The Bioavailability of Glycyrrhizinic Acid Was Enhanced by Probiotic Lactobacillus rhamnosus R0011 Supplementation in Liver Fibrosis Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
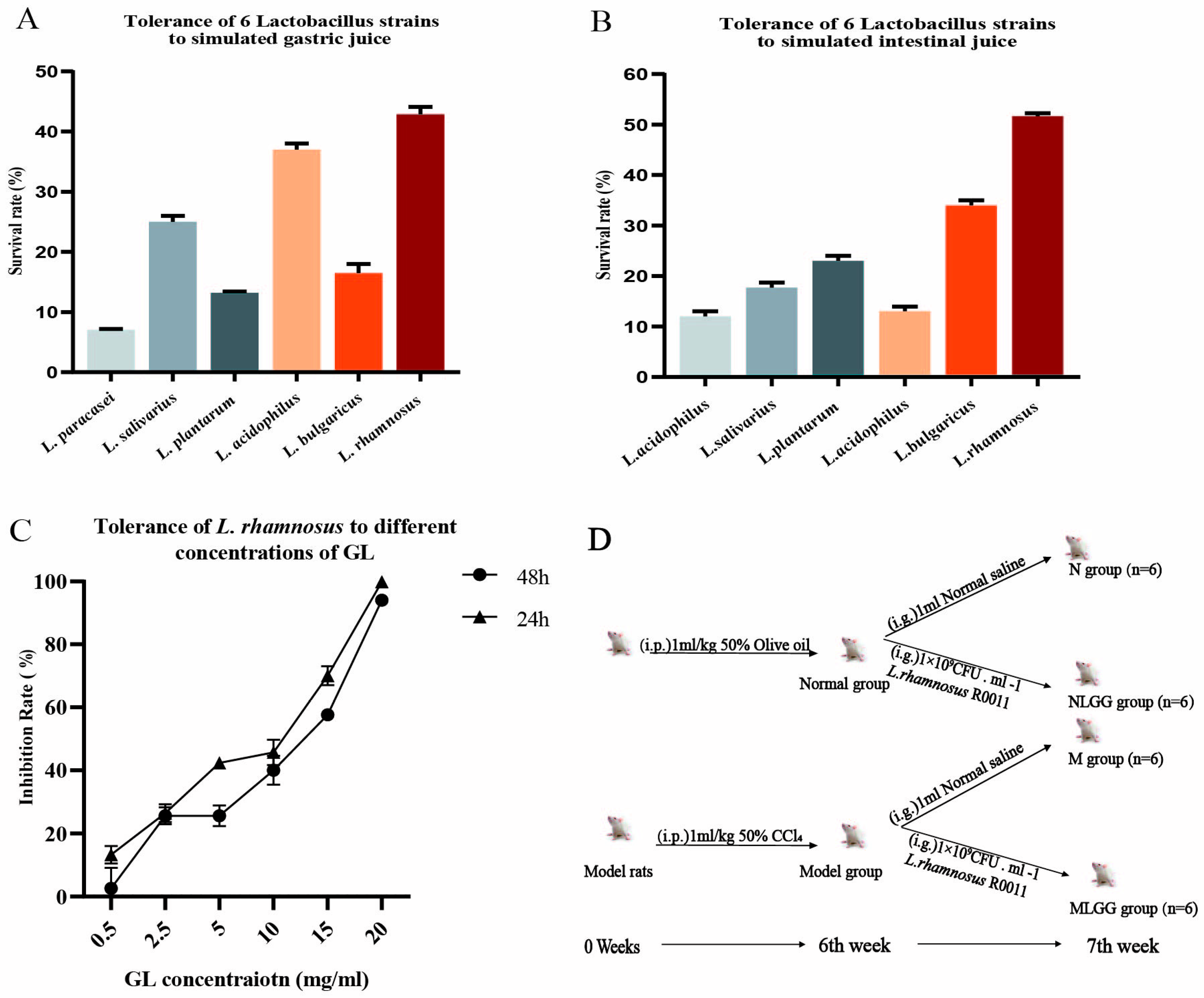
2.2. Culture and Screening of Experimental Strains
2.3. Preparation of Animal Models
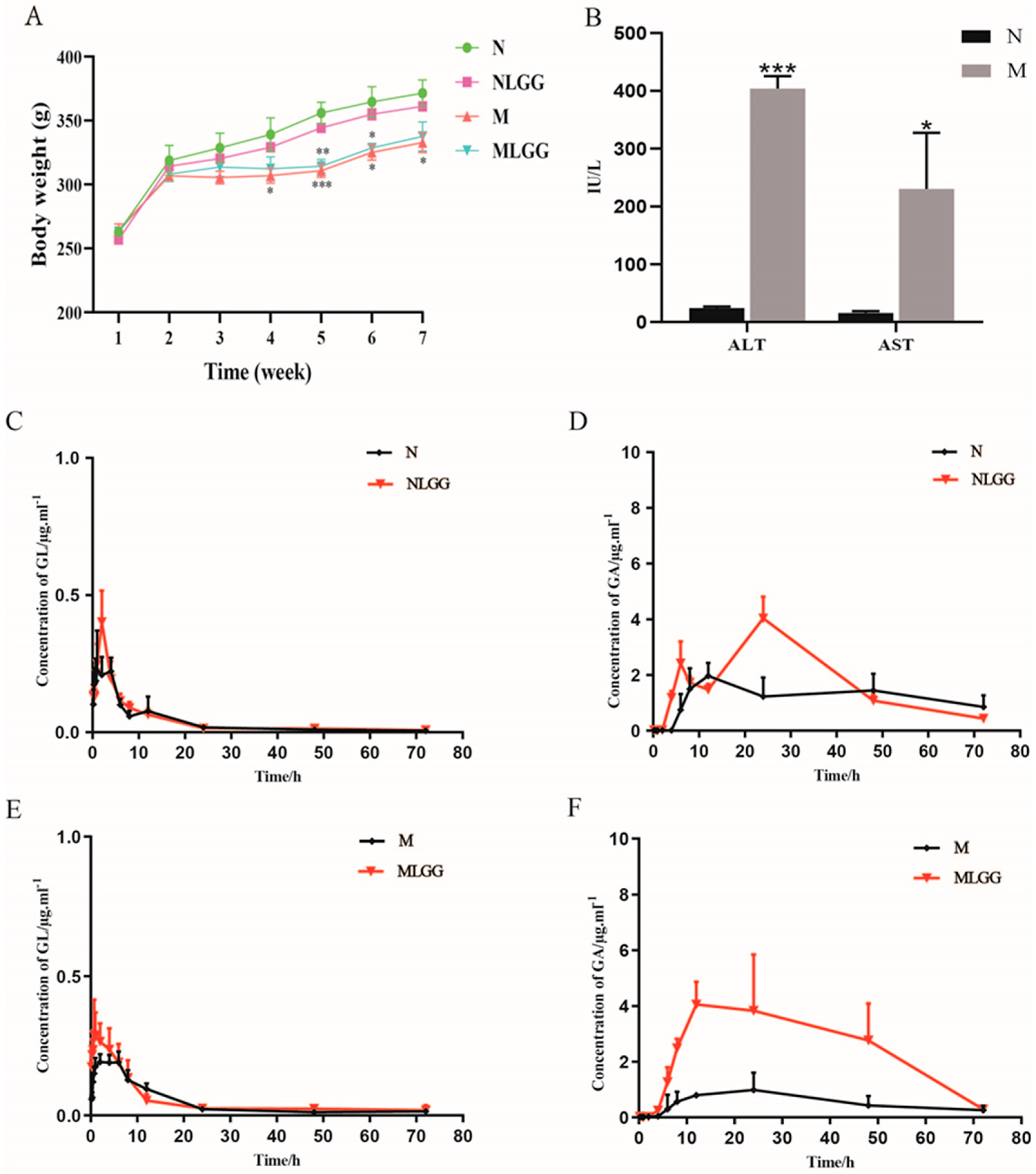
2.4. Pharmacokinetic Experiments
2.5. Quantitative Real-Time PCR (qRT-PCR)
2.6. β-Glucuronidase Enzyme Activity Assay in the Feces and Intestinal Tissues
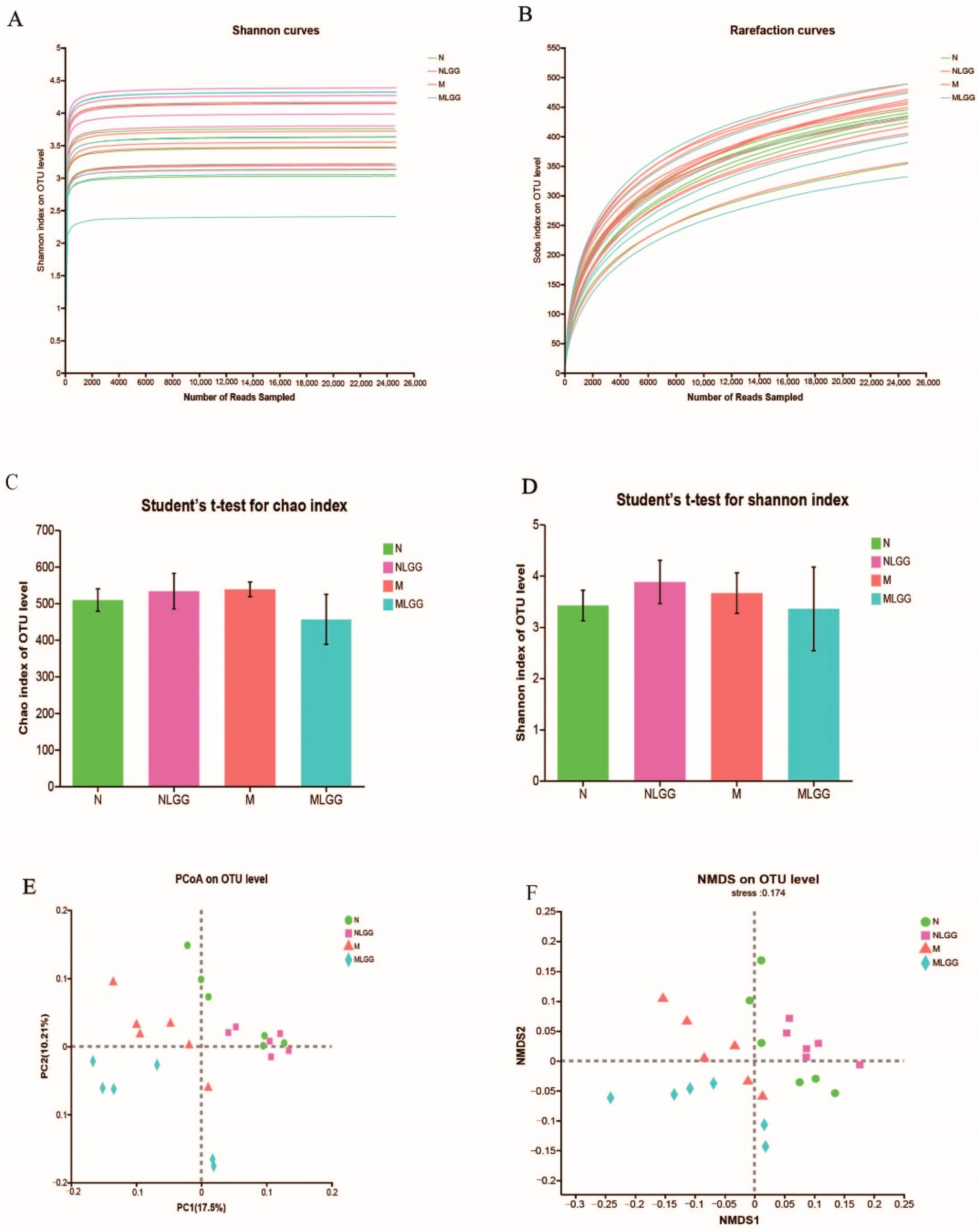
2.7. Gut Microbiota Structural Profiling
2.8. Fecal Metabolic Profiling by UPLC-MS/MS
2.9. Statistical Analysis
3. Results
3.1. In Vitro Screening of Lactobacillus Based on Tolerance to the Gastrointestinal Environment
3.2. Effects of L. rhamnosus R0011 on Pharmacokinetics of Glycyrrhizinic Acid (GL)
3.3. Effects of L. rhamnosus R0011 on Intestinal Biotransformation of Glycyrrhizinic Acid (GL)
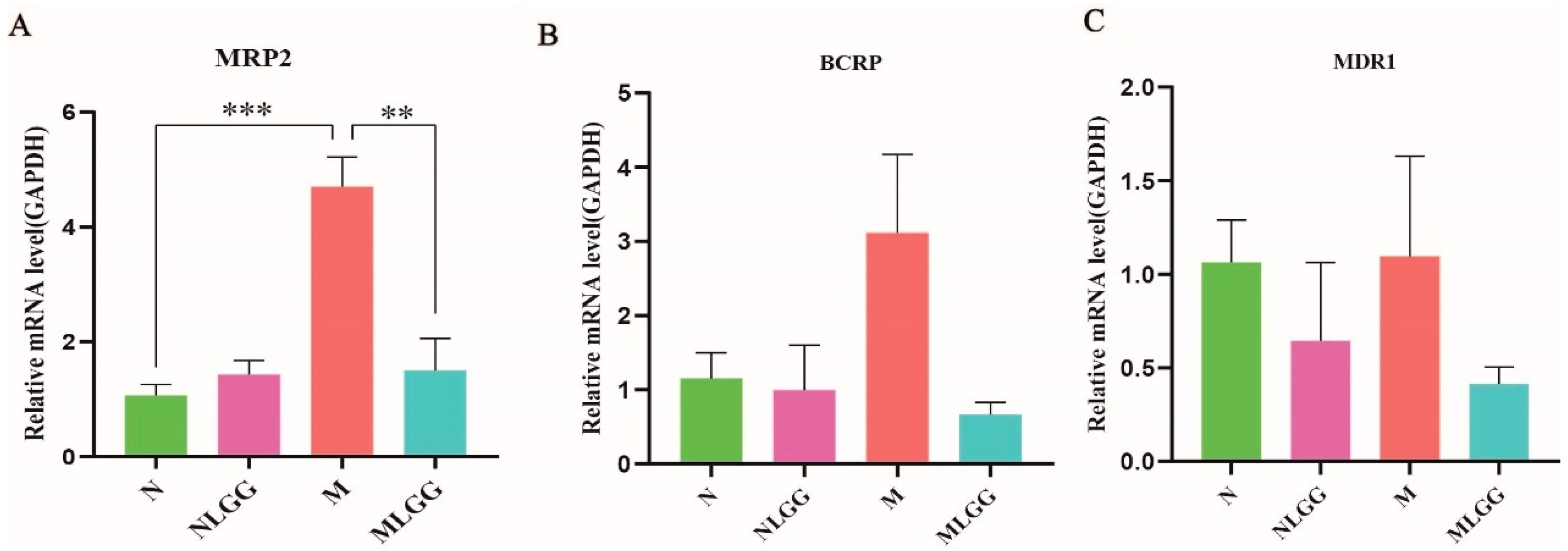
3.4. Effect of L. rhamnosus R0011 on the Gene Expression of Intestinal Drug Transporters
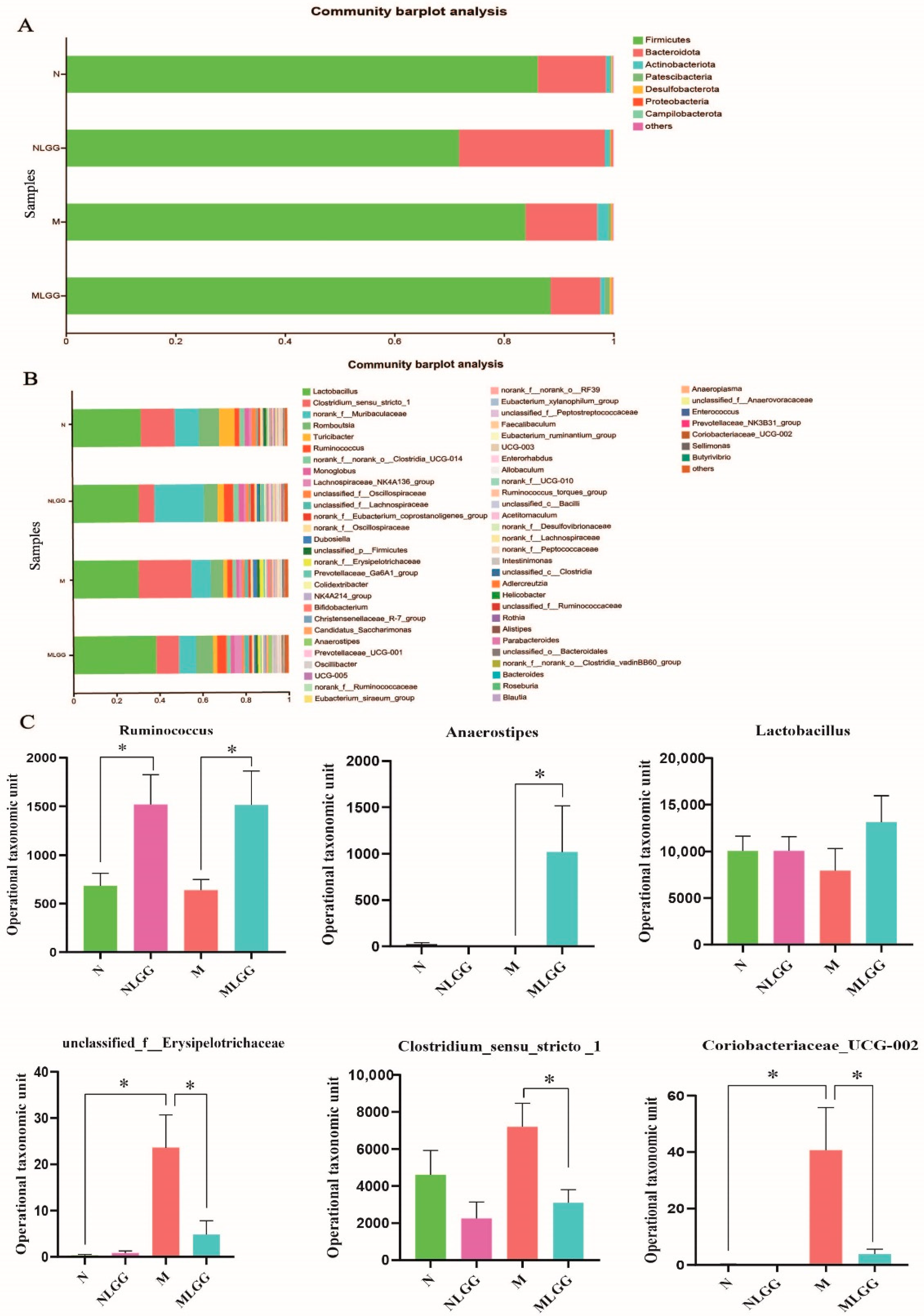
3.5. Structural Modulation of Gut Microbiota by L. rhamnosus R0011 Intervention
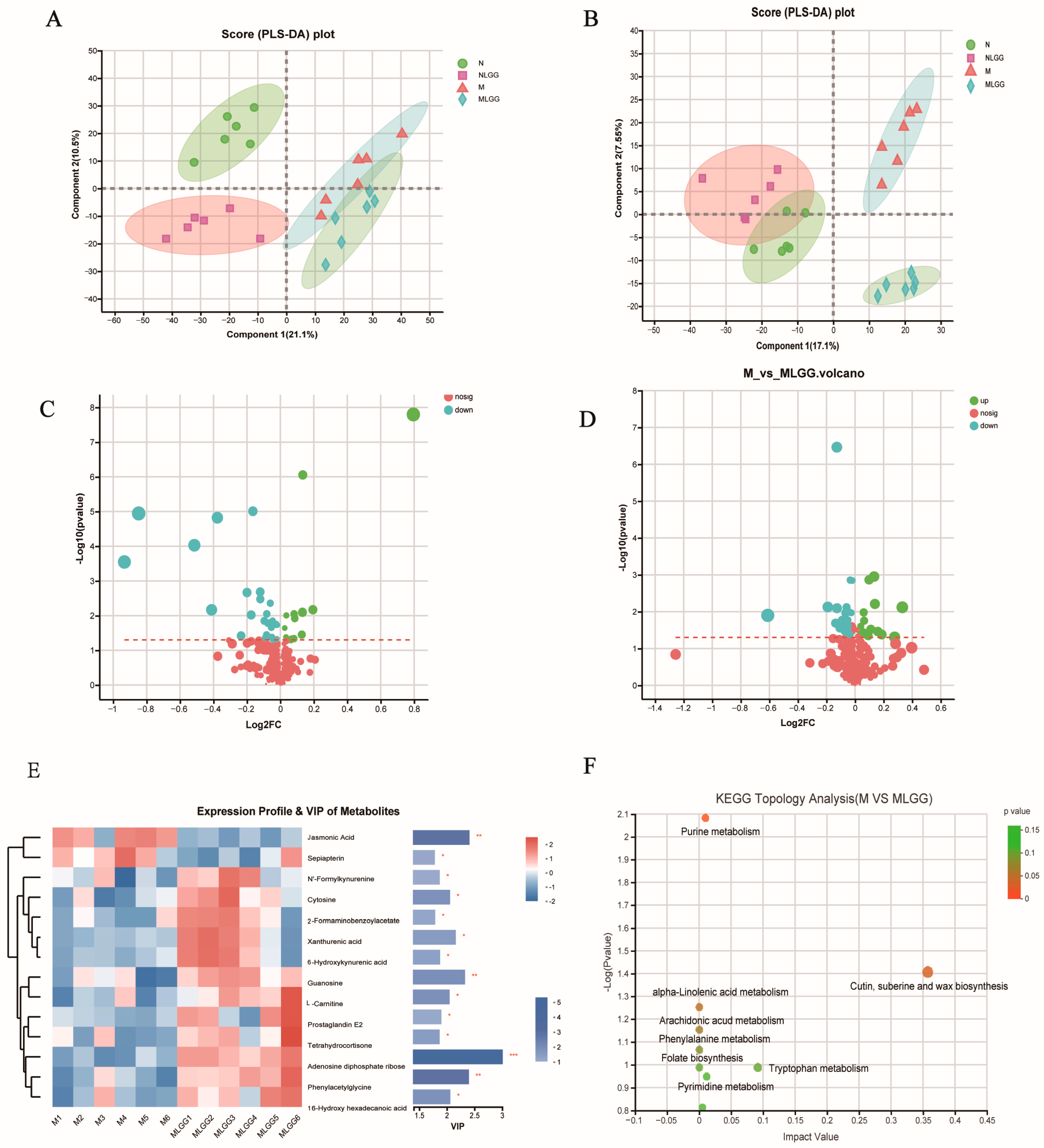
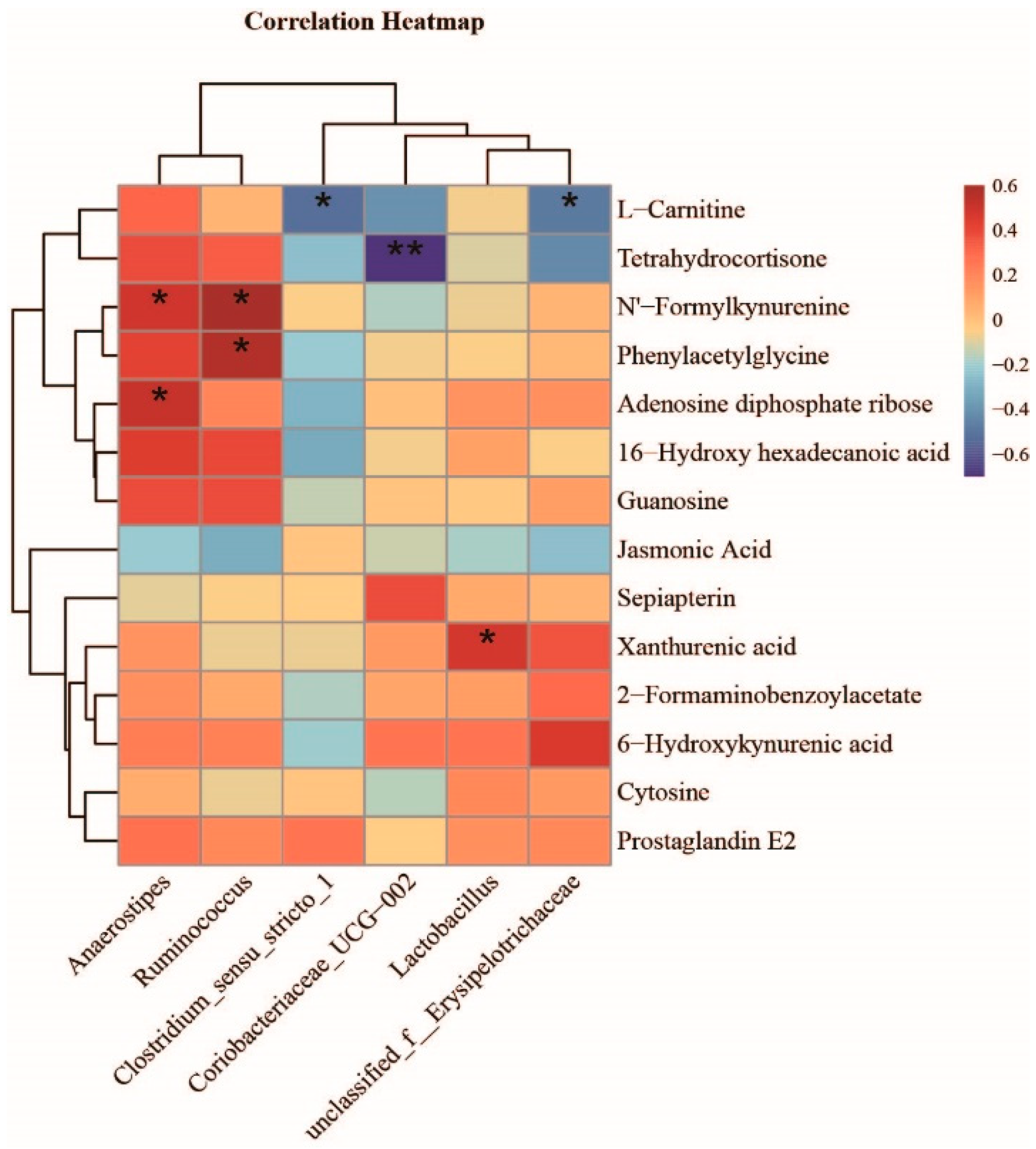
3.6. Effect of L. rhamnosus R0011 on the Modification of Microbial Metabolite Pathways
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, Y.; Gao, Z.; Chen, L.; Kang, L.; Huang, W.; Jin, M.; Wang, Q.; Bae, Y.H. Multifunctional oral delivery systems for enhanced bioavailability of therapeutic peptides/proteins. Acta Pharm. Sin. B 2019, 9, 902–922. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, H.B.; Li, S.L. Understanding the Molecular Mechanisms of the Interplay Between Herbal Medicines and Gut Microbiota. Med. Res. Rev. 2017, 37, 1140–1185. [Google Scholar] [CrossRef] [PubMed]
- Weersma, R.K.; Zhernakova, A.; Fu, J. Interaction between drugs and the gut microbiome. Gut 2020, 69, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Dey, P. Gut microbiota in phytopharmacology: A comprehensive overview of concepts, reciprocal interactions, biotransformations and mode of actions. Pharmacol. Res. 2019, 147, 104367. [Google Scholar] [CrossRef]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef]
- Zhang, F.; He, F.; Li, L.; Guo, L.; Zhang, B.; Yu, S.; Zhao, W. Bioavailability Based on the Gut Microbiota: A New Perspective. Microbiol. Mol. Biol. Rev. 2020, 84, e00072-19. [Google Scholar] [CrossRef]
- Zimmermann, M.; Zimmermann-Kogadeeva, M.; Wegmann, R.; Goodman, A.L. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature 2019, 570, 462–467. [Google Scholar] [CrossRef]
- Subramanian, V.S.; Sabui, S.; Moradi, H.; Marchant, J.S.; Said, H.M. Inhibition of intestinal ascorbic acid uptake by lipopolysaccharide is mediated via transcriptional mechanisms. Biochim. Biophys. Acta Biomembr. 2018, 1860, 556–565. [Google Scholar] [CrossRef]
- Zhang, X.; Han, Y.; Huang, W.; Jin, M.; Gao, Z. The influence of the gut microbiota on the bioavailability of oral drugs. Acta Pharm. Sin. B 2021, 11, 1789–1812. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Y.; Li, W.; You, B.; Yu, J.; Huang, X.; Yang, R. Gut Microbiota Composition Affects Procyanidin A2-Attenuated Atherosclerosis in ApoE-/-Mice by Modulating the Bioavailability of Its Microbial Metabolites. J. Agric. Food Chem. 2021, 30, 6989–6999. [Google Scholar] [CrossRef]
- Araya, M.; Morelli, L.; Reid, G.; Sanders, M.E.; Stanton, C. Guidelines for the Evaluation of Probiotics in Food; Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food London, Ontario, Canada, April 30 and May 1, 2002; Food and Agriculture Organization of the United Nations: Rome, Italy, 2002. [Google Scholar]
- Segers, M.E.; Lebeer, S. Towards a better understanding of Lactobacillus rhamnosus GG-host interactions. Microb. Cell Factories 2014, 13, S7. [Google Scholar] [CrossRef] [PubMed]
- Bharwani, A.; Mian, M.F.; Surette, M.G.; Bienenstock, J.; Forsythe, P. Oral treatment with Lactobacillus rhamnosus attenuates behavioural deficits and immune changes in chronic social stress. BMC Med. 2017, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, K.; Li, F.; Gu, Z.; Liu, Q.; He, L.; Shao, T.; Song, Q.; Zhu, F.; Zhang, L.; et al. Probiotic Lactobacillus rhamnosus GG Prevents Liver Fibrosis Through Inhibiting Hepatic Bile Acid Synthesis and Enhancing Bile Acid Excretion in Mice. Hepatology 2020, 71, 2050–2066. [Google Scholar] [CrossRef] [PubMed]
- Beneficial Microbes. Benef. Microbes 2011, 2, 1–7.
- Alemka, A.; Clyne, M.; Shanahan, F.; Tompkins, T.; Corcionivoschi, N.; Bourke, B. Probiotic colonization of the adherent mucus layer of HT29MTXE12 cells attenuates Campylobacter jejuni virulence properties. Infect. Immun. 2010, 78, 2812–2822. [Google Scholar] [CrossRef]
- Jeffrey, M.P.; Strap, J.L.; Jones, T.H.; Green-Johnson, J.M. Suppression of Intestinal Epithelial Cell Chemokine Production by Lactobacillus rhamnosus R0011 and Lactobacillus helveticus R0389 Is Mediated by Secreted Bioactive Molecules. Front. Immunol. 2018, 9, 2639. [Google Scholar] [CrossRef]
- Asl, M.N.; Hosseinzadeh, H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother. Res. 2008, 22, 709–724. [Google Scholar] [CrossRef]
- Yu, J.Y.; Ha, J.Y.; Kim, K.M.; Jung, Y.S.; Jung, J.C.; Oh, S. Anti-Inflammatory activities of licorice extract and its active compounds, glycyrrhizic acid, liquiritin and liquiritigenin, in BV2 cells and mice liver. Molecules 2015, 20, 13041–13054. [Google Scholar] [CrossRef]
- Gumpricht, E.; Dahl, R.; Devereaux, M.W.; Sokol, R.J. Licorice compounds glycyrrhizin and 18beta-glycyrrhetinic acid are potent modulators of bile acid-induced cytotoxicity in rat hepatocytes. J. Biol. Chem. 2005, 280, 10556–10563. [Google Scholar] [CrossRef]
- Kuchta, K.; Cameron, S.; Lee, M.; Cai, S.; Shoyama, Y. Which East Asian herbal medicines can decrease viral infections? Phytochem. Rev. 2022, 21, 219–237. [Google Scholar] [CrossRef]
- Ishida, T.; Jobu, K.; Kawada, K.; Morisawa, S.; Kawazoe, T.; Shiraishi, H.; Fujita, H.; Nishimura, S.; Kanno, H.; Nishiyama, M.; et al. Impact of Gut Microbiota on the Pharmacokinetics of Glycyrrhizic Acid in Yokukansan, a Kampo Medicine. Biol. Pharm. Bull. 2022, 45, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Wang, J.; Chen, L.; Shan, J.; Di, L. Glycyrrhizic acid improving the liver protective effect by restoring the composition of Lactobacillus. J. Funct. Foods 2019, 52, 219–227. [Google Scholar] [CrossRef]
- Wang, Q.; Lv, L.; Jiang, H.; Wang, K.; Yan, R.; Li, Y.; Ye, J.; Wu, J.; Wang, Q.; Bian, X.; et al. Lactobacillus helveticus R0052 alleviates liver injury by modulating gut microbiome and metabolome in D-galactosamine-treated rats. Appl. Microbiol. Biotechnol. 2019, 103, 9673–9686. [Google Scholar] [CrossRef]
- Shi, L.; Pan, R.; Lin, G.; Liang, X.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. Lactic acid bacteria alleviate liver damage caused by perfluorooctanoic acid exposure via antioxidant capacity, biosorption capacity and gut microbiota regulation. Ecotoxicol. Environ. Saf. 2021, 222, 112515. [Google Scholar] [CrossRef]
- Yuan, T.; Wang, J.; Chen, L.; Shan, J.; Di, L. Lactobacillus murinus Improved the Bioavailability of Orally Administered Glycyrrhizic Acid in Rats. Front. Microbiol. 2020, 11, 597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, S.; Duan, F.; Liu, A.; Li, S.; Zhong, W.; Sheng, W.; Chen, J.; Xu, J.; Xiao, S. Prebiotics enhance the biotransformation and bioavailability of ginsenosides in rats by modulating gut microbiota. J. Ginseng Res. 2021, 45, 334–343. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, H.; Zhu, X.; Shan, J.; Yin, A.; Cai, B.; Di, L. Improvement of intestinal absorption of forsythoside A and chlorogenic acid by different carboxymethyl chitosan and chito-oligosaccharide, application to Flos Lonicerae-Fructus Forsythiae herb couple preparations. PLoS ONE 2013, 8, e63348. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Choi, M.S.; Jeong, J.J.; Lim, S.M.; Kim, I.S.; Yoo, H.H.; Kim, D.H. Effect of Probiotics on Pharmacokinetics of Orally Administered Acetaminophen in Mice. Drug Metab. Dispos. 2018, 46, 122–130. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, C.; Wa, Y.; Qu, H.; Gu, R.; Chen, D.; Song, Z.; Chen, X. Correlation between exopolysaccharide biosynthesis and gastrointestinal tolerance of Lactiplantibacillus plantarum. J. Appl. Microbiol. 2022, 132, 584–591. [Google Scholar] [CrossRef]
- Xu, H.; Wu, L.; Pan, D.; Zeng, X.; Cai, Z.; Guo, Y.; Wang, W.; Wu, Z. Adhesion Characteristics and Dual Transcriptomic and Proteomic Analysis of Lactobacillus reuteri SH23 upon Gastrointestinal Fluid Stress. J. Proteome Res. 2021, 20, 2447–2457. [Google Scholar] [CrossRef] [PubMed]
- Akao, T. Differences in the metabolism of glycyrrhizin, glycyrrhetic acid and glycyrrhetic acid monoglucuronide by human intestinal flora. Biol. Pharm. Bull. 2000, 23, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Ma, N.; He, T.; Johnston, L.J.; Ma, X. Tryptophan (Trp) modulates gut homeostasis via aryl hydrocarbon receptor (AhR). Crit. Rev. Food Sci. Nutr. 2020, 60, 1760–1768. [Google Scholar] [CrossRef]
- Wilson, I.D.; Nicholson, J.K. The role of gut microbiota in drug response. Curr. Pharm. Des. 2009, 15, 1519–1523. [Google Scholar] [CrossRef]
- Zimmermann, M.; Zimmermann-Kogadeeva, M.; Wegmann, R.; Goodman, A.L. Separating host and microbiome contributions to drug pharmacokinetics and toxicity. Science 2019, 363, eaat9931. [Google Scholar] [CrossRef]
- Haiser, H.J.; Turnbaugh, P.J. Is it time for a metagenomic basis of therapeutics? Science 2012, 336, 1253–1255. [Google Scholar] [CrossRef]
- Saad, R.; Rizkallah, M.R.; Aziz, R.K. Gut Pharmacomicrobiomics: The tip of an iceberg of complex interactions between drugs and gut-associated microbes. Gut Pathog. 2012, 4, 16. [Google Scholar] [CrossRef]
- Liu, S.T.; Zheng, S.W.; Hou, A.J.; Zhang, J.X.; Wang, S.; Wang, X.J. A review: The phytochemistry, pharmacology, and pharmacokinetics of Curcumae Longae Rhizoma (Turmeric). World J. Tradit. Chin. Med. 2022, 8, 463–490. [Google Scholar]
- Shen, C.; Zhu, J.; Song, J.; Wang, J.; Shen, B.; Yuan, H.; Li, X. Formulation of pluronic F127/TPGS mixed micelles to improve the oral absorption of glycyrrhizic acid. Drug Dev. Ind. Pharm. 2020, 46, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Bron, P.A.; Kleerebezem, M.; Brummer, R.J.; Cani, P.D.; Mercenier, A.; MacDonald, T.T.; Garcia-Ródenas, C.L.; Wells, J.M. Can probiotics modulate human disease by impacting intestinal barrier function? Br. J. Nutr. 2017, 117, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Basturk, A.; Isik, İ.; Atalay, A.; Yılmaz, A. Investigation of the Efficacy of Lactobacillus rhamnosus GG in Infants With Cow’s Milk Protein Allergy: A Randomised Double-Blind Placebo-Controlled Trial. Probiot. Antimicrob. Proteins 2020, 12, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Q.; Gao, N.; Wang, Z.; Li, F.; Li, J.; Shan, A. Exopolysaccharides produced by Lactobacillus rhamnosus GG alleviate hydrogen peroxide-induced intestinal oxidative damage and apoptosis through the Keap1/Nrf2 and Bax/Bcl-2 pathways in vitro. Food Funct. 2021, 12, 9632–9641. [Google Scholar] [CrossRef]
- Goyal, N.; Tiwari, R.P.; Shukla, G. Lactobacillus rhamnosus GG as an Effective Probiotic for Murine Giardiasis. Interdiscip. Perspect. Infect. Dis. 2011, 2011, 795219. [Google Scholar] [CrossRef]
- Ku, S.; You, H.J.; Park, M.S.; Ji, G.E. Whole-Cell Biocatalysis for Producing Ginsenoside Rd from Rb1 Using Lactobacillus rhamnosus GG. J. Microbiol. Biotechnol. 2016, 26, 1206–1215. [Google Scholar] [CrossRef]
- Theilmann, M.C.; Goh, Y.J.; Nielsen, K.F.; Klaenhammer, T.R.; Barrangou, R.; Abou, H.M. Lactobacillus acidophilus Metabolizes Dietary Plant Glucosides and Externalizes Their Bioactive Phytochemicals. mBio 2017, 8, e01421-17. [Google Scholar] [CrossRef]
- Rossi, M.; Amaretti, A.; Leonardi, A.; Raimondi, S.; Simone, M.; Quartieri, A. Potential impact of probiotic consumption on the bioactivity of dietary phytochemicals. J. Agric. Food Chem. 2013, 61, 9551–9558. [Google Scholar] [CrossRef]
- Erdmann, P.; Bruckmueller, H.; Martin, P.; Busch, D.; Haenisch, S.; Müller, J.; Wiechowska-Kozlowska, A.; Partecke, L.I.; Heidecke, C.D.; Cascorbi, I.; et al. Dysregulation of Mucosal Membrane Transporters and Drug-Metabolizing Enzymes in Ulcerative Colitis. J. Pharm. Sci. 2019, 108, 1035–1046. [Google Scholar] [CrossRef]
- Lebeer, S.; Claes, I.; Tytgat, H.L.; Verhoeven, T.L.; Marien, E.; von Ossowski, I.; Reunanen, J.; Palva, A.; Vos, W.M.; Keersmaecker, S.C.; et al. Functional analysis of Lactobacillus rhamnosus GG pili in relation to adhesion and immunomodulatory interactions with intestinal epithelial cells. Appl. Environ. Microbiol. 2012, 78, 185–193. [Google Scholar] [CrossRef]
- Kankainen, M.; Paulin, L.; Tynkkynen, S.; von Ossowski, I.; Reunanen, J.; Partanen, P.; Satokari, R.; Vesterlund, S.; Hendrickx, A.P.; Lebeer, S.; et al. Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human- mucus binding protein. Proc. Natl. Acad. Sci. USA 2009, 106, 17193–17198. [Google Scholar] [CrossRef] [PubMed]
- Foster, L.M.; Tompkins, T.A.; Dahl, W.J. A comprehensive post-market review of studies on a probiotic product containing Lactobacillus helveticus R0052 and Lactobacillus rhamnosus R0011. Benef. Microbes 2011, 2, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.D.; Bradley, S.; Buckley, N.D.; Green-Johnson, J.M. Interactions of lactic acid bacteria with human intestinal epithelial cells: Effects on cytokine production. J. Food Prot. 2003, 66, 466–472. [Google Scholar] [CrossRef]
- Akao, T. Competition in the metabolism of glycyrrhizin with glycyrrhetic acid mono-glucuronide by mixed Eubacterium sp. GLH and Ruminococcus sp. PO1-3. Biol. Pharm. Bull. 2000, 23, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Gloux, K.; Berteau, O.; El Oumami, H.; Béguet, F.; Leclerc, M.; Doré, J. A metagenomic β-glucuronidase uncovers a core adaptive function of the human intestinal microbiome. Proc. Natl. Acad. Sci. USA 2011, 108, 4539–4546. [Google Scholar] [CrossRef] [PubMed]
- Biernat, K.A.; Pellock, S.J.; Bhatt, A.P.; Bivins, M.M.; Walton, W.G.; Tran, B.N.T.; Wei, L.; Snider, M.C.; Cesmat, A.P.; Tripathy, A.; et al. Structure, function, and inhibition of drug reactivating human gut microbial β-glucuronidases. Sci. Rep. 2019, 9, 825. [Google Scholar] [CrossRef]
- Forbes, J.D.; Chen, C.Y.; Knox, N.C.; Marrie, R.A.; El-Gabalawy, H.; de Kievit, T.; Alfa, M.; Bernstein, C.N.; Van Domselaar, G. A comparative study of the gut microbiota in immune-mediated inflammatory diseases-does a common dysbiosis exist? Microbiome 2018, 6, 221. [Google Scholar] [CrossRef]
- Wang, J.; Ji, H.; Wang, S.; Liu, H.; Zhang, W.; Zhang, D.; Wang, Y. Probiotic Lactobacillus plantarum Promotes Intestinal Barrier Function by Strengthening the Epithelium and Modulating Gut Microbiota. Front. Microbiol. 2018, 9, 1953. [Google Scholar] [CrossRef]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef]
- Natividad, J.M.; Agus, A.; Planchais, J.; Lamas, B.; Jarry, A.C.; Martin, R.; Michel, M.L.; Chong-Nguyen, C.; Roussel, R.; Straube, M.; et al. Impaired Aryl Hydrocarbon Receptor Ligand Production by the Gut Microbiota Is a Key Factor in Metabolic Syndrome. Cell Metab. 2018, 28, 737–749.e4. [Google Scholar] [CrossRef]
- da Silva Ferreira, A.R.; Märtson, A.G.; de Boer, A.; Wardill, H.R.; Alffenaar, J.W.; Harmsen, H.J.M.; Tissing, W.J.E. Does Chemotherapy-Induced Gastrointestinal Mucositis Affect the Bioavailability and Efficacy of Anti-Infective Drugs? Biomedicines 2021, 9, 1389. [Google Scholar] [CrossRef] [PubMed]

| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| MRP2 | TTCTGGATCCTCTCGGTCTTATG | ATCTGGAAACCGTAGGAGACGAA |
| BCRP | CAATGGGATCATGAAACCTG | GAGGCTGATGAATGGAGAA |
| MDR1 | GAGCCCATCCTGTTTGACTG | TGTCTCCCACTCTGGTGTTG |
| Pharmacokinetic Parameters | GL | GA | ||||||
|---|---|---|---|---|---|---|---|---|
| N | NLGG | M | MLGG | N | NLGG | M | MLGG | |
| T max (h) | 1.72 ± 1.29 | 1.53 ± 0.78 | 4.96 ± 4.14 | 1.75 ± 1.25 | 17.33 ± 15.11 | 16 ± 9.00 * | 18.00 ± 6.57 | 21.33 ± 14.68 |
| Cmax (µg/mL) | 0.29 ± 0.11 | 0.42 ± 0.28 | 0.24 ± 0.08 | 0.35 ± 0.07 | 2.05 ± 0.48 | 4.22 ± 1.85 * | 1.16 ± 0.42 | 5.93 ± 4.35 # |
| AUC.(0–t) (µg/mL h) | 2.66 ± 0.56 | 2.98 ± 1.44 | 3.22 ± 0.86 | 3.68 ± 0.70 | 88.57 ± 25.81 | 127.87 ± 4.56 | 39.83 ± 20.49 | 181.62 ± 138.60 # |
| CLz/F/L· (h kg) −1 | 17.02 ± 2.72 | 17.75 ± 7.53 | 15.64 ± 3.31 | 11.69 ± 2.43 # | 0.35 ± 0.24 | 0.41 ± 0.16 | 1.52 ± 1.08 | 0.17 ± 0.11 # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Wang, J.; Fu, Y.; Zhu, K.; Dong, Z.; Shan, J.; Di, L.; Jiang, S.; Yuan, T. The Bioavailability of Glycyrrhizinic Acid Was Enhanced by Probiotic Lactobacillus rhamnosus R0011 Supplementation in Liver Fibrosis Rats. Nutrients 2022, 14, 5278. https://doi.org/10.3390/nu14245278
Li H, Wang J, Fu Y, Zhu K, Dong Z, Shan J, Di L, Jiang S, Yuan T. The Bioavailability of Glycyrrhizinic Acid Was Enhanced by Probiotic Lactobacillus rhamnosus R0011 Supplementation in Liver Fibrosis Rats. Nutrients. 2022; 14(24):5278. https://doi.org/10.3390/nu14245278
Chicago/Turabian StyleLi, Huifang, Jing Wang, Yifan Fu, Ke Zhu, Zhiling Dong, Jinjun Shan, Liuqing Di, Shu Jiang, and Tianjie Yuan. 2022. "The Bioavailability of Glycyrrhizinic Acid Was Enhanced by Probiotic Lactobacillus rhamnosus R0011 Supplementation in Liver Fibrosis Rats" Nutrients 14, no. 24: 5278. https://doi.org/10.3390/nu14245278
APA StyleLi, H., Wang, J., Fu, Y., Zhu, K., Dong, Z., Shan, J., Di, L., Jiang, S., & Yuan, T. (2022). The Bioavailability of Glycyrrhizinic Acid Was Enhanced by Probiotic Lactobacillus rhamnosus R0011 Supplementation in Liver Fibrosis Rats. Nutrients, 14(24), 5278. https://doi.org/10.3390/nu14245278






