Bidirectional Associations between Daytime Napping Duration and Metabolic Syndrome: A Nationally Representative Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethics
2.3. Study Population
2.4. Measurements of MetS Status
2.5. Assessment of Sleep-Related Variables
2.6. Assessment of Covariates
2.6.1. Sociodemographic Characteristics
2.6.2. Health-Related Factors
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics
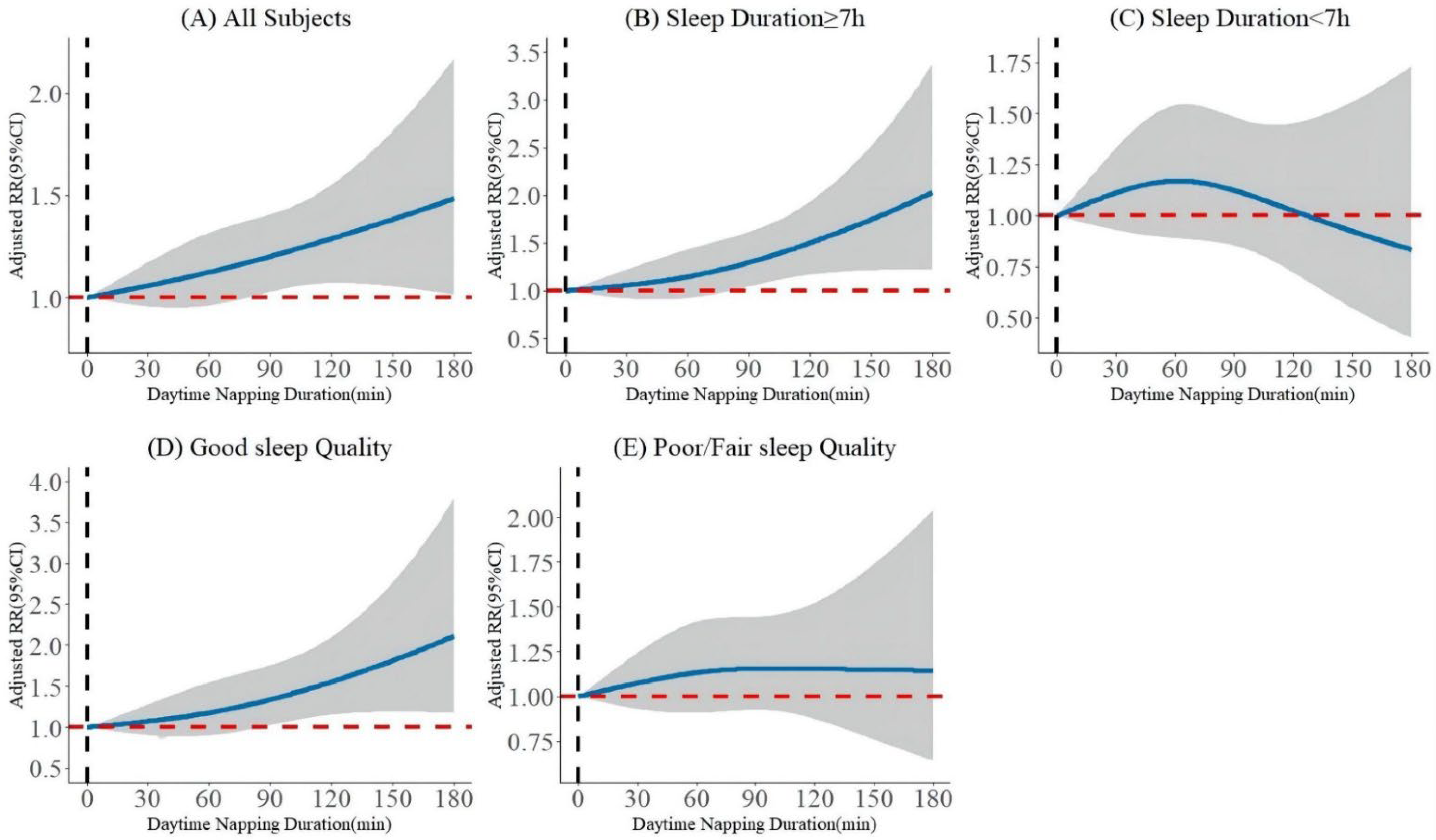
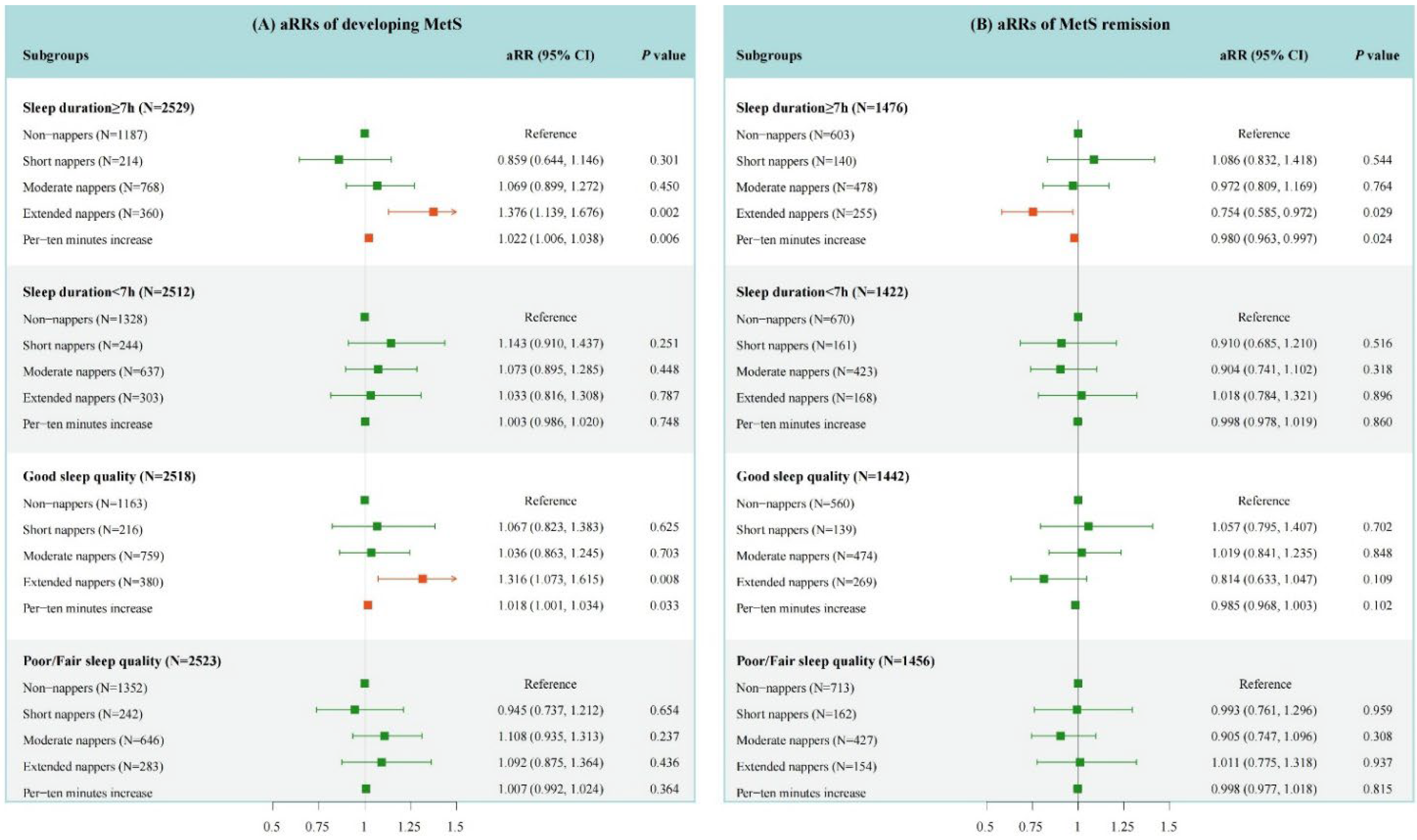
3.2. Association of Baseline Daytime Napping Duration with the Occurrence of MetS
3.3. Association of Daytime Napping Duration with the Remission of MetS
3.4. Association of Baseline MetS Status with Changes in Daytime Napping Duration
3.5. Sensitivity Analysis
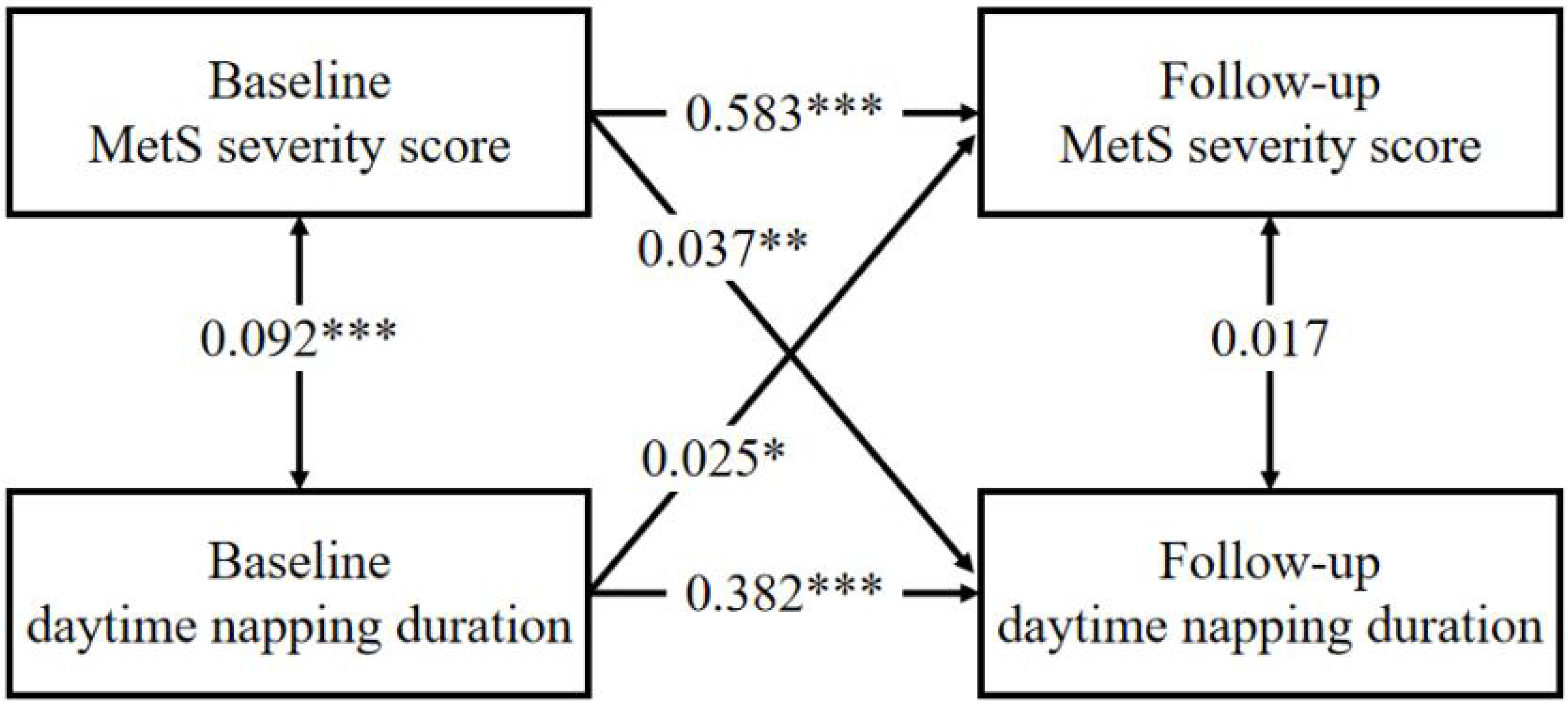
3.6. Cross-Lagged Panel Analysis
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sherling, D.H.; Perumareddi, P.; Hennekens, C.H. Metabolic Syndrome. J. Cardiovasc. Pharm. Ther. 2017, 22, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Mottillo, S.; Filion, K.B.; Genest, J.; Joseph, L.; Pilote, L.; Poirier, P.; Rinfret, S.; Schiffrin, E.L.; Eisenberg, M.J. The Metabolic Syndrome and Cardiovascular Risk: A Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 2010, 56, 1113–1132. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S. Risks for All-Cause Mortality, Cardiovascular Disease, and Diabetes Associated with the Metabolic Syndrome: A Summary of the Evidence. Diabetes Care 2005, 28, 1769–1778. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xu, Z.; He, M.; Yang, H.; Li, X.; Min, X.; Zhang, C.; Xu, C.; Angileri, F.; Légaré, S.; et al. Sleep Duration and Midday Napping with 5-Year Incidence and Reversion of Metabolic Syndrome in Middle-Aged and Older Chinese. Sleep 2016, 39, 1911–1918. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-J.; Zhang, Z.; Wang, Y.-J.; Li, J.-C.; Guo, Q.-L.; Chen, X.; Wang, E. Association of Nap Frequency with Hypertension or Ischemic Stroke Supported by Prospective Cohort Data and Mendelian Randomization in Predominantly Middle-Aged European Subjects. Hypertension 2022, 79, 1962–1970. [Google Scholar] [CrossRef]
- Li, J.; Cacchione, P.; Hodgson, N.; Riegel, B.; Ms, B.T.K.; Scharf, M.T.; Richards, K.; Gooneratne, N.S. Afternoon Napping and Cognition in Chinese Older Adults: Findings from the China Health and Retirement Longitudinal Study Baseline Assessment. J. Am. Geriatr. Soc. 2016, 65, 373–380. [Google Scholar] [CrossRef]
- He, J.; Ouyang, F.; Qiu, D.; Duan, Y.; Luo, D.; Xiao, S. Association of Nap Duration after Lunch with Prevalence of Metabolic Syndrome in a Chinese Government Employee Population. Int. J. Environ. Res. Public Health 2020, 17, 4268. [Google Scholar] [CrossRef]
- Pan, Z.; Huang, M.; Huang, J.; Yao, Z.; Lin, Z. Association of napping and all-cause mortality and incident cardiovascular diseases: A dose–response meta analysis of cohort studies. Sleep Med. 2020, 74, 165–172. [Google Scholar] [CrossRef]
- Lovato, N.; Lack, L. The effects of napping on cognitive functioning. Prog. Brain Res. 2010, 185, 155–166. [Google Scholar] [CrossRef]
- Mantua, J.; Spencer, R.M.C. Exploring the Nap Paradox: Are Mid-Day Sleep Bouts a Friend or Foe? Sleep Med. 2017, 37, 88–97. [Google Scholar] [CrossRef]
- Vgontzas, A.N.; Pejovic, S.; Zoumakis, E.; Lin, H.M.; Bixler, E.O.; Basta, M.; Fang, J.; Sarrigiannidis, A.; Chrousos, G.P. Daytime napping after a night of sleep loss decreases sleepiness, improves performance, and causes beneficial changes in cortisol and interleukin-6 secretion. Am. J. Physiol. Metab. 2007, 292, E253–E261. [Google Scholar] [CrossRef]
- Faraut, B.; Nakib, S.; Drogou, C.; Elbaz, M.; Sauvet, F.; De Bandt, J.-P.; Léger, D. Napping Reverses the Salivary Interleukin-6 and Urinary Norepinephrine Changes Induced by Sleep Restriction. J. Clin. Endocrinol. Metab. 2015, 100, E416–E426. [Google Scholar] [CrossRef]
- Wang, C.; Bangdiwala, S.I.; Rangarajan, S.; Lear, S.A.; AlHabib, K.F.; Mohan, V.; Teo, K.; Poirier, P.; Tse, L.A.; Liu, Z.; et al. Association of estimated sleep duration and naps with mortality and cardiovascular events: A study of 116,632 people from 21 countries. Eur. Heart J. 2018, 40, 1620–1629. [Google Scholar] [CrossRef]
- Li, W.; Kondracki, A.J.; Sun, N.; Gautam, P.; Kalan, M.E.; Jebai, R.; Gbadamosi, S.O.; Sun, W. Nighttime sleep duration, daytime napping, and metabolic syndrome: Findings from the China Health and Retirement Longitudinal Study. Sleep Breath. 2021, 26, 1427–1435. [Google Scholar] [CrossRef]
- Gribble, A.K.; Sayón-Orea, C.; Bes-Rastrollo, M.; Kales, S.N.; Shirahama, R.; Martínez-González, M.; Fernandez-Montero, A. Risk of Developing Metabolic Syndrome Is Affected by Length of Daily Siesta: Results from a Prospective Cohort Study. Nutrients 2021, 13, 4182. [Google Scholar] [CrossRef]
- Wu, J.; Xu, G.; Shen, L.; Zhang, Y.; Song, L.; Yang, S.; Yang, H.; Liang, Y.; Wu, T.; Wang, Y. Daily sleep duration and risk of metabolic syndrome among middle-aged and older Chinese adults: Cross-Sectional evidence from the Dongfeng-Tongji cohort study. BMC Public Health 2015, 15, 178. [Google Scholar] [CrossRef]
- Lin, D.; Sun, K.; Li, F.; Qi, Y.; Ren, M.; Huang, C.; Tang, J.; Xue, S.; Li, Y.; Yan, L. Association between habitual daytime napping and metabolic syndrome: A population-based study. Metabolism 2014, 63, 1520–1527. [Google Scholar] [CrossRef]
- Qian, Y.-X.; Liu, J.-H.; Ma, Q.-H.; Sun, H.-P.; Xu, Y.; Pan, C.-W. Associations of sleep durations and sleep-related parameters with metabolic syndrome among older Chinese adults. Endocrine 2019, 66, 240–248. [Google Scholar] [CrossRef]
- van der Pal, K.C.; Koopman, A.D.; Lakerveld, J.; van der Heijden, A.A.; Elders, P.J.; Beulens, J.W.; Rutters, F. The association between multiple sleep-related characteristics and the metabolic syndrome in the general population: The New Hoorn study. Sleep Med. 2018, 52, 51–57. [Google Scholar] [CrossRef]
- Ghazizadeh, H.; Mobarra, N.; Esmaily, H.; Seyedi, S.M.R.; Amiri, A.; Rezaeitalab, F.; Mokhber, N.; Moohebati, M.; Ebrahimi, M.; Tayebi, M.; et al. The association between daily naps and metabolic syndrome: Evidence from a population-based study in the Middle-East. Sleep Health 2020, 6, 684–689. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, Y.; Smith, J.P.; Strauss, J.; Yang, G. Cohort Profile: The China Health and Retirement Longitudinal Study (CHARLS). Int. J. Epidemiol. 2014, 43, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Crimmins, E.; Hu, P.P.; Kim, J.K.; Meng, Q.; Strauss, J.; Wang, Y.; Zeng, J.; Zhang, Y.; Zhao, Y. Venous Blood-Based Biomarkers in the China Health and Retirement Longitudinal Study: Rationale, Design, and Results from the 2015 Wave. Am. J. Epidemiol. 2019, 188, 1871–1877. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed]
- Chen, W.-Y.; Fu, Y.-P.; Zhou, M. The bidirectional relationship between metabolic syndrome and hyperuricemia in China: A longitudinal study from CHARLS. Endocrine 2022, 76, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zou, J.; Fang, S.; Zhou, J. Association between daytime napping and obesity in Chinese middle-aged and older adults. J. Glob. Health 2020, 10, 020804. [Google Scholar] [CrossRef]
- Liu, H.; Byles, J.E.; Xu, X.; Zhang, M.; Wu, X.; Hall, J.J. Association between nighttime sleep and successful aging among older Chinese people. Sleep Med. 2016, 22, 18–24. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, X.; Moore, J.; Wang, B.; Li, R. Midday Nap Duration and Hypertension among Middle-Aged and Older Chinese Adults: A Nationwide Retrospective Cohort Study. Int. J. Environ. Res. Public Health 2021, 18, 3680. [Google Scholar] [CrossRef]
- Jing, R.; Xu, T.; Rong, H.; Lai, X.; Fang, H. Longitudinal Association Between Sleep Duration and Depressive Symptoms in Chinese Elderly. Nat. Sci. Sleep 2020, 12, 737–747. [Google Scholar] [CrossRef]
- Consensus Conference Panel; Watson, N.F.; Badr, M.S.; Belenky, G.; Bliwise, D.L.; Buxton, O.M.; Buysse, D.; Dinges, D.F.; Gangwisch, J.; Grandner, M.A.; et al. Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society on the Recommended Amount of Sleep for a Healthy Adult: Methodology and Discussion. J. Clin. Sleep Med. 2015, 11, 931–952. [Google Scholar] [CrossRef]
- Andresen, E.M.; Malmgren, J.A.; Carter, W.B.; Patrick, D.L. Screening for depression in well older adults: Evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am. J. Prev. Med. 1994, 10, 77–84. [Google Scholar] [CrossRef]
- Li, H.; Zheng, D.; Li, Z.; Wu, Z.; Feng, W.; Cao, X.; Wang, J.; Gao, Q.; Li, X.; Wang, W.; et al. Association of Depressive Symptoms with Incident Cardiovascular Diseases in Middle-Aged and Older Chinese Adults. JAMA Netw. Open 2019, 2, e1916591. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, H.; Miao, X.; Li, H.; Pan, H.; Di Zhou, D.; Liu, Y.; Li, Z.; Wang, J.; Liu, X.; et al. High-Intensity physical activity is not associated with better cognition in the elder: Evidence from the China Health and Retirement Longitudinal Study. Alzheimer’s Res. Ther. 2021, 13, 182. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Yamada, T.; Shojima, N.; Yamauchi, T.; Kadowaki, T. J-curve relation between daytime nap duration and type 2 diabetes or metabolic syndrome: A dose-response meta-analysis. Sci. Rep. 2016, 6, 38075. [Google Scholar] [CrossRef]
- Lin, L.; Lu, C.; Chen, W.; Guo, V. Daytime Napping and Nighttime Sleep Duration with Incident Diabetes Mellitus: A Cohort Study in Chinese Older Adults. Int. J. Environ. Res. Public Health 2021, 18, 5012. [Google Scholar] [CrossRef]
- Shi, S.-Q.; Ansari, T.S.; McGuinness, O.P.; Wasserman, D.H.; Johnson, C.H. Circadian Disruption Leads to Insulin Resistance and Obesity. Curr. Biol. 2013, 23, 372–381. [Google Scholar] [CrossRef]
- Poggiogalle, E.; Jamshed, H.; Peterson, C.M. Circadian Regulation of Glucose, Lipid, and Energy Metabolism in Humans. Metabolism 2018, 84, 11–27. [Google Scholar] [CrossRef]
- Woods, D.L.; Kim, H.; Yefimova, M. To Nap or Not to Nap: Excessive Daytime Napping Is Associated with Elevated Evening Cortisol in Nursing Home Residents with Dementia. Biol. Res. Nurs. 2013, 15, 185–190. [Google Scholar] [CrossRef]
- Parish, J.M.; Adam, T.; Facchiano, L. Relationship of metabolic syndrome and obstructive sleep apnea. J. Clin. Sleep Med. 2007, 3, 467–472. [Google Scholar] [CrossRef]
- Drager, L.F.; Togeiro, S.M.; Polotsky, V.Y.; Lorenzi-Filho, G. Obstructive Sleep Apnea: A Cardiometabolic Risk in Obesity and the Metabolic Syndrome. J. Am. Coll. Cardiol. 2013, 62, 569–576. [Google Scholar] [CrossRef]
- Mulcahy, D.; Wright, C.; Sparrow, J.; Cunningham, D.; Curcher, D.; Purcell, H.; Fox, K. Heart rate and blood pressure consequences of an afternoon SIESTA (Snooze-Induced Excitation of Sympathetic Triggered Activity). Am. J. Cardiol. 1993, 71, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Ahmadi-Abhari, S.; Wainwright, N.W.J.; Cappuccio, F.P.; Surtees, P.G.; Luben, R.; Brayne, C.; Khaw, K.-T. Daytime napping, sleep duration and serum C reactive protein: A population-based cohort study. BMJ Open 2014, 4, e006071. [Google Scholar] [CrossRef] [PubMed]
- Lian, Y.; Yuan, Q.; Wang, G.; Tang, F. Association between sleep quality and metabolic syndrome: A systematic review and meta-analysis. Psychiatry Res. 2019, 274, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Van Cauter, E.; Spiegel, K.; Tasali, E.; Leproult, R. Metabolic consequences of sleep and sleep loss. Sleep Med. 2008, 9 (Suppl. 1), S23–S28. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Li, Y.; Zhang, Y.; Vgontzas, A.N.; Basta, M.; Chen, B.; Xu, C.; Tang, X. Sleep duration and metabolic syndrome: An updated systematic review and meta-analysis. Sleep Med. Rev. 2021, 59, 101451. [Google Scholar] [CrossRef]
- Häusler, N.; Marques-Vidal, P.; Haba-Rubio, J.; Heinzer, R. Does sleep predict next-day napping or does napping influence same-day nocturnal sleep? Results of a population-based ecological momentary assessment study. Sleep Med. 2019, 61, 31–36. [Google Scholar] [CrossRef]
- Léger, D.; Torres, M.J.; Bayon, V.; Hercberg, S.; Galan, P.; Chennaoui, M.; Andreeva, V.A. The association between physical and mental chronic conditions and napping. Sci. Rep. 2019, 9, 1795. [Google Scholar] [CrossRef]
- Pyykkönen, A.-J.; Isomaa, B.; Pesonen, A.-K.; Eriksson, J.; Groop, L.; Tuomi, T.; Räikkönen, K. Sleep duration and insulin resistance in individuals without type 2 diabetes: The PPP-Botnia Study. Ann. Med. 2014, 46, 324–329. [Google Scholar] [CrossRef]
| Characteristics | Sub-Cohort 1 | Sub-Cohort 2 | Sub-Cohort 3 |
|---|---|---|---|
| No. of participants | 5041 | 2898 | 11,390 |
| Age (years), mean (SD) | 57.89 (9.12) | 58.53 (8.77) | 58.04 (9.30) |
| Male, n (%) | 2711 (53.8) | 1006 (34.7) | 5426 (47.6) |
| Married, n (%) | 4333 (86.0) | 2458 (84.8) | 9636 (84.6) |
| Elementary school or above, n (%) | 2808 (55.7) | 1496 (51.6) | 6254 (54.9) |
| Rural residence, n (%) | 4337 (86.0) | 2345 (80.9) | 9362 (82.2) |
| Smoking status, n (%) | |||
| Current smoker | 1832 (36.3) | 656 (22.6) | 3584 (31.5) |
| Former smoker | 390 (7.7) | 199 (6.9) | 838 (7.4) |
| Non-smoker | 2819 (55.9) | 2043 (70.5) | 6968 (61.2) |
| Drinking status, n (%) | |||
| More than once a month | 1490 (29.6) | 578 (19.9) | 3000 (26.3) |
| Drink but less than once a month | 417 (8.3) | 223 (7.7) | 888 (7.8) |
| Never | 3134 (62.2) | 2097 (72.4) | 7502 (65.9) |
| Physical activity, n (%) | |||
| None | 521 (10.3) | 388 (13.4) | 1407 (12.4) |
| Mild | 1001 (19.9) | 795 (27.4) | 2433 (21.4) |
| Moderate | 1520 (30.2) | 936 (32.3) | 3424 (30.1) |
| Vigorous | 1999 (39.7) | 779 (26.9) | 4126 (36.2) |
| Depressive symptoms, n (%) | 1863 (37.0) | 1030 (35.5) | 3705 (34.4) |
| BMI (kg/m2), mean (SD) | 23.10 (4.34) | 25.83 (3.76) | 23.63 (4.31) |
| SUA (mg/dL), mean (SD) | 4.29 (1.24) | 4.58 (1.52) | 4.42 (1.23) |
| HsCRP (mg/L), median [IQR] | 0.87 [0.49, 2.07] | 1.31 [0.68, 2.50] | 0.99 [0.54, 2.12] |
| LDL-C (mg/dL), mean (SD) | 99.51 (40.41) | 131.84 (54.20) | 118.38 (35.40) |
| Antihypertensive agents, n (%) | 349 (6.9) | 855 (29.5) | 1670 (14.7) |
| Hypoglycemic agents, n (%) | 45 (0.9) | 205 (7.1) | 335 (2.9) |
| Lipid-lowering agents, n (%) | 21 (0.4) | 318 (11.0) | 426 (3.7) |
| sleeping pills/anti-depressive treatment, n (%) | 30 (0.6) | 13 (0.4) | 66 (0.6) |
| Good sleep quality, n (%) | 2518 (50.0) | 1442 (49.8) | 5770 (50.7) |
| Night-time sleep duration (h), mean (SD) | 6.40 (1.89) | 6.45 (1.85) | 6.43 (1.86) |
| Napping duration in 2011 (min/day), mean (SD) | 36.29 (44.46) | 40.27 (44.56) | 37.37 (44.07) |
| Napping duration in 2011, n (%) | |||
| 0 min/day | 2515 (49.9) | 1273 (43.9) | - |
| ≤30 min/day | 458 (9.1) | 301 (10.4) | - |
| 30–90 min/day | 1405 (27.9) | 901 (31.1) | - |
| >90 min/day | 663 (13.2) | 423 (14.6) | - |
| With MetS at baseline, n (%) | 0 (0.0) | 2898 (100.0) | 3936 (34.6) |
| Outcome variables | |||
| The incidence of MetS, n (%) | 1126 (22.3) | - | - |
| The reversion of MetS, n (%) | - | 828 (28.6) | - |
| Napping duration in 2013 (min/day), mean (SD) | - | - | 42.34 (46.30) |
| Napping duration in 2015 (min/day), mean (SD) | - | - | 42.39 (46.11) |
| N | Case, n (%) | Model 1 | Model 2 | |||
|---|---|---|---|---|---|---|
| aRR (95% CI) | p | aRR (95% CI) | p | |||
| Occurrence of MetS (N = 5041) | ||||||
| per-ten minutes increase | 1.014 (1.003, 1.026) | 0.012 | 1.013 (1.002, 1.024) | 0.027 | ||
| non-nappers a | 2515 | 556 (22.1) | Reference | - | Reference | - |
| short nappers a | 458 | 105 (22.9) | 1.033 (0.863, 1.237) | 0.726 | 1.008 (0.843, 1.205) | 0.933 |
| moderate nappers a | 1405 | 300 (21.4) | 1.087 (0.961, 1.230) | 0.185 | 1.072 (0.946, 1.215) | 0.275 |
| extended nappers a | 663 | 165 (24.9) | 1.242 (1.071, 1.441) | 0.004 | 1.216 (1.047, 1.413) | 0.011 |
| Remission of MetS (N = 2898) | ||||||
| per-ten minutes increase | 0.989 (0.976, 1.002) | 0.094 | 0.991 (0.978, 1.005) | 0.203 | ||
| non-nappers a | 1273 | 370 (29.1) | Reference | - | Reference | - |
| short nappers a | 301 | 90 (29.9) | 1.013 (0.835, 1.230) | 0.894 | 1.029 (0.847, 1.251) | 0.773 |
| moderate nappers a | 901 | 257 (28.5) | 0.949 (0.829, 1.086) | 0.445 | 0.971 (0.848, 1.111) | 0.667 |
| extended nappers a | 423 | 111 (26.2) | 0.867 (0.722, 1.040) | 0.123 | 0.892 (0.741, 1.073) | 0.224 |
| N | Model 1 | Model 2 | |||
|---|---|---|---|---|---|
| β (95% CI) | p Value | β (95% CI) | p Value | ||
| Baseline MetS status | |||||
| Without MetS | 7454 | Reference | Reference | ||
| With MetS | 3936 | 5.081 (3.524, 6.637) | <0.001 | 2.745 (1.360, 4.130) | <0.001 |
| Number of MetS components (MetS severity score) | |||||
| 0 component | 1102 | Reference | Reference | ||
| 1 component | 3204 | −0.289 (−2.984, 2.406) | 0.834 | 0.281 (−2.122, 2.683) | 0.819 |
| 2 components | 3148 | 2.411 (−0.306, 5.129) | 0.082 | 2.047 (−0.380, 4.474) | 0.098 |
| 3 components | 2192 | 4.572 (1.704, 7.440) | 0.002 | 2.837 (0.266, 5.408) | 0.031 |
| 4 components | 1160 | 6.454 (3.212, 9.696) | <0.001 | 4.032 (1.146, 6.918) | 0.006 |
| 5 components | 584 | 10.608 (6.533, 14.683) | <0.001 | 7.053 (3.435, 10.670) | <0.001 |
| Baseline MetS components status | |||||
| Without hyperglycemia | 7018 | Reference | Reference | ||
| With hyperglycemia | 4372 | 3.897 (2.382, 5.412) | <0.001 | 2.236 (0.903, 3.570) | 0.001 |
| Without hypertriglyceridemia | 8666 | Reference | Reference | ||
| With hypertriglyceridemia | 2724 | 3.393 (1.682, 5.103) | <0.001 | 2.426 (0.903, 3.948) | 0.002 |
| Without low HDL-C | 4759 | Reference | Reference | ||
| With low HDL-C | 6631 | 0.989 (−0.515, 2.494) | 0.197 | 0.278 (−1.062, 1.618) | 0.684 |
| Without hypertension | 6126 | Reference | Reference | ||
| With hypertension | 5264 | 2.742 (1.238, 4.246) | <0.001 | 1.967 (0.633, 3.301) | 0.004 |
| Without central obesity | 6745 | Reference | Reference | ||
| With central obesity | 4645 | 5.368 (3.795, 6.940) | <0.001 | 2.710 (1.309, 4.111) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wu, Z.; Jin, X.; Jin, R.; Han, Z.; Zhang, H.; Xu, Z.; Liu, Y.; Guo, X.; Tao, L. Bidirectional Associations between Daytime Napping Duration and Metabolic Syndrome: A Nationally Representative Cohort Study. Nutrients 2022, 14, 5292. https://doi.org/10.3390/nu14245292
Wang J, Wu Z, Jin X, Jin R, Han Z, Zhang H, Xu Z, Liu Y, Guo X, Tao L. Bidirectional Associations between Daytime Napping Duration and Metabolic Syndrome: A Nationally Representative Cohort Study. Nutrients. 2022; 14(24):5292. https://doi.org/10.3390/nu14245292
Chicago/Turabian StyleWang, Jinqi, Zhiyuan Wu, Xiaohan Jin, Rui Jin, Ze Han, Haiping Zhang, Zongkai Xu, Yue Liu, Xiuhua Guo, and Lixin Tao. 2022. "Bidirectional Associations between Daytime Napping Duration and Metabolic Syndrome: A Nationally Representative Cohort Study" Nutrients 14, no. 24: 5292. https://doi.org/10.3390/nu14245292
APA StyleWang, J., Wu, Z., Jin, X., Jin, R., Han, Z., Zhang, H., Xu, Z., Liu, Y., Guo, X., & Tao, L. (2022). Bidirectional Associations between Daytime Napping Duration and Metabolic Syndrome: A Nationally Representative Cohort Study. Nutrients, 14(24), 5292. https://doi.org/10.3390/nu14245292







