The Impact of Serum Levels of Vitamin D3 and Its Metabolites on the Prognosis and Disease Severity of COVID-19
Abstract
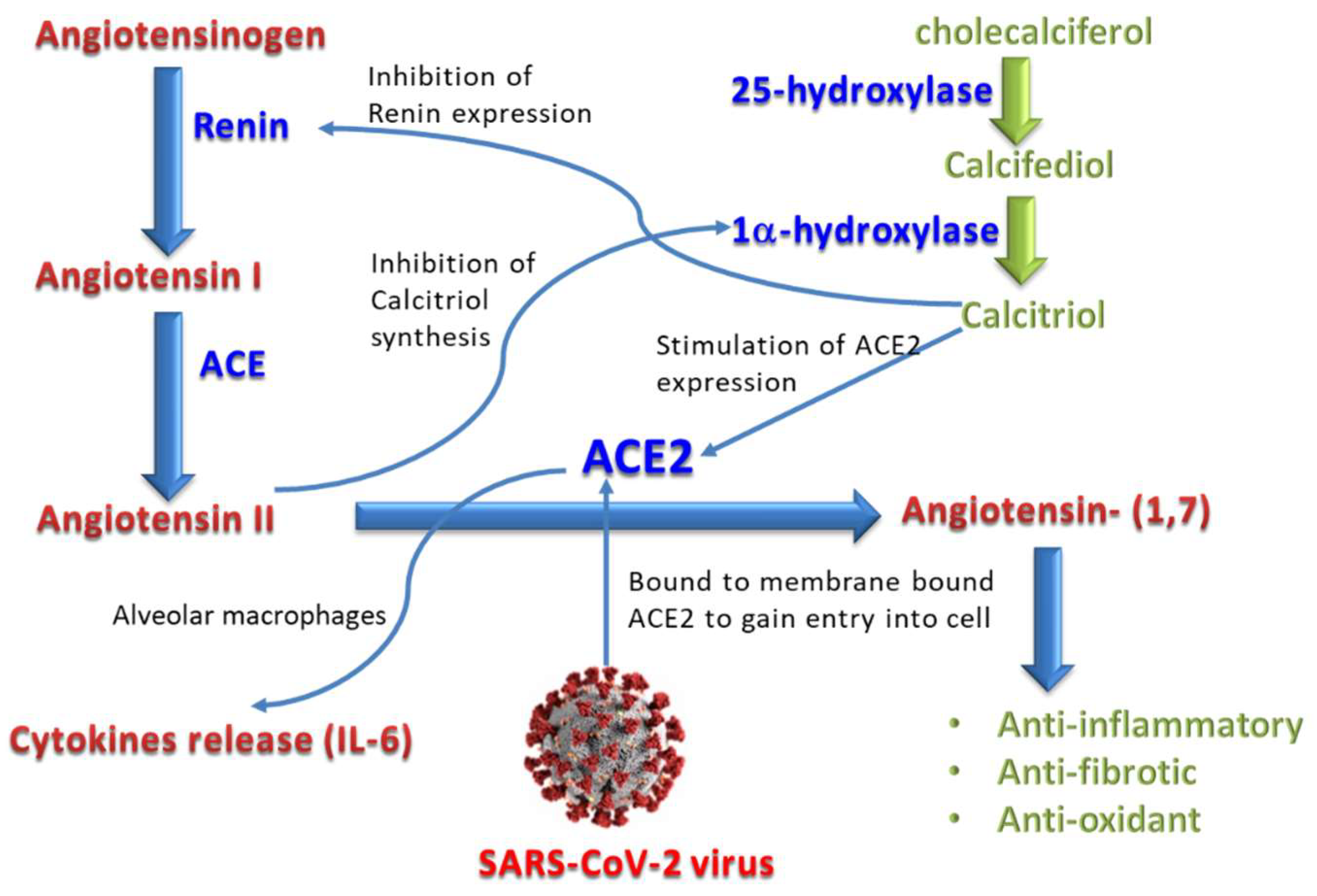
1. Introduction
2. Materials and Methods
2.1. Selection of Study Subjects
2.2. Sample Collection and Handling
2.3. Specimen Testing for COVID-19
2.4. Blood Sample Analyses
2.5. Statistical Analysis
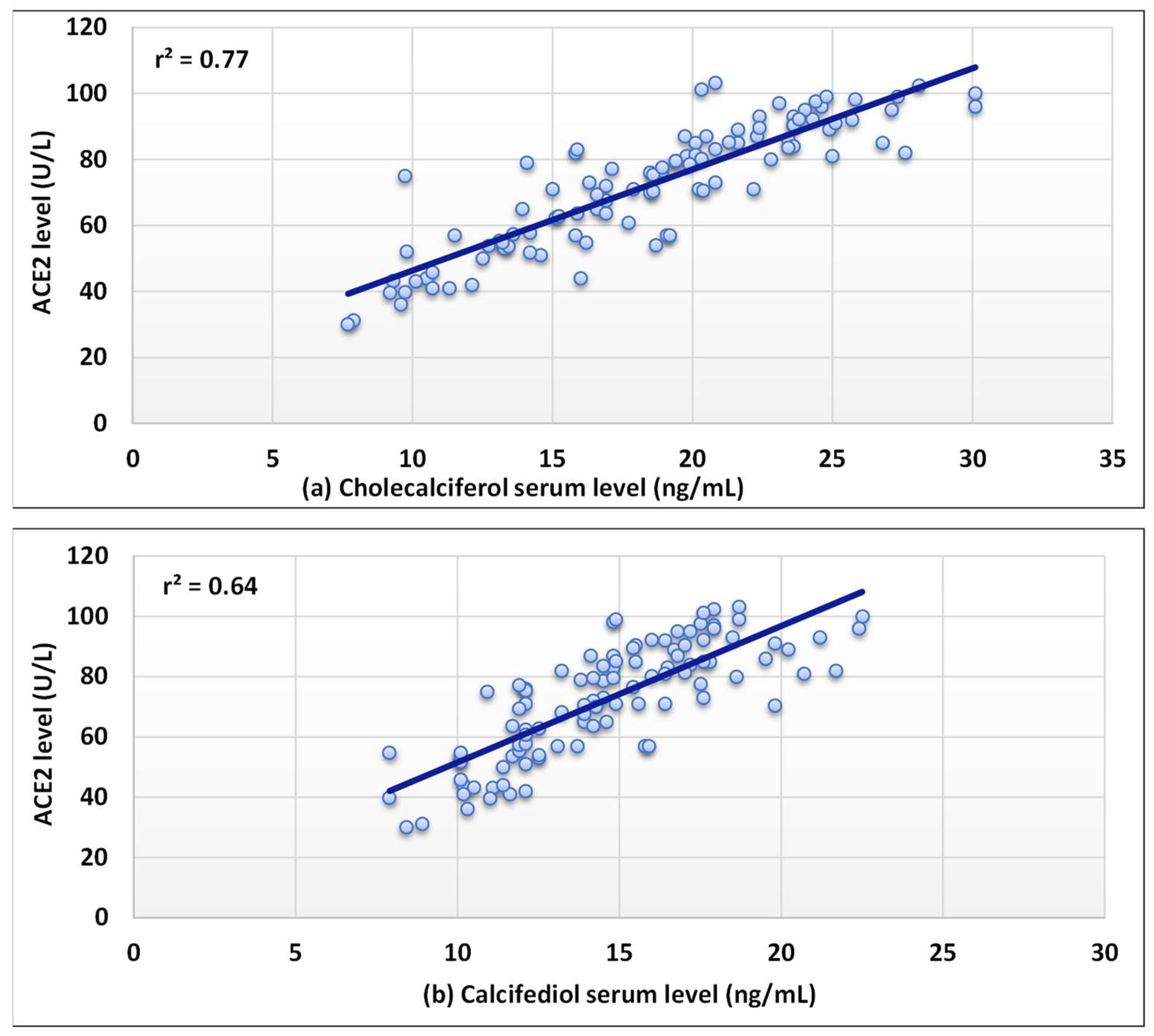
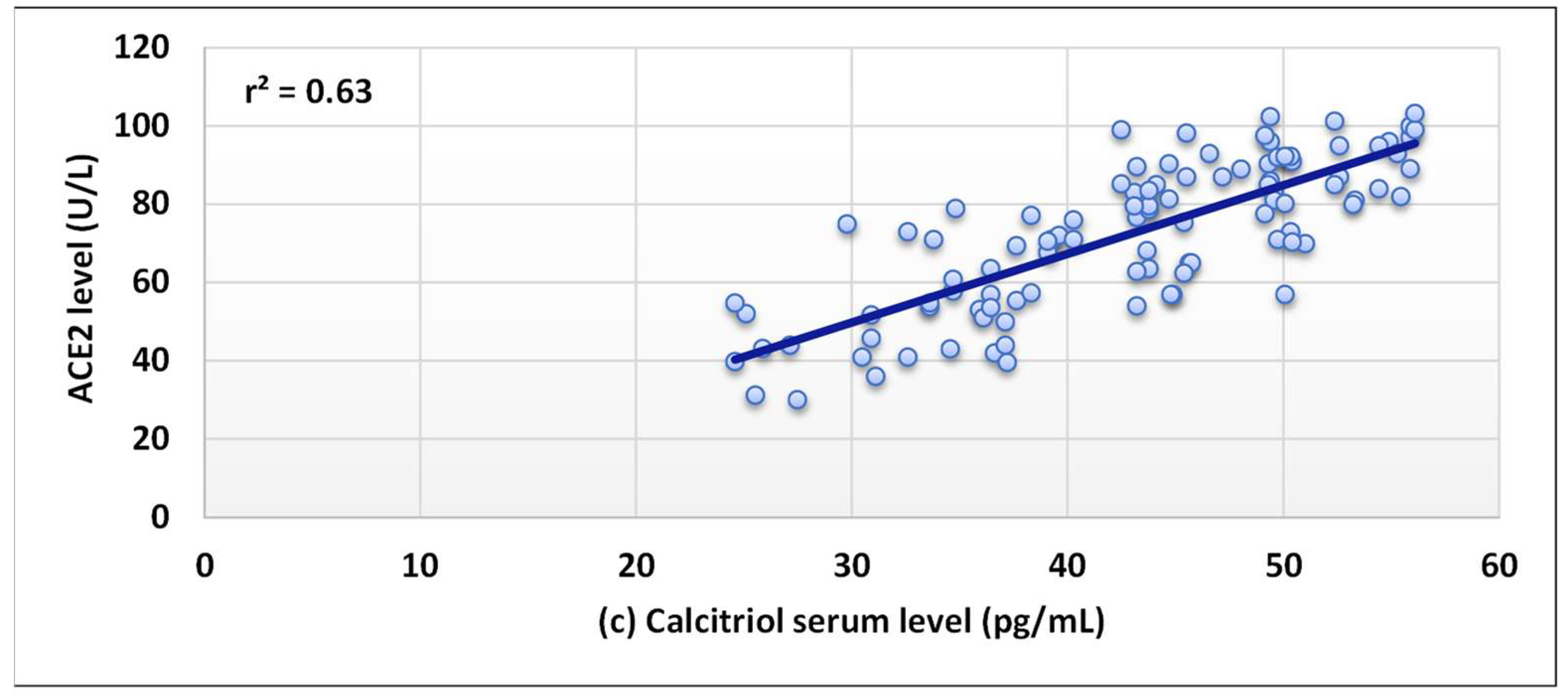
3. Results
4. Discussion
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pollard, C.A.; Morran, M.P.; Nestor-Kalinoski, A.L. The COVID-19 pandemic: A global health crisis. Physiol. Genom. 2020, 52, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Bartoszko, J.J.; Siemieniuk, R.A.C.; Kum, E.; Qasim, A.; Zeraatkar, D.; Ge, L.; Han, M.A.; Sadeghirad, B.; Agarwal, A.; Agoritsas, T.; et al. Prophylaxis against covid-19: Living systematic review and network meta-analysis. BMJ 2021, 373, n949. [Google Scholar] [CrossRef] [PubMed]
- Smit, M.; Marinosci, A.; Agoritsas, T.; Calmy, A. Prophylaxis for COVID-19: A systematic review. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2021, 27, 532–537. [Google Scholar] [CrossRef]
- Khojah, H.M.J. Community pharmacy services and preparedness during COVID-19 outbreak in Madinah, Saudi Arabia. Saudi Pharm. J. 2020, 28, 1402–1407. [Google Scholar] [CrossRef] [PubMed]
- Mukattash, T.L.; Alkhalidy, H.; Alzu’Bi, B.; Abu-Farha, R.; Itani, R.; Karout, S.; Khojah, H.M.J.; Khdour, M.; El-Dahiyat, F.; Jarab, A. Dietary supplements intake during the second wave of COVID-19 pandemic: A multinational Middle Eastern study. Eur. J. Integr. Med. 2022, 49, 102102. [Google Scholar] [CrossRef] [PubMed]
- Bartley, J. Vitamin D: Emerging roles in infection and immunity. Expert Rev. Anti-Infect. Ther. 2010, 8, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Vanherwegen, A.-S.; Gysemans, C.; Mathieu, C. Regulation of Immune Function by Vitamin D and Its Use in Diseases of Immunity. Endocrinol. Metab. Clin. N. Am. 2017, 46, 1061–1094. [Google Scholar] [CrossRef]
- Ismailova, A.; White, J.H. Vitamin D, infections and immunity. Rev. Endocr. Metab. Disord. 2021, 23, 265–277. [Google Scholar] [CrossRef]
- Malek Mahdavi, A. A brief review of interplay between vitamin D and angiotensin-converting enzyme 2: Implications for a potential treatment for COVID-19. Rev. Med. Virol. 2020, 30, e2119. [Google Scholar] [CrossRef]
- Beyerstedt, S.; Casaro, E.B.; Rangel, É.B. COVID-19: Angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2021, 40, 905–919. [Google Scholar] [CrossRef]
- Kuhn, J.H.; Li, W.; Choe, H.; Farzan, M. Angiotensin-Converting Enzyme 2: A Functional Receptor for SARS Coronavirus. Cell Mol. Life Sci. 2004, 61, 2738–2743. [Google Scholar] [CrossRef] [PubMed]
- Warner, F.J.; Rajapaksha, H.; Shackel, N.; Herath, C.B. ACE2: From protection of liver disease to propagation of COVID-19. Clin. Sci. 2020, 134, 3137–3158. [Google Scholar] [CrossRef]
- Turner, A.J.; Hiscox, J.A.; Hooper, N.M. ACE2: From vasopeptidase to SARS virus receptor. Trends Pharmacol. Sci. 2004, 25, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Scialo, F.; Daniele, A.; Amato, F.; Pastore, L.; Matera, M.G.; Cazzola, M.; Castaldo, G.; Bianco, A. ACE2: The Major Cell Entry Receptor for SARS-CoV-2. Lung 2021, 198, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Li, X.; Su, X.; Mu, D.; Qu, Y. Could SARS-CoV-2-induced lung injury be attenuated by vitamin D? Int. J. Infect. Dis. 2020, 102, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Rafiullah, M. Can a Combination of AT1R Antagonist and Vitamin D Treat the Lung Complication of COVID-19? Am. J. Med. Sci. 2020, 360, 338–341. [Google Scholar] [CrossRef]
- Honardoost, M.; Ghavideldarestani, M.; Khamseh, M.E. Role of vitamin D in pathogenesis and severity of COVID-19 infection. Arch. Physiol. Biochem. 2020, 126, 1–7. [Google Scholar] [CrossRef]
- Urena-Torres, P.; Souberbielle, J.C. Pharmacologic role of vitamin D natural products. Curr. Vasc. Pharmacol. 2014, 12, 278–285. [Google Scholar] [CrossRef]
- Kulda, V. Vitamin D metabolism. Vnitr. Lek. 2012, 58, 400–404. [Google Scholar]
- Brown, A.J. Therapeutic uses of vitamin D analogues. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2001, 38, S3–S19. [Google Scholar] [CrossRef]
- Jones, G.; Prosser, D.E. Chapter 3-The Activating Enzymes of Vitamin D Metabolism (25- and 1α-Hydroxylases); Feldman, D., Pike, J.W., Adams, J.S.B.T.-V.D., Third, E., Eds.; Academic Press: San Diego, CA, USA, 2011; pp. 23–42. ISBN 978-0-12-381978-9. [Google Scholar]
- Takayama, K.; Kato, S. The Vitamin D3 1alpha-Hydroxylase Gene and Its Regulation by Active Vitamin D3. Biosci. Biotechnol. Biochem. 2011, 75, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, L.H.M.; Butler, A.E.; Dargham, S.R.; Latif, A.; Chidiac, O.M.; Atkin, S.L.; Abi Khalil, C. Vitamin D3 metabolite ratio as an indicator of vitamin D status and its association with diabetes complications. BMC Endocr. Disord. 2020, 20, 161. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, M.; Tartaglione, L.; Rotondi, S.; Muci, M.L.; Mandanici, G.; Farcomeni, A.; Marangella, M.; Mazzaferro, S. Calcitriol/calcifediol ratio: An indicator of vitamin D hydroxylation efficiency? BBA Clin. 2015, 3, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Mardani, R.; Alamdary, A.; Nasab, S.M.; Gholami, R.; Ahmadi, N.; Gholami, A. Association of vitamin D with the modulation of the disease severity in COVID-19. Virus Res. 2020, 289, 198148. [Google Scholar] [CrossRef]
- Raisi-Estabragh, Z.; Martineau, A.R.; Curtis, E.M.; Moon, R.J.; Darling, A.; Lanham-New, S.; Ward, K.A.; Cooper, C.; Munroe, P.B.; Petersen, S.E.; et al. Vitamin D and coronavirus disease 2019 (COVID-19): Rapid evidence review. Aging Clin. Exp. Res. 2021, 33, 2031–2041. [Google Scholar] [CrossRef]
- Szarpak, L.; Rafique, Z.; Gasecka, A.; Chirico, F.; Gawel, W.; Hernik, J.; Kaminska, H.; Filipiak, K.J.; Jaguszewski, M.J.; Szarpak, L. A systematic review and meta-analysis of effect of vitamin D levels on the incidence of COVID-19. Cardiol. J. 2021, 28, 647–654. [Google Scholar] [CrossRef]
- Munshi, R.; Hussein, M.H.; Toraih, E.A.; Elshazli, R.M.; Jardak, C.; Sultana, N.; Youssef, M.R.; Omar, M.; Attia, A.S.; Fawzy, M.S.; et al. Vitamin D insufficiency as a potential culprit in critical COVID-19 patients. J. Med. Virol. 2021, 93, 733–740. [Google Scholar] [CrossRef]
- Teymoori-Rad, M.; Shokri, F.; Salimi, V.; Marashi, S.M. The interplay between vitamin D and viral infections. Rev. Med. Virol. 2019, 29, e2032. [Google Scholar] [CrossRef]
- Guo, Y.-R.; Cao, Q.-D.; Hong, Z.-S.; Tan, Y.-Y.; Chen, S.-D.; Jin, H.-J.; Tan, K.-S.; Wang, D.-Y.; Yan, Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—An update on the status. Mil. Med. Res. 2020, 7, 11. [Google Scholar] [CrossRef]
- Ali, N. Role of vitamin D in preventing of COVID-19 infection, progression and severity. J. Infect. Public Health 2020, 13, 1373–1380. [Google Scholar] [CrossRef]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [PubMed]
- Cantorna, M.T.; Snyder, L.; Lin, Y.-D.; Yang, L. Vitamin D and 1,25(OH)2D Regulation of T cells. Nutrients 2015, 7, 3011–3021. [Google Scholar] [CrossRef] [PubMed]
- Sabaka, P.; Koščálová, A.; Straka, I.; Hodosy, J.; Lipták, R.; Kmotorková, B.; Kachlíková, M.; Kušnírová, A. Role of interleukin 6 as a predictive factor for a severe course of Covid-19: Retrospective data analysis of patients from a long-term care facility during Covid-19 outbreak. BMC Infect. Dis. 2021, 21, 308. [Google Scholar] [CrossRef] [PubMed]
- Magro, G. SARS-CoV-2 and COVID-19: Is interleukin-6 (IL-6) the ‘culprit lesion’ of ARDS onset? What is there besides Tocilizumab? SGP130Fc. Cytokine X 2020, 2, 100029. [Google Scholar] [CrossRef]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef]
- Lagunas-Rangel, F.A. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis. J. Med. Virol. 2020, 92, 1733–1734. [Google Scholar] [CrossRef]
- Demir, A.D.; Durmaz, Z.H. A Comparison of vitamin D deficiency with neutrophil lymphocyte ratio and CRP levels in Covid-19 patients. Med. Sci. Discov. 2021, 8, 306–309. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Khojah, H.M.J.; Al-Thagfan, S.S.; Alahmadi, Y.M.; Mohammed, Y.A. An ultrasensitive UHPLC-ESI-MS/MS method augmented with a controlled microwave derivatization reaction for quantitation of vitamin D3 and its major metabolites in COVID-19 patients. Talanta 2022, 246, 123497. [Google Scholar] [CrossRef]
- Al-Thagfan, S.S.; Alolayan, S.O.; Ahmed, S.; Emara, M.M.; Awadallah, M.F. Impacts of deficiency in vitamin D derivatives on disease severity in adult bronchial asthma patients. Pulm. Pharmacol. Ther. 2021, 70, 102073. [Google Scholar] [CrossRef]
- Wacker, M.; Holick, M.F. Sunlight and Vitamin D. Dermato-Endocrinol. 2013, 5, 51–108. [Google Scholar] [CrossRef]
- Smolders, J.; van den Ouweland, J.; Geven, C.; Pickkers, P.; Kox, M. Letter to the Editor: Vitamin D deficiency in COVID-19: Mixing up cause and consequence. Metabolism 2021, 115, 154434. [Google Scholar] [CrossRef] [PubMed]
- Słomka, A.; Kowalewski, M.; Żekanowska, E. Coronavirus Disease 2019 (COVID-19): A Short Review on Hematological Manifestations. Pathogens 2020, 9, 493. [Google Scholar] [CrossRef]
- Thomas, T.; Stefanoni, D.; Dzieciatkowska, M.; Issaian, A.; Nemkov, T.; Hill, R.C.; Francis, R.O.; Hudson, K.E.; Buehler, P.W.; Zimring, J.C.; et al. Evidence of Structural Protein Damage and Membrane Lipid Remodeling in Red Blood Cells from COVID-19 Patients. J. Proteome Res. 2020, 19, 4455–4469. [Google Scholar] [CrossRef] [PubMed]
- Kamphuis, E.; Junt, T.; Waibler, Z.; Forster, R.; Kalinke, U. Type I interferons directly regulate lymphocyte recirculation and cause transient blood lymphopenia. Blood 2006, 108, 3253–3261. [Google Scholar] [CrossRef]
- Shenoy, V.; Ferreira, A.J.; Qi, Y.; Fraga-Silva, R.A.; Díez-Freire, C.; Dooies, A.; Jun, J.Y.; Sriramula, S.; Mariappan, N.; Pourang, D.; et al. The Angiotensin-Converting Enzyme 2/Angiogenesis-(1–7)/Mas Axis Confers Cardiopulmonary Protection against Lung Fibrosis and Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2010, 182, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Shroff, R.; Wan, M.; Rees, L. Can vitamin D slow down the progression of chronic kidney disease? Pediatr. Nephrol. 2012, 27, 2167–2173. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D in Health and Disease: Vitamin D for Health and in Chronic Kidney Disease. Semin. Dial. 2005, 18, 266–275. [Google Scholar] [CrossRef]


| Demographic Features | COVID-19 Group (n = 103) | Control Group (n = 50) | p-Value |
|---|---|---|---|
| Age (years) a | 47.32 ± 12.44 | 49.26 ± 9.94 | NS |
| Sex b | |||
| Male | 80 (77.67%) | 37 (74.0%) | - |
| Female | 23 (22.33%) | 13 (26.0%) | - |
| Weight (kg) a | 73.90 ± 12.30 | 72.12 ± 12.89 | NS |
| Height (cm) a | 165.86 ± 9.40 | 165.06 ± 7.52 | NS |
| BMI (kg/m2) a | 26.74 ± 3.38 | 26.47 ± 4.43 | NS |
| Test | COVID-19 Group * (n = 103) | Control Group * (n = 50) | p-Value |
|---|---|---|---|
| ACE2 (U/L) | 72.37 ± 18.67 | 32.36 ± 11.27 | ≤0.001 (HS) |
| IL-6 (pg/mL) | 95.84 ± 25.23 | 2.76 ± 0.62 | ≤0.001 (HS) |
| Glucose (mg/dL) | 188.42 ± 37.62 | 125.75 ± 25.49 | ≤0.001 (HS) |
| Total WBCs (×103/μL) | 7.86 ± 2.59 | 5.18 ± 0.76 | ≤0.05 (S) |
| Neutrophils (%) | 57.22 ± 2.27 | 47.81 ± 6.17 | ≤0.001 (HS) |
| Eosinophils (%) | 5.1 ± 0.16 | 5.08 ± 0.17 | NS |
| Lymphocytes (%) | 36.63 ± 6.14 | 45.02 ± 2.64 | ≤0.001 (HS) |
| Platelets (×103/μL) | 264.42 ± 72.30 | 215.10 ± 25.38 | ≤0.001 (HS) |
| NLR | 1.61 ± 0.30 | 1.07 ± 0.16 | ≤0.001 (HS) |
| RBCs (×106/µL) | 4.54 ± 0.56 | 5.09 ± 0.64 | ≤0.05 (S) |
| Hb (g/dL) | 11.98 ± 1.78 | 13.25 ± 0.9 | ≤0.05 (S) |
| HCT (%) | 36.25 ± 5.44 | 40.92 ± 2.21 | ≤0.05 (S) |
| MCV (fL) | 83.79 ± 5.34 | 81.35 ± 3.47 | NS |
| MCH (pg) | 30.40 ± 3.46 | 27.21 ± 2.82 | ≤0.05 (S) |
| MCHC (g/dL) | 34.00 ± 1.31 | 30.12 ± 1.75 | ≤0.05 (S) |
| Vitamin D and Its Metabolites | COVID-19 Group * (n = 103) | Control Group * (n = 50) | p-Value |
|---|---|---|---|
| Cholecalciferol (ng/mL) | 18.50 ± 5.36 | 29.13 ± 4.94 | ≤0.001 (HS) |
| Calcifediol (ng/mL) | 14.60 ± 3.30 | 23.10 ± 3.02 | ≤0.001 (HS) |
| Calcitriol (pg/mL) | 42.90 ± 8.44 | 65.15 ± 7.11 | ≤0.001 (HS) |
| Cacitriol/Calcifediol ratio (VMR1, pg/ng) | 2.97 ± 0.30 | 2.86 ± 0.46 | NS |
| Calcifediol/Cholecalciferol ratio (VMR2, ng/ng) | 0.82 ± 0.14 | 0.81 ± 0.17 | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khojah, H.M.J.; Ahmed, S.A.; Al-Thagfan, S.S.; Alahmadi, Y.M.; Abdou, Y.A. The Impact of Serum Levels of Vitamin D3 and Its Metabolites on the Prognosis and Disease Severity of COVID-19. Nutrients 2022, 14, 5329. https://doi.org/10.3390/nu14245329
Khojah HMJ, Ahmed SA, Al-Thagfan SS, Alahmadi YM, Abdou YA. The Impact of Serum Levels of Vitamin D3 and Its Metabolites on the Prognosis and Disease Severity of COVID-19. Nutrients. 2022; 14(24):5329. https://doi.org/10.3390/nu14245329
Chicago/Turabian StyleKhojah, Hani M. J., Sameh A. Ahmed, Sultan S. Al-Thagfan, Yaser M. Alahmadi, and Yasser A. Abdou. 2022. "The Impact of Serum Levels of Vitamin D3 and Its Metabolites on the Prognosis and Disease Severity of COVID-19" Nutrients 14, no. 24: 5329. https://doi.org/10.3390/nu14245329
APA StyleKhojah, H. M. J., Ahmed, S. A., Al-Thagfan, S. S., Alahmadi, Y. M., & Abdou, Y. A. (2022). The Impact of Serum Levels of Vitamin D3 and Its Metabolites on the Prognosis and Disease Severity of COVID-19. Nutrients, 14(24), 5329. https://doi.org/10.3390/nu14245329






