Bioactive Oligopeptides from Ginseng (Panax ginseng Meyer) Suppress Oxidative Stress-Induced Senescence in Fibroblasts via NAD+/SIRT1/PGC-1α Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture and Treatments
2.3. Cell Viability Assay
2.4. Flow Cytometry
2.5. Biochemical Analysis
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
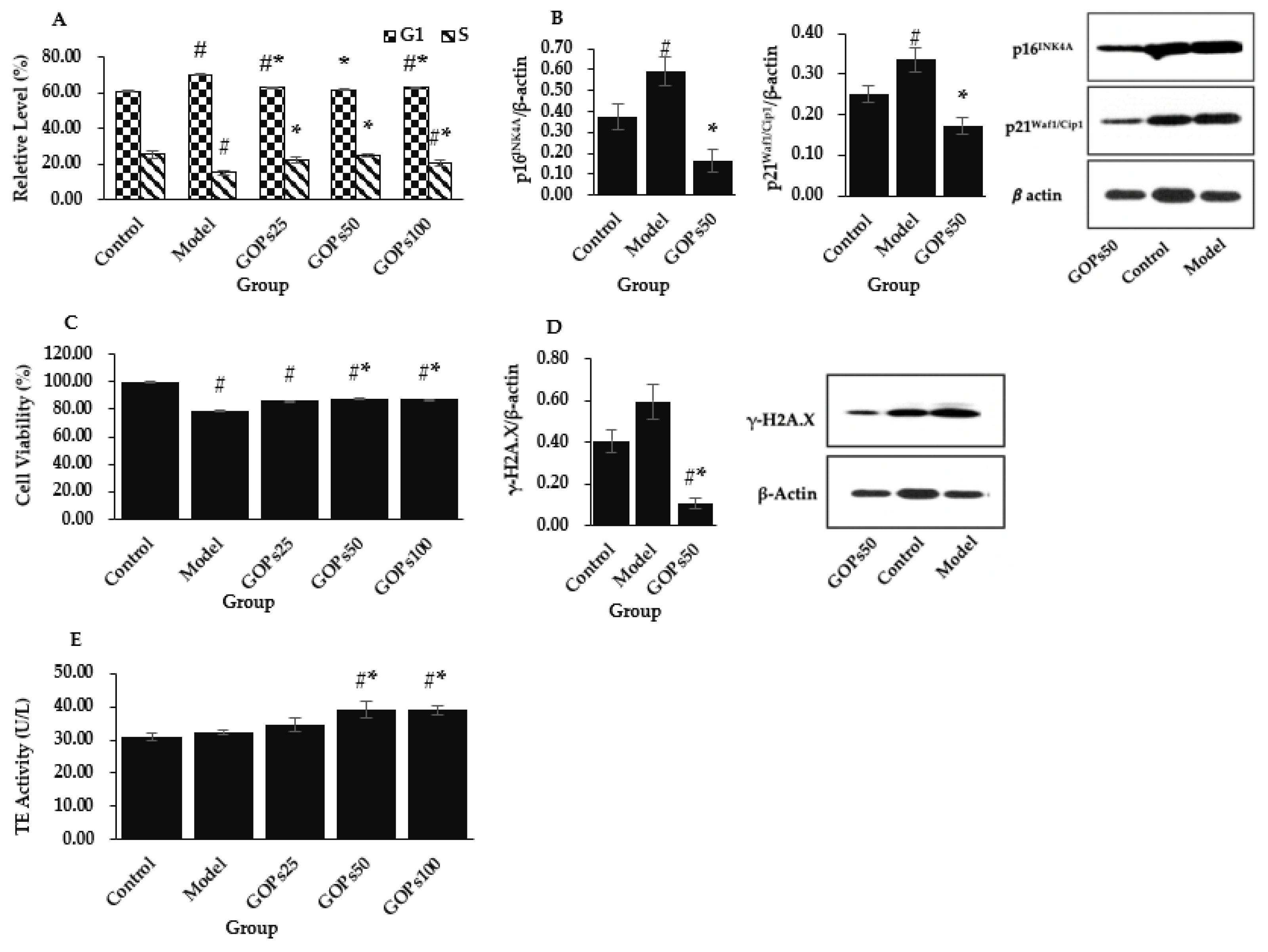
3.1. Effect of GOPs on Hallmarks of NIH/3T3 Senescence
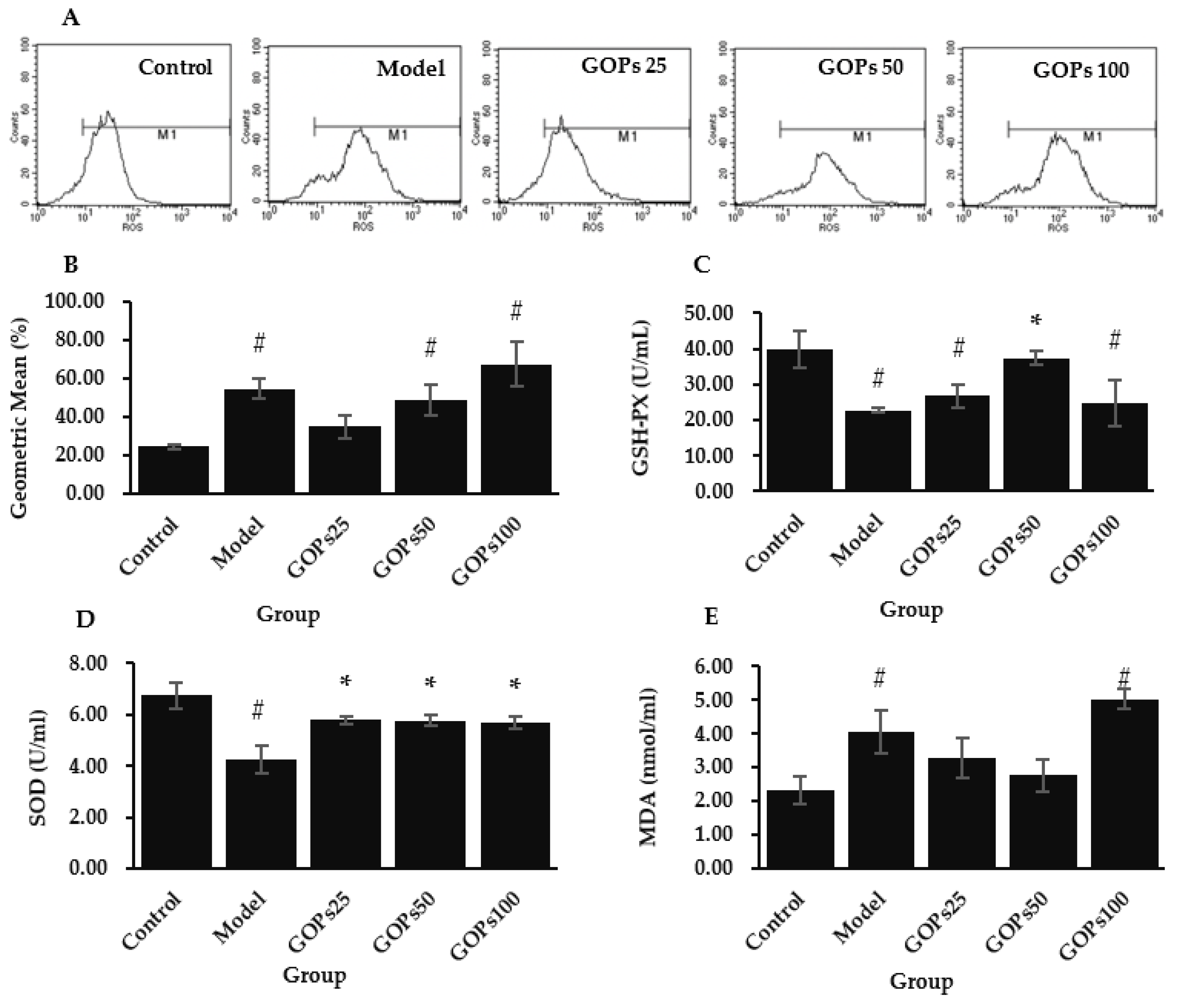
3.2. Effect of GOPs on Oxidative Stress Status
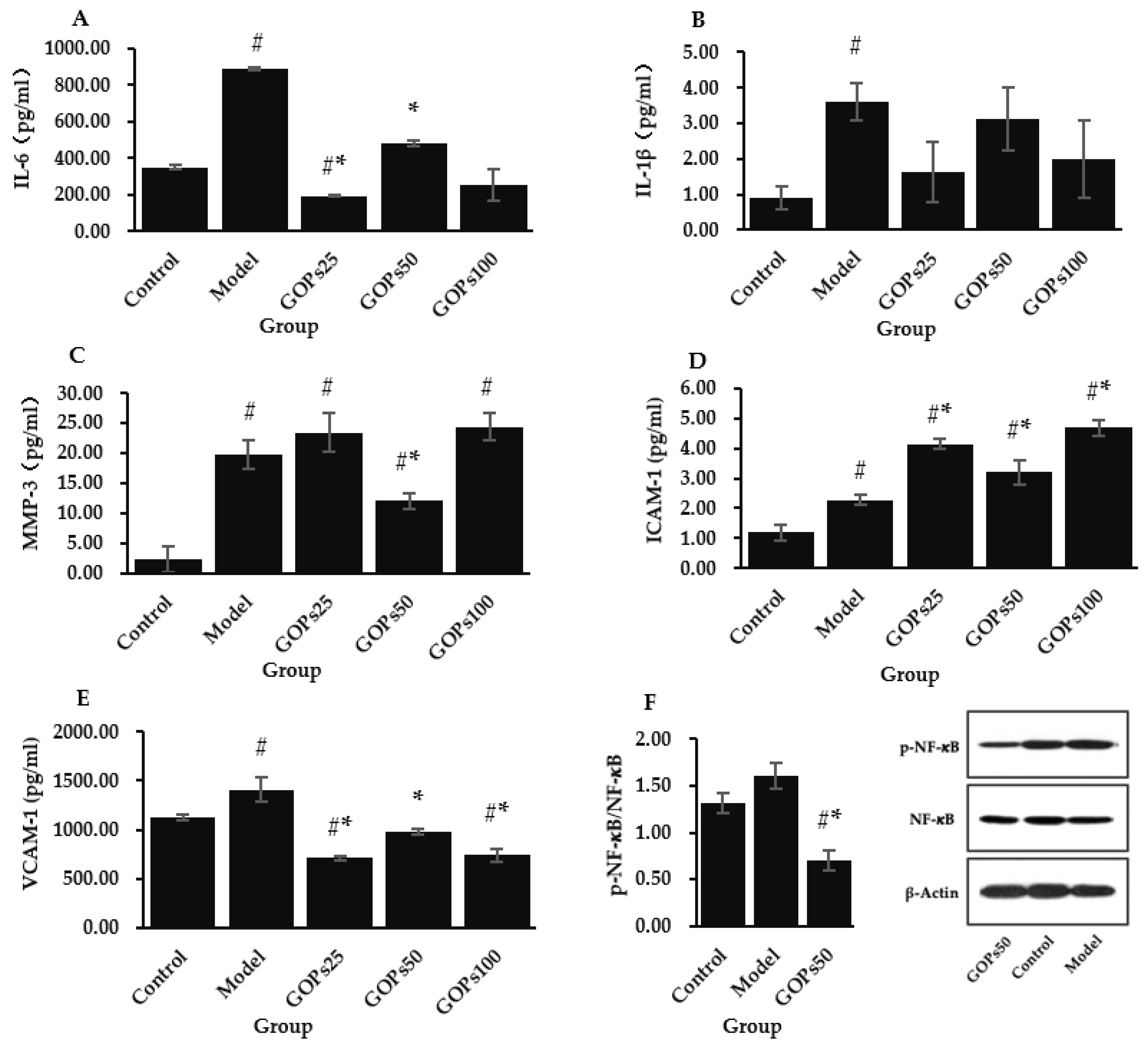
3.3. Effect of GOPs on Senescence-Associated Secretory Phenotype (SASP)
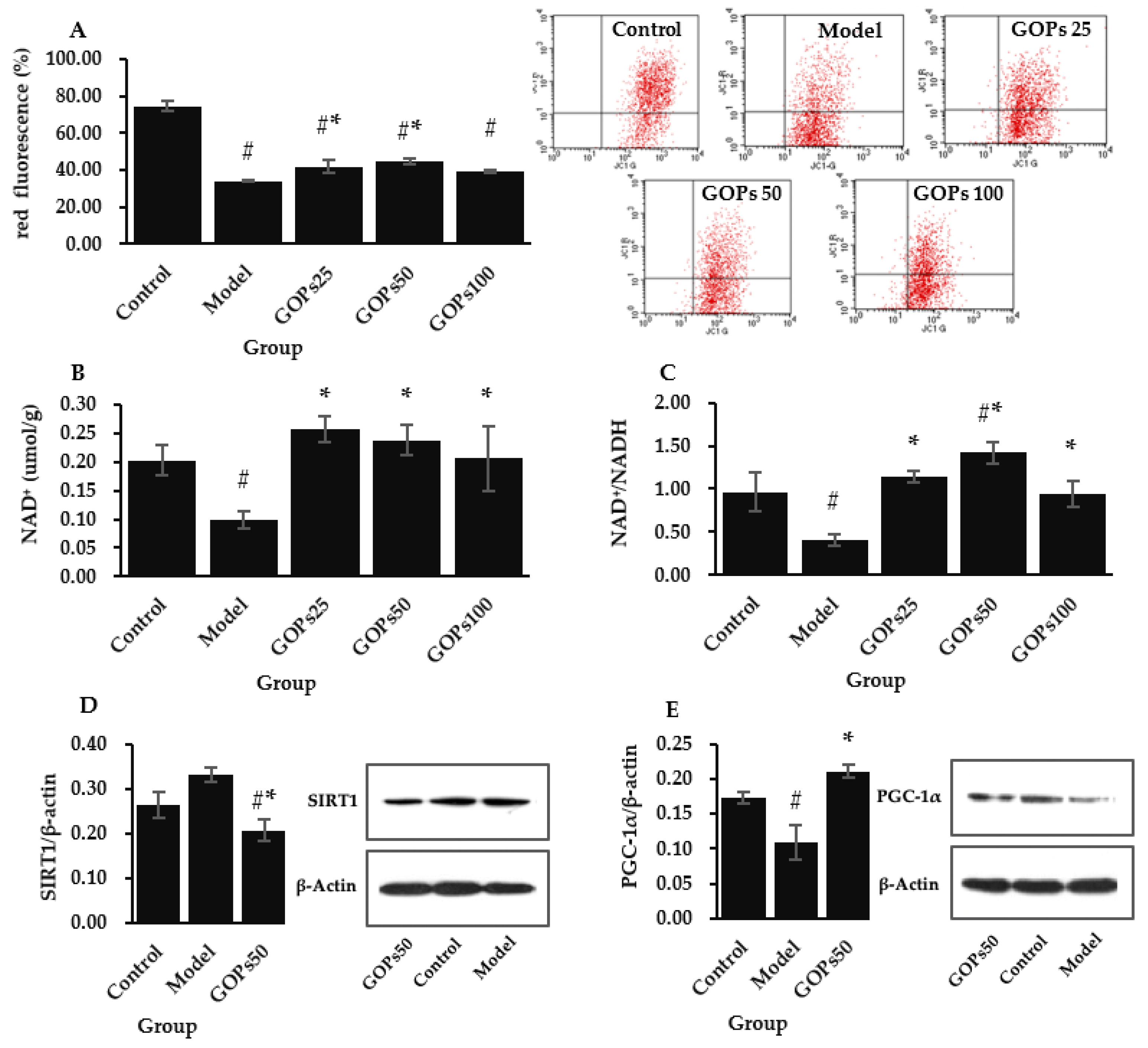
3.4. Effect of GOPs on Mitochondrial Function and Biogenesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Kitada, M.; Ogura, Y.; Monno, I.; Koya, D. The impact of dietary protein intake on longevity and metabolic health. EBioMedicine 2019, 43, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Shoveller, A.K.; McKnight, L.M.; Wood, K.M.; Cant, J.P. Lessons from animal nutritionists: Dietary amino acid requirement studies and considerations for healthy aging studies. Ann. N. Y. Acad. Sci. 2018, 1418, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.E.; Suarez, J.A.; Brandhorst, S.; Balasubramanian, P.; Cheng, C.W.; Madia, F.; Fontana, L.; Mirisola, M.G.; Guevara-Aguirre, J.; Wan, J.; et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014, 19, 407–417. [Google Scholar] [CrossRef]
- Ortolá, R.; Struijk, E.A.; García-Esquinas, E.; Rodríguez-Artalejo, F.; Lopez-Garcia, E. Changes in Dietary Intake of Animal and Vegetable Protein and Unhealthy Aging. Am. J. Med. 2020, 133, 231–239.e237. [Google Scholar] [CrossRef]
- Romano, C.; Corsetti, G.; Flati, V.; Pasini, E.; Picca, A.; Calvani, R.; Marzetti, E.; Dioguardi, F.S. Influence of Diets with Varying Essential/Nonessential Amino Acid Ratios on Mouse Lifespan. Nutrients 2019, 11, 1367. [Google Scholar] [CrossRef]
- Yoon, M.S. The Emerging Role of Branched-Chain Amino Acids in Insulin Resistance and Metabolism. Nutrients 2016, 8, 405. [Google Scholar] [CrossRef] [PubMed]
- Traylor, D.A.; Gorissen, S.H.M.; Phillips, S.M. Perspective: Protein Requirements and Optimal Intakes in Aging: Are We Ready to Recommend More Than the Recommended Daily Allowance? Adv. Nutr. 2018, 9, 171–182. [Google Scholar] [CrossRef]
- Rafii, M.; Chapman, K.; Elango, R.; Campbell, W.W.; Ball, R.O.; Pencharz, P.B.; Courtney-Martin, G. Dietary Protein Requirement of Men >65 Years Old Determined by the Indicator Amino Acid Oxidation Technique Is Higher than the Current Estimated Average Requirement. J. Nutr. 2015, 146, 681–687. [Google Scholar] [CrossRef]
- Vogler, B.K.; Pittler, M.H.; Ernst, E. The efficacy of ginseng. A systematic review of randomised clinical trials. Eur. J. Clin. Pharmacol. 1999, 55, 567–575. [Google Scholar] [CrossRef]
- Kim, Y.J.; Zhang, D.; Yang, D.C. Biosynthesis and biotechnological production of ginsenosides. Biotechnol. Adv. 2015, 33, 717–735. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ren, C.; Zhang, Y.; Wu, X. Ginseng: An Nonnegligible Natural Remedy for Healthy Aging. Aging Dis. 2017, 8, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S. Korean Red Ginseng Tonic Extends Lifespan in D. melanogaster. Biomol. Ther. 2013, 21, 241–245. [Google Scholar] [CrossRef]
- Lee, J.H.; Choi, S.H.; Kwon, O.S.; Shin, T.J.; Lee, J.H.; Lee, B.H.; Yoon, I.S.; Pyo, M.K.; Rhim, H.; Lim, Y.H.; et al. Effects of ginsenosides, active ingredients of Panax ginseng, on development, growth, and life span of Caenorhabditis elegans. Biol. Pharm. Bull. 2007, 30, 2126–2134. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Chen, Q.H.; Ren, J.W.; Sun, B.; Cai, X.X.; Li, D.; Mao, R.X.; Wu, X.; Li, Y. Ginseng (Panax ginseng Meyer) Oligopeptides Protect against Binge Drinking-Induced Liver Damage through Inhibiting Oxidative Stress and Inflammation in Rats. Nutrients 2018, 10, 1665. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Cai, X.; Wang, J.; Zhang, Y.; Sun, B.; Li, Y. Anti-Fatigue Effects of Small Molecule Oligopeptides Isolated from Panax ginseng C. A. Meyer in Mice. Nutrients 2016, 8, 807. [Google Scholar] [CrossRef]
- He, L.X.; Ren, J.W.; Liu, R.; Chen, Q.H.; Zhao, J.; Wu, X.; Zhang, Z.F.; Wang, J.B.; Pettinato, G.; Li, Y. Ginseng (Panax ginseng Meyer) oligopeptides regulate innate and adaptive immune responses in mice via increased macrophage phagocytosis capacity, NK cell activity and Th cells secretion. Food Funct. 2017, 8, 3523–3532. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Sun, B.; Li, D.; Mao, R.; Li, H.; Li, Y.; Wang, J. Beneficial Effects of Small Molecule Oligopeptides Isolated from Panax ginseng Meyer on Pancreatic Beta-Cell Dysfunction and Death in Diabetic Rats. Nutrients 2017, 9, 1061. [Google Scholar] [CrossRef]
- Schaafsma, G. Safety of protein hydrolysates, fractions thereof and bioactive peptides in human nutrition. Eur. J. Clin. Nutr. 2009, 63, 1161–1168. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Chen, Z.; Chen, Y.; Lin, Q.; Liang, Y. Exogenous Bioactive Peptides Have a Potential Therapeutic Role in Delaying Aging in Rodent Models. Int. J. Mol. Sci. 2022, 23, 1421. [Google Scholar] [CrossRef]
- Zhai, L.; Xu, X.; Liu, J.; Jing, C.; Yang, X.; Zhao, D.; Jiang, R.; Sun, L.W. A Novel Biochemical Study of Anti-Dermal Fibroblast Replicative Senescence Potential of Panax notoginseng Oligosaccharides. Front. Pharmacol. 2021, 12, 690538. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Cheng, L.; Quek, C.; Bellingham, S.A.; Hill, A.F. Novel miR-29b target regulation patterns are revealed in two different cell lines. Sci. Rep. 2019, 9, 17449. [Google Scholar] [CrossRef] [PubMed]
- Guimarães-Pinto, K.; Nascimento, D.O.; Corrêa-Ferreira, A.; Morrot, A.; Freire-de-Lima, C.G.; Lopes, M.F.; DosReis, G.A.; Filardy, A.A. Trypanosoma cruzi Infection Induces Cellular Stress Response and Senescence-Like Phenotype in Murine Fibroblasts. Front. Immunol. 2018, 9, 1569. [Google Scholar] [CrossRef]
- Kuilman, T.; Michaloglou, C.; Mooi, W.J.; Peeper, D.S. The essence of senescence. Genes Dev. 2010, 24, 2463–2479. [Google Scholar] [CrossRef] [PubMed]
- D’Adda di Fagagna, F. Living on a break: Cellular senescence as a DNA-damage response. Nat. Rev. Cancer 2008, 8, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Xu, M.; Yu, H.; Wang, L.; Li, X.; Rak, J.; Wang, S.; Zhao, R.C. Mesenchymal stem cell-derived small extracellular vesicles mitigate oxidative stress-induced senescence in endothelial cells via regulation of miR-146a/Src. Signal Transduct. Target Ther. 2021, 6, 354. [Google Scholar] [CrossRef]
- Wang, B.; Ke, W.; Wang, K.; Li, G.; Ma, L.; Lu, S.; Xiang, Q.; Liao, Z.; Luo, R.; Song, Y.; et al. Mechanosensitive Ion Channel Piezo1 Activated by Matrix Stiffness Regulates Oxidative Stress-Induced Senescence and Apoptosis in Human Intervertebral Disc Degeneration. Oxidative Med. Cell. Longev. 2021, 2021, 8884922. [Google Scholar] [CrossRef]
- Li, Y.F.; Ouyang, S.H.; Tu, L.F.; Wang, X.; Yuan, W.L.; Wang, G.E.; Wu, Y.P.; Duan, W.J.; Yu, H.M.; Fang, Z.Z.; et al. Caffeine Protects Skin from Oxidative Stress-Induced Senescence through the Activation of Autophagy. Theranostics 2018, 8, 5713–5730. [Google Scholar] [CrossRef]
- Muñoz-Espín, D.; Serrano, M. Cellular senescence: From physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014, 15, 482–496. [Google Scholar] [CrossRef]
- Pintado-Berninches, L.; Fernandez-Varas, B.; Benitez-Buelga, C.; Manguan-Garcia, C.; Serrano-Benitez, A.; Iarriccio, L.; Carrillo, J.; Guenechea, G.; Egusquiaguirre, S.P.; Pedraz, J.L.; et al. GSE4 peptide suppresses oxidative and telomere deficiencies in ataxia telangiectasia patient cells. Cell Death Differ. 2019, 26, 1998–2014. [Google Scholar] [CrossRef]
- Takubo, K.; Aida, J.; Izumiyama-Shimomura, N.; Ishikawa, N.; Sawabe, M.; Kurabayashi, R.; Shiraishi, H.; Arai, T.; Nakamura, K. Changes of telomere length with aging. Geriatr. Gerontol. Int. 2010, 10, S197–S206. [Google Scholar] [CrossRef] [PubMed]
- Nicolas-Espinosa, J.; Yepes-Molina, L.; Carvajal, M. Bioactive peptides from broccoli stems strongly enhance regenerative keratinocytes by stimulating controlled proliferation. Pharm. Biol. 2022, 60, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Edgar, S.; Hopley, B.; Genovese, L.; Sibilla, S.; Laight, D.; Shute, J. Effects of collagen-derived bioactive peptides and natural antioxidant compounds on proliferation and matrix protein synthesis by cultured normal human dermal fibroblasts. Sci. Rep. 2018, 8, 10474. [Google Scholar] [CrossRef] [PubMed]
- Moskalev, A.A.; Shaposhnikov, M.V.; Plyusnina, E.N.; Zhavoronkov, A.; Budovsky, A.; Yanai, H.; Fraifeld, V.E. The role of DNA damage and repair in aging through the prism of Koch-like criteria. Ageing Res. Rev. 2013, 12, 661–684. [Google Scholar] [CrossRef]
- Xia, E.; Zhu, X.; Gao, X.; Ni, J.; Guo, H. Antiaging Potential of Peptides from Underused Marine Bioresources. Mar. Drugs 2021, 19, 513. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.Y.; Kennedy, B.K. SIRT6, oxidative stress, and aging. Cell Res. 2016, 26, 143–144. [Google Scholar] [CrossRef]
- Shang, N.; Meram, C.; Bandara, N.; Wu, J. Protein and Peptides for Elderly Health. Adv. Protein Chem. Struct. Biol. 2018, 112, 265–308. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Q.; Kong, D.; Xu, P. Production and Functionality of Food-derived Bioactive Peptides: A Review. Mini Rev. Med. Chem. 2018, 18, 1524–1535. [Google Scholar] [CrossRef]
- Chen, S.; Yang, Q.; Chen, X.; Tian, Y.; Liu, Z.; Wang, S. Bioactive peptides derived from crimson snapper and in vivo anti-aging effects on fat diet-induced high fat Drosophila melanogaster. Food Funct. 2020, 11, 524–533. [Google Scholar] [CrossRef]
- Chi, C.F.; Cao, Z.H.; Wang, B.; Hu, F.Y.; Li, Z.R.; Zhang, B. Antioxidant and functional properties of collagen hydrolysates from Spanish mackerel skin as influenced by average molecular weight. Molecules 2014, 19, 11211–11230. [Google Scholar] [CrossRef]
- Wiley, C.D.; Campisi, J. From Ancient Pathways to Aging Cells-Connecting Metabolism and Cellular Senescence. Cell Metab. 2016, 23, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Wiggins, K.A.; Parry, A.J.; Cassidy, L.D.; Humphry, M.; Webster, S.J.; Goodall, J.C.; Narita, M.; Clarke, M.C.H. IL-1α cleavage by inflammatory caspases of the noncanonical inflammasome controls the senescence-associated secretory phenotype. Aging Cell 2019, 18, e12946. [Google Scholar] [CrossRef] [PubMed]
- Ohanna, M.; Giuliano, S.; Bonet, C.; Imbert, V.; Hofman, V.; Zangari, J.; Bille, K.; Robert, C.; Bressac-de Paillerets, B.; Hofman, P.; et al. Senescent cells develop a PARP-1 and nuclear factor-{kappa}B-associated secretome (PNAS). Genes Dev. 2011, 25, 1245–1261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, H.; Wang, L.; Zhang, S.; Wang, F.; Lin, H.; Gao, S.; Li, X.; Liu, K. Anti-inflammatory peptides and metabolomics-driven biomarkers discovery from sea cucumber protein hydrolysates. J. Food Sci. 2021, 86, 3540–3549. [Google Scholar] [CrossRef]
- Yuan, L.; Chu, Q.; Wu, X.; Yang, B.; Zhang, W.; Jin, W.; Gao, R. Anti-inflammatory and Antioxidant Activity of Peptides From Ethanol-Soluble Hydrolysates of Sturgeon (Acipenser schrenckii) Cartilage. Front. Nutr. 2021, 8, 689648. [Google Scholar] [CrossRef]
- Chatterjee, C.; Gleddie, S.; Xiao, C.W. Soybean Bioactive Peptides and Their Functional Properties. Nutrients 2018, 10, 1211. [Google Scholar] [CrossRef]
- Dou, Z.; Ghosh, K.; Vizioli, M.G.; Zhu, J.; Sen, P.; Wangensteen, K.J.; Simithy, J.; Lan, Y.; Lin, Y.; Zhou, Z.; et al. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature 2017, 550, 402–406. [Google Scholar] [CrossRef]
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018, 28, 436–453. [Google Scholar] [CrossRef]
- Chen, H.; Ruiz, P.D.; McKimpson, W.M.; Novikov, L.; Kitsis, R.N.; Gamble, M.J. MacroH2A1 and ATM Play Opposing Roles in Paracrine Senescence and the Senescence-Associated Secretory Phenotype. Mol. Cell 2015, 59, 719–731. [Google Scholar] [CrossRef]
- Oh, Y.; Ahn, C.B.; Je, J.Y. Cytoprotective Role of Edible Seahorse (Hippocampus abdominalis)-Derived Peptides in H(2)O(2)-Induced Oxidative Stress in Human Umbilical Vein Endothelial Cells. Mar. Drugs 2021, 19, 86. [Google Scholar] [CrossRef]
- Komakula, S.S.B.; Tumova, J.; Kumaraswamy, D.; Burchat, N.; Vartanian, V.; Ye, H.; Dobrzyn, A.; Lloyd, R.S.; Sampath, H. The DNA Repair Protein OGG1 Protects Against Obesity by Altering Mitochondrial Energetics in White Adipose Tissue. Sci. Rep. 2018, 8, 14886. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Ahn, C.B.; Nam, K.H.; Kim, Y.K.; Yoon, N.Y.; Je, J.Y. Amino Acid Composition, Antioxidant, and Cytoprotective Effect of Blue Mussel (Mytilus edulis) Hydrolysate through the Inhibition of Caspase-3 Activation in Oxidative Stress-Mediated Endothelial Cell Injury. Mar. Drugs 2019, 17, 135. [Google Scholar] [CrossRef] [PubMed]
- Rera, M.; Bahadorani, S.; Cho, J.; Koehler, C.L.; Ulgherait, M.; Hur, J.H.; Ansari, W.S.; Lo, T., Jr.; Jones, D.L.; Walker, D.W. Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog. Cell Metab. 2011, 14, 623–634. [Google Scholar] [CrossRef]
- Ho, J.H.; Baskaran, R.; Wang, M.F.; Yang, H.S.; Lo, Y.H.; Mohammedsaleh, Z.M.; Lin, W.T. Bioactive Peptides and Exercise Modulate the AMPK/SIRT1/PGC-1α/FOXO3 Pathway as a Therapeutic Approach for Hypertensive Rats. Pharmaceuticals 2022, 15, 819. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Wei, Y.; Xu, Y.; Zhang, R.; Li, M.; Qin, H.; Gu, R.; Cai, M. The anti-fatigue activity of corn peptides and their effect on gut bacteria. J. Sci. Food Agric. 2022, 102, 3456–3466. [Google Scholar] [CrossRef]
- Kim, M.J.; Koo, Y.D.; Kim, M.; Lim, S.; Park, Y.J.; Chung, S.S.; Jang, H.C.; Park, K.S. Rg3 Improves Mitochondrial Function and the Expression of Key Genes Involved in Mitochondrial Biogenesis in C2C12 Myotubes. Diabetes Metab. J. 2016, 40, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, R.Y.; Zhao, J.; Dong, Z.; Feng, D.Y.; Wu, R.; Shi, M.; Zhao, G. Ginsenoside Rd Protects SH-SY5Y Cells against 1-Methyl-4-phenylpyridinium Induced Injury. Int. J. Mol. Sci. 2015, 16, 14395–14408. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Li, Y.; Zhai, X.; Zheng, B.; Banbury, L.; Zhao, X.; Li, R. Comparison of Inhibitory Effects of Safflower Decoction and Safflower Injection on Protein and mRNA Expressions of iNOS and IL-1β in LPS-Activated RAW264.7 Cells. J. Immunol. Res. 2019, 2019, 1018274. [Google Scholar] [CrossRef]
| Amino Acid | Content (g/100 g) | Amino Acid | Content (g/100 g) |
|---|---|---|---|
| Aspartic Acid | 0.19 | Cystine | 0.01 |
| Glutamic Acid | 0.12 | Valine | 0.06 |
| Serine | 0.02 | Methionine | 0.02 |
| histidine | 0.06 | Phenylalanine | 0.09 |
| glycine | 0.02 | Isoleucine | 0.04 |
| Threonine | 0.05 | Leucine | 0.08 |
| Arginine | 2.26 | Lysine | 0.06 |
| Alanine | 0.13 | Proline | 0.65 |
| Tyrosine | 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, N.; Xu, M.-H.; Li, Y. Bioactive Oligopeptides from Ginseng (Panax ginseng Meyer) Suppress Oxidative Stress-Induced Senescence in Fibroblasts via NAD+/SIRT1/PGC-1α Signaling Pathway. Nutrients 2022, 14, 5289. https://doi.org/10.3390/nu14245289
Zhu N, Xu M-H, Li Y. Bioactive Oligopeptides from Ginseng (Panax ginseng Meyer) Suppress Oxidative Stress-Induced Senescence in Fibroblasts via NAD+/SIRT1/PGC-1α Signaling Pathway. Nutrients. 2022; 14(24):5289. https://doi.org/10.3390/nu14245289
Chicago/Turabian StyleZhu, Na, Mei-Hong Xu, and Yong Li. 2022. "Bioactive Oligopeptides from Ginseng (Panax ginseng Meyer) Suppress Oxidative Stress-Induced Senescence in Fibroblasts via NAD+/SIRT1/PGC-1α Signaling Pathway" Nutrients 14, no. 24: 5289. https://doi.org/10.3390/nu14245289
APA StyleZhu, N., Xu, M.-H., & Li, Y. (2022). Bioactive Oligopeptides from Ginseng (Panax ginseng Meyer) Suppress Oxidative Stress-Induced Senescence in Fibroblasts via NAD+/SIRT1/PGC-1α Signaling Pathway. Nutrients, 14(24), 5289. https://doi.org/10.3390/nu14245289







