Sex-Specific Associations of Red Meat and Processed Meat Consumption with Serum Metabolites in the UK Biobank
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Metabolomic Profiling
2.3. Assessment of Dietary Intake
2.4. Assessment of Potential Confounders
2.5. Statistical Analysis
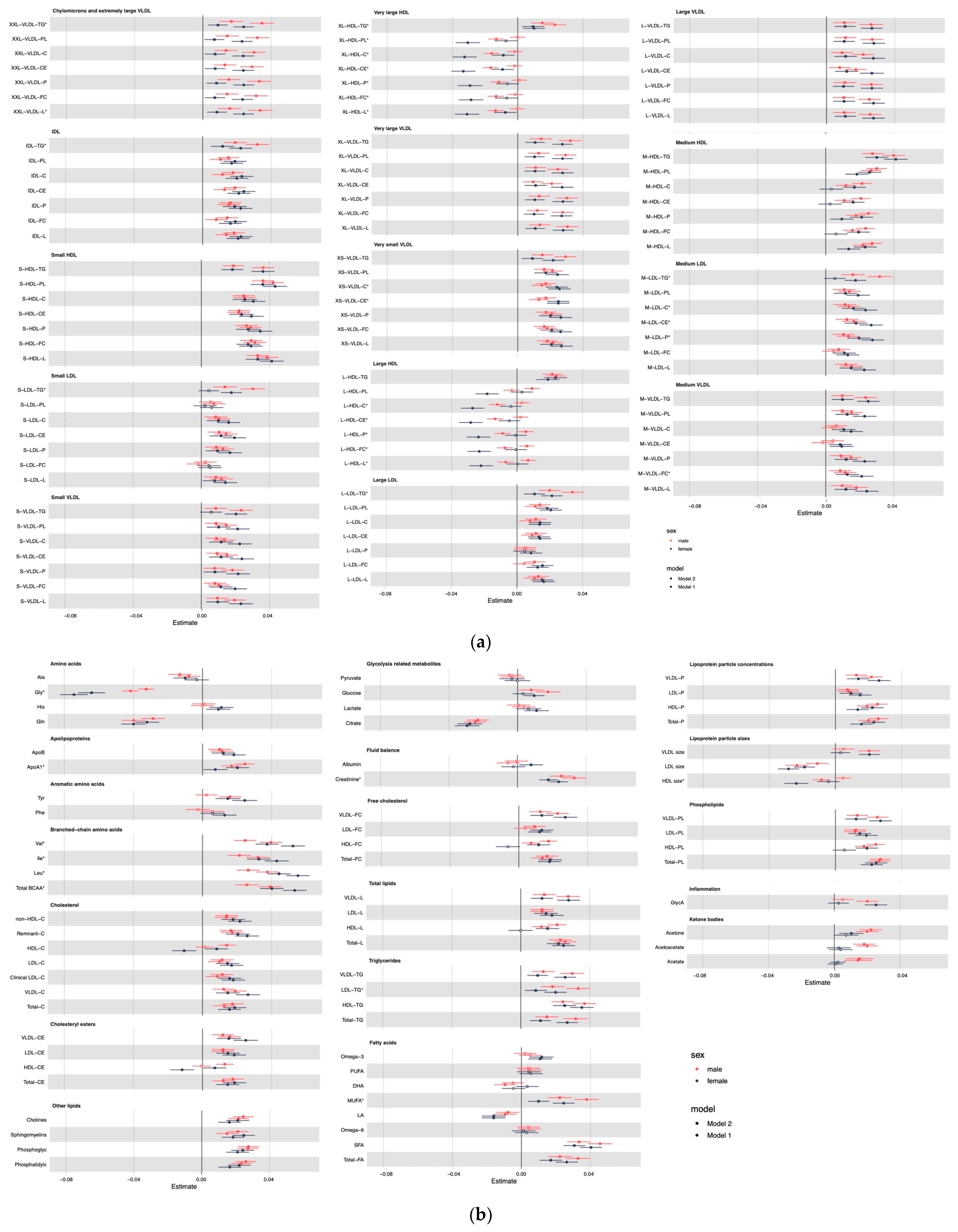
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [CrossRef]
- Dietary Guidelines for Americans, 2020–2025, 9th ed.; US Department of Health and Human Services, US Department of Agriculture: Washington, DC, USA, 2020.
- Zhong, V.W.; Van Horn, L.; Greenland, P.; Carnethon, M.R.; Ning, H.; Wilkins, J.T.; Lloyd-Jones, D.M.; Allen, N.B. Associations of Processed Meat, Unprocessed Red Meat, Poultry, or Fish Intake With Incident Cardiovascular Disease and All-Cause Mortality. JAMA Intern. Med. 2020, 180, 503. [Google Scholar] [CrossRef] [PubMed]
- Micha, R.; Peñalvo, J.L.; Cudhea, F.; Imamura, F.; Rehm, C.D.; Mozaffarian, D. Association Between Dietary Factors and Mortality from Heart Disease, Stroke, and Type 2 Diabetes in the United States. JAMA 2017, 317, 912. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Bergeron, N.; Levison, B.S.; Li, X.S.; Chiu, S.; Jia, X.; Koeth, R.A.; Li, L.; Wu, Y.; Tang, W.H.W.; et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur. Heart J. 2019, 40, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Chen, L.; Lv, J.; Pang, Y.; Guo, Y.; Pei, P.; Du, H.; Yang, L.; Millwood, I.Y.; Walters, R.G.; et al. Association of Red Meat Consumption, Metabolic Markers, and Risk of Cardiovascular Diseases. Front. Nutr. 2022, 9, 703. [Google Scholar] [CrossRef] [PubMed]
- Wittenbecher, C.; Mühlenbruch, K.; Kröger, J.; Jacobs, S.; Kuxhaus, O.; Floegel, A.; Fritsche, A.; Pischon, T.; Prehn, C.; Adamski, J.; et al. Amino acids, lipid metabolites, and ferritin as potential mediators linking red meat consumption to type 2 diabetes. Am. J. Clin. Nutr. 2015, 101, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Soininen, P.; Kangas, A.J.; Würtz, P.; Suna, T.; Ala-Korpela, M. Quantitative Serum Nuclear Magnetic Resonance Metabolomics in Cardiovascular Epidemiology and Genetics. Circ. Cardiovasc. Genet. 2015, 8, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef] [PubMed]
- Würtz, P.; Kangas, A.J.; Soininen, P.; Lawlor, D.A.; Davey Smith, G.; Ala-Korpela, M. Quantitative Serum Nuclear Magnetic Resonance Metabolomics in Large-Scale Epidemiology: A Primer on-Omic Technologies. Am. J. Epidemiol. 2017, 186, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, K.E.; Young, H.J.; Guo, W.; Key, T.J. Dietary assessment in UK Biobank: An evaluation of the performance of the touchscreen dietary questionnaire. J. Nutr. Sci. 2018, 7, E6. [Google Scholar] [CrossRef] [PubMed]
- Townsend, P.; Phillimore, P.; Beattie, A. Health and Deprivation: Inequality and the North; Croom Helm: London, UK, 1988. [Google Scholar]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med. Sci. Sport. Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, K.; Liu, F.; Lu, X.; Huang, J.; Gu, D. Association of circulating branched-chain amino acids with risk of cardiovascular disease: A systematic review and meta-analysis. Atherosclerosis 2022, 350, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hayden, K.; Jackson, R.; Schutte, R. Association of red and processed meat consumption with cardiovascular morbidity and mortality in participants with and without obesity: A prospective cohort study. Clin. Nutr. 2021, 40, 3643–3649. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, B.T.; Zhu, L.; Eckel, R.H.; Stafford, J.M. Sex differences in lipid and lipoprotein metabolism. Mol. Metab. 2018, 15, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and Creatinine Metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef] [PubMed]
- Sebranek, J.G. An overview of functional non-meat ingredients in meat processing: The current toolbox. In Proceedings of the 68th Annual Reciprocal Meat Conference, Lincoln, NE, USA, 18 December 2015; pp. 42–46. [Google Scholar]
- Kettunen, J.; Ritchie, S.C.; Anufrieva, O.; Lyytikäinen, L.-P.; Hernesniemi, J.; Karhunen, P.J.; Kuukasjärvi, P.; Laurikka, J.; Kähönen, M.; Lehtimäki, T.; et al. Biomarker Glycoprotein Acetyls Is Associated with the Risk of a Wide Spectrum of Incident Diseases and Stratifies Mortality Risk in Angiography Patients. Circ. Genom. Precis. Med. 2018, 11, e002234. [Google Scholar] [CrossRef]
- Coffey, D.S. Similarities of prostate and breast cancer: Evolution, diet, and estrogens. Urology 2001, 57, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Fry, A.; Littlejohns, T.J.; Sudlow, C.; Doherty, N.; Adamska, L.; Sprosen, T.; Collins, R.; Allen, N.E. Comparison of Sociodemographic and Health-Related Characteristics of UK Biobank Participants with Those of the General Population. Am. J. Epidemiol. 2017, 186, 1026–1034. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, B.; Zhao, J.V. Sex-Specific Associations of Red Meat and Processed Meat Consumption with Serum Metabolites in the UK Biobank. Nutrients 2022, 14, 5306. https://doi.org/10.3390/nu14245306
Fan B, Zhao JV. Sex-Specific Associations of Red Meat and Processed Meat Consumption with Serum Metabolites in the UK Biobank. Nutrients. 2022; 14(24):5306. https://doi.org/10.3390/nu14245306
Chicago/Turabian StyleFan, Bohan, and Jie V. Zhao. 2022. "Sex-Specific Associations of Red Meat and Processed Meat Consumption with Serum Metabolites in the UK Biobank" Nutrients 14, no. 24: 5306. https://doi.org/10.3390/nu14245306
APA StyleFan, B., & Zhao, J. V. (2022). Sex-Specific Associations of Red Meat and Processed Meat Consumption with Serum Metabolites in the UK Biobank. Nutrients, 14(24), 5306. https://doi.org/10.3390/nu14245306






