Effects of Different Doses, Forms, and Frequencies of Zinc Supplementation on Biomarkers of Iron and Zinc Status among Young Children in Dhaka, Bangladesh
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Interventions
2.4. Study Procedures
2.5. Laboratory Analysis Methods
2.6. Statistical Analyses
2.7. Ethical Considerations
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wessells, K.R.; Brown, K.H. Estimating the global prevalence of zinc deficiency: Results based on zinc availability in national food supplies and the prevalence of stunting. PLoS ONE 2012, 7, e50568. [Google Scholar] [CrossRef]
- Gupta, S.; Brazier, A.K.M.; Lowe, N.M. Zinc deficiency in low- and middle-income countries: Prevalence and approaches for mitigation. J. Hum. Nutr. Diet 2020, 33, 624–643. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.H.; Peerson, J.M.; Baker, S.K.; Hess, S.Y. Preventive zinc supplementation among infants, preschoolers, and older prepubertal children. Food Nutr. Bull. 2009, 30, S12–S40. [Google Scholar] [CrossRef] [PubMed]
- Mayo-Wilson, E.; Junior, J.A.; Imdad, A.; Dean, S.; Chan, X.H.; Chan, E.S.; Jaswal, A.; Bhutta, Z.A. Zinc supplementation for preventing mortality, morbidity, and growth failure in children aged 6 months to 12 years of age. Cochrane Database Syst. Rev. 2014, 15, CD009384. [Google Scholar] [CrossRef] [PubMed]
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef]
- Pelletier, D.; DePee, S. Micronutrient powder programs: New findings and future directions for implementation science. Matern. Child. Nutr. 2019, 15, e12802. [Google Scholar] [CrossRef]
- World Health Organization. Guideline: Use of Multiple Micronutrient Powders for Home Fortification of Foods Consumed by Infants and Children 6–23 Months of Age; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- World Health Organization. WHO Guideline: Use of Multiple Micronutrient Powders for Point-Of-Use Fortification of Foods Consumed by Infants and Young Children Aged 6–23 Months and Children Aged 2–12 Years; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Schauer, C.; Zlotkin, S. Home fortification with micronutrient sprinkles—A new approach for the prevention and treatment of nutritional anemias. Paediatr. Child Health 2003, 8, 87–90. [Google Scholar] [CrossRef]
- UNICEF Supply Catalogue. S1580201: Multiple Micronutrient Powder, Sach./PAC-30. Available online: https://supply.unicef.org/s1580201.html (accessed on 2 December 2022).
- De-Regil, L.M.; Jefferds, M.E.D.; Pena-Rosas, J.P. Point-of-use fortification of foods with micronutrient powders containing iron in children of preschool and school-age. Cochrane Database Syst. Rev. 2017, 11, CD009666. [Google Scholar] [CrossRef]
- Suchdev, P.S.; Jefferds, M.E.D.; Ota, E.; da Silva Lopes, K.; De-Regil, L.M. Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age. Cochrane Database Syst. Rev. 2020, 2, CD008959. [Google Scholar] [CrossRef]
- Salam, R.A.; MacPhail, C.; Das, J.K.; Bhutta, Z.A. Effectiveness of Micronutrient Powders (MNP) in women and children. BMC Public Health 2013, 13, S22. [Google Scholar] [CrossRef]
- Paganini, D.; Zimmermann, M.B. The effects of iron fortification and supplementation on the gut microbiome and diarrhea in infants and children: A review. Am. J. Clin. Nutr. 2017, 106, 1688S–1693S. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; McDonald, C.M.; Krebs, N.F.; Westcott, J.; Rahman, A.E.; El Arifeen, S.; Ahmed, T.; King, J.C.; Black, R.E. Study Protocol for a Randomized, Double-Blind, Community-Based Efficacy Trial of Various Doses of Zinc in Micronutrient Powders or Tablets in Young Bangladeshi Children. Nutrients 2018, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Black, R.E.; Krebs, N.F.; Westcott, J.; Long, J.; Islam, K.M.; Peerson, J.M.; Sthity, R.A.; Khandaker, A.M.; Hasan, M.; et al. Different Doses, Forms, and Frequencies of Zinc Supplementation for the Prevention of Diarrhea and Promotion of Linear Growth among Young Bangladeshi Children: A Six-Arm, Randomized, Community-Based Efficacy Trial. J. Nutr. 2022, 152, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The Treatment of Diarrhoea: A Manual for Physicians and Other Senior Health Workers—4th Revision; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- World Health Organization. National Policy/Guideline on Management of Child Diarrhea Recommends Treatment with ORS, Zinc Ad Fluid. Available online: https://platform.who.int/data/maternal-newborn-child-adolescent-ageing/national-policies/mca/national-policy-guideline-on-management-of-childhood-diarrhea-recommends-treatment-with-ors-zinc-and-fluid?themeId=b2cd94e3-2cf2-496a-9525-a5665feda624 (accessed on 5 December 2022).
- World Health Organization. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Height and Body Mass Index-for-Age: Methods and Development; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- International Zinc Nutrition Consultative Group. IZiNCG Practical Tips: Collecting Blood in the Field for Assessment of Plasma or Serum Zinc Concentration. 2019. Available online: https://www.izincg.org/new-blog-1/practical-tips-blood-collection (accessed on 5 December 2022).
- Erhardt, J.G.; Estes, J.E.; Pfeiffer, C.M.; Biesalski, H.K.; Craft, N.E. Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C-reactive protein by an inexpensive, sensitive, and simple sandwich enzyme-linked immunosorbent assay technique. J. Nutr. 2004, 134, 3127–3132. [Google Scholar] [CrossRef]
- Haas, J.D.; Brownlie, T. Iron deficiency and reduced work capacity: A critical review of the research to determine a causal relationship. J. Nutr. 2001, 131, 676S–688S. [Google Scholar] [CrossRef]
- Walker, S.P.; Wachs, T.D.; Gardner, J.M.; Lozoff, B.; Wasserman, G.A.; Pollitt, E.; Carter, J.A.; International Child Development Steering, G. Child development: Risk factors for adverse outcomes in developing countries. Lancet 2007, 369, 145–157. [Google Scholar] [CrossRef]
- Aaron, G.J.; Lo, N.B.; Hess, S.Y.; Guiro, A.T.; Wade, S.; Brown, K.H. Plasma zinc concentration increases within 2 weeks in healthy Senegalese men given liquid supplemental zinc, but not zinc-fortified wheat bread. J. Nutr. 2011, 141, 1369–1374. [Google Scholar] [CrossRef][Green Version]
- Lo, N.B.; Aaron, G.J.; Hess, S.Y.; Dossou, N.I.; Guiro, A.T.; Wade, S.; Brown, K.H. Plasma zinc concentration responds to short-term zinc supplementation, but not zinc fortification, in young children in Senegal1,2. Am. J. Clin. Nutr. 2011, 93, 1348–1355. [Google Scholar] [CrossRef]
- Long, J.M.; Khandaker, A.M.; Sthity, R.A.; Westcott, J.E.; Matveev, A.; Black, R.E.; King, J.C.; Islam, K.M.; El Arifeen, S.; Ahmed, T.; et al. Exchangeable Zinc Pool Size Reflects Form of Zinc Supplementation in Young Children and Is Not Associated with Markers of Inflammation. Nutrients 2022, 14, 481. [Google Scholar] [CrossRef]
- Barffour, M.A.; Hinnouho, G.M.; Kounnavong, S.; Wessells, K.R.; Ratsavong, K.; Bounheuang, B.; Chanhthavong, B.; Sitthideth, D.; Sengnam, K.; Arnold, C.D.; et al. Effects of Daily Zinc, Daily Multiple Micronutrient Powder, or Therapeutic Zinc Supplementation for Diarrhea Prevention on Physical Growth, Anemia, and Micronutrient Status in Rural Laotian Children: A Randomized Controlled Trial. J. Pediatr. 2019, 207, 80–89e82. [Google Scholar] [CrossRef]
- Long, J.M.; Mondal, P.; Westcott, J.E.; Miller, L.V.; Islam, M.M.; Ahmed, M.; Mahfuz, M.; Ahmed, T.; Krebs, N.F. Zinc Absorption from Micronutrient Powders Is Low in Bangladeshi Toddlers at Risk of Environmental Enteric Dysfunction and May Increase Dietary Zinc Requirements. J. Nutr. 2019, 149, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Jaeggi, T.; Kortman, G.A.; Moretti, D.; Chassard, C.; Holding, P.; Dostal, A.; Boekhorst, J.; Timmerman, H.M.; Swinkels, D.W.; Tjalsma, H.; et al. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut 2015, 64, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Geros, A.S.; Simmons, A.; Drakesmith, H.; Aulicino, A.; Frost, J.N. The battle for iron in enteric infections. Immunology 2020, 161, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Fahim, S.M.; Das, S.; Sanin, K.I.; Gazi, M.A.; Mahfuz, M.; Islam, M.M.; Ahmed, T. Association of Fecal Markers of Environmental Enteric Dysfunction with Zinc and Iron Status among Children at First Two Years of Life in Bangladesh. Am. J. Trop. Med. Hyg. 2018, 99, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Lauer, J.M.; Ghosh, S.; Ausman, L.M.; Webb, P.; Bashaasha, B.; Agaba, E.; Turyashemererwa, F.M.; Tran, H.Q.; Gewirtz, A.T.; Erhardt, J.; et al. Markers of Environmental Enteric Dysfunction Are Associated with Poor Growth and Iron Status in Rural Ugandan Infants. J. Nutr. 2020, 150, 2175–2182. [Google Scholar] [CrossRef]
- Soofi, S.; Cousens, S.; Iqbal, S.P.; Akhund, T.; Khan, J.; Ahmed, I.; Zaidi, A.K.; Bhutta, Z.A. Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: A cluster-randomised trial. Lancet 2013, 382, 29–40. [Google Scholar] [CrossRef]
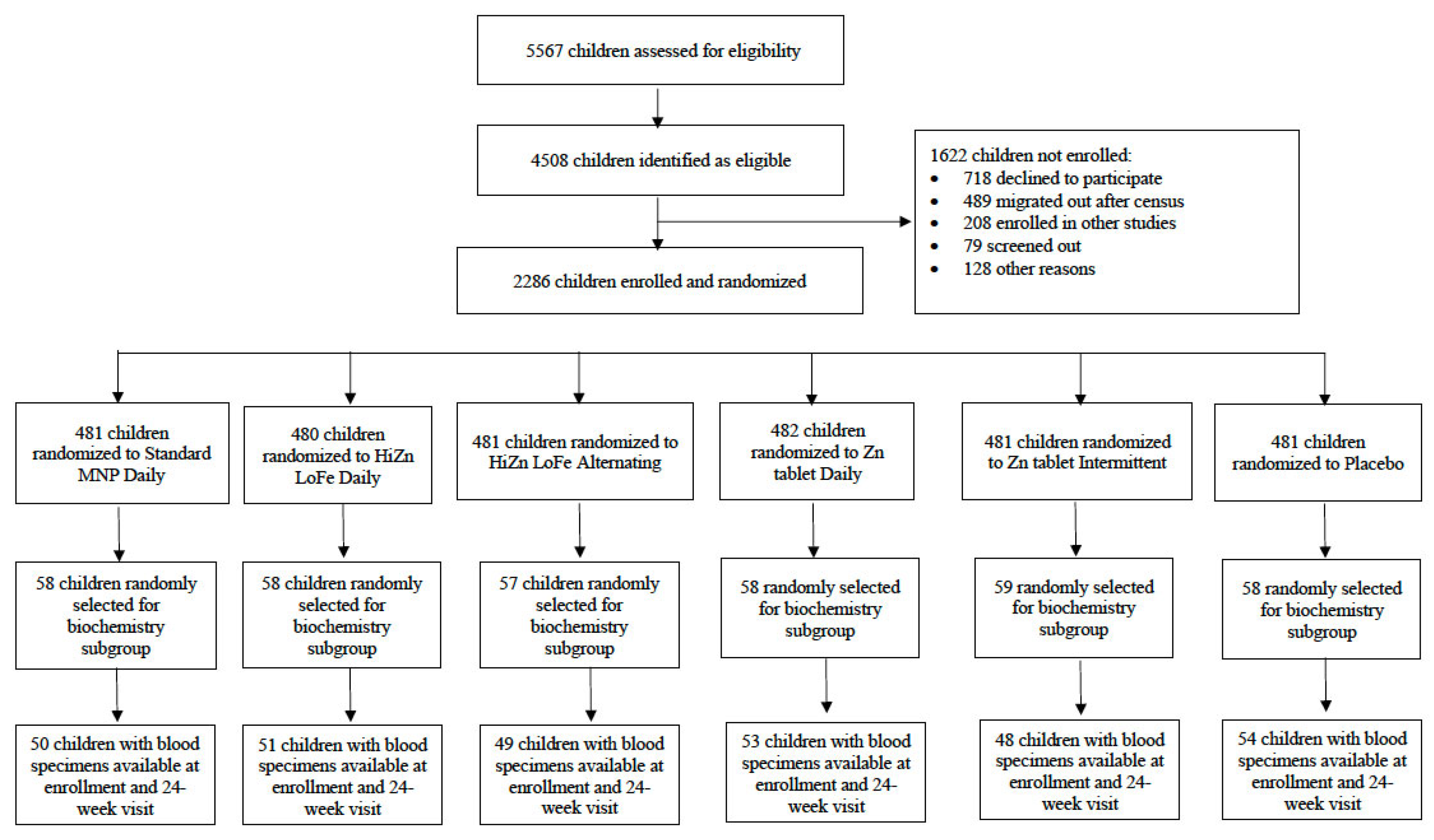
| Standard MNP | HiZn LoFe Daily (n = 58) | HiZn LoFe Alternating (n = 57) | Zn Tablet Daily (n = 58) | Zn Tablet Intermittent (n = 59) | Placebo Control (n = 58) | |
|---|---|---|---|---|---|---|
| Child characteristics | ||||||
| Age, months | 9.72 ± 0.82 | 9.83 ± 0.92 | 9.76 ± 0.83 | 9.70 ± 0.93 | 9.77 ± 0.82 | 9.86 ± 0.86 |
| Male | 29 (50.0) | 29 (50.0) | 29 (50.9) | 29 (50.0) | 29 (49.2) | 29 (50.0) |
| Breastfeeding | 58 (100) | 52 (89.7) | 54 (94.7) | 54 (93.1) | 57 (96.6) | 53 (91.4) |
| Length-for-age Z-score | −1.08 ± 1.01 | −1.43 ± 1.00 | −1.16 ± 1.19 | −1.21 ± 1.06 | −1.22 ± 1.02 | −1.45 ± 1.12 |
| Weight-for-age Z-score | −0.73 ± 1.02 | −1.39 ± 0.96 | −0.89 ± 1.07 | −0.91 ± 1.17 | −0.96 ± 0.93 | −1.29 ± 1.06 |
| Weight-for-length-Z score | −0.17 ± 1.00 | −0.80 ± 0.88 | −0.32 ± 0.85 | −0.32 ± 1.06 | −0.39 ± 0.86 | −0.64 ± 0.92 |
| MUAC, cm | 14.1 ± 0.96 | 13.6 ± 0.83 | 14.0 ± 0.94 | 14.1 ± 1.13 | 14.1 ± 0.90 | 13.7 ± 0.93 |
| Child healthy at enrollment | 14 (24.1) | 15 (25.9) | 21 (36.8) | 11 (19.0) | 22 (37.3) | 15 (25.9) |
| Maternal characteristics | ||||||
| Age, years | 23.2 ± 5.35 | 24.8 ± 4.44 | 25.2 ± 6.17 | 25.4 ± 5.06 | 24.9 ± 5.39 | 24.4 ± 4.96 |
| Currently married | 58 (100) | 58 (100) | 57 (100) | 58 (100) | 59 (100) | 58 (100) |
| Occupation | ||||||
| Housewife | 55 (94.8) | 56 (96.6) | 54 (94.7) | 55 (94.8) | 53 (89.8) | 54 (93.1) |
| Work outside home | 2 (3.4) | 2 (3.4) | 3 (5.3) | 2 (3.4) | 6 (10.2) | 4 (6.9) |
| Work from home | 1 (1.7) | 0 (0) | 0 (0) | 1 (1.7) | 0 (0) | 0 (0) |
| Years of education completed | 6.83 ± 4.34 | 5.76 ± 3.81 | 6.60 ± 4.44 | 5.62 ± 3.65 | 7.07 ± 4.01 | 6.40 ± 3.80 |
| Number of live births in lifetime | 1.72 ± 0.83 | 1.91 ± 0.88 | 1.88 ± 1.10 | 2.07 ± 1.06 | 1.63 ± 0.79 | 1.86 ± 1.08 |
| Socioeconomic characteristics | ||||||
| Number of people who sleep in the household | 4.47 ± 2.50 | 4.83 ± 1.68 | 4.58 ± 1.99 | 4.67 ± 1.77 | 4.42 ± 1.62 | 4.72 ± 1.70 |
| Main source of drinking water | ||||||
| Piped into dwelling | 52 (89.7) | 1 (1.7) | 47 (82.5) | 3 (5.2) | 3 (5.2) | |
| Piped to yard/plot | 4 (6.9) | 33 (56.9) | 9 (15.8) | 33 (56.9) | 52 (88.1) | 36 (62.1) |
| Public tap/stand pipe | 1 (1.7) | 18 (31.0) | 0 (0) | 19 (32.8) | 7 (11.9) | 15 (25.9) |
| Tube well or borehole | 1 (1.7) | 4 (6.9) | 1 (1.8) | 3 (5.2) | 0 (0) | 3 (5.2) |
| Other | 0 (0) | 2 (3.4) | 0 (0) | 0 (0) | 0 (0) | 1 (1.7) |
| Flush toilet use | 57 (98.3) | 50 (86.2) | 57 (100) | 55 (94.8) | 59 (100) | 50 (86.2) |
| Protected toilet use 3 | 7 (12.1) | 5 (8.6) | 8 (14.0) | 4 (6.9) | 7 (11.9) | 3 (5.2) |
| Hygiene score 4 | 12.4 ± 2.38 | 12.9 ± 2.33 | 12.7 ± 2.29 | 12.9 ± 2.55 | 12.7 ± 2.19 | 12.5 ± 2.42 |
| Asset score 5 | 5.02 ± 2.21 | 5.48 ± 2.09 | 5.49 ± 2.77 | 5.14 ± 2.38 | 5.59 ± 2.45 | 5.16 ± 2.38 |
| Average monthly income for the entire household, BDT 6 | 18,078 ± 8443 | 13,853 ± 8325 | 22,000 ± 11,720 | 16,621 ± 12,421 | 18,864 ± 11,264 | 15,388 ± 9618 |
| HFIAS classification 7 | ||||||
| Food-secure | 44 (75.9) | 46 (79.3) | 46 (80.7) | 47 (81.0) | 41 (69.5) | 53 (91.4) |
| Mildly food-insecure | 3 (5.2) | 2 (3.4) | 3 (5.3) | 2 (3.4) | 7 (11.9) | 0 (0) |
| Moderately food-insecure | 6 (10.3) | 5 (8.6) | 1 (1.8) | 4 (6.9) | 3 (5.1) | 3 (5.2) |
| Severely food-insecure | 5 (8.6) | 5 (8.6) | 7 (12.3) | 5 (8.6) | 8 (13.6) | 2 (3.4) |
| Standard MNP | HiZn LoFe Daily | HiZn LoFe Alternating | Zn Tablet Daily | Zn Tablet Intermittent | Placebo Control | p Value | |
|---|---|---|---|---|---|---|---|
| Biomarker at enrollment | n = 58 | n = 58 | n = 57 | n = 58 | n = 59 | n = 57 | |
| Serum zinc, ug/dL 3 | 70.0 ± 9.97 | 70.5 ± 10.09 | 67.8 ± 12.2 | 66.7 ± 12.8 | 70.2 ± 12.0 | 67.8 ± 14.4 | 0.40 |
| Serum zinc < 65 ug/dL 3 | 15 (25.9) | 17 (29.3) | 24 (42.9) | 28 (48.3) | 15 (25.4) | 24 (42.1) | 0.03 |
| Hemoglobin, g/dL 4 | 10.4 ± 1.16 | 10.3 ± 1.00 | 10.3 ± 1.12 | 10.3 ± 1.04 | 10.4 ± 1.19 | 10.3 ± 1.05 | 0.48 |
| Hemoglobin < 11 g/dL 4 | 334 (69.4) | 345 (71.9) | 333 (69.2) | 357 (74.1) | 322 (66.9) | 347 (72.1) | 0.18 |
| Adjusted ferritin, ug/L 5 | 6.26 ± 7.02 b | 10.9 ± 9.91 a | 6.05 ± 5.47 b | 9.87 ± 9.65 a,b | 7.69 ± 9.26 a,b | 9.15 ± 9.08 a,b | 0.007 |
| Adjusted ferritin < 12 ug/L 5 | 38 (65.5) | 29 (50.0) | 43 (75.4) | 34 (58.6) | 35 (59.3) | 31 (54.4) | 0.10 |
| sTfR, mg/L 6 | 12.2 ± 5.31 a,b | 9.71 ± 3.49 b | 10.4 ± 4.66 a,b | 11.1 ± 5.00 a,b | 10.5 ± 4.95 a,b | 12.4 ± 6.43 a | 0.03 |
| sTfR 6 > 8.3 mg/L 6 | 45 (77.6) | 38 (65.5) | 33 (57.9) | 40 (69.0) | 33 (55.9) | 41 (71.9) | 0.12 |
| Adjusted BIS, mg/kg body weight 6,7 | −3.69 ± 5.15 b | −0.93 ± 3.98 a | −3.24 ± 4.23 a,b | −1.69 ± 4.44 a,b | −2.38 ± 5.54 a,b | −2.33 ± 4.63 a,b | 0.02 |
| Adjusted BIS < 0 6,7 | 43 (74.1) | 30 (51.7) | 42 (73.7) | 34 (58.6) | 36 (61.0) | 34 (59.6) | 0.09 |
| Iron deficiency anemia | 34 (58.6) | 25 (43.1) | 30 (52.6) | 32 (55.2) | 26 (44.1) | 24 (42.1) | 0.33 |
| RBP, umol/L 6 | 1.08 ± 0.22 a,b | 0.98 ± 0.23 b | 1.14 ± 0.23 a | 0.96 ± 0.30 b | 1.12 ± 0.25 a | 0.95 ± 0.28 b | <0.0001 |
| RBP < 0.81 umol/L 6 | 4 (6.9) a,b | 10 (17.2) a,b | 2 (3.5) a | 14 (24.1) a,b | 4 (6.8) a,b | 16 (28.1) b | 0.001 |
| CRP, mg/L 6 | 0.23 ± 0.46 | 0.23 ± 0.41 | 0.22 ± 0.38 | 0.32 ± 0.63 | 0.14 ± 0.25 | 0.21 ± 0.43 | 0.31 |
| CRP> 5 mg/L 6 | 5 (8.6) | 4 (6.9) | 2 (3.5) | 5 (8.6) | 2 (3.4) | 6 (10.5) | 0.61 |
| AGP, g/L 6 | 0.68 ± 0.30 | 0.62 ± 0.33 | 0.66 ± 0.30 | 0.67 ± 0.38 | 0.614 ± 0.27 | 0.604 ± 0.33 | 0.66 |
| AGP > 1 g/L 6 | 9 (15.5) | 13 (22.4) | 11 (19.3) | 14 (24.1) | 7 (11.9) | 9 (15.8) | 0.53 |
| Biomarker at 24 week visit | n = 50 | n = 51 | n = 49 | n = 53 | n = 48 | n = 54 | |
| Serum zinc, ug/dL 7 | 71.2 ± 2.38 c,d | 80.7 ± 2.56 b,c | 81.3 ± 2.60 a,b | 92.5 ± 2.86 a | 66.7 ± 2.28 d | 73.7 ± 2.22 b–d | <0.0001 |
| Serum zinc < 65 ug/dL | 9 (18.0) a,b | 6 (12.2) a,b | 7 (14.3) a,b | 3 (5.8) a | 17 (35.4) b | 18 (32.7) b | 0.0003 |
| Hemoglobin, g/dL 3 | 11.0 ± 0.05 a | 10.7 ± 0.05 b | 10.7 ± 0.05 b | 10.2 ± 0.05 c | 10.21 ± 0.0 c | 10.2 ± 0.05 c | <0.0001 |
| Hemoglobin < 11 g/dL | 203 (45.9) a | 247 (54.8) a,b | 264 (60.4) b | 324 (73.8) c | 303 (69.5) c | 340 (75.2) c | <0.0001 |
| Adjusted ferritin, ug/L 4 | 14.4 ± 2.01 a | 10.8 ± 1.42 a | 10.9 ± 1.44 a | 5.10 ± 0.65 b | 5.09 ± 0.73 b | 5.68 ± 0.72 b | <0.0001 |
| Adjusted ferritin < 12 ug/L | 23 (46.0) a | 22 (43.1) a | 23 (46.9) a,b | 42 (79.2) c | 36 (75.0) b,c | 46 (83.6) c | <0.0001 |
| sTfR6, mg/L | 7.72 ± 0.2 d | 10.0 ± 0.56 b–d | 9.95 ± 0.56 c,d | 12.9 ± 0.62 a | 12.3 ± 0.68 a,b | 12.2 ± 0.60 a–c | <0.0001 |
| sTfR6 > 8.3 mg/L | 19 (38.0) a | 28 (54.9) a | 31 (63.3) a–c | 45 (84.9) c | 28 (58.3) a,b | 45 (81.8) b,c | <0.0001 |
| Adjusted BIS, mg/kg body weight 6,7 | 0.78 ± 0.60 a | −0.95 ± 0.56 a | −0.87 ± 0.57 a | −4.73 ± 0.55 b | −4.40 ± 0.61 b | −4.04 ± 0.54 b | <0.0001 |
| Adjusted BIS < 0 mg/kg body weight 6,7 | 20 (40.0) a | 24 (47.1) a | 27 (55.1) a | 44 (83.0) b | 33 (68.8) a,b | 47 (85.5) b | <0.0001 |
| Iron deficiency anemia | 14 (28.0) a | 17 (33.3) a,b | 15 (30.6) a–c | 32 (61.5) a–c | 26 (54.2) b,c | 39 (70.9) c | <0.0001 |
| RBP, umol/L 6 | 0.93 ± 0.04 a,b | 1.09 ± 0.04 a | 0.91 ± 0.04 a,b | 0.97 ± 0.04 a,b | 0.84 ± 0.04 b | 1.02 ± 0.04 a,b | 0.008 |
| RBP < 0.81 umol/L 6 | 8 (16.0) | 4 (7.8) | 8 (16.3) | 8 (15.1) | 12 (25.0) | 6 (10.9) | 0.20 |
| CRP, mg/L 6 | 0.37 ± 0.15 | 0.17 ± 0.06 | 0.60 ± 0.23 | 0.17 ± 0.06 | 0.46 ± 0.19 | 0.32 ± 0.12 | 0.28 |
| CRP > 5 mg/L 6 | 6 (12.0) | 7 (13.7) | 8 (16.3) | 8 (15.1) | 9 (18.8) | 10 (18.2) | 0.94 |
| AGP, g/L 6 | 0.60 ± 0.06 b | 0.69 ± 0.06 a,b | 0.83 ± 0.07 a | 0.64 ± 0.05 a,b | 0.67 ± 0.06 a,b | 0.76 ± 0.06 a,b | 0.03 |
| AGP > 1 g/L 6 | 10 (20.0) a,b | 11 (21.6) a,b | 20 (40.8) b | 7 (13.2) a | 15 (31.3) a,b | 13 (23.6) a,b | 0.04 |
| Variable | Effect Modifier (X) | Standard MNP | HiZn LoFe Daily | HiZn LoFe Alternating | Zn Tablet Daily | Zn Tablet Intermittent | Placebo Control | Group by X p-Value | Group within X p-Value |
|---|---|---|---|---|---|---|---|---|---|
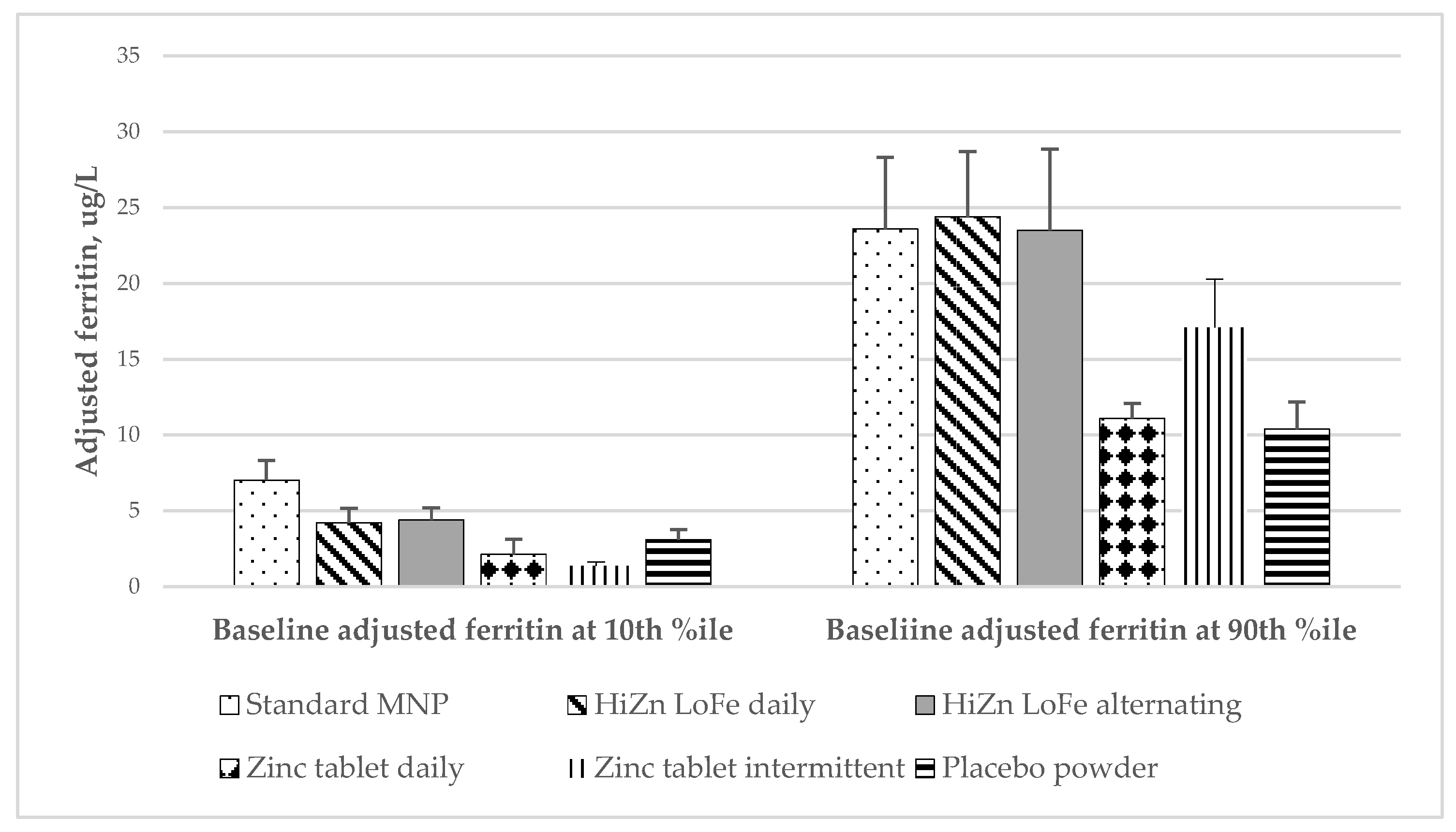
| Adj. ferritin at 24 weeks, ug/L 3 | Baseline adj. ferritin at 10th %ile 3 | 7.04 ± 1.29 a | 4.22 ± 0.96 a,b | 4.39 ± 0.81 a,b | 2.14 ± 0.48 b,c | 1.37 ± 0.25 c | 3.10 ± 0.66 a–c | 0.004 | <0.0001 |
| Baseline adj. ferritin at 90th %ile 3 | 23.6 ± 4.71 a,b | 24.4 ± 4.31 a | 23.5 ± 5.36 a–c | 11.1 ± 1.84 b,c | 17.1 ± 3.19 a–c | 10.4 ± 1.78 c | 0.0002 | ||
| Lowest tertile of consumption | 7.61 ± 1.90 | 6.66 ± 1.61 | 9.56 ± 2.0 | 5.63 ± 1.48 | 6.27 ± 1.68 | 5.79 ± 1.31 | 0.01 | 0.62 | |
| Mid tertile of consumption | 11.8 ± 2.61 a | 12.02 ± 2.08 a | 10.2 ± 2.06 a | 5.51 ± 0.93 a | 5.48 ± 1.04 a | 6.48 ± 1.17 a | 0.0003 | ||
| Highest tertile of consumption | 22.0 ± 3.89 a | 12.3 ± 2.44 a | 12.8 ± 2.55 a | 4.19 ± 0.83 b | 4.24 ± 0.85 b | 4.85 ± 0.89 b | <0.0001 | ||
| Adj. ferritin < 12 ug/L at 24 weeks 3 | Baseline adj. ferritin at 10th %ile 3 | 63.5 ± 10.3 a | 88.9 ± 7.75 a | 83.7 ± 8.64 a,b | 99.9 ± 0.15 b | 98.8 ± 1.65 a,b | 98.9 ± 1.56 a,b | 0.03 | 0.004 |
| Baseline adj. ferritin at 90th %ile 3 | 25.9 ± 10.0 a,b | 13.6 ± 7.01 a | 5.73 ± 4.92 a | 46.0 ± 13.4 a,b | 37.4 ± 14.5 a,b | 64.3 ± 11.02 b | 0.001 | ||
| sTfR at 24 weeks, mg/L 3,4 | Baseline sTfR at 10th %ile 3 | 6.99 ± 0.65 a,b | 6.86 ± 0.59 b | 7.19 ± 0.63 a,b | 9.39 ± 0.68 a | 7.83 ± 0.63 a,b | 9.28 ± 0.68 a | <0.0001 | 0.007 |
| Baseline sTfR at 90th %ile 3 | 10.6 ± 0.87 c | 15.3 ± 1.47 b,c | 15.1 ± 1.14 b,c | 19.2 ± 1.37 a,b | 23.2 ± 1.52 a | 17.3 ± 0.98 b | <0.0001 | ||
| BIS (mg/kg body weight) 4,5 | Baseline body iron at 10th %ile | −1.81 ± 0.88 a | −5.41 ± 1.15 a–c | −4.44 ± 0.91 a,b | −8.98 ± 1.08 c,d | −10.9 ± 0.89 d | −7.23 ± 0.96 b–d | 0.0002 | <0.0001 |
| Baseline body iron at 90th %ile | 3.48 ± 0.89 a,b | 4.07 ± 0.81 a | 3.83 ± 0.97 a,b | −0.15 ± 0.74 c | 2.62 ± 0.83 a–c | 0.75 ± 0.82 b,c | 0.0002 | ||
| Lowest tertile of consumption | −0.84 ± 1.15 | −2.24 ± 1.12 | 0.21 ± 0.98 | −2.51 ± 1.21 | −2.75 ± 1.24 | −2.46 ± 1.05 | 0.007 | 0.32 | |
| Mid tertile of consumption | 1.02 ± 1.02 a | 0.29 ± 0.80 a | 0.49 ± 0.93 a | −3.46 ± 0.78 b | −2.47 ± 0.87 a,b | −2.62 ± 0.83 a,b | <0.0001 | ||
| Highest tertile of consumption | 3.20 ± 0.81 a | 0.89 ± 0.91 a | 0.23 ± 0.92 a,b | −5.90 ± 0.91 c | −4.70 ± 0.93 c | −3.06 ± 0.84 b,c | <0.0001 | ||
| BIS < 0 4,5 | Baseline BIS at 10th %ile | 42.9 ± 11.2 a | 88.9 ± 7.92 a,b | 85.2 ± 9.14 a,b | 99.8 ± 0.37 b | 99.6 ± 0.83 b | 97.4 ± 2.77 a,b | 0.0291 | 0.0004 |
| Baseline body iron at 90th %ile | 17.5 ± 8.25 a,b | 12.0 ± 6.33 a | 6.50 ± 5.04 a | 46.3 ± 11.6 b | 15.3 ± 9.40 a,b | 42.8 ± 11.9 a,b | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.M.; Black, R.E.; Krebs, N.F.; Westcott, J.; Long, J.M.; Islam, K.M.; Peerson, J.M.; Sthity, R.A.; Khandaker, A.M.; Hasan, M.; et al. Effects of Different Doses, Forms, and Frequencies of Zinc Supplementation on Biomarkers of Iron and Zinc Status among Young Children in Dhaka, Bangladesh. Nutrients 2022, 14, 5334. https://doi.org/10.3390/nu14245334
Islam MM, Black RE, Krebs NF, Westcott J, Long JM, Islam KM, Peerson JM, Sthity RA, Khandaker AM, Hasan M, et al. Effects of Different Doses, Forms, and Frequencies of Zinc Supplementation on Biomarkers of Iron and Zinc Status among Young Children in Dhaka, Bangladesh. Nutrients. 2022; 14(24):5334. https://doi.org/10.3390/nu14245334
Chicago/Turabian StyleIslam, M. Munirul, Robert E. Black, Nancy F. Krebs, Jamie Westcott, Julie M. Long, Kazi M. Islam, Janet M. Peerson, Rahvia Alam Sthity, Afsana Mim Khandaker, Mehedi Hasan, and et al. 2022. "Effects of Different Doses, Forms, and Frequencies of Zinc Supplementation on Biomarkers of Iron and Zinc Status among Young Children in Dhaka, Bangladesh" Nutrients 14, no. 24: 5334. https://doi.org/10.3390/nu14245334
APA StyleIslam, M. M., Black, R. E., Krebs, N. F., Westcott, J., Long, J. M., Islam, K. M., Peerson, J. M., Sthity, R. A., Khandaker, A. M., Hasan, M., El Arifeen, S., Ahmed, T., King, J. C., & McDonald, C. M. (2022). Effects of Different Doses, Forms, and Frequencies of Zinc Supplementation on Biomarkers of Iron and Zinc Status among Young Children in Dhaka, Bangladesh. Nutrients, 14(24), 5334. https://doi.org/10.3390/nu14245334






