2647. [Google Scholar] [CrossRef] [PubMed]
- Salvi, R.J.; Wang, J.; Ding, D. Auditory plasticity and hyperactivity following cochlear damage. Hear. Res. 2000, 147, 261–274. [Google Scholar] [CrossRef]
- Bhatt, J.M.; Lin, H.W.; Bhattacharyya, N. Prevalence, Severity, Exposures, and Treatment Patterns of Tinnitus in the United States. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 959–965. [Google Scholar] [CrossRef]
- Simoes, J.; Neff, P.; Schoisswohl, S.; Bulla, J.; Schecklmann, M.; Harrison, S.; Vesala, M.; Langguth, B.; Schlee, W. Toward Personalized Tinnitus Treatment: An Exploratory Study Based on Internet Crowdsensing. Front. Public Health 2019, 7, 157. [Google Scholar] [CrossRef] [PubMed]
- Luetzenberg, F.S.; Babu, S.; Seidman, M.D. Alternative Treatments of Tinnitus: Alternative Medicine. Otolaryngol. Clin. North Am. 2020, 53, 637–650. [Google Scholar] [CrossRef]
- Hall, D.A.; Mohamad, N.; Firkins, L.; Fenton, M.; Stockdale, D. Identifying and prioritizing unmet research questions for people with tinnitus: The James Lind Alliance Tinnitus Priority Setting Partnership. Clin. Investig. 2013, 3, 21–28. [Google Scholar] [CrossRef]
- Tapsell, L.C.; Neale, E.P.; Satija, A.; Hu, F.B. Foods, Nutrients, and Dietary Patterns: Interconnections and Implications for Dietary Guidelines. Adv. Nutr. (Bethesda, Md.) 2016, 7, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O. Nutrition and Chronic Conditions. Nutrients 2019, 11, 459. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.L. Back to Basics: The Effect of Healthy Diet and Exercise on Chronic Disease Management. S. D. Med. 2017, 10–18. [Google Scholar]
- Wang, L.L.; Wang, Q.; Hong, Y.; Ojo, O.; Jiang, Q.; Hou, Y.Y.; Huang, Y.H.; Wang, X.H. The Effect of Low-Carbohydrate Diet on Glycemic Control in Patients with Type 2 Diabetes Mellitus. Nutrients 2018, 10, 661. [Google Scholar] [CrossRef] [PubMed]
- World Heath Organization. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016. Available online: https://www.who.int/publications/i/item/9789241565257 (accessed on 10 October 2022).
- Miller, V.; Micha, R.; Choi, E.; Karageorgou, D.; Webb, P.; Mozaffarian, D. Evaluation of the Quality of Evidence of the Association of Foods and Nutrients With Cardiovascular Disease and Diabetes: A Systematic Review. JAMA Netw. Open 2022, 5, e2146705. [Google Scholar] [CrossRef]
- Petimar, J.; Park, Y.M.; Smith-Warner, S.A.; Fung, T.T.; Sandler, D.P. Dietary index scores and invasive breast cancer risk among women with a family history of breast cancer. Am. J. Clin. Nutr. 2019, 109, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Petimar, J.; Smith-Warner, S.A.; Fung, T.T.; Rosner, B.; Chan, A.T.; Hu, F.B.; Giovannucci, E.L.; Tabung, F.K. Recommendation-based dietary indexes and risk of colorectal cancer in the Nurses’ Health Study and Health Professionals Follow-up Study. Am. J. Clin. Nutr. 2018, 108, 1092–1103. [Google Scholar] [CrossRef]
- Puzzono, M.; Mannucci, A.; Grannò, S.; Zuppardo, R.A.; Galli, A.; Danese, S.; Cavestro, G.M. The Role of Diet and Lifestyle in Early-Onset Colorectal Cancer: A Systematic Review. Cancers 2021, 13, 5933. [Google Scholar] [CrossRef]
- Potter, J.; Brown, L.; Williams, R.L.; Byles, J.; Collins, C.E. Diet Quality and Cancer Outcomes in Adults: A Systematic Review of Epidemiological Studies. Int. J. Mol. Sci. 2016, 17, 1052. [Google Scholar] [CrossRef]
- Movassagh, E.Z.; Vatanparast, H. Current Evidence on the Association of Dietary Patterns and Bone Health: A Scoping Review. Adv. Nutr. 2017, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Melaku, Y.A.; Gill, T.K.; Adams, R.; Shi, Z. Association between dietary patterns and low bone mineral density among adults aged 50 years and above: Findings from the North West Adelaide Health Study (NWAHS). Br. J. Nutr. 2016, 116, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, K.; Maruyama, K.; Ikeda, A.; Nagao, M.; Noda, H.; Umesawa, M.; Hayama-Terada, M.; Muraki, I.; Okada, C.; Tanaka, M.; et al. Dietary fiber intake and risk of incident disabling dementia: The Circulatory Risk in Communities Study. Nutr. Neurosci. 2022, 1–8. [Google Scholar] [CrossRef]
- Frausto, D.M.; Forsyth, C.B.; Keshavarzian, A.; Voigt, R.M. Dietary Regulation of Gut-Brain Axis in Alzheimer’s Disease: Importance of Microbiota Metabolites. Front. Neurosci. 2021, 15, 736814. [Google Scholar] [CrossRef] [PubMed]
- Spankovich, C.; Le Prell, C.G. The role of diet in vulnerability to noise-induced cochlear injury and hearing loss. J. Acoust. Soc. Am. 2019, 146, 4033. [Google Scholar] [CrossRef]
- Dawes, P.; Cruickshanks, K.J.; Marsden, A.; Moore, D.R.; Munro, K.J. Relationship Between Diet, Tinnitus, and Hearing Difficulties. Ear Hear. 2020, 41, 289–299. [Google Scholar] [CrossRef]
- Huang, Q.; Jin, Y.; Reed, N.S.; Ma, Y.; Power, M.C.; Talegawkar, S.A. Diet quality and hearing loss among middle-older aged adults in the USA: Findings from National Health and Nutrition Examination Survey. Public Health Nutr. 2020, 23, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Puga, A.M.; Pajares, M.A.; Varela-Moreiras, G.; Partearroyo, T. Interplay between Nutrition and Hearing Loss: State of Art. Nutrients 2018, 11, 35. [Google Scholar] [CrossRef]
- Curhan, S.G.; Wang, M.; Eavey, R.D.; Stampfer, M.J.; Curhan, G.C. Adherence to Healthful Dietary Patterns Is Associated with Lower Risk of Hearing Loss in Women. J. Nutr. 2018, 148, 944–951. [Google Scholar] [CrossRef]
- Curhan, S.G.; Halpin, C.; Wang, M.; Eavey, R.D.; Curhan, G.C. Prospective Study of Dietary Patterns and Hearing Threshold Elevation. Am. J. Epidemiol. 2020, 189, 204–214. [Google Scholar] [CrossRef]
- Spankovich, C.; Bishop, C.; Johnson, M.F.; Elkins, A.; Su, D.; Lobarinas, E.; Le Prell, C.G. Relationship between dietary quality, tinnitus and hearing level: Data from the national health and nutrition examination survey, 1999-2002. Int. J. Audiol. 2017, 56, 716–722. [Google Scholar] [CrossRef]
- Lee, D.Y.; Kim, Y.H. Relationship Between Diet and Tinnitus: Korea National Health and Nutrition Examination Survey. Clin. Exp. Otorhinolaryngol. 2018, 11, 158–165. [Google Scholar] [CrossRef]
- Spankovich, C.; Le Prell, C.G. Healthy diets, healthy hearing: National Health and Nutrition Examination Survey, 1999–2002. Int. J. Audiol. 2013, 52, 369–376. [Google Scholar] [CrossRef]
- Spankovich, C. The role of nutrition in healthy hearing: Human evidence. In Free Radicals in ENT Pathology; Springer: Berlin, Germany, 2015; pp. 111–126. [Google Scholar]
- Tang, D.; Tran, Y.; Shekhawat, G.S.; Burlutsky, G.; Mitchell, P.; Gopinath, B. Dietary Fibre Intake and the 10-Year Incidence of Tinnitus in Older Adults. Nutrients 2021, 13, 4126. [Google Scholar] [CrossRef]
- Aljuaid, S.M.; Mirza, A.A.; Habib, L.A.; AlHarthi, L.A.; Alansari, B.M.; AlQahtani, B.G.; Althobaiti, Y.A. Does Caffeine Intake Increase the Incidence of Tinnitus? A Systematic Review. Int. Arch. Otorhinolaryngol. 2021, 25, e628–e632. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.-H.; Ma, Z.-C.; Sun, Y.-N. A Risk Assessment Approach of Hypertension Based on Mobile Crowd Sensing. J. Inf. Sci. Eng. 2020, 36, 1107–1124. [Google Scholar]
- Gardašević, G.; Katzis, K.; Bajić, D.; Berbakov, L. Emerging Wireless Sensor Networks and Internet of Things Technologies-Foundations of Smart Healthcare. Sensors 2020, 20, 3619. [Google Scholar] [CrossRef] [PubMed]
- Kraft, R.; Schlee, W.; Stach, M.; Reichert, M.; Langguth, B.; Baumeister, H.; Probst, T.; Hannemann, R.; Pryss, R. Combining Mobile Crowdsensing and Ecological Momentary Assessments in the Healthcare Domain. Front. Neurosci. 2020, 14, 164. [Google Scholar] [CrossRef] [PubMed]
- Simsek, M.; Kantarci, B. Artificial Intelligence-Empowered Mobilization of Assessments in COVID-19-like Pandemics: A Case Study for Early Flattening of the Curve. Int. J. Environ. Res. Public Health 2020, 17, 3437. [Google Scholar] [CrossRef] [PubMed]
- Pryss, R.; Schobel, J.; Hoppenstedt, B.; Spiliopoulou, M.; Langguth, B.; Probst, T.; Schlee, W.; Reichert, M.; Kurthen, I.; Giroud, N.; et al. Ecological Momentary Assessment based Differences between Android and iOS Users of the TrackYourHearing mHealth Crowdsensing Platform. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society Annual International Conference, Berlin, Germany, 23–27 July 2019; Volume 2019, pp. 3951–3955. [Google Scholar] [CrossRef]
- Vogel, C.; Schobel, J.; Schlee, W.; Engelke, M.; Pryss, R. UNITI Mobile-EMI-Apps for a Large-Scale European Study on Tinnitus. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual International Conference, Jalisco, Mexico, 1–5 November 2021; Volume 2021, pp. 2358–2362. [Google Scholar] [CrossRef]
- Probst, T.; Pryss, R.C.; Langguth, B.; Spiliopoulou, M.; Landgrebe, M.; Vesala, M.; Harrison, S.; Schobel, J.; Reichert, M.; Stach, M.; et al. Outpatient Tinnitus Clinic, Self-Help Web Platform, or Mobile Application to Recruit Tinnitus Study Samples? Front. Aging Neurosci. 2017, 9, 113. [Google Scholar] [CrossRef]
- Tang, D.; Tran, Y.; Lewis, J.R.; Bondonno, N.P.; Bondonno, C.P.; Hodgson, J.M.; Domingo, D.; McAlpine, D.; Burlutsky, G.; Mitchell, P.; et al. Associations between intake of dietary flavonoids and the 10-year incidence of tinnitus in older adults. Eur. J. Nutr. 2022, 61, 1957–1964. [Google Scholar] [CrossRef] [PubMed]
- Longadge, R.; Dongre, S. Class imbalance problem in data mining review. arXiv 2013, arXiv:1305.1707. [Google Scholar]
- Malarvizhi, R.; Thanamani, A.S. K-nearest neighbor in missing data imputation. Int. J. Eng. Res. Dev. 2012, 5, 5–7. [Google Scholar]
- Kuhn, M.; Johnson, K. Feature Engineering and Selection: A Practical Approach for Predictive Models; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic minority over-sampling technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Greenwell, B.M.; Boehmke, B.C.; Gray, B. Variable Importance Plots-An Introduction to the vip Package. R J. 2020, 12, 343. [Google Scholar] [CrossRef]
- Holm, S. A Simple Sequentially Rejective Multiple Test Procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
- Ellis, P.D. The Essential Guide to Effect Sizes: Statistical Power, Meta-Analysis, and the Interpretation of Research Results; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2018; Available online: https://www.R-project.org (accessed on 10 October 2022).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.A.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Kuhn, M.; Wickham, H. Tidymodels: A Collection of Packages for Modeling and Machine Learning Using Tidyverse Principles. Boston, MA, USA. 2020. Available online: https://tidymodels.org (accessed on 10 December 2020).
- Hofmeister, M. Do dietary factors significantly influence tinnitus? Aust. J. Gen. Pract. 2019, 48, 153–157. [Google Scholar] [CrossRef]
- Ledesma, A.L.L.; Leite Rodrigues, D.; Monteiro de Castro Silva, I.; Oliveira, C.A.; Bahmad, F., Jr. The effect of caffeine on tinnitus: Randomized triple-blind placebo-controlled clinical trial. PLoS ONE 2021, 16, e0256275. [Google Scholar] [CrossRef] [PubMed]
- Stephens, D. Detrimental effects of alcohol on tinnitus. Clin. Otolaryngol. Allied Sci. 1999, 24, 114–116. [Google Scholar] [CrossRef]
- Pugh, R.; Budd, R.J.; Stephens, S.D. Patients’ reports of the effect of alcohol on tinnitus. Br. J. Audiol. 1995, 29, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Mazzoli, M. Complementary Tinnitus Therapies. In Textbook of Tinnitus; Møller, A.R., Langguth, B., De Ridder, D., Kleinjung, T., Eds.; Springer: New York, NY, USA, 2011; pp. 733–747. [Google Scholar] [CrossRef]
- Trinidade, A.; Robinson, T.; Phillips, J.S. The role of caffeine in otorhinolaryngology: Guilty as charged? Eur. Arch. Otohinolaryngol. 2014, 271, 2097–2102. [Google Scholar] [CrossRef] [PubMed]
- Claire, L.S.; Stothart, G.; McKenna, L.; Rogers, P.J. Caffeine abstinence: An ineffective and potentially distressing tinnitus therapy. Int. J. Audiol. 2010, 49, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Hussain, K.; Murdin, L.; Schilder, A.G.M. Restriction of salt, caffeine and alcohol intake for the treatment of Ménière’s disease or syndrome. Cochrane Database Syst. Rev. 2018, 12, CD012173. [Google Scholar] [CrossRef]
- Biswas, R.; Lugo, A.; Genitsaridi, E.; Trpchevska, N.; Akeroyd, M.A.; Cederroth, C.R.; Liu, X.; Schlee, W.; Garavello, W.; Gallus, S.; et al. Chapter 1—Modifiable lifestyle-related risk factors for tinnitus in the general population: An overview of smoking, alcohol, body mass index and caffeine intake. In Progress in Brain Research; Langguth, B., Kleinjung, T., Ridder, D.D., Schlee, W., Vanneste, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 263, pp. 1–24. [Google Scholar]
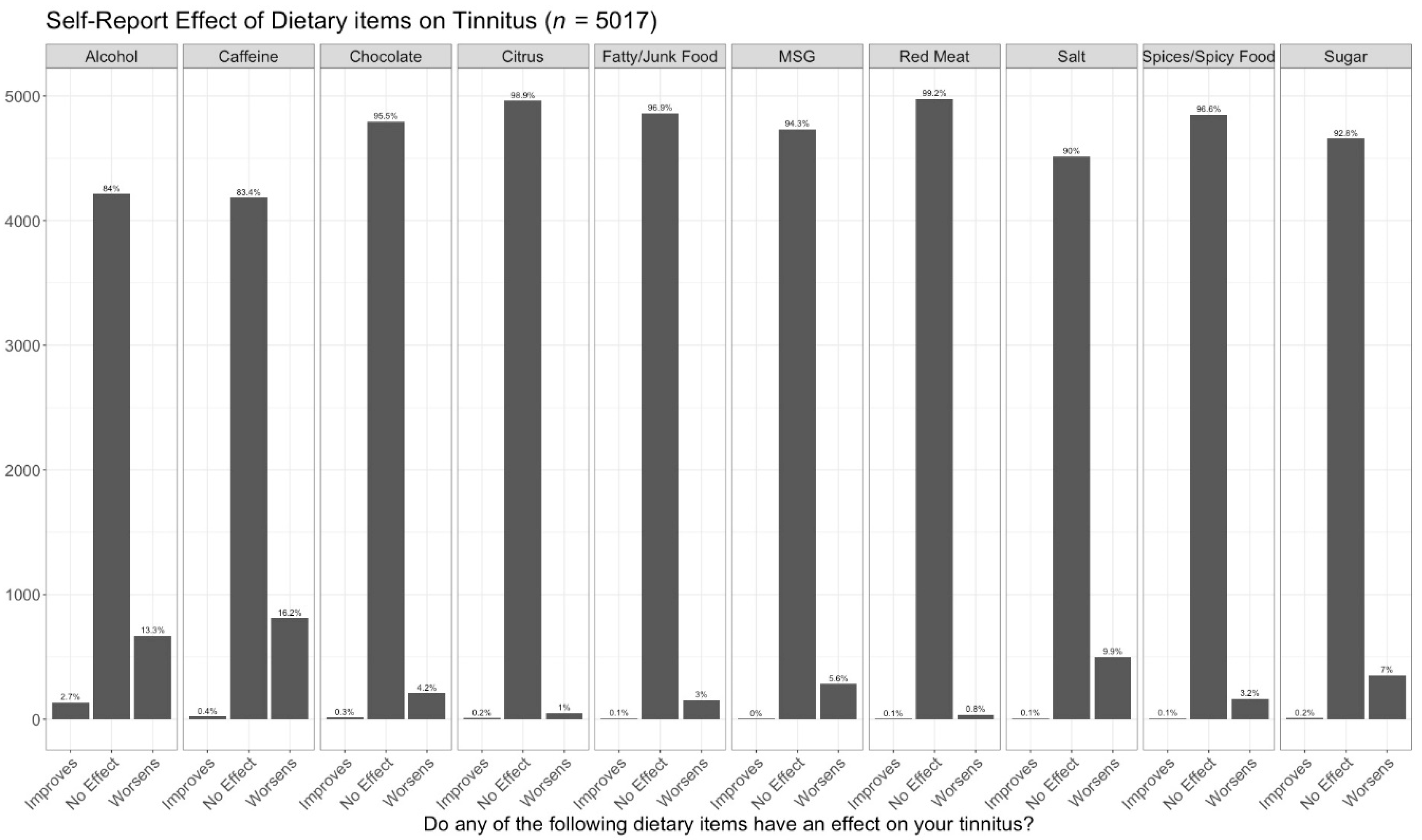
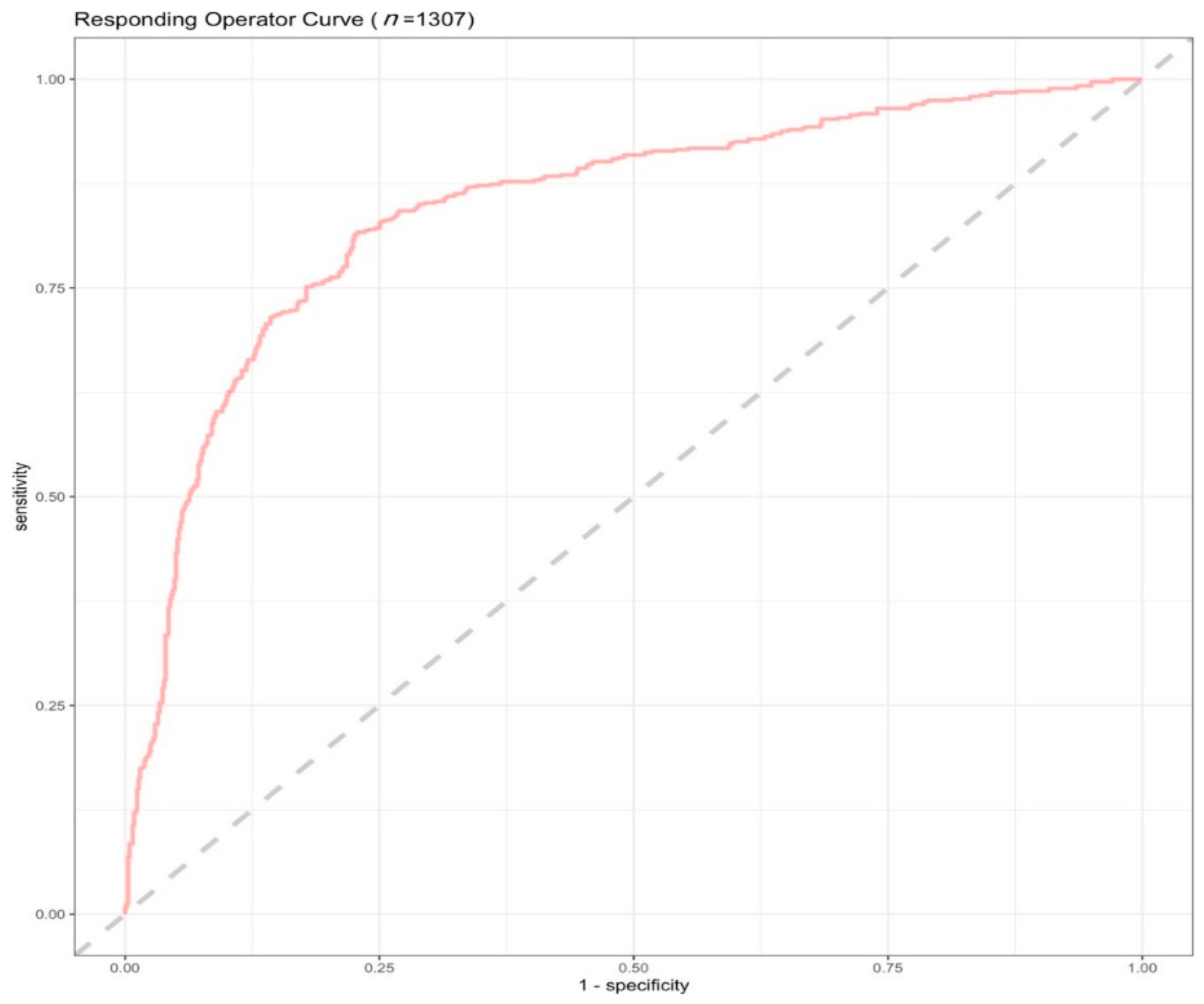
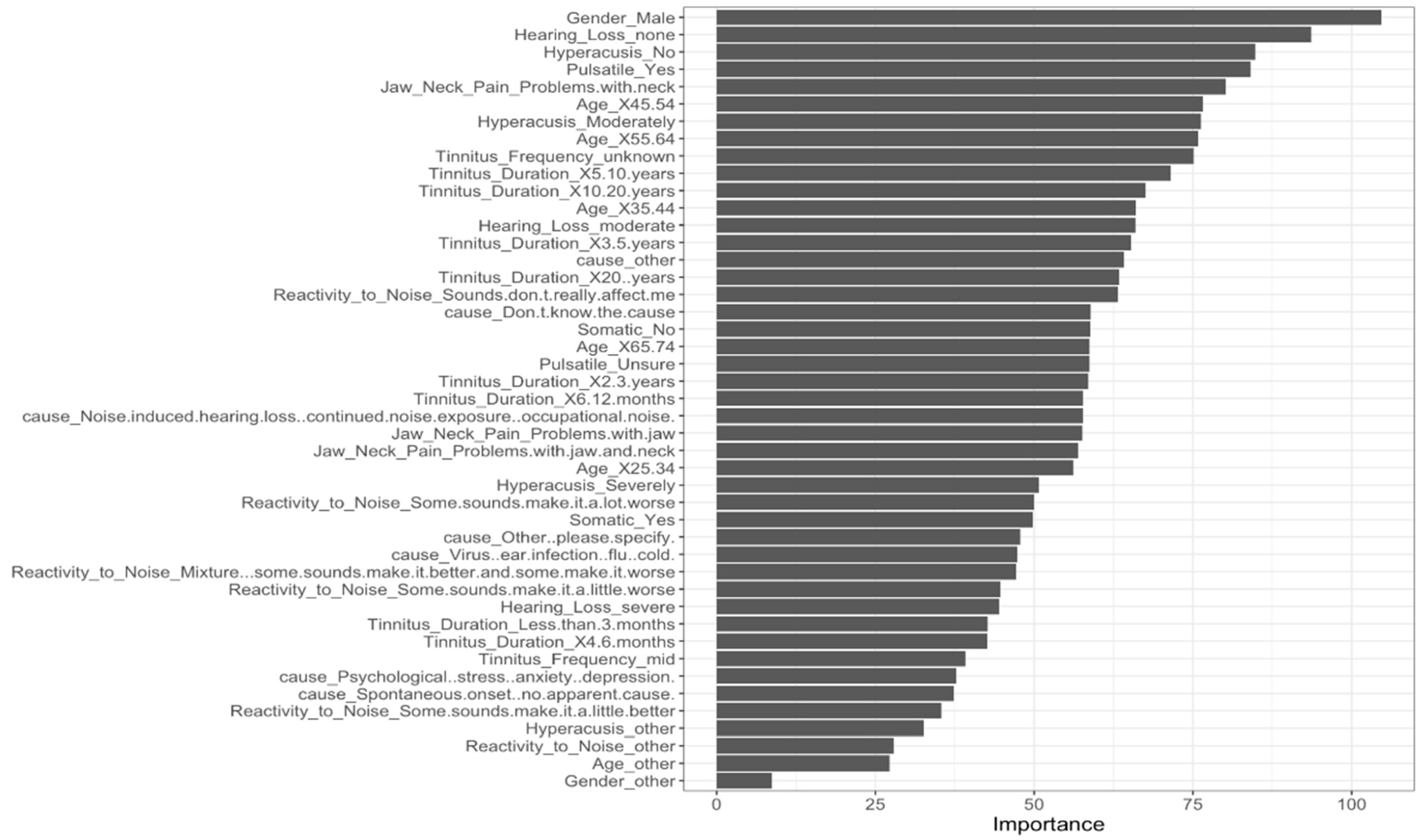
| Effect (n = 1621) | No Effect (n = 3396) | Adjusted p-Values | Effect Size | |
|---|---|---|---|---|
| Age | <0.001 | 0.09 | ||
| Under 18 years of age | 13 (0.8%) | 39 (1.1%) | ||
| 18–24 | 99 (6.1%) | 171 (5.0%) | ||
| 25–34 | 211 (13.0%) | 360 (10.6%) | ||
| 35–44 | 256 (15.8%) | 423 (12.5%) | ||
| 45–54 | 353 (21.8%) | 694 (20.4%) | ||
| 55–64 | 462 (28.5%) | 1046 (30.8%) | ||
| 65–74 | 198 (12.2%) | 558 (16.4%) | ||
| 75+ | 27 (1.7%) | 91 (2.7%) | ||
| Prefer not to say | 2 (0.1%) | 14 (0.4%) | ||
| Gender | 1 | 0.02 | ||
| Female | 688 (42.4%) | 1444 (42.5%) | ||
| Male | 925 (57.1%) | 1927 (56.7%) | ||
| Transgender | 6 (0.4%) | 11 (0.3%) | ||
| Prefer not to say | 2 (0.1%) | 14 (0.4%) | ||
| Duration of tinnitus | 0.01 | 0.07 | ||
| Less than 3 months | 81 (5.0%) | 205 (6.0%) | ||
| 4–6 months | 75 (4.6%) | 182 (5.4%) | ||
| 6–12 months | 163 (10.1%) | 288 (8.5%) | ||
| 1–2 years | 245 (15.1%) | 414 (12.2%) | ||
| 2–3 years | 183 (11.3%) | 346 (10.2%) | ||
| 3–5 years | 194 (12.0%) | 395 (11.6%) | ||
| 5–10 years | 250 (15.4%) | 571 (16.8%) | ||
| 10–20 years | 225 (13.9%) | 463 (13.6%) | ||
| 20+ years | 205 (12.6%) | 532 (15.7%) | ||
| Effect of noise on tinnitus | <0.001 | 0.18 | ||
| I don’t know | 162 (10.0%) | 490 (14.4%) | ||
| Both improve and worsen tinnitus | 437 (27.0%) | 625 (18.4%) | ||
| Some sounds make it a little better | 102 (6.3%) | 260 (7.7%) | ||
| Some sounds make it a little worse | 204 (12.6%) | 365 (10.7%) | ||
| Some sounds make it a lot better | 72 (4.4%) | 127 (3.7%) | ||
| Some sounds make it a lot worse | 400 (24.7%) | 611 (18.0%) | ||
| Sounds don’t really affect me | 244 (15.1%) | 918 (27.0%) | ||
| Hyperacusis | <0.001 | 0.10 | ||
| Don’t know | 48 (3.0%) | 152 (4.5%) | ||
| Mildly | 472 (29.1%) | 829 (24.4%) | ||
| Moderately | 430 (26.5%) | 737 (21.7%) | ||
| No | 509 (31.4%) | 1384 (40.8%) | ||
| Severely | 162 (10.0%) | 294 (8.7%) | ||
| Pulsatile | 1 | 0.02 | ||
| Yes | 299 (18.4%) | 590 (17.4%) | ||
| No | 1165 (71.9%) | 2486 (73.2%) | ||
| Unsure | 157 (9.7%) | 320 (9.4%) | ||
| Somatic tinnitus | <0.001 | 0.09 | ||
| Yes | 628 (38.7%) | 1019 (30.0%) | ||
| No | 857 (52.9%) | 2093 (61.6%) | ||
| Don’t know | 136 (8.4%) | 284 (8.4%) | ||
| Jaw/neck problems | <0.001 | 0.08 | ||
| Problems with jaw | 160 (9.9%) | 277 (8.2%) | ||
| Problems with neck | 284 (17.5%) | 601 (17.7%) | ||
| Problems with jaw and neck | 249 (15.4%) | 350 (10.3%) | ||
| None | 928 (57.2%) | 2168 (63.8%) | ||
| Hearing loss | 1 | 0.02 | ||
| Mild | 702 (43.3%) | 1437 (42.3%) | ||
| Moderate | 234 (14.4%) | 508 (15.0%) | ||
| Severe | 103 (6.4%) | 196 (5.8%) | ||
| None known of | 582 (35.9%) | 1255 (37.0%) | ||
| Tinnitus frequency | 0.001 | 0.06 | ||
| high | 300 (18.5%) | 531 (15.6%) | ||
| mid | 107 (6.6%) | 157 (4.6%) | ||
| unknown | 1214 (74.9%) | 2708 (79.7%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcrum, S.C.; Engelke, M.; Goedhart, H.; Langguth, B.; Schlee, W.; Vesala, M.; Simoes, J.P. The Influence of Diet on Tinnitus Severity: Results of a Large-Scale, Online Survey. Nutrients 2022, 14, 5356. https://doi.org/10.3390/nu14245356
Marcrum SC, Engelke M, Goedhart H, Langguth B, Schlee W, Vesala M, Simoes JP. The Influence of Diet on Tinnitus Severity: Results of a Large-Scale, Online Survey. Nutrients. 2022; 14(24):5356. https://doi.org/10.3390/nu14245356
Chicago/Turabian StyleMarcrum, Steven C., Milena Engelke, Hazel Goedhart, Berthold Langguth, Winfried Schlee, Markku Vesala, and Jorge P. Simoes. 2022. "The Influence of Diet on Tinnitus Severity: Results of a Large-Scale, Online Survey" Nutrients 14, no. 24: 5356. https://doi.org/10.3390/nu14245356
APA StyleMarcrum, S. C., Engelke, M., Goedhart, H., Langguth, B., Schlee, W., Vesala, M., & Simoes, J. P. (2022). The Influence of Diet on Tinnitus Severity: Results of a Large-Scale, Online Survey. Nutrients, 14(24), 5356. https://doi.org/10.3390/nu14245356









