Can Lactoferrin, a Natural Mammalian Milk Protein, Assist in the Battle against COVID-19?
Abstract
1. Introduction
2. COVID-19: A Continuous Burden on Mankind
2.1. Pathogenesis
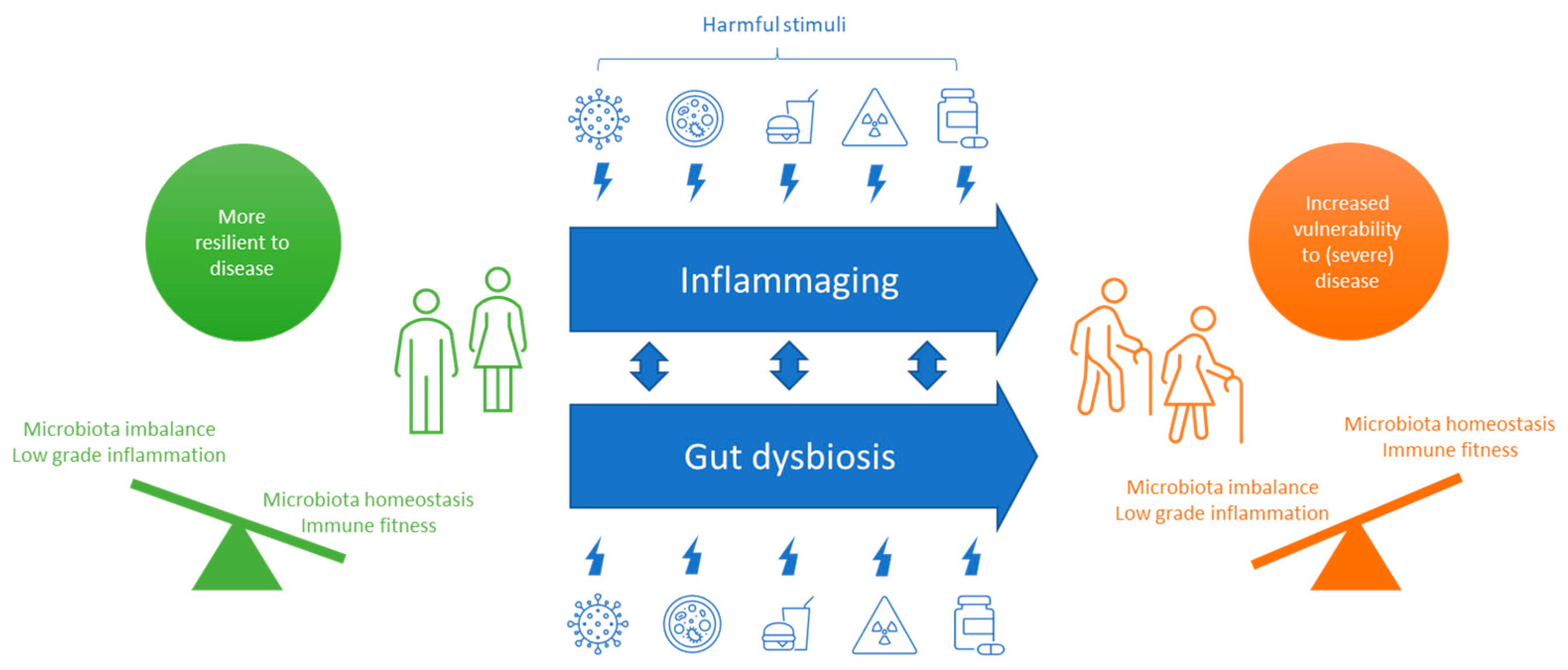
2.2. COVID-19 in the Aging Population
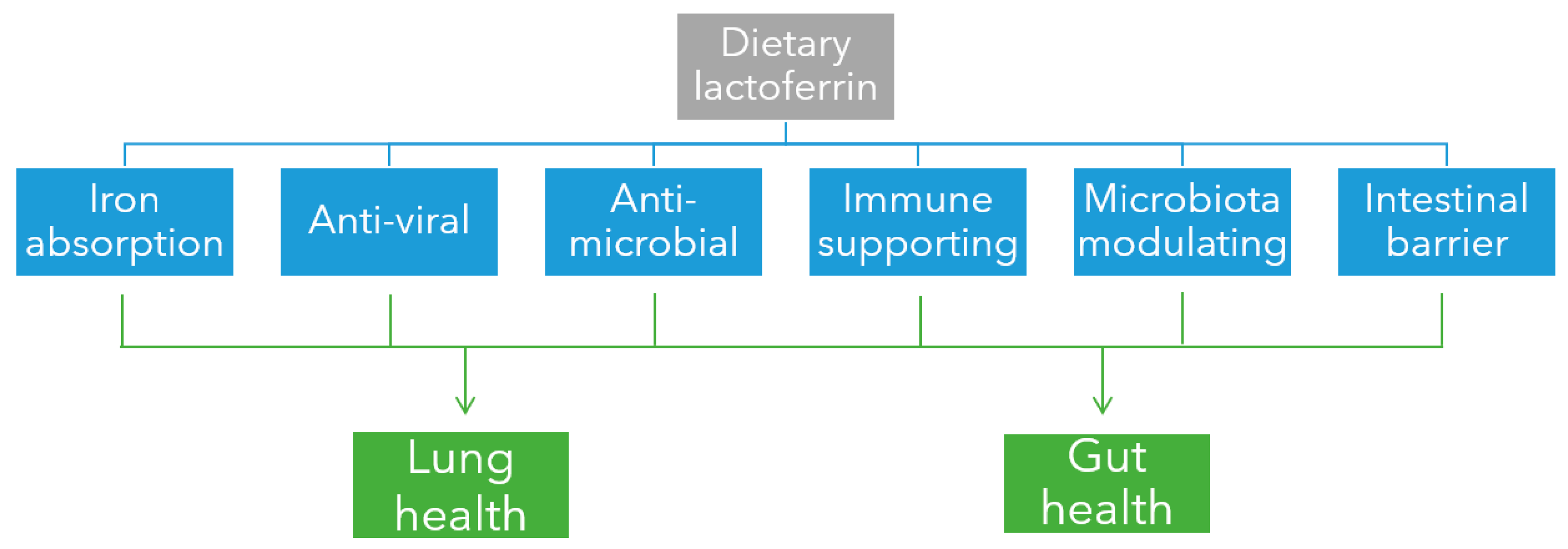
3. Lactoferrin’s Protective Effects against SARS-CoV-2 Infection
3.1. Iron-Binding and Absorption
3.2. Anti-Viral Activity
3.3. Anti-Microbial Activity
3.4. Immune Modulation
3.5. Microbiota Modulation
3.6. Intestinal Barrier Function
4. Lactoferrin Intervention Studies in COVID-19 Patients
5. Information Gaps and Research Opportunities
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dong, E.; Du, H.; Gardner, L. An Interactive Web-Based Dashboard to Track COVID-19 in Real Time. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef]
- Ochani, R.K.; Asad, A.; Yasmin, F.; Shaikh, S.; Khalid, H.; Batra, S.; Sohail, M.R.; Mahmood, S.F.; Ochani, R.; Arshad, M.H.; et al. COVID-19 Pandemic: From Origins to Outcomes. A Comprehensive Review of Viral Pathogenesis, Clinical Manifestations, Diagnostic Evaluation, and Management. Infez. Med. 2021, 29, 20–36. [Google Scholar]
- Wu, Y.; Kang, L.; Guo, Z.; Liu, J.; Liu, M.; Liang, W. Incubation Period of COVID-19 Caused by Unique SARS-CoV-2 Strains: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2022, 5, e2228008. [Google Scholar] [CrossRef]
- Seyed Hosseini, E.; Riahi Kashani, N.; Nikzad, H.; Azadbakht, J.; Hassani Bafrani, H.; Haddad Kashani, H. The Novel Coronavirus Disease-2019 (COVID-19): Mechanism of Action, Detection and Recent Therapeutic Strategies. Virology 2020, 551, 1–9. [Google Scholar] [CrossRef]
- Baj, J.; Karakuła-Juchnowicz, H.; Teresiński, G.; Buszewicz, G.; Ciesielka, M.; Sitarz, R.; Forma, A.; Karakuła, K.; Flieger, W.; Portincasa, P.; et al. COVID-19: Specific and Non-Specific Clinical Manifestations and Symptoms: The Current State of Knowledge. J. Clin. Med. 2020, 9, 1753. [Google Scholar] [CrossRef]
- Gold, J.E.; Okyay, R.A.; Licht, W.E.; Hurley, D.J. Investigation of Long COVID Prevalence and Its Relationship to Epstein-Barr Virus Reactivation. Pathogens 2021, 10, 763. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.S.; Hung, I.F.N.; Chan, P.P.Y.; Lung, K.C.; Tso, E.; Liu, R.; Ng, Y.Y.; Chu, M.Y.; Chung, T.W.H.; Tam, A.R.; et al. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-Analysis. Gastroenterology 2020, 159, 81–95. [Google Scholar] [CrossRef]
- Calder, P.C. Nutrition, Immunity and COVID-19. BMJ Nutr. Prev. Health 2020, 3, 74–92. [Google Scholar] [CrossRef]
- Zabetakis, I.; Lordan, R.; Norton, C.; Tsoupras, A. COVID-19: The Inflammation Link and the Role of Nutrition in Potential Mitigation. Nutrients 2020, 12, 1466. [Google Scholar] [CrossRef] [PubMed]
- Galmés, S.; Serra, F.; Palou, A. Current State of Evidence: Influence of Nutritional and Nutrigenetic Factors on Immunity in the COVID-19 Pandemic Framework. Nutrients 2020, 12, 2738. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, Y.; Gong, C.; Wang, J.; Liu, B.; Shi, L.; Duan, J. Prevalence of Malnutrition and Analysis of Related Factors in Elderly Patients with COVID-19 in Wuhan, China. Eur. J. Clin. Nutr. 2020, 74, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Fedele, D.; de Francesco, A.; Riso, S.; Collo, A. Obesity, Malnutrition, and Trace Element Deficiency in the Coronavirus Disease (COVID-19) Pandemic: An Overview. Nutrition 2021, 81, 111016. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Ng, T.B.; Sun, W.Z. Lactoferrin as Potential Preventative and Adjunct Treatment for COVID-19. Int. J. Antimicrob. Agents 2020, 56, 106118. [Google Scholar] [CrossRef]
- Sorensen, M.; Sorensen, S. Sorensen: Compte Rendu Des Travaux Du Laboratoire...—Google Scholar. Available online: https://scholar.google.com/scholar_lookup?author=M.+Sorensen&author=S.+Sorensen+&publication_year=1939&title=Compte+Rendu+des+Travaux+du+Laboratoire+de+Carlsberg#d=gs_cit&t=1666697945791&u=%2Fscholar%3Fq%3Dinfo%3AjYIBnF-QaZQJ%3Ascholar.google.com%2F%26output%3Dcite%26scirp%3D0%26hl%3Dnl (accessed on 25 October 2022).
- Czosnykowska-Łukacka, M.; Orczyk-Pawiłowicz, M.; Broers, B.; Królak-Olejnik, B. Lactoferrin in Human Milk of Prolonged Lactation. Nutrients 2019, 11, 2350. [Google Scholar] [CrossRef]
- Yang, Z.; Jiang, R.; Chen, Q.; Wang, J.; Duan, Y.; Pang, X.; Jiang, S.; Bi, Y.; Zhang, H.; Lönnerdal, B.; et al. Concentration of Lactoferrin in Human Milk and Its Variation during Lactation in Different Chinese Populations. Nutrients 2018, 10, 1235. [Google Scholar] [CrossRef]
- Rai, D.; Adelman, A.S.; Zhuang, W.; Rai, G.P.; Boettcher, J.; Lönnerdal, B. Longitudinal Changes in Lactoferrin Concentrations in Human Milk: A Global Systematic Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1539–1547. [Google Scholar] [CrossRef]
- Cheng, J.B.; Wang, J.Q.; Bu, D.P.; Liu, G.L.; Zhang, C.G.; Wei, H.Y.; Zhou, L.Y.; Wang, J.Z. Factors Affecting the Lactoferrin Concentration in Bovine Milk. J. Dairy Sci. 2008, 91, 970–976. [Google Scholar] [CrossRef]
- Peroni, D.G.; Fanos, V. Lactoferrin Is an Important Factor When Breastfeeding and COVID-19 Are Considered. Acta Paediatr. 2020, 109, 2139. [Google Scholar] [CrossRef] [PubMed]
- Telang, S. Lactoferrin: A Critical Player in Neonatal Host Defense. Nutrients 2018, 10, 1228. [Google Scholar] [CrossRef]
- Legrand, D. Overview of Lactoferrin as a Natural Immune Modulator. J. Pediatr. 2016, 173, S10–S15. [Google Scholar] [CrossRef]
- Pammi, M.; Suresh, G. Enteral Lactoferrin Supplementation for Prevention of Sepsis and Necrotizing Enterocolitis in Preterm Infants. Cochrane Database Syst. Rev. 2020, 3, CD007137. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B.; Heyden, E.L.; Pretorius, E. The Biology of Lactoferrin, an Iron-Binding Protein That Can Help Defend Against Viruses and Bacteria. Front. Immunol. 2020, 11, 1221. [Google Scholar] [CrossRef] [PubMed]
- Guillén, C.; McInnes, I.B.; Kruger, H.; Brock, J.H. Iron, Lactoferrin and Iron Regulatory Protein Activity in the Synovium; Relative Importance of Iron Loading and the Inflammatory Response. Ann. Rheum. Dis. 1998, 57, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Recio, I.; Moreno, F.J.; López-Fandiño, R. Glycosylated Dairy Components: Their Roles in Nature and Ways to Make Use of Their Biofunctionality in Dairy Products. In Dairy-Derived Ingredients: Food and Nutraceutical Uses; Elsevier: Amsterdam, The Netherlands, 2009; pp. 170–211. [Google Scholar] [CrossRef]
- McGrath, B.A.; Fox, P.F.; McSweeney, P.L.H.; Kelly, A.L. Composition and Properties of Bovine Colostrum: A Review. Dairy Sci. Technol. 2015, 96, 133–158. [Google Scholar] [CrossRef]
- Steijns, J.M.; van Hooijdonk, A.C.M. Occurrence, Structure, Biochemical Properties and Technological Characteristics of Lactoferrin. Br. J. Nutr. 2000, 84, 11–17. [Google Scholar] [CrossRef]
- Superti, F. Lactoferrin from Bovine Milk: A Protective Companion for Life. Nutrients 2020, 12, 2562. [Google Scholar] [CrossRef]
- Rosa, L.; Cutone, A.; Conte, M.P.; Campione, E.; Bianchi, L.; Valenti, P. An Overview on in Vitro and in Vivo Antiviral Activity of Lactoferrin: Its Efficacy against SARS-CoV-2 Infection. Biometals 2022, 1, 1–20. [Google Scholar] [CrossRef]
- Cascella, M.; Rajnik, M.; Cuomo, A.; Dulebohn, S.C.; di Napoli, R. Features, Evaluation, and Treatment of Coronavirus (COVID-19); StatPearls: Tampa, FL, USA, 2022. [Google Scholar]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural Basis for the Recognition of SARS-CoV-2 by Full-Length Human ACE2. Science 2020, 367, 1444. [Google Scholar] [CrossRef]
- Nitin, P.; Nandhakumar, R.; Vidhya, B.; Rajesh, S.; Sakunthala, A. COVID-19: Invasion, Pathogenesis and Possible Cure—A Review. J. Virol. Methods 2022, 300, 114434. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed]
- van de Veerdonk, F.L.; Giamarellos-Bourboulis, E.; Pickkers, P.; Derde, L.; Leavis, H.; van Crevel, R.; Engel, J.J.; Wiersinga, W.J.; Vlaar, A.P.J.; Shankar-Hari, M.; et al. A Guide to Immunotherapy for COVID-19. Nat. Med. 2022, 28, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Kotagiri, P.; Mescia, F.; Hanson, A.L.; Turner, L.; Bergamaschi, L.; Peñalver, A.; Richoz, N.; Moore, S.D.; Ortmann, B.M.; Dunmore, B.J.; et al. The Impact of Hypoxia on B Cells in COVID-19. EBioMedicine 2022, 77, 103878. [Google Scholar] [CrossRef] [PubMed]
- Parasher, A. COVID-19: Current Understanding of Its Pathophysiology, Clinical Presentation and Treatment. Postgrad Med. J. 2021, 97, 312–320. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef]
- Habib, H.M.; Ibrahim, S.; Zaim, A.; Ibrahim, W.H. The Role of Iron in the Pathogenesis of COVID-19 and Possible Treatment with Lactoferrin and Other Iron Chelators. Biomed Pharm. 2021, 136, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A.L.; Mcnamara, M.S.; Sinclair, D.A. Why Does COVID-19 Disproportionately Affect Older People? Aging (Albany NY) 2020, 12, 9959. [Google Scholar] [CrossRef]
- Boechat, J.L.; Chora, I.; Morais, A.; Delgado, L. The Immune Response to SARS-CoV-2 and COVID-19 Immunopathology—Current Perspectives. Pulmonology 2021, 27, 423. [Google Scholar] [CrossRef]
- Bajaj, V.; Gadi, N.; Spihlman, A.P.; Wu, S.C.; Choi, C.H.; Moulton, V.R. Aging, Immunity, and COVID-19: How Age Influences the Host Immune Response to Coronavirus Infections? Front. Physiol. 2021, 11, 1793. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822. [Google Scholar] [CrossRef]
- Wiertsema, S.P.; van Bergenhenegouwen, J.; Garssen, J.; Knippels, L.M.J. The Interplay between the Gut Microbiome and the Immune System in the Context of Infectious Diseases throughout Life and the Role of Nutrition in Optimizing Treatment Strategies. Nutrients 2021, 13, 886. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jazwinski, S.M. The Gut Microbiota and Healthy Aging: A Mini-Review. Gerontology 2018, 64, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Babaei, P.; Ji, B.; Nielsen, J. Human Gut Microbiota and Healthy Aging: Recent Developments and Future Prospective. Nutr. Healthy Aging 2016, 4, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.B.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut Microbiota Composition Correlates with Diet and Health in the Elderly. Nature 2016, 488, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Zhang, F.; Lui, G.C.Y.; Yeoh, Y.K.; Li, A.Y.L.; Zhan, H.; Wan, Y.; Chung, A.C.K.; Cheung, C.P.; Chen, N.; et al. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology 2020, 159, 944–955.e8. [Google Scholar] [CrossRef]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.Y.; Zhang, F.; Liu, Q.; Li, A.Y.L.; Chung, A.C.K.; Cheung, C.P.; Tso, E.Y.K.; Fung, K.S.C.; et al. Gut Microbiota Composition Reflects Disease Severity and Dysfunctional Immune Responses in Patients with COVID-19. Gut 2021, 70, 698–706. [Google Scholar] [CrossRef]
- Robinson, L.A.; Pierce, C.M. Is ‘Inflammaging’ Fuelling Severe COVID-19 Disease? J. R. Soc. Med. 2020, 113, 346–349. [Google Scholar] [CrossRef]
- Bukowska-Ośko, I.; Sulejczak, D.; Kaczyńska, K.; Kleczkowska, P.; Kramkowski, K.; Popiel, M.; Wietrak, E.; Kowalczyk, P. Lactoferrin as a Human Genome “Guardian”—An Overall Point of View. Int. J. Mol. Sci. 2022, 23, 5248. [Google Scholar] [CrossRef]
- Brink, L.R.; Chichlowski, M.; Pastor, N.; Narayanappa, A.T.; Shah, N. In the Age of Viral Pandemic, Can Ingredients Inspired by Human Milk and Infant Nutrition Be Repurposed to Support the Immune System? Nutrients 2021, 13, 870. [Google Scholar] [CrossRef]
- Gallo, V.; Giansanti, F.; Arienzo, A.; Antonini, G. Antiviral Properties of Whey Proteins and Their Activity against SARS-CoV-2 Infection. J. Funct. Foods 2022, 89, 104932. [Google Scholar] [CrossRef]
- Ochoa, T.J.; Pezo, A.; Cruz, K.; Chea-Woo, E.; Cleary, T.G. Clinical Studies of Lactoferrin in Children. Biochem. Cell Biol. 2012, 90, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.C.; Pereira, B.F.M.; Leandro, K.C.; Costa, M.P.; Spisso, B.F.; Conte-Junior, C.A. Bioactive Compounds in Infant Formula and Their Effects on Infant Nutrition and Health: A Systematic Literature Review. Int. J. Food Sci. 2021, 2021, 8850080. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Guo, C. A Review on Lactoferrin and Central Nervous System Diseases. Cells 2021, 10, 1810. [Google Scholar] [CrossRef]
- Karav, S.; German, J.B.; Rouquie, C.; le Parc, A.; Barile, D. Studying Lactoferrin N-Glycosylation. Int. J. Mol. Sci. 2017, 18, 870. [Google Scholar] [CrossRef] [PubMed]
- Demmelmair, H.; Prell, C.; Timby, N.; Lönnerdal, B. Benefits of Lactoferrin, Osteopontin and Milk Fat Globule Membranes for Infants. Nutrients 2017, 9, 817. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, P. Clinical Benefits of Lactoferrin for Infants and Children. J. Pediatr. 2016, 173, S43–S52. [Google Scholar] [CrossRef]
- Pammi, M.; Abrams, S.A. Oral Lactoferrin for the Prevention of Sepsis and Necrotizing Enterocolitis in Preterm Infants. Cochrane Database of Systematic Reviews 2015, 12, CD007137. [Google Scholar]
- Campione, E.; Cosio, T.; Rosa, L.; Lanna, C.; Girolamo, S.D.; Gaziano, R.; Valenti, P.; Bianchi, L. Lactoferrin as Protective Natural Barrier of Respiratory and Intestinal Mucosa against Coronavirus Infection and Inflammation. Int. J. Mol. Sci. 2020, 21, 4903. [Google Scholar] [CrossRef]
- Franco, I.; Pérez, M.D.; Conesa, C.; Calvo, M.; Sánchez, L. Effect of Technological Treatments on Bovine Lactoferrin: An Overview. Food Res. Int. 2018, 106, 173–182. [Google Scholar] [CrossRef]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Lactoferrin: Structure, Function, Denaturation and Digestion. Crit. Rev. Food Sci. Nutr. 2019, 59, 580–596. [Google Scholar] [CrossRef]
- Pasricha, S.R.; Tye-Din, J.; Muckenthaler, M.U.; Swinkels, D.W. Iron Deficiency. Lancet 2021, 397, 233–248. [Google Scholar] [CrossRef]
- Girelli, D.; Marchi, G.; Busti, F.; Vianello, A. Iron Metabolism in Infections: Focus on COVID-19. Semin. Hematol. 2021, 58, 182. [Google Scholar] [CrossRef]
- Taneri, P.E.; Gómez-Ochoa, S.A.; Llanaj, E.; Raguindin, P.F.; Rojas, L.Z.; Roa-Díaz, Z.M.; Salvador, D.; Groothof, D.; Minder, B.; Kopp-Heim, D.; et al. Anemia and Iron Metabolism in COVID-19: A Systematic Review and Meta-Analysis. Eur. J. Epidemiol. 2020, 35, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Suriawinata, E.; Mehta, K.J. Iron and Iron-Related Proteins in COVID-19. Clin. Exp. Med. 2022, 18, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Sienkiewicz, M.; Jaśkiewicz, A.; Tarasiuk, A.; Fichna, J. Lactoferrin: An Overview of Its Main Functions, Immunomodulatory and Antimicrobial Role, and Clinical Significance. Crit. Rev. Food Sci. Nutr. 2022, 62, 6016–6033. [Google Scholar] [CrossRef] [PubMed]
- Campione, E.; Lanna, C.; Cosio, T.; Rosa, L.; Conte, M.P.; Iacovelli, F.; Romeo, A.; Falconi, M.; del Vecchio, C.; Franchin, E.; et al. Lactoferrin as Antiviral Treatment in COVID-19 Management: Preliminary Evidence. Int. J. Environ. Res. Public Health 2021, 18, 10985. [Google Scholar] [CrossRef]
- Rosa, L.; Cutone, A.; Lepanto, M.S.; Paesano, R.; Valenti, P. Lactoferrin: A Natural Glycoprotein Involved in Iron and Inflammatory Homeostasis. Int. J. Mol. Sci. 2017, 18, 1985. [Google Scholar] [CrossRef]
- Mancinelli, R.; Rosa, L.; Cutone, A.; Lepanto, M.S.; Franchitto, A.; Onori, P.; Gaudio, E.; Valenti, P. Viral Hepatitis and Iron Dysregulation: Molecular Pathways and the Role of Lactoferrin. Molecules 2020, 25, 1997. [Google Scholar] [CrossRef]
- Lönnerdal, B. Infant Formula and Infant Nutrition: Bioactive Proteins of Human Milk and Implications for Composition of Infant Formulas. American Journal of Clinical Nutrition 2014, 99, 712S–717S. [Google Scholar] [CrossRef]
- Mulder, A.M.; Connellan, P.A.; Oliver, C.J.; Morris, C.A.; Stevenson, L.M. Bovine Lactoferrin Supplementation Supports Immune and Antioxidant Status in Healthy Human Males. Nutr. Res. 2008, 28, 583–589. [Google Scholar] [CrossRef]
- Mrityunjaya, M.; Pavithra, V.; Neelam, R.; Janhavi, P.; Halami, P.M.; Ravindra, P.V. Immune-Boosting, Antioxidant and Anti-Inflammatory Food Supplements Targeting Pathogenesis of COVID-19. Front. Immunol. 2020, 11, 2337. [Google Scholar] [CrossRef] [PubMed]
- Mayeur, S.; Spahis, S.; Pouliot, Y.; Levy, E. Lactoferrin, a Pleiotropic Protein in Health and Disease. Antioxid Redox Signal 2016, 24, 813–836. [Google Scholar] [CrossRef] [PubMed]
- Mikulic, N.; Uyoga, M.A.; Mwasi, E.; Stoffel, N.U.; Zeder, C.; Karanja, S.; Zimmermann, M.B. Iron Absorption Is Greater from Apo-Lactoferrin and Is Similar Between Holo-Lactoferrin and Ferrous Sulfate: Stable Iron Isotope Studies in Kenyan Infants. J. Nutr. 2020, 150, 3200–3207. [Google Scholar] [CrossRef] [PubMed]
- el Amrousy, D.; El-Afify, D.; Elsawy, A.; Elsheikh, M.; Donia, A.; Nassar, M. Lactoferrin for Iron-Deficiency Anemia in Children with Inflammatory Bowel Disease: A Clinical Trial. Pediatr. Res. 2022, 92, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Artym, J.; Zimecki, M.; Kruzel, M.L. Lactoferrin for Prevention and Treatment of Anemia and Inflammation in Pregnant Women: A Comprehensive Review. Biomedicines 2021, 9, 898. [Google Scholar] [CrossRef]
- Abu Hashim, H.; Foda, O.; Ghayaty, E. Lactoferrin or Ferrous Salts for Iron Deficiency Anemia in Pregnancy: A Meta-Analysis of Randomized Trials. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 219, 45–52. [Google Scholar] [CrossRef]
- Lepanto, M.S.; Rosa, L.; Cutone, A.; Conte, M.P.; Paesano, R.; Valenti, P. Efficacy of Lactoferrin Oral Administration in the Treatment of Anemia and Anemia of Inflammation in Pregnant and Non-Pregnant Women: An Interventional Study. Front. Immunol. 2018, 9, 2123. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, S.; Nekhai, S.; Liu, S. Depriving Iron Supply to the Virus Represents a Promising Adjuvant Therapeutic Against Viral Survival. Curr. Clin. Microbiol. Rep. 2020, 7, 13. [Google Scholar] [CrossRef]
- Engin, A.B.; Engin, E.D.; Engin, A. Can Iron, Zinc, Copper and Selenium Status Be a Prognostic Determinant in COVID-19 Patients? Environ. Toxicol. Pharmacol. 2022, 95, 103937. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Oda, H.; Yamauchi, K.; Abe, F. Lactoferrin for Prevention of Common Viral Infections. J. Infect. Chemother. 2014, 20, 666–671. [Google Scholar] [CrossRef]
- Berlutti, F.; Pantanella, F.; Natalizi, T.; Frioni, A.; Paesano, R.; Polimeni, A.; Valenti, P. Antiviral Properties of Lactoferrin—A Natural Immunity Molecule. Molecules 2011, 16, 6992–7018. [Google Scholar] [CrossRef] [PubMed]
- Sinopoli, A.; Isonne, C.; Santoro, M.M.; Baccolini, V. The Effects of Orally Administered Lactoferrin in the Prevention and Management of Viral Infections: A Systematic Review. Rev. Med. Virol. 2022, 32, e2261. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.S.; Hasan, S.S.; Kow, C.S.; Merchant, H.A. Lactoferrin Reduces the Risk of Respiratory Tract Infections: A Meta-Analysis of Randomized Controlled Trials. Clin. Nutr. ESPEN 2021, 45, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Chai, L.; Li, H.; Zhang, Y.; Xie, H.M.; Shang, J.; Tian, W.; Yang, P.; Jiang, A.C. Effect of Bovine Lactoferrin from Iron-Fortified Formulas on Diarrhea and Respiratory Tract Infections of Weaned Infants in a Randomized Controlled Trial. Nutrition 2016, 32, 222–227. [Google Scholar] [CrossRef]
- Motoki, N.; Mizuki, M.; Tsukahara, T.; Miyakawa, M.; Kubo, S.; Oda, H.; Tanaka, M.; Yamauchi, K.; Abe, F.; Nomiyama, T. Effects of Lactoferrin-Fortified Formula on Acute Gastrointestinal Symptoms in Children Aged 12–32 Months: A Randomized, Double-Blind, Placebo-Controlled Trial. Front. Pediatr. 2020, 8, 233. [Google Scholar] [CrossRef]
- Li, F.; Wu, S.S.; Berseth, C.L.; Harris, C.L.; Richards, J.D.; Wampler, J.L.; Zhuang, W.; Cleghorn, G.; Rudolph, C.D.; Liu, B.; et al. Improved Neurodevelopmental Outcomes Associated with Bovine Milk Fat Globule Membrane and Lactoferrin in Infant Formula: A Randomized, Controlled Trial. J. Pediatr. 2019, 215, 24–31.e8. [Google Scholar] [CrossRef]
- King, J.C.; Cummings, G.E.; Guo, N.; Trivedi, L.; Readmond, B.X.; Keane, V.; Feigelman, S.; de Waard, R. A Double-Blind, Placebo-Controlled, Pilot Study of Bovine Lactoferrin Supplementation in Bottle-Fed Infants. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 245–251. [Google Scholar] [CrossRef]
- Vitetta, L.; Coulson, S.; Beck, S.L.; Gramotnev, H.; Du, S.; Lewis, S. The Clinical Efficacy of a Bovine Lactoferrin/Whey Protein Ig-Rich Fraction (Lf/IgF) for the Common Cold: A Double Blind Randomized Study. Complement Ther. Med. 2013, 21, 164–171. [Google Scholar] [CrossRef]
- Oda, H.; Wakabayashi, H.; Tanaka, M.; Yamauchi, K.; Sugita, C.; Yoshida, H.; Abe, F.; Sonoda, T.; Kurokawa, M. Effects of Lactoferrin on Infectious Diseases in Japanese Summer: A Randomized, Double-Blinded, Placebo-Controlled Trial. J. Microbiol. Immunol. Infect. 2021, 54, 566–574. [Google Scholar] [CrossRef]
- Ochoa, T.J.; Chea-Woo, E.; Baiocchi, N.; Pecho, I.; Campos, M.; Prada, A.; Valdiviezo, G.; Lluque, A.; Lai, D.; Cleary, T.G. Randomized Double-Blind Controlled Trial of Bovine Lactoferrin for Prevention of Diarrhea in Children. J. Pediatr. 2013, 162, 349–356. [Google Scholar] [CrossRef]
- Oda, H. The Researches on the Body Defense Effect...—Google Scholar. Available online: https://scholar.google.com/scholar?hl=nl&as_sdt=0%2C5&q=Oda+H.+The+researches+on+the+body+defense+effect+of+lctoferrin.+Milk+Sci.+2013%3B62%3A105%E2%80%93109.+&btnG= (accessed on 26 October 2022).
- Tsukahara, T.; Fujimori, A.; Misawa, Y.; Oda, H.; Yamauchi, K.; Abe, F.; Nomiyama, T. The Preventive Effect of Lactoferrin-Containing Yogurt on Gastroenteritis in Nursery School Children—Intervention Study for 15 Weeks. Int. J. Environ. Res. Public Health 2020, 17, 2534. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.; Yalda, D.; Lu, S.; Nikolov, Z.; Misaki, R.; Fujiyama, K.; Huang, N. Process Development and Economic Evaluation of Recombinant Human Lactoferrin Expressed in Rice Grain. Transgenic. Res. 2005, 14, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Zavaleta, N.; Figueroa, D.; Rivera, J.; Sánchez, J.; Alfaro, S.; Lönnerdal, B. Efficacy of Rice-Based Oral Rehydration Solution Containing Recombinant Human Lactoferrin and Lysozyme in Peruvian Children with Acute Diarrhea. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 258–264. [Google Scholar] [CrossRef] [PubMed]
- de Pasquale, V.; Quiccione, M.S.; Tafuri, S.; Avallone, L.; Pavone, L.M. Heparan Sulfate Proteoglycans in Viral Infection and Treatment: A Special Focus on SARS-CoV-2. Int. J. Mol. Sci. 2021, 22, 6574. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.; Yang, N.; Deng, J.; Liu, K.; Yang, P.; Zhang, G.; Jiang, C. Inhibition of SARS Pseudovirus Cell Entry by Lactoferrin Binding to Heparan Sulfate Proteoglycans. PLoS ONE 2011, 6, e23710. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 Entry into Cells. Nat. Rev. Mol. Cell Biol. 2021, 23, 3–20. [Google Scholar] [CrossRef]
- Azzi, L.; Carcano, G.; Gianfagna, F.; Grossi, P.; Gasperina, D.D.; Genoni, A.; Fasano, M.; Sessa, F.; Tettamanti, L.; Carinci, F.; et al. Saliva Is a Reliable Tool to Detect SARS-CoV-2. J. Infect. 2020, 81, e45–e50. [Google Scholar] [CrossRef]
- Zhu, F.; Zhong, Y.; Ji, H.; Ge, R.; Guo, L.; Song, H.; Wu, H.; Jiao, P.; Li, S.; Wang, C.; et al. ACE2 and TMPRSS2 in Human Saliva Can Adsorb to the Oral Mucosal Epithelium. J. Anat. 2022, 240, 398–409. [Google Scholar] [CrossRef]
- Meng, B.; Abdullahi, A.; Ferreira, I.A.T.M.; Goonawardane, N.; Saito, A.; Kimura, I.; Yamasoba, D.; Gerber, P.P.; Fatihi, S.; Rathore, S.; et al. Altered TMPRSS2 Usage by SARS-CoV-2 Omicron Impacts Infectivity and Fusogenicity. Nature 2022, 603, 706–714. [Google Scholar] [CrossRef]
- Wotring, J.W.; Fursmidt, R.; Ward, L.; Sexton, J.Z. Evaluating the in Vitro Efficacy of Bovine Lactoferrin Products against SARS-CoV-2 Variants of Concern. J. Dairy Sci. 2022, 105, 2791–2802. [Google Scholar] [CrossRef]
- Hu, Y.; Meng, X.; Zhang, F.; Xiang, Y.; Wang, J. The in Vitro Antiviral Activity of Lactoferrin against Common Human Coronaviruses and SARS-CoV-2 Is Mediated by Targeting the Heparan Sulfate Co-Receptor. Emerg. Microbes. Infect. 2021, 10, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Ostrov, D.A.; Bluhm, A.P.; Li, D.; Khan, J.Q.; Rohamare, M.; Rajamanickam, K.; Bhanumathy, K.K.; Lew, J.; Falzarano, D.; Vizeacoumar, F.J.; et al. Highly Specific Sigma Receptor Ligands Exhibit Anti-Viral Properties in SARS-CoV-2 Infected Cells. Pathogens 2021, 10, 1514. [Google Scholar] [CrossRef]
- Mirabelli, C.; Wotring, J.W.; Zhang, C.J.; McCarty, S.M.; Fursmidt, R.; Pretto, C.D.; Qiao, Y.; Zhang, Y.; Frum, T.; Kadambi, N.S.; et al. Morphological Cell Profiling of SARS-CoV-2 Infection Identifies Drug Repurposing Candidates for COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2105815118. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Y.; He, J.; Zhu, W. Antibacterial Properties of Lactoferrin: A Bibliometric Analysis from 2000 to Early 2022. Front. Microbiol. 2022, 13, 2928. [Google Scholar] [CrossRef]
- Arnold, R.R.; Cole, M.F.; McGhee, J.R. A Bactericidal Effect for Human Lactoferrin. Science 1977, 197, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, K.E.; Carter, D.A. The Antifungal Activity of Lactoferrin and Its Derived Peptides: Mechanisms of Action and Synergy with Drugs against Fungal Pathogens. Front. Microbiol. 2017, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, R.; Goyal, K.; Sehgal, A. Trichomoniasis and Lactoferrin: Future Prospects. Infect. Dis. Obstet. Gynecol. 2012, 2012, 536037. [Google Scholar] [CrossRef] [PubMed]
- Okuda, M.; Nakazawa, T.; Yamauchi, K.; Miyashiro, E.; Koizumi, R.; Booka, M.; Teraguchi, S.; Tamura, Y.; Yoshikawa, N.; Adachi, Y.; et al. Bovine Lactoferrin Is Effective to Suppress Helicobacter Pylori Colonization in the Human Stomach: A Randomized, Double-Blind, Placebo-Controlled Study. J. Infect. Chemother. 2005, 11, 265–269. [Google Scholar] [CrossRef]
- Kim, W.S.; Ohashi, M.; Shimazaki, K.I. Inhibitory Effects of Synthetic Peptides Containing Bovine Lactoferrin C-Lobe Sequence on Bacterial Growth. Food Sci. Anim. Resour. 2016, 36, 452–457. [Google Scholar] [CrossRef]
- Nakamura, M.; Tsuda, N.; Miyata, T.; Ikenaga, M. Antimicrobial Effect and Mechanism of Bovine Lactoferrin against the Potato Common Scab Pathogen Streptomyces Scabiei. PLoS ONE 2022, 17, e0264094. [Google Scholar] [CrossRef]
- Nairz, M.; Schroll, A.; Sonnweber, T.; Weiss, G. The Struggle for Iron—A Metal at the Host-Pathogen Interface. Cell Microbiol. 2010, 12, 1691–1702. [Google Scholar] [CrossRef] [PubMed]
- Appelmelk, B.J.; An, Y.Q.; Geerts, M.; Thijs, B.G.; de Boer, H.A.; MacLaren, D.M.; de Graaff, J.; Nuijens, J.H. Lactoferrin Is a Lipid A-Binding Protein. Infect. Immun. 1994, 62, 2628–2632. [Google Scholar] [CrossRef] [PubMed]
- Brock, J.H. Lactoferrin—50 years on. Biochem. Cell Biol. 2012, 90, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Teraguchi, S.; Wakabayashi, H.; Kuwata, H.; Yamauchi, K.; Tamura, Y. Protection against Infections by Oral Lactoferrin: Evaluation in Animal Models. Biometals 2004, 17, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa, M.; Oda, H.; Tanaka, M. Clinical Research Review: Usefulness of Bovine Lactoferrin in Child Health. BioMetals 2022, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ajello, M.; Greco, R.; Giansanti, F.; Massucci, M.T.; Antonini, G.; Valenti, P. Anti-Invasive Activity of Bovine Lactoferrin towards Group A Streptococci. Biochem. Cell Biol. 2002, 80, 119–124. [Google Scholar] [CrossRef]
- Laffan, A.M.; McKenzie, R.; Forti, J.; Conklin, D.; Marcinko, R.; Shrestha, R.; Bellantoni, M.; Greenough, W.B. Lactoferrin for the Prevention of Post-Antibiotic Diarrhoea. J. Health Popul. Nutr. 2011, 29, 547–551. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.P.R.; Daneman, N. Bacterial Co-Infection and Secondary Infection in Patients with COVID-19: A Living Rapid Review and Meta-Analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Kaczyńska, K.; Kleczkowska, P.; Bukowska-ośko, I.; Kramkowski, K.; Sulejczak, D. The Lactoferrin Phenomenon—A Miracle Molecule. Molecules 2022, 27, 2941. [Google Scholar] [CrossRef]
- Santoro, A.; Zhao, J.; Wu, L.; Carru, C.; Biagi, E.; Franceschi, C. Microbiomes Other than the Gut: Inflammaging and Age-Related Diseases. Semin. Immunopathol. 2020, 42, 589–605. [Google Scholar] [CrossRef]
- Conneely, O.M. Antiinflammatory Activities of Lactoferrin. J. Am. Coll Nutr. 2001, 20, 389S–395S. [Google Scholar] [CrossRef] [PubMed]
- Reseco, L.; Atienza, M.; Fernandez-Alvarez, M.; Carro, E.; Cantero, J.L. Salivary Lactoferrin Is Associated with Cortical Amyloid-Beta Load, Cortical Integrity, and Memory in Aging. Alzheimers Res. Ther. 2021, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Donovan, S.M. The Role of Lactoferrin in Gastrointestinal and Immune Development and Function: A Preclinical Perspective. J. Pediatr. 2016, 173, S16–S28. [Google Scholar] [CrossRef]
- Liu, N.; Feng, G.; Zhang, X.; Hu, Q.; Sun, S.; Sun, J.; Sun, Y.; Wang, R.; Zhang, Y.; Wang, P.; et al. The Functional Role of Lactoferrin in Intestine Mucosal Immune System and Inflammatory Bowel Disease. Front. Nutr. 2021, 8, 914. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Francis, J.; Doster, R.S.; Haley, K.P.; Craft, K.M.; Moore, R.E.; Chambers, S.A.; Aronoff, D.M.; Osteen, K.; Damo, S.M.; et al. Lactoferrin: A Critical Mediator of Both Host Immune Response and Antimicrobial Activity in Response to Streptococcal Infections. ACS Infect. Dis. 2020, 6, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Comstock, S.S.; Reznikov, E.A.; Contractor, N.; Donovan, S.M. Dietary Bovine Lactoferrin Alters Mucosal and Systemic Immune Cell Responses in Neonatal Piglets. J. Nutr. 2014, 144, 525–532. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, J.; Xiao, D.; Shu, G.; Gu, L. Bovine Lactoferrin Protects Dextran Sulfate Sodium Salt Mice Against Inflammation and Impairment of Colonic Epithelial Barrier by Regulating Gut Microbial Structure and Metabolites. Front. Nutr. 2021, 8, 660598. [Google Scholar] [CrossRef]
- Ishikado, A.; Imanaka, H.; Kotani, M.; Fujita, A.; Mitsuishi, Y.; Kanemitsu, T.; Tamura, Y.; Makino, T. Liposomal Lactoferrin Induced Significant Increase of the Interferon-Alpha (IFN-α) Producibility in Healthy Volunteers. BioFactors 2004, 21, 69–72. [Google Scholar] [CrossRef]
- Bharadwaj, S.; Naidu, T.A.G.; Betageri, G.V.; Prasadarao, N.V.; Naidu, A.S. Inflammatory Responses Improve with Milk Ribonuclease-Enriched Lactoferrin Supplementation in Postmenopausal Women. Inflamm. Res. 2010, 59, 971–978. [Google Scholar] [CrossRef]
- Legrand, D. Lactoferrin, a Key Molecule in Immune and Inflammatory Processes. Biochem. Cell Biol. 2012, 90, 252–268. [Google Scholar] [CrossRef]
- Liu, C.; Peng, Q.; Wei, L.; Li, Z.; Zhang, X.; Wu, Y.; Wang, J.; Zheng, X.; Wen, Y.; Zheng, R.; et al. Deficiency of Lactoferrin Aggravates Lipopolysaccharide-Induced Acute Inflammation via Recruitment Macrophage in Mice. BioMetals 2022, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Liu, C.; Wang, J.; Zheng, X.; Peng, Q.; Ye, Q.; Qin, Z.; Li, Z.; Zhang, X.; Wu, Y.; et al. Lactoferrin Is Required for Early B Cell Development in C57BL/6 Mice. J. Hematol. Oncol. 2021, 14, 58. [Google Scholar] [CrossRef]
- Reghunathan, R.; Jayapal, M.; Hsu, L.Y.; Chng, H.H.; Tai, D.; Leung, B.P.; Melendez, A.J. Expression Profile of Immune Response Genes in Patients with Severe Acute Respiratory Syndrome. BMC Immunol. 2005, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Abers, M.S.; Delmonte, O.M.; Ricotta, E.E.; Fintzi, J.; Fink, D.L.; Almeida de Jesus, A.A.; Zarember, K.A.; Alehashemi, S.; Oikonomou, V.; Desai, J.V.; et al. An Immune-Based Biomarker Signature Is Associated with Mortality in COVID-19 Patients. JCI Insight 2021, 6, e144455. [Google Scholar] [CrossRef] [PubMed]
- del Valle, D.M.; Kim-Schulze, S.; Huang, H.H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An Inflammatory Cytokine Signature Predicts COVID-19 Severity and Survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.M.; de Oliveira, M.H.S.; Benoit, S.; Plebani, M.; Lippi, G. Hematologic, Biochemical and Immune Biomarker Abnormalities Associated with Severe Illness and Mortality in Coronavirus Disease 2019 (COVID-19): A Meta-Analysis. Clin. Chem. Lab. Med. 2020, 58, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.; Fatima, R.; Assaly, R. Elevated Interleukin-6 and Severe COVID-19: A Meta-Analysis. J. Med. Virol. 2020, 92, 2283–2285. [Google Scholar] [CrossRef]
- Udomsinprasert, W.; Jittikoon, J.; Sangroongruangsri, S.; Chaikledkaew, U. Circulating Levels of Interleukin-6 and Interleukin-10, But Not Tumor Necrosis Factor-Alpha, as Potential Biomarkers of Severity and Mortality for COVID-19: Systematic Review with Meta-Analysis. J. Clin. Immunol. 2021, 41, 11–22. [Google Scholar] [CrossRef]
- Zimecki, M.; Actor, J.K.; Kruzel, M.L. The Potential for Lactoferrin to Reduce SARS-CoV-2 Induced Cytokine Storm. Int. Immunopharmacol. 2021, 95, 107571. [Google Scholar] [CrossRef]
- Lepanto, M.S.; Rosa, L.; Paesano, R.; Valenti, P.; Cutone, A. Lactoferrin in Aseptic and Septic Inflammation. Molecules 2019, 24, 1323. [Google Scholar] [CrossRef]
- Welsh, K.J.; Hwang, S.A.; Boyd, S.; Kruzel, M.L.; Hunter, R.L.; Actor, J.K. Influence of Oral Lactoferrin on Mycobacterium Tuberculosis Induced Immunopathology. Tuberculosis 2011, 91 (Suppl. S1), S105–S113. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.A.; Kruzel, M.L.; Actor, J.K. Oral Recombinant Human or Mouse Lactoferrin Reduces Mycobacterium Tuberculosis TDM Induced Granulomatous Lung Pathology. Biochem. Cell Biol. 2017, 95, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.K.T.; Niaz, Z.; Kruzel, M.L.; Actor, J.K. Recombinant Human Lactoferrin Reduces Inflammation and Increases Fluoroquinolone Penetration to Primary Granulomas During Mycobacterial Infection of C57Bl/6 Mice. Arch. Immunol. Ther. Exp. 2022, 70, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.K.T.; Niaz, Z.; D’Aigle, J.; Hwang, S.A.; Kruzel, M.L.; Actor, J.K. Lactoferrin Reduces Mycobacterial M1-Type Inflammation Induced with Trehalose 6,6’-Dimycolate and Facilitates the Entry of Fluoroquinolone into Granulomas. Biochem. Cell Biol. 2021, 99, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Salaris, C.; Scarpa, M.; Elli, M.; Bertolini, A.; Guglielmetti, S.; Pregliasco, F.; Blandizzi, C.; Brun, P.; Castagliuolo, I. Protective Effects of Lactoferrin against SARS-CoV-2 Infection In Vitro. Nutrients 2021, 13, 328. [Google Scholar] [CrossRef]
- Artym, J.; Zimecki, M. Antimicrobial and Prebiotic Activity of Lactoferrin in the Female Reproductive Tract: A Comprehensive Review. Biomedicines 2021, 9, 1940. [Google Scholar] [CrossRef]
- Liepke, C.; Adermann, K.; Raida, M.; Mägert, H.J.; Forssmann, W.G.; Zucht, H.D. Human Milk Provides Peptides Highly Stimulating the Growth of Bifidobacteria. Eur. J. Biochem. 2002, 269, 712–718. [Google Scholar] [CrossRef]
- Oda, H.; Wakabayashi, H.; Yamauchi, K.; Abe, F. Lactoferrin and Bifidobacteria. BioMetals 2014, 27, 915–922. [Google Scholar] [CrossRef]
- Chen, Y.; Gu, S.; Chen, Y.; Lu, H.; Shi, D.; Guo, J.; Wu, W.R.; Yang, Y.; Li, Y.; Xu, K.J.; et al. Letter: Six-Month Follow-up of Gut Microbiota Richness in Patients with COVID-19. Gut 2022, 71, 222. [Google Scholar] [CrossRef]
- de Oliveira, G.L.V.; Oliveira, C.N.S.; Pinzan, C.F.; de Salis, L.V.V.; Cardoso, C.R.D.B. Microbiota Modulation of the Gut-Lung Axis in COVID-19. Front. Immunol. 2021, 12, 635471. [Google Scholar] [CrossRef]
- Wischmeyer, P.E.; Tang, H.; Ren, Y.; Bohannon, L.; Ramirez, Z.E.; Andermann, T.M.; Messina, J.A.; Sung, J.A.; Jensen, D.; Jung, S.-H.; et al. Daily Lactobacillus Probiotic versus Placebo in COVID-19-Exposed Household Contacts (PROTECT-EHC): A Randomized Clinical Trial. medRxiv 2022. [Google Scholar] [CrossRef]
- Buccigrossi, V.; de Marco, G.; Bruzzese, E.; Ombrato, L.; Bracale, I.; Polito, G.; Guarino, A. Lactoferrin Induces Concentration-Dependent Functional Modulation of Intestinal Proliferation and Differentiation. Pediatr. Res. 2007, 61, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B. Nutritional and Physiologic Significance of Human Milk Proteins. Am. J. Clin. Nutr. 2003, 77, 1537S–1543S. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B.; Du, X.; Jiang, R. Biological Activities of Commercial Bovine Lactoferrin Sources. Biochem. Cell Biol. 2021, 99, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Troost, F.J.; Saris, W.; Brummer, R.-J. Original Communication Recombinant Human Lactoferrin Ingestion Attenuates Indomethacin-Induced Enteropathy in Vivo in Healthy Volunteers. Eur. J. Clin. Nutr. 2003, 57, 1579–1585. [Google Scholar] [CrossRef]
- Calitri, C.; Fumi, I.; Ignaccolo, M.G.; Banino, E.; Benetti, S.; Lupica, M.M.; Fantone, F.; Pace, M.; Garofalo, F. Gastrointestinal Involvement in Paediatric COVID-19—From Pathogenesis to Clinical Management: A Comprehensive Review. World J. Gastroenterol. 2021, 27, 3303. [Google Scholar] [CrossRef]
- Yamada, S.; Noda, T.; Okabe, K.; Yanagida, S.; Nishida, M.; Kanda, Y. SARS-CoV-2 Induces Barrier Damage and Inflammatory Responses in the Human IPSC-Derived Intestinal Epithelium. J. Pharmacol. Sci. 2022, 149, 139–146. [Google Scholar] [CrossRef]
- Yu, W.; Ou, X.; Liu, X.; Zhang, S.; Gao, X.; Cheng, H.; Zhu, B.; Yan, J. ACE2 Contributes to the Maintenance of Mouse Epithelial Barrier Function. Biochem. Biophys. Res. Commun. 2020, 533, 1276. [Google Scholar] [CrossRef]
- Kucia, M.; Wietrak, E.; Szymczak, M.; Majchrzak, M.; Kowalczyk, P. Protective Action of L. Salivarius SGL03 and Lactoferrin against COVID-19 Infections in Human Nasopharynx. Materials 2021, 14, 3086. [Google Scholar] [CrossRef]
- Serrano, G.; Kochergina, I.; Albors, A.; Diaz, E.; Hueso, G.; Serrano, J.M. Liposomal Lactoferrin as Potential Preventative and Cure for COVID-19. Int. J. Res. Health Sci. 2020, 8, 8–15. [Google Scholar] [CrossRef]
- Rosa, L.; Tripepi, G.; Naldi, E.; Aimati, M.; Santangeli, S.; Venditto, F.; Caldarelli, M.; Valenti, P. Ambulatory Covid-19 Patients Treated with Lactoferrin as a Supplementary Antiviral Agent: A Preliminary Study. J. Clin. Med. 2021, 10, 4276. [Google Scholar] [CrossRef] [PubMed]
- Dix, C.; Wright, O. Bioavailability of a Novel Form of Microencapsulated Bovine Lactoferrin and Its Effect on Inflammatory Markers and the Gut Microbiome: A Pilot Study. Nutrients 2018, 10, 1115. [Google Scholar] [CrossRef] [PubMed]
- Algahtani, F.D.; Elabbasy, M.T.; Samak, M.A.; Adeboye, A.A.; Yusuf, R.A.; Ghoniem, M.E. The Prospect of Lactoferrin Use as Adjunctive Agent in Management of SARS-CoV-2 Patients: A Randomized Pilot Study. Medicina 2021, 57, 842. [Google Scholar] [CrossRef] [PubMed]
- Valenti, P.; Rosa, L.; Capobianco, D.; Lepanto, M.S.; Schiavi, E.; Cutone, A.; Paesano, R.; Mastromarino, P. Role of Lactobacilli and Lactoferrin in the Mucosal Cervicovaginal Defense. Front. Immunol. 2018, 9, 376. [Google Scholar] [CrossRef] [PubMed]
- Bermejo-Pareja, F.; del Ser, T.; Valentí, M.; de la Fuente, M.; Bartolome, F.; Carro, E. Salivary Lactoferrin as Biomarker for Alzheimer’s Disease: Brain-Immunity Interactions. Alzheimer’s Dement. 2020, 16, 1196–1204. [Google Scholar] [CrossRef]
- Jiang, R.; Liu, L.; Liu, L.; Du, X.; Lönnerdal, B. Evaluation of Bioactivities of the Bovine Milk Lactoferrin-Osteopontin Complex in Infant Formulas. J. Agric. Food Chem. 2020, 68, 6104–6111. [Google Scholar] [CrossRef]
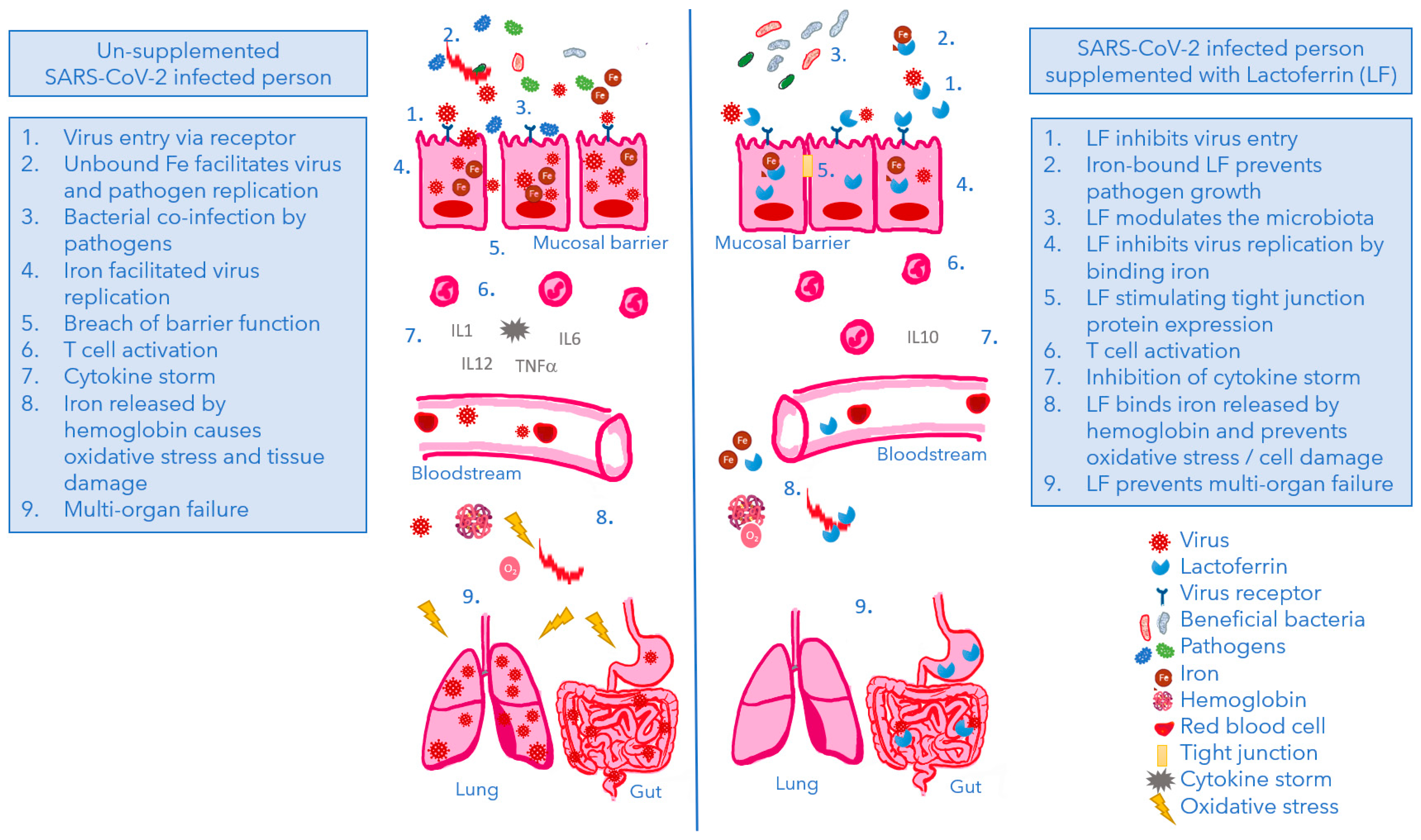
| COVID-19 Pathogenesis | LF’s Iron-Related Effect |
|---|---|
| Iron deficiency risk increases | LF increases iron absorption thereby lowering the risk of iron deficiency 2 |
| Ferritin and IL6 levels increase | LF decreases ferritin and IL6 levels 1 |
| Intracellular iron overload increases viral replication | LF decreases the intracellular iron level 2 resulting in reduced viral replication 1 |
| Virus attacks hemoglobin leading to iron and oxygen release thereby inducing oxidative stress | LF chelates iron thereby reducing oxidative stress 2 |
| COVID-19 Pathogenesis | LF’s Anti-Viral Effect |
|---|---|
| SARS-CoV-2 attaches to HSPG, concentrates on the cell surface and subsequently binds to ACE2 for cell entry | LF blocks binding of SARS-CoV-2 to HSPG in an ACE2 and iron independent manner |
| LF inhibits virus infection of different SARS-CoV-2 variants. Its efficacy resides in LF’s N-terminus | |
| Virus replication is enhanced by intracellular iron. The virus hijacks the cell’s stress response system involving sigma receptors | Iron-binding LF inhibits virus replication and can complement specific anti-viral drugs |
| COVID-19 Pathogenesis | LF’s Anti-Microbial Effect |
|---|---|
| About 72% of COVID-19 patients in health care settings received antibiotics | LF inhibits growth of gram-negative and gram-positive bacteria, fungi, and protozoa |
| Prevalence of bacterial co-infection is ~7% and of secondary bacterial infection is ~14% | LF selectively inhibits pathogens, whereas it does not inhibit beneficial microbes |
| The oral cavity plays an important role in SARS-CoV-2 infection and transmission | LF is naturally present in the oral cavity providing microbial homeostasis |
| Elderly are more susceptible to (severe) COVID-19 | LF levels in saliva decrease with age, leading to a dysbiosis and susceptibility to disease |
| COVID-19 Pathogenesis | LF’s Immune Effect |
|---|---|
| Prominent early features of COVID-19 include a pronounced reduction in B cells important in defense against SARS-CoV-2 | LF has a profound modulatory action by the differentiation of immature B-cells into efficient antigen presenting cells |
| Immune system may over-react sending in neutrophils, T-helper- (CD4) and cytotoxic T-cells (CD8) that release pro-inflammatory cytokines, especially IL-1 and IL-6 | LF increases total T-cell activation, T-helper cell activation and cytotoxic T-cell activation, and suppresses cytokines levels including IL-6 and TNF-α |
| The ‘cytokine storm’ damages normal lung cells more than the virus it targets leading to acute respiratory distress syndrome (ARDS) | In a model of pulmonary acute respiratory distress syndrome (ARDS) in granulomatous inflammation, LF can reduce pulmonary pathological features |
| Some cytokines, including IL-6, IL-10, and TNF-α, have been described as biomarkers related to severe SARS-CoV-2 infection | LF supplementation decreases levels of cytokines including IL-6 and TNF-α, and increases IL-10 |
| Reference | Title | Dose (mg/d) | Intervention | Population | Country | Main Outcomes |
|---|---|---|---|---|---|---|
| Serrano et al., 2020 [164] | Liposomal Lactoferrin as Potential Preventative and Cure for COVID-19 | 120–180 and 60–90 | 10 days | 75 patients + 256 family members | Spain | Faster recovery time and symptom relieve from e.g., weakness, loss of smell and taste, cough and muscular pain. |
| Algahtani et al., 2021 [167] | The Prospect of Lactoferrin Use as Adjunctive Agent in Management of SARS-CoV-2 Patients: A Randomized Pilot Study | 200 | 7 days | 54 patients (mild-moderate) | Egypt | No statistically significant difference among studied groups regarding recovery of symptoms or blood/immune parameters |
| Campione et al., 2021 [69] | Lactoferrin as Antiviral Treatment in COVID-19 Management: Preliminary Evidence | 1000 + 16 | 30 days | 92 patients (mild-moderate) | Italy | Reduction in symptoms and shortening of illness duration by about 13 days. Some COVID19-biomarkers improved (IL-6, D-Dimer, ferritin) |
| Rosa et al., 2021 [165] | Ambulatory COVID-19 Patients Treated with Lactoferrin as a Supplementary Antiviral Agent: A Preliminary Study | 200–1000 | 50 days | 121 patients (mild-moderate) | Italy | The time required to achieve a negative SARS-CoV-2 PCR result was significantly lower. The effectiveness on symptom resolution was progressively higher in older adults. |
| Trial ID | Title | Age | Country | Primary Outcome |
|---|---|---|---|---|
| NCT 04412395 | Clinical Assessment of Oral Lactoferrin as a Safe Antiviral and Immunoregulatory in Treating COVID-19 Disease | 18–80 | Egypt | Survival rate; Rate of disease remission; The number of patients with PCR negative results. |
| NL9742 | Lactoferrin in the treatment of Long COVID | 18–60 | The Netherlands | Do fatigue symptoms diminish faster with the use of lactoferrin combined with usual care compared to usual care solely? |
| CTRI/2021/ 12/038672 | Clinical trial on Mild and Moderate COVID-19 | >18 | India | Change from baseline in CRP, D-dimer and other biomarkers; Proportion of patients and time to progress from mild to critical grade; Reduction in viral shedding; Improvements in COVID-19 symptoms |
| NCT 04713735 | Impact of Lactoferrin vs. Placebo on Respiratory Tract Infections | >55 | USA | Number of Respiratory Tract Infections |
| NCT 04847791 | Lactoferrin in COVID-19 Hospitalized Patients | 18–99 | Italy | Intensive care unit hospitalization rate; death; proportion of discharged patients; National Early Warning Score (NEWS) |
| NCT 04621149 | An Outpatient Study Investigating Non-prescription Treatments for COVID-19 | 20–70 | USA | Reduction in Participant Symptoms of COVID-19 |
| PER-064-20 | Lactoferrin for Prevention of COVID-19 in Health Care Personnel | 18–59 | Peru | Serology (IgM or IgG) or RT-PCR for COVID-19; Number of COVID-19 infections |
| NCT 04427865 | Utility of Lactoferrin as a Preventive Agent for Healthcare Workers Exposed to COVID-19 | 18–65 | Egypt | Incidence of SARS-CoV-2 |
| NCT 04421534 | Utility of Lactoferrin as an Adjunct Therapeutic Agent for COVID-19 | 18–65 | Egypt | Time to clinical improvement |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Einerhand, A.W.C.; van Loo-Bouwman, C.A.; Weiss, G.A.; Wang, C.; Ba, G.; Fan, Q.; He, B.; Smit, G. Can Lactoferrin, a Natural Mammalian Milk Protein, Assist in the Battle against COVID-19? Nutrients 2022, 14, 5274. https://doi.org/10.3390/nu14245274
Einerhand AWC, van Loo-Bouwman CA, Weiss GA, Wang C, Ba G, Fan Q, He B, Smit G. Can Lactoferrin, a Natural Mammalian Milk Protein, Assist in the Battle against COVID-19? Nutrients. 2022; 14(24):5274. https://doi.org/10.3390/nu14245274
Chicago/Turabian StyleEinerhand, Alexandra Wilhelmina Carla, Carolien Annika van Loo-Bouwman, Gisela Adrienne Weiss, Caiyun Wang, Genna Ba, Qicheng Fan, Baoping He, and Gerrit Smit. 2022. "Can Lactoferrin, a Natural Mammalian Milk Protein, Assist in the Battle against COVID-19?" Nutrients 14, no. 24: 5274. https://doi.org/10.3390/nu14245274
APA StyleEinerhand, A. W. C., van Loo-Bouwman, C. A., Weiss, G. A., Wang, C., Ba, G., Fan, Q., He, B., & Smit, G. (2022). Can Lactoferrin, a Natural Mammalian Milk Protein, Assist in the Battle against COVID-19? Nutrients, 14(24), 5274. https://doi.org/10.3390/nu14245274








