The Role and Mechanism of Polysaccharides in Anti-Aging
Abstract
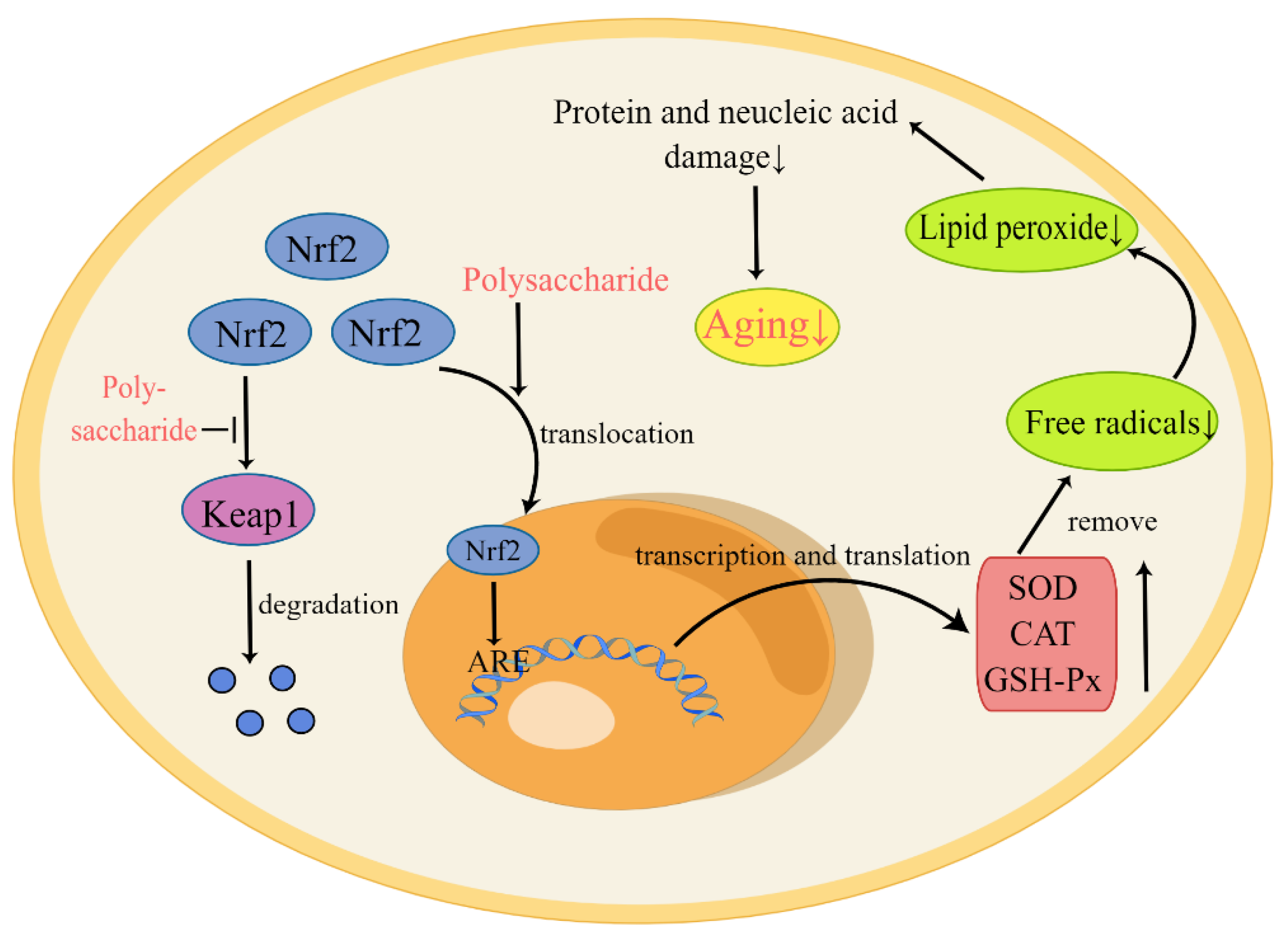
1. Introduction
2. Research on Anti-Aging Polysaccharides
2.1. C. elegans and D. melanogaster
2.2. Mice
| Polysaccharides | Main Aging Indicators | Mechanism | Reference |
|---|---|---|---|
| ASP | Cell analysis (cell cycle and the propotion of senescent cell); age-related genes (p53); Telomere and telomerase | ASP can antagonize X-ray-induced senescence of HSC, possibly by affecting telomere and p53 expression | [17] |
| ASP | Antioxidant indexes (SOD, GSH-Px, MDA, AGEs); liver tissue markers (ALT, AST, TBil, histomorphology) | ASP can antagonize D-Gal induced liver injury in aging mice, possibly by inhibiting oxidative stress | [38] |
| ASP | Antioxidant indexes (SOD, GSH-Px, MDA, AGEs); DNA damage markers (8-OH-DG); renal tissue markers (BUN, Crea, UA, Cysc, histomorphology) | ASP can antagonize D-Gal induced subacute kidney injury in mice, possibly by inhibiting oxidative stress injury | [39] |
| ASP | DNA damage markers (ROS, 8-OHdG, 4-HNE and γ-H2A.X); age-related pathways (P16Ink4a-Rb, p19Arf-Mdm2-p53-p21CIP1/Waf and Wnt/β-catenin) | ASP has antioxidant ability but the effect is not as good as VE; ASP delays senescence by affecting the expression of senescence signaling pathway factors | [34] |
| ASP | Age antioxidant indexes (SOD, CAT, GSH-Px); organ indexes; immune modulatory (inflammatory factors) | ASP effectively protects liver and kidney from D-Gal-induced injury in mice, which may be related to the reduction of oxidative response and inflammatory stress | [18] |
| ASP | Cell analysis (cell proliferation and the propotion of senescent cell); antioxidant indexes (SOD, MDA, T-AOC, ROS); age-related genes (P53, P21); immune modulatory (inflammatory factors) | ASP may delay brain aging in mice by regulating the number and function of hippocampal neural stem cells, reducing the oxidative damage, inhibiting the expression of inflammatory cytokines and aging genes | [19] |
| AcAPS 7 and its major purified fractions (AcAPS-1, AcAPS-2 and AcAPS-3) | Liver and kidney tissues damage markers (AST, ALT, ALP, BUN, CRE, ALB, histomorphology); antioxidant indexes (SOD, CAT, GSH-Px, MDA) | AcAPS-2 has a good protective effect on liver and kidney, among which rhamnose and glucose play a more important role | [40] |
| IZPS 8 | Antioxidant indexes (SOD, MDA, T-AOC); brain tissue damage markers (histomorphology) | IZPS can increase the antioxidant activity | [37] |
| MWP 9 | Antioxidant index (SOD, CAT, GSH-Px, MDA); neuronal apoptosis | MWP can improve antioxidant ability and inhibit neuronal apoptosis | [41] |
| APS | Antioxidant indexes (SOD, CAT, GSH-Px, MDA, ROS); mitochondrial damage markers (permeability) | APS can improve antioxidant capacity, inhibit mitochondrial damage and swelling | [20] |
| APS | Cell analysis (propotion of senescent cell); age-related genes (P16, P21, P53); mitochondrial damage marker (NCLX, ATP, cytochrome C oxidase activity and the oxygen consumption rate); immune modulatory (inflammatory factors) | APS can regulate the senescence of vascular endothelial cells induced by high glucose through enhancing the expression of NCLX, inhibiting inflammasome activation, improving mitochondrial dysfunction and promoting autophagy | [42] |
| YLSP 10 | Antioxidant indexes (SOD, CAT, GSH-Px, MDA and AGEs); immune modulatory (cytokine levels, organ indexes); aging-related genes (P21, P53) | YLSP may inhibit the aging process by enhancing antioxidant activity and immune function and regulating the expression of aging-related genes | [43] |
2.3. Cell Lines Studies
3. Anti-Aging Mechanism of Polysaccharides
3.1. Oxidative Damage
3.2. Age-Related Genes and Pathways
3.3. Immune Modulation
3.4. Telomere Attrition
4. Conclusions and Perspective
- Most polysaccharides used in the literature are extracted from plants. As such, their components are complex and unclear, and may include components other than pure polysaccharides. Extraction and purification methods have a great influence on the experimental results.
- The absorption mechanism and anti-aging mechanism of polysaccharides requires further exploration.
- Aging is a process of many physiological changes, and it involves multiple factors and organs. For animals with short life cycles such as C. elegans and D. melanogaster, life span can be directly detected. For other organisms with longer life spans, there are fewer intuitive indicators to characterize aging, which is an indirect representation of one or several factors or organs.
- There remains a lack of long-term and large-scale clinical testing of polysaccharides as potential anti-aging drugs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Dziechciaz, M.; Filip, R. Biological psychological and social determinants of old age: Bio-psycho-social aspects of human aging. Ann. Agr. Env. Med. 2014, 21, 835–838. [Google Scholar] [CrossRef] [PubMed]
- United Nations Department of Economic and Social Affairs. World Population Prospects 2022. Online Edition ed. 2022. Available online: https://population.un.org/wpp/ (accessed on 15 September 2022).
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxidative Med. Cell. Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef] [PubMed]
- Da, C.J.; Vitorino, R.; Silva, G.M.; Vogel, C.; Duarte, A.C.; Rocha-Santos, T. A synopsis on aging-Theories, mechanisms and future prospects. Ageing Res. Rev. 2016, 29, 90–112. [Google Scholar]
- Kraig, E.; Linehan, L.A.; Liang, H.; Romo, T.Q.; Liu, Q.; Wu, Y.; Benavides, A.D.; Curiel, T.J.; Javors, M.A.; Musi, N.; et al. A randomized control trial to establish the feasibility and safety of rapamycin treatment in an older human cohort: Immunological, physical performance, and cognitive effects. Exp. Gerontol. 2018, 105, 53–69. [Google Scholar] [CrossRef]
- de Kreutzenberg, S.V.; Ceolotto, G.; Cattelan, A.; Pagnin, E.; Mazzucato, M.; Garagnani, P.; Borelli, V.; Bacalini, M.G.; Franceschi, C.; Fadini, G.P.; et al. Metformin improves putative longevity effectors in peripheral mononuclear cells from subjects with prediabetes. A randomized controlled trial. Nutr. Metab. Carbiovasc. Dis. 2015, 25, 686–693. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Wang, S. The Role of Rapamycin in Healthspan Extension via the Delay of Organ Aging. Ageing Res. Rev. 2021, 70, 101376. [Google Scholar] [CrossRef]
- Hu, D.; Xie, F.; Xiao, Y.; Lu, C.; Zhong, J.; Huang, D.; Chen, J.; Wei, J.; Jiang, Y.; Zhong, T. Metformin: A Potential Candidate for Targeting Aging Mechanisms. Aging Dis. 2021, 12, 480–493. [Google Scholar] [CrossRef]
- Li, J.; Kim, S.G.; Blenis, J. Rapamycin: One drug, many effects. Cell Metab. 2014, 19, 373–379. [Google Scholar] [CrossRef]
- Soukas, A.A.; Hao, H.; Wu, L. Metformin as Anti-Aging Therapy: Is It for Everyone? Trends Endocrinol. Metab. 2019, 30, 745–755. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Hao, C.; Sun, M.; Wang, H.; Zhang, L.; Wang, W. Low molecular weight heparins and their clinical applications. Prog. Mol. Biol. Transl. Sci. 2019, 163, 21–39. [Google Scholar]
- Li, X.; Ma, L.; Zhang, L. Molecular basis for Poria cocos mushroom polysaccharide used as an antitumor drug in China. Prog. Mol. Biol. Transl. Sci. 2019, 163, 263–296. [Google Scholar]
- Wang, N.; Liu, J.; Xie, F.; Gao, X.; Ye, J.H.; Sun, L.Y.; Wei, R.; Ai, J. miR-124/ATF-6, a novel lifespan extension pathway of Astragalus polysaccharide in Caenorhabditis elegans. J. Cell. Biochem. 2015, 116, 242–251. [Google Scholar] [CrossRef]
- Yang, F.; Xiu, M.; Yang, S.; Li, X.; Tuo, W.; Su, Y.; He, J.; Liu, Y. Extension of Drosophila Lifespan by Astragalus polysaccharide through a Mechanism Dependent on Antioxidant and Insulin/IGF-1 Signaling. Evid.-Based Complement. Altern. Med. 2021, 2021, 6686748. [Google Scholar] [CrossRef]
- Zhang, X.P.; Liu, J.; Xu, C.Y.; Wei, Q.; Li, J.; Wang, L.; Wang, J.W.; Wang, Y.P. Effect of Angelica sinensis polysaccharide on expression of telomere, telomerase and P53 in mice aging hematopoietic stem cells. Zhongguo Zhong Yao Za Zhi 2013, 38, 2354–2358. [Google Scholar]
- Mo, Z.Z.; Lin, Z.X.; Su, Z.R.; Zheng, L.; Li, H.L.; Xie, J.H.; Xian, Y.F.; Yi, T.G.; Huang, S.Q.; Chen, J.P. Angelica sinensis Supercritical Fluid CO2 Extract Attenuates D-Galactose-Induced Liver and Kidney Impairment in Mice by Suppressing Oxidative Stress and Inflammation. J. Med. Food 2018, 21, 887–898. [Google Scholar] [CrossRef]
- Cheng, X.; Yao, H.; Xiang, Y.; Chen, L.; Xiao, M.; Wang, Z.; Xiao, H.; Wang, L.; Wang, S.; Wang, Y. Effect of Angelica polysaccharide on brain senescence of Nestin-GFP mice induced by D-galactose. Neurochem. Int. 2019, 122, 149–156. [Google Scholar] [CrossRef]
- Li, X.T.; Zhang, Y.K.; Kuang, H.X.; Jin, F.X.; Liu, D.W.; Gao, M.B.; Liu, Z.; Xin, X.J. Mitochondrial protection and anti-aging activity of Astragalus polysaccharides and their potential mechanism. Int. J. Mol. Sci. 2012, 13, 1747–1761. [Google Scholar] [CrossRef]
- Park, H.H.; Jung, Y.; Lee, S.V. Survival assays using Caenorhabditis elegans. Mol. Cells 2017, 40, 90–99. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, T.; Li, M.; Xue, T.; Liu, H.; Zhang, W.; Ding, X.; Zhuang, Z. Anti-aging effect of polysaccharide from Bletilla striata on nematode Caenorhabditis elegans. Pharmacogn. Mag. 2015, 11, 449–454. [Google Scholar] [PubMed]
- Staats, S.; Luersen, K.; Wagner, A.E.; Rimbach, G. Drosophila melanogaster as a Versatile Model Organism in Food and Nutrition Research. J. Agric. Food Chem. 2018, 66, 3737–3753. [Google Scholar] [CrossRef] [PubMed]
- Azman, K.F.; Zakaria, R. D-Galactose-induced accelerated aging model: An overview. Biogerontology 2019, 20, 763–782. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Hui, H.; Xin, A.; Cui, H.; Jin, H.; Yang, X.; Liu, H.; Qin, B. Anti-aging effects on Caenorhabditis elegans of a polysaccharide, O-acetyl glucomannan, from roots of Lilium davidii var. unicolor Cotton. Int. J. Biol. Macromol. 2020, 155, 846–852. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, Y.; Fan, H.; Billy, K.J.; Zhao, Y.; Zhan, X.; Yang, L.; Jia, Y. Effects of Lycium barbarum Polysaccharides on Health and Aging of C. elegans Depend on daf-12/daf-16. Oxidative Med. Cell. Longev. 2019, 2019, 6379493. [Google Scholar] [CrossRef]
- Yuan, Y.; Kang, N.; Li, Q.; Zhang, Y.; Liu, Y.; Tan, P. Study of the Effect of Neutral Polysaccharides from Rehmannia glutinosa on Lifespan of Caenorhabditis elegans. Molecules 2019, 24, 4592. [Google Scholar] [CrossRef]
- Tang, R.; Chen, X.; Dang, T.; Deng, Y.; Zou, Z.; Liu, Q.; Gong, G.; Song, S.; Ma, F.; Huang, L.; et al. Lycium barbarum polysaccharides extend the mean lifespan of Drosophila melanogaster. Food Funct. 2019, 10, 4231–4241. [Google Scholar] [CrossRef]
- Li, Y.; Guan, S.; Liu, C.; Chen, X.; Zhu, Y.; Xie, Y.; Wang, J.; Ji, X.; Li, L.; Li, Z.; et al. Neuroprotective effects of Coptis chinensis Franch polysaccharide on amyloid-beta (Abeta)-induced toxicity in a transgenic Caenorhabditis elegans model of Alzheimer’s disease (AD). Int. J. Biol. Macromol. 2018, 113, 991–995. [Google Scholar] [CrossRef]
- Feng, S.; Cheng, H.; Xu, Z.; Shen, S.; Yuan, M.; Liu, J.; Ding, C. Thermal stress resistance and aging effects of Panax notoginseng polysaccharides on Caenorhabditis elegans. Int. J. Biol. Macromol. 2015, 81, 188–194. [Google Scholar] [CrossRef]
- Zhu, Y.; Yu, X.; Ge, Q.; Li, J.; Wang, D.; Wei, Y.; Ouyang, Z. Antioxidant and anti-aging activities of polysaccharides from Cordyceps cicadae. Int. J. Biol. Macromol. 2020, 157, 394–400. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, J.Z.; Lin, Z.X.; Yuan, Q.J.; Li, Y.C.; Liang, J.L.; Zhan, J.Y.; Xie, Y.L.; Su, Z.R.; Liu, Y.H. Ameliorative effect of supercritical fluid extract of Chrysanthemum indicum Linnen against D-galactose induced brain and liver injury in senescent mice via suppression of oxidative stress, inflammation and apoptosis. J. Ethnopharmacol. 2019, 234, 44–56. [Google Scholar] [CrossRef]
- Mu, X.; Zhang, Y.; Li, J.; Xia, J.; Chen, X.; Jing, P.; Song, X.; Wang, L.; Wang, Y. Angelica Sinensis Polysaccharide Prevents Hematopoietic Stem Cells Senescence in D-Galactose-Induced Aging Mouse Model. Stem Cells Int. 2017, 2017, 3508907. [Google Scholar] [CrossRef]
- Rodier, F.; Campisi, J. Four faces of cellular senescence. J. Cell Biol. 2011, 192, 547–556. [Google Scholar] [CrossRef]
- Huelsken, J.; Birchmeier, W. New aspects of Wnt signaling pathways in higher vertebrates. Curr. Opin. Genet. Dev. 2001, 11, 547–553. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, Z.; Hu, C.; Zhang, J.; Sun, X.; Rong, C.; Jia, L. Antioxidant, antibacterial and anti-aging activities of intracellular zinc polysaccharides from Grifola frondosa SH-05. Int. J. Biol. Macromol. 2017, 95, 778–787. [Google Scholar] [CrossRef]
- Xia, J.Y.; Fan, Y.L.; Jia, D.Y.; Zhang, M.S.; Zhang, Y.Y.; Li, J.; Jing, P.W.; Wang, L.; Wang, Y.P. Protective effect of Angelica sinensis polysaccharide against liver injury induced by D-galactose in aging mice and its mechanisms. Zhonghua Gan Zang Bing Za Zhi 2016, 24, 214–219. [Google Scholar]
- Fan, Y.L.; Xia, J.Y.; Jia, D.Y.; Zhang, M.S.; Zhang, Y.Y.; Wang, L.; Huang, G.N.; Wang, Y.P. Protective effect of Angelica sinensis polysaccharides on subacute renal damages induced by D-galactose in mice and its mechanism. Zhongguo Zhong Yao Za Zhi 2015, 40, 4229–4233. [Google Scholar]
- Li, S.; Liu, H.; Wang, W.; Wang, X.; Zhang, C.; Zhang, J.; Jing, H.; Ren, Z.; Gao, Z.; Song, X.; et al. Antioxidant and anti-aging effects of acidic-extractable polysaccharides by Agaricus bisporus. Int. J. Biol. Macromol. 2018, 106, 1297–1306. [Google Scholar] [CrossRef]
- Hui, Y.; Jun-li, H.; Chuang, W. Anti-oxidation and anti-aging activity of polysaccharide from Malus micromalus Makino fruit wine. Int. J. Biol. Macromol. 2019, 121, 1203–1212. [Google Scholar] [CrossRef]
- Miao, X.Y.; Zhu, X.X.; Gu, Z.Y.; Fu, B.; Cui, S.Y.; Zu, Y.; Rong, L.J.; Hu, F.; Chen, X.M.; Gong, Y.P.; et al. Astragalus Polysaccharides Reduce High-glucose-induced Rat Aortic Endothelial Cell Senescence and Inflammasome Activation by Modulating the Mitochondrial Na(+)/Ca(2+) Exchanger. Cell Biochem. Biophys. 2022, 80, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Doan, V.M.; Chen, C.; Lin, X.; Nguyen, V.P.; Nong, Z.; Li, W.; Chen, Q.; Ming, J.; Xie, Q.; Huang, R. Yulangsan polysaccharide improves redox homeostasis and immune impairment in D-galactose-induced mimetic aging. Food Funct. 2015, 6, 1712–1718. [Google Scholar] [CrossRef]
- Xiao, H.; Xiong, L.; Song, X.; Jin, P.; Chen, L.; Chen, X.; Yao, H.; Wang, Y.; Wang, L. Angelica sinensis Polysaccharides Ameliorate Stress-Induced Premature Senescence of Hematopoietic Cell via Protecting Bone Marrow Stromal Cells from Oxidative Injuries Caused by 5-Fluorouracil. Int. J. Mol. Sci. 2017, 18, 2265. [Google Scholar] [CrossRef] [PubMed]
- Avadhani, K.S.; Manikkath, J.; Tiwari, M.; Chandrasekhar, M.; Godavarthi, A.; Vidya, S.M.; Hariharapura, R.C.; Kalthur, G.; Udupa, N.; Mutalik, S. Skin delivery of epigallocatechin-3-gallate (EGCG) and hyaluronic acid loaded nano-transfersomes for antioxidant and anti-aging effects in UV radiation induced skin damage. Drug Deliv. 2017, 24, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Duan, C.; Chen, B.; Li, M.; Ruan, Y.; Xu, D.; Shi, D.; Yu, D.; Li, J.; Wang, C. Tremella fuciformis polysaccharide suppresses hydrogen peroxide-triggered injury of human skin fibroblasts via upregulation of SIRT1. Mol. Med. Rep. 2017, 16, 1340–1346. [Google Scholar] [CrossRef]
- Mu, S.; Yang, W.; Huang, G. Antioxidant activities and mechanisms of polysaccharides. Chem. Biol. Drug Des. 2021, 97, 628–632. [Google Scholar] [CrossRef]
- Kong, Y.; Trabucco, S.E.; Zhang, H. Oxidative stress, mitochondrial dysfunction and the mitochondria theory of aging. Interdiscip. Top. Gerontol. 2014, 39, 86–107. [Google Scholar]
- Ou, H.L.; Schumacher, B. DNA damage responses and p53 in the aging process. Blood 2018, 131, 488–495. [Google Scholar] [CrossRef]
- Dutto, I.; Tillhon, M.; Cazzalini, O.; Stivala, L.A.; Prosperi, E. Biology of the cell cycle inhibitor p21(CDKN1A): Molecular mechanisms and relevance in chemical toxicology. Arch. Toxicol. 2015, 89, 155–178. [Google Scholar] [CrossRef]
- Roitenberg, N.; Bejerano-Sagie, M.; Boocholez, H.; Moll, L.; Marques, F.C.; Golodetzki, L.; Nevo, Y.; Elami, T.; Cohen, E. Modulation of caveolae by insulin/IGF-1 signaling regulates aging of Caenorhabditis elegans. Embo Rep. 2018, 19, e45673. [Google Scholar] [CrossRef]
- Sen, I.; Zhou, X.; Chernobrovkin, A.; Puerta-Cavanzo, N.; Kanno, T.; Salignon, J.; Stoehr, A.; Lin, X.; Baskaner, B.; Brandenburg, S.; et al. DAF-16/FOXO requires Protein Phosphatase 4 to initiate transcription of stress resistance and longevity promoting genes. Nat. Commun. 2020, 11, 138. [Google Scholar] [CrossRef]
- Maiese, K.; Li, F.; Chong, Z.Z.; Shang, Y.C. The Wnt signaling pathway: Aging gracefully as a protectionist? Pharmacol. Ther. 2008, 118, 58–81. [Google Scholar] [CrossRef]
- Lezzerini, M.; Budovskaya, Y. A dual role of the Wnt signaling pathway during aging in Caenorhabditis elegans. Aging Cell 2014, 13, 8–18. [Google Scholar] [CrossRef]
- Weng, N.P. Aging of the immune system: How much can the adaptive immune system adapt? Immunity 2006, 24, 495–499. [Google Scholar] [CrossRef]
- Zhang, W.; Hwang, J.; Park, H.B.; Lim, S.M.; Go, S.; Kim, J.; Choi, I.; You, S.; Jin, J.O. Human Peripheral Blood Dendritic Cell and T Cell Activation by Codium fragile Polysaccharide. Mar. Drugs 2020, 18, 535. [Google Scholar] [CrossRef]
- Budamagunta, V.; Manohar-Sindhu, S.; Yang, Y.; He, Y.; Traktuev, D.O.; Foster, T.C.; Zhou, D. Senescence-associated hyper-activation to inflammatory stimuli in vitro. Aging (Albany NY). 2021, 13, 19088–19107. [Google Scholar] [CrossRef]
- Uribarri, J.; Cai, W.; Peppa, M.; Goodman, S.; Ferrucci, L.; Striker, G.; Vlassara, H. Circulating Glycotoxins and Dietary Advanced Glycation Endproducts: Two Links to Inflammatory Response, Oxidative Stress, and Aging. J. Gerontol. Ser. A 2007, 62, 427–433. [Google Scholar] [CrossRef]
- Gautieri, A.; Passini, F.S.; Silván, U.; Guizar-Sicairos, M.; Carimati, G.; Volpi, P.; Moretti, M.; Schoenhuber, H.; Redaelli, A.; Berli, M.; et al. Advanced glycation end-products: Mechanics of aged collagen from molecule to tissue. Matrix Biol. 2017, 59, 95–108. [Google Scholar] [CrossRef]
- Kim, M.T.; Harty, J.T. Impact of inflammatory cytokines on effector and memory CD8+T cells. Front. Immunol. 2014, 5, 295. [Google Scholar] [CrossRef]
- Kale, A.; Sharma, A.; Stolzing, A.; Desprez, P.Y.; Campisi, J. Role of immune cells in the removal of deleterious senescent cells. Immun. Ageing 2020, 17, 16. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, J.; Zhang, T. Immunomodulatory activities of polysaccharides from Ganoderma on immune effector cells. Food Chem. 2021, 340, 127933. [Google Scholar] [CrossRef]
- Jacczak, B.; Rubis, B.; Toton, E. Potential of Naturally Derived Compounds in Telomerase and Telomere Modulation in Skin Senescence and Aging. Int. J. Mol. Sci. 2021, 22, 6381. [Google Scholar] [CrossRef]
- de Lange, T. Shelterin-Mediated Telomere Protection. Annu. Rev. Genet. 2018, 52, 223–247. [Google Scholar] [CrossRef]
- Zhang, F.; Song, X.; Li, L.; Wang, J.; Lin, L.; Li, C.; Li, H.; Lv, Y.; Jin, Y.; Liu, Y.; et al. Polygala tenuifolia polysaccharide (PTP) inhibits cell proliferation by repressing Bmi-1 expression and downregulating telomerase activity. Tumour Biol. 2015, 36, 2907–2912. [Google Scholar] [CrossRef]
- Han, M.H.; Lee, D.S.; Jeong, J.W.; Hong, S.H.; Choi, I.W.; Cha, H.J.; Kim, S.; Kim, H.S.; Park, C.; Kim, G.Y.; et al. Fucoidan Induces ROS-Dependent Apoptosis in 5637 Human Bladder Cancer Cells by Downregulating Telomerase Activity via Inactivation of the PI3K/Akt Signaling Pathway. Drug Dev. Res. 2017, 78, 37–48. [Google Scholar] [CrossRef]
| Polysaccharides | Main Aging Indicators | Mechanism | Reference |
|---|---|---|---|
| APS | Lifespan; age-related genes (miR-124 and atf-6) | The lifespan of C. elegans is prolonged by APS with the regulation atf-6 by miR-124 | [15] |
| LBP 1 | Lifespan under normal and stress conditions; age-related genes (sir-2.1, daf-12, and daf-16) | The effects of LBP on C. elegans health and aging were modulated by sir-2.1, daf-12, and daf-16 | [27] |
| CCP 2 | Lifespan; age-related genes (HSP) | CCP can protect nerves and delay aging | [30] |
| BSP 3 | Lifespan under normal and stress conditions; age-related pathway (IIS pathway) | BSP affects nematode life through the IIS pathway | [22] |
| PRG 4 | Aging pigment (lipofuscin); antioxidant enzymes (SOD and CAT and AGEs); age-related pathway (IIS pathway) | PRG can enhance the ability of nematodes to resist oxidative stress and delay senescence through IIS | [28] |
| LPR 5 | Lifespan under normal and stress conditions; aging pigment; antioxidant indexes (SOD, CAT, MDA, ROS) | LPR can improve the antioxidant defense system and scavenge free radicals of nematodes to extend the lifespan without toxicity | [26] |
| Panax notoginseng polysaccharide | Lifespan under normal and heat stress conditions; antioxidant indexes (SOD, CAT, MDA, ROS) | The scavenging ability of it is weak, but it can improve the activity of antioxidant enzymes, reduce the formation of lipid peroxides, and significantly prolong the life span | [31] |
| Polysaccharides | Main Aging Indicators | Mechanism | Reference |
|---|---|---|---|
| CP 6 | Lifespan under normal and oxidative stress conditions; antioxidant indexes (GSH-Px, MDA, CAT, SOD1 and MTH) | CP70 can up-regulate the antioxidant related genes CAT, SOD1 and MTH to prolong the lifespan of Drosophila | [32] |
| APS | Lifespan under normal and oxidative stress; antioxidant indexes (Sod1, Sod2, Cat); age-related pathway (IIS pathway) | APS can extend the lifespan of Drosophila by affecting antioxidant capacity and IIS pathway | [16] |
| LBP | Lifespan under normal and stress conditions; antioxidant indexes (SOD, CAT, MDA); expression of aging-related pathways (MAPK, TOR, S6K) and genes (Hep, MTH, and Rpn11) | The anti-aging activity of LBP is related to the expression of aging related pathways and longevity genes | [29] |
| Polysaccharides | Objects | Main Aging Indicators | Mechannism | Reference |
|---|---|---|---|---|
| ASP | Homo sapiens bone marrow/stroma cell line | Cell analysis; antioxidant indexes (ROS, SOD, GSH-Px); DNA damage markers (8-OHdG, γH2AX) | ASP protects cells from chemotherapy injury by reducing the oxidative damage and improving hematopoietic function | [44] |
| Transfersomes containing EGCG and hyaluronic acid | Human keratinocyte cell lines | Cell analysis (viability); antioxidant indexes (ROS and MDA); skin aging genes (MMP2 and MMP9) | Transfersomes have excellent antioxidant ability, inhibit collagen degradation, and enhance cell viability and skin penetration | [45] |
| TFPS 11 | Human skin fibroblasts | Cell analysis (viability and apoptosis); ROS; aging-related genes (p16, p21, p53, SIRT-1) | TFPS attenuates oxidative stress and apoptosis induced by hydrogen peroxide in skin fibroblasts by upregulating Sirt1 expression | [46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Luo, J.; Qi, J.; Zhao, X.; An, P.; Luo, Y.; Wang, G. The Role and Mechanism of Polysaccharides in Anti-Aging. Nutrients 2022, 14, 5330. https://doi.org/10.3390/nu14245330
Guo X, Luo J, Qi J, Zhao X, An P, Luo Y, Wang G. The Role and Mechanism of Polysaccharides in Anti-Aging. Nutrients. 2022; 14(24):5330. https://doi.org/10.3390/nu14245330
Chicago/Turabian StyleGuo, Xinlu, Junjie Luo, Jingyi Qi, Xiya Zhao, Peng An, Yongting Luo, and Guisheng Wang. 2022. "The Role and Mechanism of Polysaccharides in Anti-Aging" Nutrients 14, no. 24: 5330. https://doi.org/10.3390/nu14245330
APA StyleGuo, X., Luo, J., Qi, J., Zhao, X., An, P., Luo, Y., & Wang, G. (2022). The Role and Mechanism of Polysaccharides in Anti-Aging. Nutrients, 14(24), 5330. https://doi.org/10.3390/nu14245330











