Risk of Malnutrition in Hospitalized COVID-19 Patients: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection
2.4. Quality Assessment
2.5. Data Extraction
2.6. Statistical Analysis
3. Results
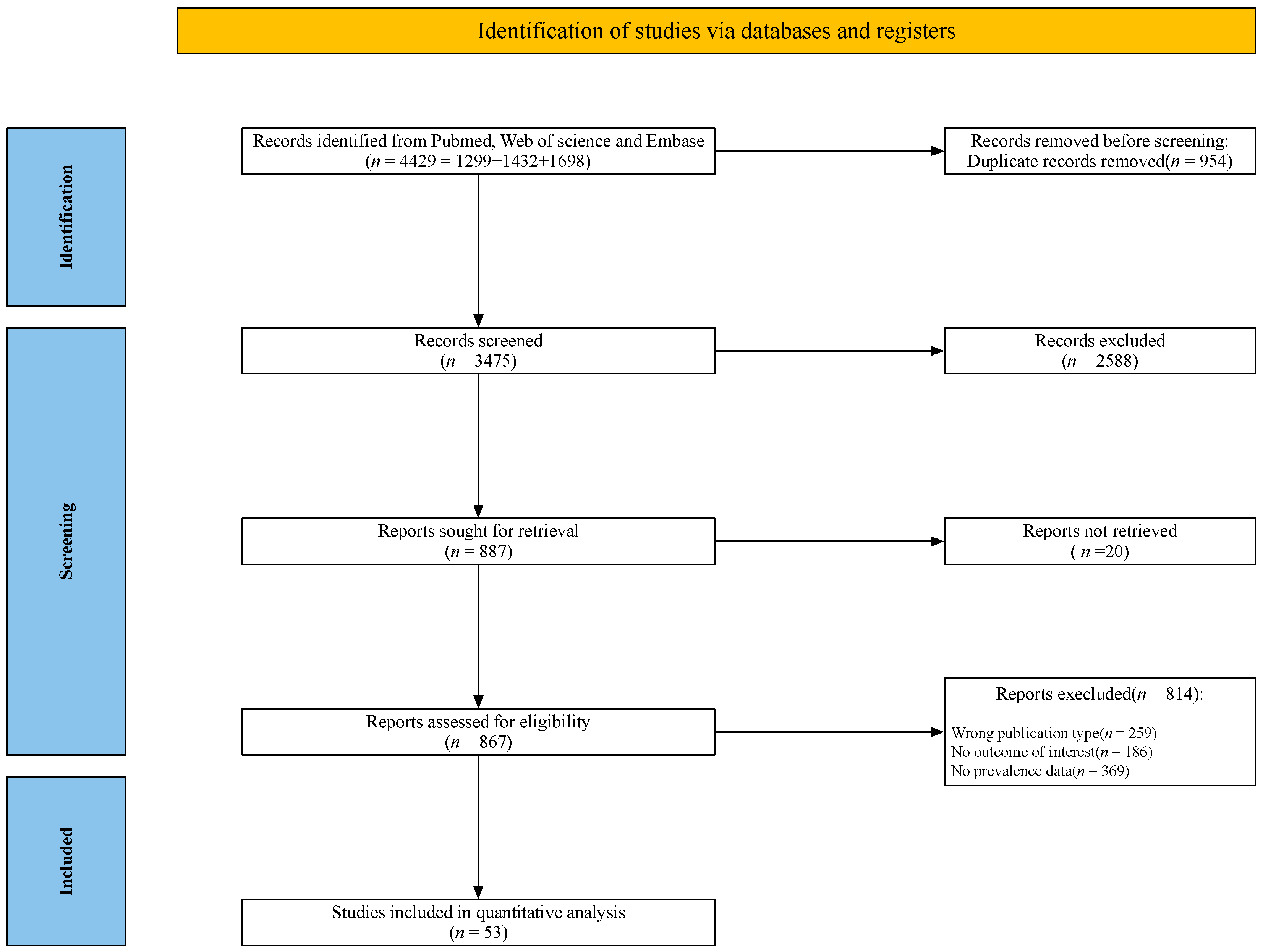
3.1. Search Results
3.2. Study Characteristics
3.3. Quality Assessment
3.4. Prevalence of Risk of Malnutrition among Hospitalized Patients with COVID-19
3.4.1. Overall Results
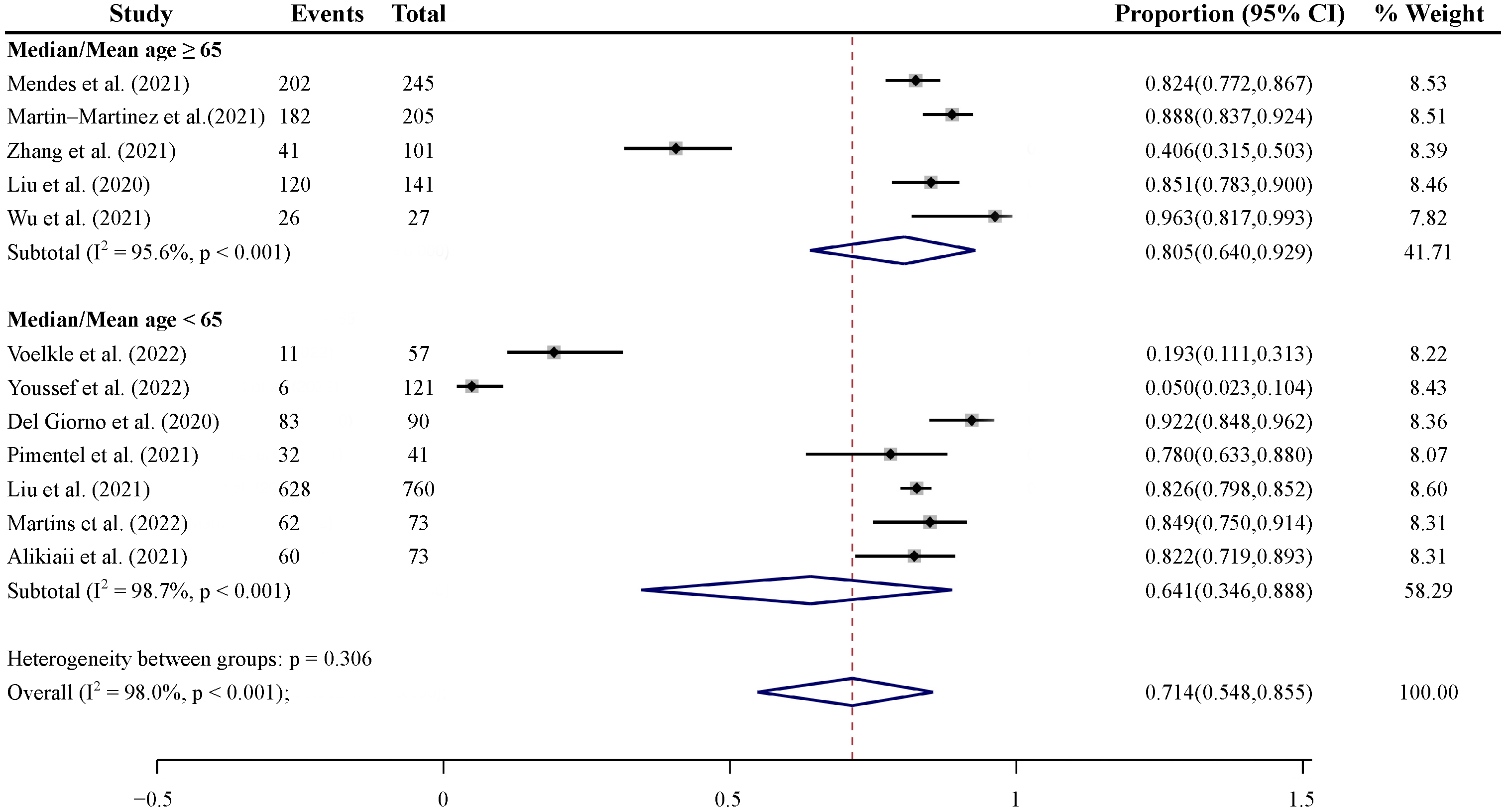
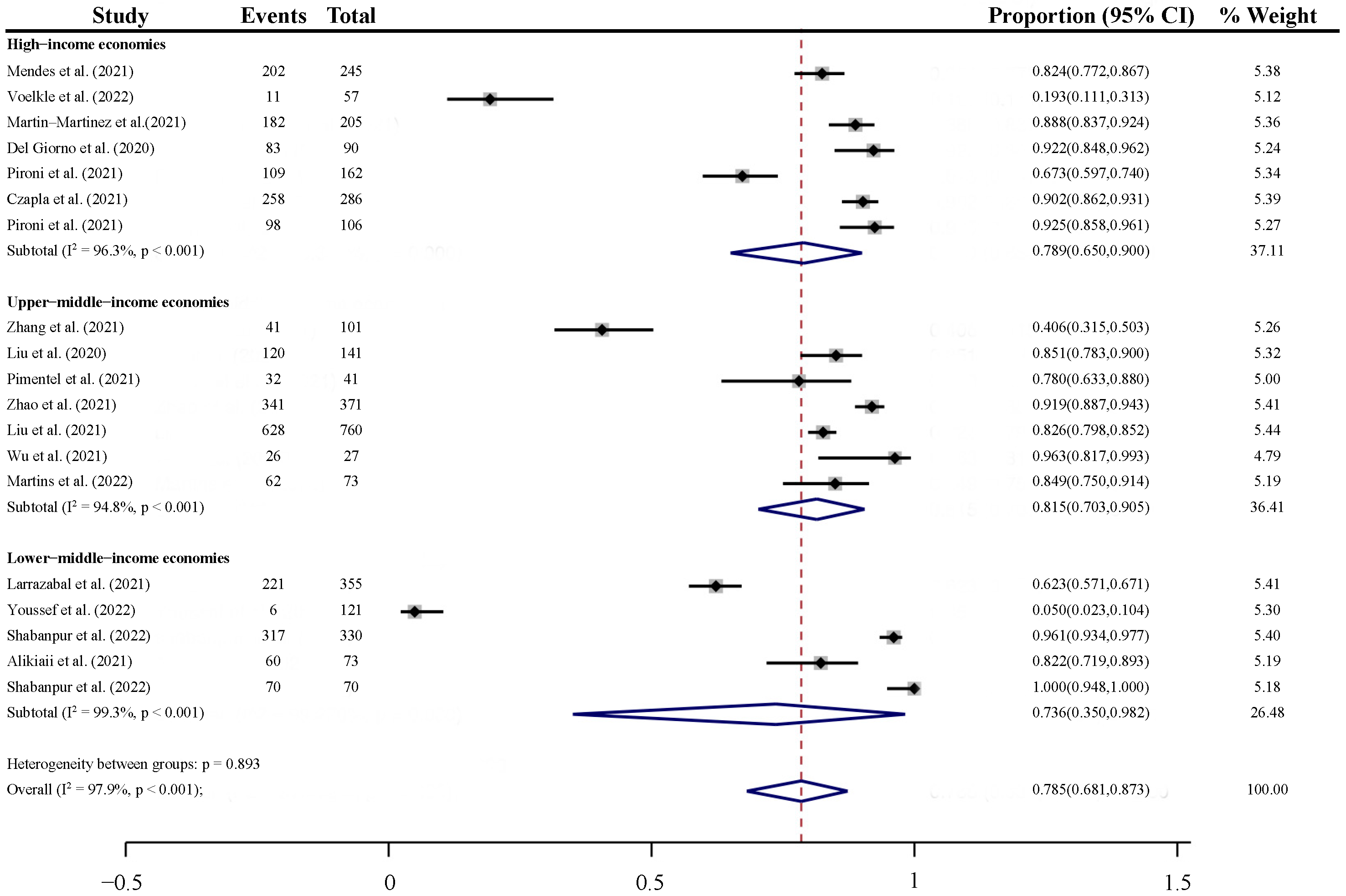
3.4.2. Subgroup Analyses
3.5. Publication Bias
4. Discussion
4.1. Main Findings
4.2. Assessment Tools for Malnutrition and Risk of Malnutrition
4.3. Recommendations following Malnutrition Screening
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Demasi, M. COVID-19 and Metabolic Syndrome: Could Diet Be the Key? Royal Society of Medicine: London, UK, 2021; Volume 26, pp. 1–2. [Google Scholar]
- Beck, M.A.; Handy, J.; Levander, O.A. Host nutritional status: The neglected virulence factor. Trends Microbiol. 2004, 12, 417–423. [Google Scholar] [CrossRef]
- Savino, W.; Dardenne, M.; Velloso, L.A.; Silva-Barbosa, S.D. The thymus is a common target in malnutrition and infection. Br. J. Nutr. 2007, 98, S11–S16. [Google Scholar] [CrossRef]
- Taylor, A.K.; Cao, W.; Vora, K.P.; Cruz, J.D.L.; Shieh, W.-J.; Zaki, S.R.; Katz, J.M.; Sambhara, S.; Gangappa, S. Protein Energy Malnutrition Decreases Immunity and Increases Susceptibility to Influenza Infection in Mice. J. Infect. Dis. 2012, 207, 501–510. [Google Scholar] [CrossRef]
- Skrajnowska, D.; Brumer, M.; Kankowska, S.; Matysek, M.; Miazio, N.; Bobrowska-Korczak, B. COVID-19: Diet Composition and Health. Nutrients 2021, 13, 2980. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F. Persistent symptoms in patients after acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Chopra, V.; Flanders, S.A.; O’Malley, M.; Malani, A.N.; Prescott, H.C. Sixty-day outcomes among patients hospitalized with COVID-19. Ann. Intern. Med. 2021, 174, 576–578. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Van Kampen, J.J.A.; Van De Vijver, D.A.M.C.; Fraaij, P.L.A.; Haagmans, B.L.; Lamers, M.M.; Okba, N.; van den Akker, J.P.C.; Endeman, H.; Gommers, D.A.M.P.J.; Cornelissen, J.J.; et al. Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19). Nat. Commun. 2021, 12, 267. [Google Scholar] [CrossRef]
- Hosey, M.M.; Needham, D.M. Survivorship after COVID-19 ICU stay. Nat. Rev. Dis. Prim. 2020, 6, 60. [Google Scholar] [CrossRef]
- Lescure, F.-X.; Bouadma, L.; Nguyen, D.; Parisey, M.; Wicky, P.-H.; Behillil, S.; Gaymard, A.; Bouscambert-Duchamp, M.; Donati, F.; Le Hingrat, Q.; et al. Clinical and virological data of the first cases of COVID-19 in Europe: A case series. Lancet Infect. Dis. 2020, 20, 697–706. [Google Scholar] [CrossRef]
- Chen, J.; Wu, B.; Liu, F.; Jiao, G.; Lu, R.; Li, H.; Zhao, J.; Yang, Y.; Lu, X.; Li, J.; et al. Lung transplantation for an ARDS patient post-COVID-19 infection. Chest 2020, 157, A453. [Google Scholar] [CrossRef]
- George, P.M.; Wells, A.U.; Jenkins, R.G. Pulmonary fibrosis and COVID-19: The potential role for antifibrotic therapy. Lancet Respir. Med. 2020, 8, 807–815. [Google Scholar] [CrossRef]
- Ramlall, V.; Thangaraj, P.M.; Meydan, C.; Foox, J.; Butler, D.; Kim, J.; May, B.; De Freitas, J.K.; Glicksberg, B.S.; Mason, C.E. Immune complement and coagulation dysfunction in adverse outcomes of SARS-CoV-2 infection. Nat. Med. 2020, 26, 1609–1615. [Google Scholar] [CrossRef]
- Nougier, C.; Benoit, R.; Simon, M.; Desmurs-Clavel, H.; Marcotte, G.; Argaud, L.; David, J.S.; Bonnet, A.; Negrier, C.; Dargaud, Y. Hypofibrinolytic state and high thrombin generation may play a major role in SARS-CoV2 associated thrombosis. J. Thromb. Haemost. 2020, 18, 2215–2219. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265–1273. [Google Scholar] [CrossRef]
- Bai, C.; Chotirmall, S.H.; Rello, J.; Alba, G.A.; Ginns, L.C.; Krishnan, J.A.; Rogers, R.; Bendstrup, E.; Burgel, P.-R.; Chalmers, J.D.; et al. Updated guidance on the management of COVID-19: From an American Thoracic Society/European Respiratory Society coordinated international task force (29 July 2020). Eur. Respir. Rev. 2020, 29, 200287. [Google Scholar] [CrossRef]
- Luks, A.M.; Swenson, E.R. Pulse oximetry for monitoring patients with COVID-19 at home. Potential pitfalls and practical guidance. Ann. Am. Thorac. Soc. 2020, 17, 1040–1046. [Google Scholar] [CrossRef]
- Yeo, H.J.; Byun, K.S.; Han, J.; Kim, J.H.; Lee, S.E.; Yoon, S.H.; Jeon, D.; Kim, Y.S.; Cho, W.H. Prognostic significance of malnutrition for long-term mortality in community-acquired pneumonia: A propensity score matched analysis. Korean J. Intern. Med. 2019, 34, 841. [Google Scholar] [CrossRef]
- Laviano, A.; Koverech, A.; Zanetti, M. Nutrition support in the time of SARS-CoV-2 (COVID-19). Nutrition 2020, 74, 110834. [Google Scholar] [CrossRef]
- Bedock, D.; Lassen, P.B.; Mathian, A.; Moreau, P.; Couffignal, J.; Ciangura, C.; Poitou-Bernert, C.; Jeannin, A.-C.; Mosbah, H.; Fadlallah, J.; et al. Prevalence and severity of malnutrition in hospitalized COVID-19 patients. Clin. Nutr. ESPEN 2020, 40, 214–219. [Google Scholar] [CrossRef]
- Browne, N.T.; Snethen, J.A.; Greenberg, C.S.; Frenn, M.; Kilanowski, J.F.; Gance-Cleveland, B.; Burke, P.J.; Lewandowski, L. When Pandemics Collide: The Impact of COVID-19 on Childhood Obesity. J Pediatr Nurs 2021, 56, 90–98. [Google Scholar] [CrossRef]
- Yu, Y.; Ye, J.; Chen, M.; Jiang, C.; Lin, W.; Lu, Y.; Ye, H.; Li, Y.; Wang, Y.; Liao, Q.; et al. Malnutrition prolongs the hospitalization of patients with COVID-19 infection: A clinical epidemiological analysis. J. Nutr. Health Aging 2021, 25, 369–373. [Google Scholar] [CrossRef]
- Spolidoro, G.C.; Azzolino, D.; Shamir, R.; Cesari, M.; Agostoni, C. Joint effort towards preventing nutritional deficiencies at the extremes of life during COVID-19. Nutrients 2021, 13, 1616. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Qu, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Stachowska, E.; Folwarski, M.; Jamioł-Milc, D.; Maciejewska, D.; Skonieczna-Żydecka, K. Nutritional support in coronavirus 2019 disease. Medicina 2020, 56, 289. [Google Scholar] [CrossRef]
- Kurtz, A.; Grant, K.; Marano, R.; Arrieta, A.; Feaster, W.; Steele, C.; Ehwerhemuepha, L. Long-term effects of malnutrition on severity of COVID-19. Sci. Rep. 2021, 11, 14974. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Y.; Gong, C.; Wang, J.; Liu, B.; Shi, L.; Duan, J. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur. J. Clin. Nutr. 2020, 74, 871–875. [Google Scholar] [CrossRef]
- National Health Commission. National Administration of Traditional Chinese Medicine on March 15. Diagnosis and treatment protocol for COVID-19 patients (Trial Version 9). Health Care Sci. 2022, 1, 14–28. [Google Scholar] [CrossRef]
- National Institutes of Health. COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 18 October 2022).
- Agarwal, A.; Rochwerg, B.; Lamontagne, F.; Siemieniuk, R.A.; Agoritsas, T.; Askie, L.; Lytvyn, L.; Leo, Y.-S.; Macdonald, H.; Zeng, L. A living WHO guideline on drugs for COVID-19. BMJ 2020, 370, m3379. [Google Scholar] [CrossRef]
- Nyaga, V.N.; Arbyn, M.; Aerts, M. Metaprop: A Stata command to perform meta-analysis of binomial data. Arch. Public Health 2014, 72, 39. [Google Scholar] [CrossRef]
- Cheema, H.A.; Shahid, A.; Ehsan, M.; Ayyan, M. The misuse of funnel plots in meta-analyses of proportions: Are they really useful? Clin. Kidney J. 2022, 15, 1209–1210. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.P.; Saratzis, A.; Sutton, A.J.; Boucher, R.H.; Sayers, R.D.; Bown, M.J. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J. Clin. Epidemiol. 2014, 67, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Furuya-Kanamori, L.; Barendregt, J.J.; Doi, S.A. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int. J. Evid.-Based Healthc. 2018, 16, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Del Giorno, R.; Quarenghi, M.; Stefanelli, K.; Capelli, S.; Giagulli, A.; Quarleri, L.; Stehrenberger, D.; Ossola, N.; Monotti, R.; Gabutti, L. Nutritional Risk Screening and Body Composition in COVID-19 Patients Hospitalized in an Internal Medicine Ward. Int. J. Gen. Med. 2020, 13, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Larrazabal, R.B., Jr.; Chiu, H.H.C.; Palileo-Villanueva, L.A.M. Outcomes of nutritionally at-risk Coronavirus Disease 2019 (COVID 19) patients admitted in a tertiary government hospital: A follow-up study of the MalnutriCoV study. Clin. Nutr. ESPEN 2021, 43, 239–244. [Google Scholar] [CrossRef]
- Martin–Martinez, A.; Ortega, O.; Viñas, P.; Arreola, V.; Nascimento, W.; Costa, A.; Riera, S.A.; Alarcón, C.; Clavé, P. COVID-19 is associated with oropharyngeal dysphagia and malnutrition in hospitalized patients during the spring 2020 wave of the pandemic. Clin. Nutr. 2021, 41, 2996–3006. [Google Scholar] [CrossRef]
- Mendes, A.; Serratrice, C.; Herrmann, F.R.; Gold, G.; Graf, C.E.; Zekry, D.; Genton, L. Nutritional risk at hospital admission is associated with prolonged length of hospital stay in old patients with COVID-19. Clin. Nutr. 2021, 41, 3085–3088. [Google Scholar] [CrossRef]
- Voelkle, M.; Gregoriano, C.; Neyer, P.; Koch, D.; Kutz, A.; Bernasconi, L.; Conen, A.; Mueller, B.; Schuetz, P. Prevalence of Micronutrient Deficiencies in Patients Hospitalized with COVID-19: An Observational Cohort Study. Nutrients 2022, 14, 1862. [Google Scholar] [CrossRef]
- Youssef, N.; Elbadry, M.; Al Shafie, A.; Abdalazeem, A.; Hasan, S.; Tahoon, M.; Omran, D.; El Kassas, M.; Msc, A.A.S.; Msc, A.A.; et al. Nutritional status associated with clinical outcomes among patients hospitalized with COVID-19: A multicenter prospective study in Egypt. Nurs. Health Sci. 2022, 24, 204–213. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Y.; Ge, Y.; Shi, Y.; Lv, P.; Zhang, J.; Fu, G.; Zhou, Y.; Jiang, K.; Lin, N.; et al. Evaluation of Nutrition Risk and Its Association With Mortality Risk in Severely and Critically Ill COVID-19 Patients. JPEN J. Parenter. Enter. Nutr. 2021, 45, 32–42. [Google Scholar] [CrossRef]
- Zhang, K.; Qin, W.; Zheng, Y.; Pang, J.; Zhong, N.; Fei, J.; Li, Y.; Jian, X.; Hou, X.; Hu, Z.; et al. Malnutrition Contributes to Low Lymphocyte Count in Early-Stage Coronavirus Disease-2019. Front. Nutr. 2021, 8, 739216. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, S.; Mao, Z.; Wang, W.; Hu, H. Clinical significance of nutritional risk screening for older adult patients with COVID-19. Eur. J. Clin. Nutr. 2020, 74, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Cong, J.; Wang, Q.; Mei, Y.; Peng, Y.; Zhou, M.; Zhu, W.; Chen, X.; Guan, W.; He, P. Risk of Malnutrition Is Common in Patients with Coronavirus Disease 2019 (COVID-19) in Wuhan, China: A Cross-sectional Study. J. Nutr. 2021, 151, 1591–1596. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, R.F.W.; Moraes, G.C.D.; Barcelos, S.G.C.; Figueiredo, P.C.M.D.; Das Merces, M.C. Evaluation of Nutritional Risk and Prevalence of Obesity in Patients with COVID-19 in A Reference Hospital in Salvador, Bahia, Brazil: A Cross-Sectional Study. Int. J. Nutrol. 2021, 14, 11–15. [Google Scholar] [CrossRef]
- Pironi, L.; Sasdelli, A.S.; Ravaioli, F.; Baracco, B.; Battaiola, C.; Bocedi, G.; Brodosi, L.; Leoni, L.; Mari, G.A.; Musio, A. Malnutrition and nutritional therapy in patients with SARS-CoV-2 disease. Clin Nutr 2021, 40, 1330–1337. [Google Scholar] [CrossRef]
- Shabanpur, M.; Pourmahmoudi, A.; Nicolau, J.; Veronese, N.; Roustaei, N.; Jahromi, A.J.; Hosseinikia, M. The importance of nutritional status on clinical outcomes among both ICU and Non-ICU patients with COVID-19. Clin. Nutr. ESPEN 2022, 49, 225–231. [Google Scholar] [CrossRef]
- Alikiaii, B.; Heidari, Z.; Fazeli, A.; Rahimi Varposhti, M.; Moradi Farsani, D.; Fattahpour, S.; Rafiee, S.; Bagherniya, M. Evaluation of the effectiveness of the Nutritional Risk Screening System 2002 (NRS-2002) in COVID-19 patients admitted to the intensive care unit. Int. J. Clin. Pr. 2021, 75, e14934. [Google Scholar] [CrossRef]
- Czapla, M.; Juárez-Vela, R.; Gea-Caballero, V.; Zieliński, S.; Zielińska, M. The Association between Nutritional Status and In-Hospital Mortality of COVID-19 in Critically-Ill Patients in the ICU. Nutrients 2021, 13, 3302. [Google Scholar] [CrossRef]
- Martins, P.M.; Gomes, T.L.N.; Franco, E.P.; Vieira, L.L.; Pimentel, G.D. High neutrophil-to-lymphocyte ratio at intensive care unit admission is associated with nutrition risk in patients with COVID-19. J. Parenter. Enter. Nutr. 2022, 46, 1441–1448. [Google Scholar] [CrossRef]
- Wu, S.; Lou, J.; Xu, P.; Luo, R.; Li, L. Early enteral nutrition improves the outcome of critically ill patients with COVID-19: A retrospective study. Asia Pac. J. Clin. Nutr. 2021, 30, 192–198. [Google Scholar] [CrossRef]
- Thomas, S.; Alexander, C.; Cassady, B.A. Nutrition risk prevalence and nutrition care recommendations for hospitalized and critically-ill patients with COVID-19. Clin. Nutr. ESPEN 2021, 44, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Hawryłkowicz, V.; Lietz-Kijak, D.; Kaźmierczak-Siedlecka, K.; Sołek-Pastuszka, J.; Stachowska, L.; Folwarski, M.; Parczewski, M.; Stachowska, E. Patient Nutrition and Probiotic Therapy in COVID-19: What Do We Know in 2021? Nutrients 2021, 13, 3385. [Google Scholar] [CrossRef] [PubMed]
- Vong, T.; Yanek, L.R.; Wang, L.; Yu, H.; Fan, C.; Zhou, E.; Oh, S.J.; Szvarca, D.; Kim, A.; Potter, J.J.; et al. Malnutrition Increases Hospital Length of Stay and Mortality among Adult Inpatients with COVID-19. Nutrients 2022, 14, 1310. [Google Scholar] [CrossRef]
- Mohammadi, P.; Varpaei, H.A.; Mohammadi, M.; Rahimi, M.; Orandi, A. Evaluation of the Relationship between Nutritional Status of COVID-19 Patients Admitted to the ICU and Patients’ Prognosis: A Cohort Study. J. Nutr. Metab. 2022, 2022, 5016649. [Google Scholar] [CrossRef]
- Kang, M.C.; Kim, J.H.; Ryu, S.-W.; Moon, J.Y.; Park, J.H.; Park, J.K.; Baik, H.-W.; Seo, J.-M.; Son, M.-W.; Song, G.A.; et al. Prevalence of malnutrition in hospitalized patients: A multicenter cross-sectional study. J. Korean Med. Sci. 2018, 33, e10. [Google Scholar] [CrossRef]
- van der Meij, B.S.; Ligthart-Melis, G.C.; de van der Schueren, M.A. Malnutrition in patients with COVID-19: Assessment and consequences. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 543–554. [Google Scholar] [CrossRef]
- Sulli, A.; Gotelli, E.; Casabella, A.; Paolino, S.; Pizzorni, C.; Alessandri, E.; Grosso, M.; Ferone, D.; Smith, V.; Cutolo, M. Vitamin D and lung outcomes in elderly COVID-19 patients. Nutrients 2021, 13, 717. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Approaches to nutritional screening in patients with Coronavirus Disease 2019 (COVID-19). Int. J. Environ. Res. Public Health 2021, 18, 2772. [Google Scholar] [CrossRef]
- Kroc, Ł.; Fife, E.; Piechocka-Wochniak, E.; Sołtysik, B.; Kostka, T. Comparison of nutrition risk screening 2002 and subjective global assessment form as short nutrition assessment tools in older hospitalized adults. Nutrients 2021, 13, 225. [Google Scholar] [CrossRef]
- Paulsen, M.M.; Hagen, M.L.L.; Frøyen, M.H.; Foss-Pedersen, R.J.; Bergsager, D.; Tangvik, R.J.; Andersen, L.F. A dietary assessment app for hospitalized patients at nutritional risk: Development and evaluation of the MyFood app. JMIR Mhealth Uhealth 2018, 6, e9953. [Google Scholar] [CrossRef]
- de Bruin, J.S.; Schuh, C.; Seeling, W.; Luger, E.; Gall, M.; Hütterer, E.; Kornek, G.; Ludvik, B.; Hoppichler, F.; Schindler, K. Assessing the feasibility of a mobile health-supported clinical decision support system for nutritional triage in oncology outpatients using Arden Syntax. Artif. Intell. Med. 2018, 92, 34–42. [Google Scholar] [CrossRef]
- Barazzoni, R.; Bischoff, S.C.; Breda, J.; Wickramasinghe, K.; Krznaric, Z.; Nitzan, D.; Pirlich, M.; Singer, P. ESPEN Expert Statements and Practical Guidance for Nutritional Management of Individuals with SARS-CoV-2 Infection; Elsevier: Amsterdam, The Netherlands, 2020; Volume 39, pp. 1631–1638. [Google Scholar]
- Caccialanza, R.; Laviano, A.; Lobascio, F.; Montagna, E.; Bruno, R.; Ludovisi, S.; Corsico, A.G.; Di Sabatino, A.; Belliato, M.; Calvi, M.; et al. Early nutritional supplementation in non-critically ill patients hospitalized for the 2019 novel coronavirus disease (COVID-19): Rationale and feasibility of a shared pragmatic protocol. Nutrition 2020, 74, 110835. [Google Scholar] [CrossRef] [PubMed]
- Martindale, R.; Patel, J.J.; Taylor, B.; Arabi, Y.M.; Warren, M.; McClave, S.A. Nutrition therapy in critically ill patients with coronavirus disease 2019. J. Parenter. Enter. Nutr. 2020, 44, 1174–1184. [Google Scholar] [CrossRef] [PubMed]
- Terblanche, E.; Hills, J.; Russell, E.; Lewis, R.; Rose, L. Dietetic-Led Nutrition Interventions in Patients with COVID-19 during Intensive Care and Ward-Based Rehabilitation: A Single-Center Observational Study. Nutrients 2022, 14, 1062. [Google Scholar] [CrossRef] [PubMed]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Goisser, S.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.C.; et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin. Nutr. 2019, 38, 10–47. [Google Scholar] [CrossRef]
- Thomson, K.H.; Rice, S.; Arisa, O.; Johnson, E.; Tanner, L.; Marshall, C.; Sotire, T.; Richmond, C.; O’Keefe, H.; Mohammed, W.; et al. Effectiveness and cost-effectiveness of oral nutritional supplements in frail older people who are malnourished or at risk of malnutrition: A systematic review and meta-analysis. Lancet Healthy Longev. 2022, 3, e654–e666. [Google Scholar] [CrossRef]
- Da Porto, A.; Tascini, C.; Peghin, M.; Sozio, E.; Colussi, G.; Casarsa, V.; Bulfone, L.; Graziano, E.; De Carlo, C.; Catena, C.; et al. Prognostic Role of Malnutrition Diagnosed by Bioelectrical Impedance Vector Analysis in Older Adults Hospitalized with COVID-19 Pneumonia: A Prospective Study. Nutrients 2021, 13, 4085. [Google Scholar] [CrossRef]
- Wierdsma, N.J.; Kruizenga, H.M.; Konings, L.A.; Krebbers, D.; Jorissen, J.R.; Joosten, M.I.; van Aken, L.H.; Tan, F.M.; van Bodegraven, A.A.; Soeters, M.R.; et al. Poor nutritional status, risk of sarcopenia and nutrition related complaints are prevalent in COVID-19 patients during and after hospital admission. Clin. Nutr. ESPEN 2021, 43, 369–376. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, Y.; Liu, Y.; Xiang, Y.; Tao, C.; Yu, H.; Huang, J. A Correlation Analysis between the Nutritional Status and Prognosis of COVID-19 Patients. J. Nutr. Health Aging 2021, 25, 84–93. [Google Scholar] [CrossRef]
- Song, F.; Ma, H.; Wang, S.; Qin, T.; Xu, Q.; Yuan, H.; Li, F.; Wang, Z.; Liao, Y.; Tan, X.; et al. Nutritional screening based on objective indices at admission predicts in-hospital mortality in patients with COVID-19. Nutr. J. 2021, 20, 46. [Google Scholar] [CrossRef]
- You, Y.; Chen, M.; Chen, X.; Yu, W. Diaphragm thickness on computed tomography for nutritional assessment and hospital stay prediction in critical COVID-19. Asia Pac. J. Clin. Nutr. 2022, 31, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Recinella, G.; Marasco, G.; Serafini, G.; Maestri, L.; Bianchi, G.; Forti, P.; Zoli, M. Prognostic role of nutritional status in elderly patients hospitalized for COVID-19: A monocentric study. Aging Clin. Exp. Res. 2020, 32, 2695–2701. [Google Scholar] [CrossRef] [PubMed]
- Otero, J.A.; Figuero, L.S.B.; Mattin, M.G.; Martin, I.U.; Morais, P.C.; Olmedo, L.C.; Galiana, L.I.; Gutierrez, C.D.; Gomez, J.C.; Gudino, L.C.; et al. The nutritional status of the elderly patient infected with COVID-19: The forgotten risk factor? Figshare 2021, 37, 549–554. [Google Scholar] [CrossRef]
- McGovern, J.; Al-Azzawi, Y.; Kemp, O.; Moffitt, P.; Richards, C.; Dolan, R.D.; Laird, B.J.; McMillan, D.C.; Maguire, D. The relationship between frailty, nutritional status, co-morbidity, CT-body composition and systemic inflammation in patients with COVID-19. J. Transl. Med. 2022, 20, 98. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Tong, H.; Li, Y.; Ge, Y.; Shi, Y.; Lv, P.; Zhao, X.; Zhang, J.; Fu, G.; Zhou, Y.; et al. Role of Prealbumin in Predicting the Prognosis of Severely and Critically Ill COVID-19 Patients. Am. J. Trop. Med. Hyg. 2021, 105, 718–726. [Google Scholar] [CrossRef]
- Fernandes, A.L.; Reis, B.Z.; Murai, I.H.; Pereira, R.M.R. Prognostic Nutritional Index and Oxygen Therapy Requirement Associated With Longer Hospital Length of Stay in Patients With Moderate to Severe COVID-19: Multicenter Prospective Cohort Analyses. Front. Nutr. 2022, 9, 802562. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, L.; Wang, H.; Wang, X.; Qu, G.; Cai, J.; Zhang, H. Malnutrition is associated with hyperinflammation and immunosuppression in COVID-19 patients: A prospective observational study. Nutr. Clin. Pract. 2021, 36, 863–871. [Google Scholar] [CrossRef]
- Allard, L.; Ouedraogo, E.; Molleville, J.; Bihan, H.; Giroux-Leprieur, B.; Sutton, A.; Baudry, C.; Josse, C.; Didier, M.; Deutsch, D.; et al. Malnutrition: Percentage and Association with Prognosis in Patients Hospitalized for Coronavirus Disease 2019. Nutrients 2020, 12, 3679. [Google Scholar] [CrossRef]
- Wei, C.; Liu, Y.; Li, Y.; Zhang, Y.; Zhong, M.; Meng, X. Evaluation of the nutritional status in patients with COVID-19. J. Clin. Biochem. Nutr. 2020, 67, 116–121. [Google Scholar] [CrossRef]
- Rouget, A.; Vardon-Bounes, F.; Lorber, P.; Vavasseur, A.; Marion, O.; Marcheix, B.; Lairez, O.; Balardy, L.; Fourcade, O.; Conil, J.-M. Prevalence of malnutrition in coronavirus disease 19: The NUTRICOV study. Br. J. Nutr. 2021, 126, 1296–1303. [Google Scholar] [CrossRef]
- Vaillant, M.-F.; Agier, L.; Martineau, C.; Philipponneau, M.; Romand, D.; Masdoua, V.; Behar, M.; Nesseler, C.; Achamrah, N.; Laubé, V. Food intake and weight loss of surviving inpatients in the course of COVID-19 infection: A longitudinal study of the multicenter NutriCoviD30 cohort. Nutrition 2022, 93, 111433. [Google Scholar] [CrossRef] [PubMed]
- Thiam, C.N.; Mathavan, S.; Abdullah, A.; Chong, E.G.M. Malnutrition among patients admitted to the subacute geriatric ward during the COVID-19 pandemic era: A cross-sectional study in a tertiary hospital in Malaysia. Med. J. Malays. 2022, 77, 313–319. [Google Scholar]
- Farina, N.; Nordbeck, S.; Montgomery, M.; Cordwin, L.; Blair, F.; Cherry-Bukowiec, J.; Kraft, M.D.; Pleva, M.R.; Raymond, E. Early Enteral Nutrition in Mechanically Ventilated Patients With COVID-19 Infection. Nutr. Clin. Pract. 2021, 36, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Fiorindi, C.; Campani, F.; Rasero, L.; Campani, C.; Livi, L.; Giovannoni, L.; Amato, C.; Giudici, F.; Bartoloni, A.; Fattirolli, F.; et al. Prevalence of nutritional risk and malnutrition during and after hospitalization for COVID-19 infection: Preliminary results of a single-centre experience. Clin. Nutr. ESPEN 2021, 45, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Stefano, M.; Andrea, B.; Daniela, C.; Emanuela, M.; Lorena, P.; Daniela, D.; Sökeland, F.; Azzolini, E.; Beatrice, M. Malnutrition risk as a negative prognostic factor in COVID-19 patients. Clin. Nutr. ESPEN 2021, 45, 369–373. [Google Scholar] [CrossRef]
- Ansu, V.; Papoutsakis, C.; Gletsu-Miller, N.; Spence, L.A.; Kelley, K.; Woodcock, L.; Wallace, T.C.; Steiber, A. Nutrition care practice patterns for patients with COVID-19—A preliminary report. J. Parenter. Enter. Nutr. 2021, 45, 1774–1778. [Google Scholar] [CrossRef]
- Nicolau, J.; Ayala, L.; Sanchis, P.; Olivares, J.; Dotres, K.; Soler, A.-G.; Rodriguez, I.; Gomez, L.-A.; Masmiquel, L. Influence of nutritional status on clinical outcomes among hospitalized patients with COVID-19. Clin. Nutr. ESPEN 2021, 43, 223–229. [Google Scholar] [CrossRef]
- Rives-Lange, C.; Zimmer, A.; Merazka, A.; Carette, C.; Martins-Bexinga, A.; Hauw-Berlemont, C.; Guerot, E.; Jannot, A.S.; Diehl, J.L.; Czernichow, S.; et al. Evolution of the nutritional status of COVID-19 critically-ill patients: A prospective observational study from ICU admission to three months after ICU discharge. Clin. Nutr. 2021, 41, 3026–3031. [Google Scholar] [CrossRef]
- Shahbazi, S.; Hajimohammadebrahim-Ketabforoush, M.; Shariatpanahi, M.V.; Shahbazi, E.; Shariatpanahi, Z.V. The validity of the global leadership initiative on malnutrition criteria for diagnosing malnutrition in critically ill patients with COVID-19: A prospective cohort study. Clin. Nutr. ESPEN 2021, 43, 377–382. [Google Scholar] [CrossRef]
- Gómez-Uranga, A.; Guzmán-Martínez, J.; Esteve-Atiénzar, P.J.; Wikman-Jorgensen, P.; Núñez-Cruz, J.M.; Espinosa-Del-Barrio, L.; Hernández-Isasi, I.; Pomares-Gómez, F.J.; Perelló-Camacho, E.; Fernández-García, N.; et al. Nutritional and Functional Impact of Acute SARS-CoV-2 Infection in Hospitalized Patients. J. Clin. Med. 2022, 11, 2424. [Google Scholar] [CrossRef]
- Martinuzzi, A.L.N.; Manzanares, W.; Quesada, E.; Reberendo, M.J.; Baccaro, F.; Aversa, I.; Kecskes, C.E.; Magnífico, L.; González, V.; Bolzico, D.; et al. Nutritional risk and clinical outcomes in critically ill adult patients with COVID-19. Nutr. Hosp. 2021, 38, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Vahdat Shariatpanahi, Z.; Vahdat Shariatpanahi, M.; Shahbazi, E.; Shahbazi, S. Refeeding Syndrome and Its Related Factors in Critically Ill Coronavirus Disease 2019 Patients: A Prospective Cohort Study. Front. Nutr. 2022, 9, 830457. [Google Scholar] [CrossRef] [PubMed]
- Chadli, A.; Haraj, N.E.; El Aziz, S.; Laidi, S.; Mounir, A.; Bensbaa, S.; Mjabber, A.; Barrou, L.; El Kettani El Hamidi, C.; Nsiri, A.; et al. COVID-19: Patient care after discharge from the Intensive Care Unit. Int. J. Clin. Pract. 2021, 75, e14270. [Google Scholar] [CrossRef]
- Leoni, M.L.G.; Moschini, E.; Beretta, M.; Zanello, M.; Nolli, M. The modified NUTRIC score (mNUTRIC) is associated with increased 28-day mortality in critically ill COVID-19 patients: Internal validation of a prediction model. Clin. Nutr. ESPEN 2022, 48, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; He, Z.; Yu, G.; Peng, D.; Feng, Y.; Ling, J.; Wang, Y.; Li, S.; Bian, Y. The modified NUTRIC score can be used for nutritional risk assessment as well as prognosis prediction in critically ill COVID-19 patients. Clin. Nutr. 2021, 40, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhou, C.-L.; Ba, Y.-M.; Wang, Y.-M.; Song, B.; Cheng, X.-B.; Dong, Q.-F.; Wang, L.-L.; You, S.-S. Nutritional risk and therapy for severe and critical COVID-19 patients: A multicenter retrospective observational study. Clin. Nutr. 2021, 40, 2154–2161. [Google Scholar] [CrossRef] [PubMed]
- Cuerda, C.; Sánchez López, I.; Gil Martínez, C.; Merino Viveros, M.; Velasco, C.; Cevallos Peñafiel, V.; Maíz Jiménez, M.; Gonzalo, I.; González-Sánchez, V.; Ramos Carrasco, A.; et al. Impact of COVID-19 in nutritional and functional status of survivors admitted in intensive care units during the first outbreak. Preliminary results of the NUTRICOVID study. Clin. Nutr. 2021, 41, 2934–2939. [Google Scholar] [CrossRef]


| Author | Country | Time Frame | Sample Size | Age 1 | Sex | COVID-19 Confirmation | Timing of Assessment | Specific Time of Evaluation | Outcome 2 | Prevalence 3 |
|---|---|---|---|---|---|---|---|---|---|---|
| General Ward | ||||||||||
| Del Giorno et al. (2020) [36] | Switzerland | 2020/3 | 90 | 64.5 ± 13.7 | Male (67.8%) | PCR 4/CT 5 | At admission | the first 24 h of admission | Malnutrition risk (Y/N) | 92.0% |
| Larrazabal et al. (2021) [37] | Philippines | 2020/7/15–2020/9/15 | 355 | NR | NR | RT-PCR 6 | At admission | NR 7 | Malnutrition risk (low/moderate/high) | 37.7% low; 47.3% moderate; 14.9% high risk |
| Martin–Martinez et al. (2021) [38] | Spain | 2020/4/14–2020/7/30 | 205 | 69.3 ± 17.5 | Male (47.8%) | PCR | At admission | NR | Malnutrition risk (Y/N) | 88.7% |
| Mendes et al. (2021) [39] | Switzerland | 2020/3/13–2020/5/17 | 245 | 86.1 ± 6.4 | Male (42%) | RT-PCR | At admission | NR | Malnutrition risk (no/at risk/high risk) | 17.6% no risk; 32.2% at risk; 50.2% high risk |
| Voelkle et al. (2022) [40] | Switzerland | 2020/3/17–2020/4/30 | 57 | 67.0 (60.0, 74.2) | Male (60%) | RT-PCR | At admission | NR | Malnutrition risk (Y/N) | 19.0% |
| Youssef et al. (2022) [41] | Egypt | 2020/7–2020/12 | 121 | 52.4 ± 10.5 | Male (84.3%) | NR | At admission | on day 1 of admission | Malnutrition risk (Mild–moderate/Severe) | 94.9% Mild–moderate; 5.1% Severe |
| Zhao et al. (2021) [42] | China | 2020/1/29–2020/2/19 | 371 | NR | NR | NR | At admission | on the first day of hospitalization | Malnutrition risk (no/low/high) | 8% no risk; 76% low; 16% high risk |
| Zhang et al. (2021) [43] | China | 2020/2/6-2020/3/20 | 101 | 65.3 ± 13 | Male (59.4%) | NR | At admission | on the first day of hospitalization | Malnutrition risk(Y/N) | 40.5% |
| Liu et al. (2020) [44] | China | 2020/1/28–2020/3/28 | 141 | 71.7 ± 5.9 | Male (48.2%) | RT-PCR/CT | During hospitaliza-tion | NR | Malnutrition risk (Y/N) | 85.1% |
| Liu et al. (2021) [45] | China | 2020/1/29–2020/3/15 | 760 | 60 (46, 74) | Male (50%) | RT-PCR/CT | During hospitaliza-tion | NR | Malnutrition risk (Y/N) | 82.6% |
| Pimentel et al. (2021) [46] | Brazil | NR | 41 | 45.7 ± 12.4 | Male (61.0%) | RT-PCR | During hospitaliza-tion | NR | Malnutrition risk (low/high) | 22.0% low risk; 78.0% high risk |
| Pironi et al. (2021) [47] | Italy | 2020/4 | 162 | NR | NR | NR | During hospitaliza-tion | a one-day clinical audit | Malnutrition risk (Y/N) | 67.3% |
| Shabanpur et al. (2022) [48] | Iran | 2021/5–2021/7 | 330 | NR | NR | NR | During hospitaliza-tion | in a 6-week period | Malnutrition (risk/moderate/severe) | 4% at risk; 69% moderate; 27% severe risk |
| ICU | ||||||||||
| Alikiaii et al. (2021) [49] | Iran | 2021/1/1 | 73 | 58.9 ± 18.8 | Male (63%) | RT-PCR | During hospitaliza-tion | NR | Malnutrition risk (low/moderate/high) | 17.8% low; 69.9% moderate; 12.3% high risk |
| Czapla et al. (2021) [50] | Poland | 2020/9–2021/6 | 286 | NR | Male (67.8%) | RT-PCR | At admission | at the time of admission to ICU 8 | Malnutrition risk(Y/N) | 90.2% |
| Martins et al. (2022) [51] | Brazil | 2020/3–2020/10 | 73 | 56 | Male (63%) | PCR | At admission | the first 48 h of admission to the ICU | Malnutrition risk(Y/N) | 85.0% |
| Pironi et al. (2021) [47] | Italy | 2020/4 | 106 | NR | NR | NR | During hospitaliza-tion | a one-day clinical audit | Malnutrition risk(Y/N) | 92.5% |
| Shabanpur et al. (2022) [48] | Iran | 2021/5–2021/7 | 70 | NR | NR | NR | During hospitaliza-tion | in a 6-week period | Malnutrition (risk/moderate/severe) | 0% at risk; 20% moderate; 80% severe risk |
| Wu et al. (2021) [52] | China | 2020/1/15–2020/2/29 | 27 | 74.9 ± 10.5 | Male (66.7%) | NR | During hospitaliza-tion | on the seventh day of admission | Malnutrition risk (potential/ high) | 3.7% potential risk; 96.3% high risk |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, X.; Liu, Z.; He, X.; Wang, X.; Yuan, C.; Huang, L.; Song, R.; Wu, Y. Risk of Malnutrition in Hospitalized COVID-19 Patients: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 5267. https://doi.org/10.3390/nu14245267
Feng X, Liu Z, He X, Wang X, Yuan C, Huang L, Song R, Wu Y. Risk of Malnutrition in Hospitalized COVID-19 Patients: A Systematic Review and Meta-Analysis. Nutrients. 2022; 14(24):5267. https://doi.org/10.3390/nu14245267
Chicago/Turabian StyleFeng, Xiaoru, Zeqi Liu, Xiaotong He, Xibiao Wang, Changzheng Yuan, Liyan Huang, Rui Song, and You Wu. 2022. "Risk of Malnutrition in Hospitalized COVID-19 Patients: A Systematic Review and Meta-Analysis" Nutrients 14, no. 24: 5267. https://doi.org/10.3390/nu14245267
APA StyleFeng, X., Liu, Z., He, X., Wang, X., Yuan, C., Huang, L., Song, R., & Wu, Y. (2022). Risk of Malnutrition in Hospitalized COVID-19 Patients: A Systematic Review and Meta-Analysis. Nutrients, 14(24), 5267. https://doi.org/10.3390/nu14245267





