Resveratrol against Cervical Cancer: Evidence from In Vitro and In Vivo Studies
Abstract
1. Introduction
1.1. Cervical Cancer
1.2. Resveratrol
2. Resveratrol against Cervical Cancer
2.1. Resveratrol against Cervical Cancer: In Vitro Studies
2.2. Resveratrol Analogs against Cervical Cancer: In Vitro
2.3. Resveratrol against Cervical Cancer: In Vivo Animal Studies
3. Limitations and Controversies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cervical Cancer. Available online: https://www.who.int/health-topics/cervical-cancer (accessed on 25 November 2022).
- Parkin, D.M.; Whelan, S.L.; Ferlay, J.; Teppo, L.; Thomas, D.B.E. Cancer Incidence in Five Continents: Volume VIII. Available online: https://hero.epa.gov/hero/index.cfm/reference/details/reference_id/729990 (accessed on 25 November 2022).
- Muthusami, S.; Sabanayagam, R.; Periyasamy, L.; Muruganantham, B.; Park, W.Y. A Review on the Role of Epidermal Growth Factor Signaling in the Development, Progression and Treatment of Cervical Cancer. Int. J. Biol. Macromol. 2022, 194, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, J.; Lei, Y.; Cong, C.; Tan, D.; Zhou, X. Research Progress on the PI3K/AKT Signaling Pathway in Gynecological Cancer. Mol. Med. Rep. 2019, 19, 4529–4535. [Google Scholar] [CrossRef] [PubMed]
- Mitra, T.; Elangovan, S. Cervical Cancer Development, Chemoresistance, and Therapy: A Snapshot of Involvement of MicroRNA. Mol. Cell. Biochem. 2021, 476, 4363–4385. [Google Scholar] [CrossRef] [PubMed]
- George, I.A.; Chauhan, R.; Dhawale, R.E.; Iyer, R.; Limaye, S.; Sankaranarayanan, R.; Venkataramanan, R.; Kumar, P. Insights into Therapy Resistance in Cervical Cancer. Adv. Cancer Biol.-Metastasis 2022, 6, 100074. [Google Scholar] [CrossRef]
- Morgan, E.L.; Scarth, J.A.; Patterson, M.R.; Wasson, C.W.; Hemingway, G.C.; Barba-Moreno, D.; Macdonald, A. E6-Mediated Activation of JNK Drives EGFR Signalling to Promote Proliferation and Viral Oncoprotein Expression in Cervical Cancer. Cell Death Differ. 2021, 28, 1669–1687. [Google Scholar] [CrossRef]
- Yang, Q.; Al-Hendy, A. The Regulatory Functions and the Mechanisms of Long Non-Coding RNAs in Cervical Cancer. Cells 2022, 11, 1149. [Google Scholar] [CrossRef]
- Smola, S. Immunopathogenesis of HPV-Associated Cancers and Prospects for Immunotherapy. Viruses 2017, 9, 254. [Google Scholar] [CrossRef]
- He, S.; Li, Q.; Huang, Q.; Cheng, J. Targeting Protein Kinase C for Cancer Therapy. Cancers 2022, 14, 1104. [Google Scholar] [CrossRef]
- Tilborghs, S.; Corthouts, J.; Verhoeven, Y.; Arias, D.; Rolfo, C.; Trinh, X.B.; van Dam, P.A. The Role of Nuclear Factor-Kappa B Signaling in Human Cervical Cancer. Crit. Rev. Oncol. Hematol. 2017, 120, 141–150. [Google Scholar] [CrossRef]
- Allouch, S.; Malki, A.; Allouch, A.; Gupta, I.; Vranic, S.; Al Moustafa, A.-E. High-Risk HPV Oncoproteins and PD-1/PD-L1 Interplay in Human Cervical Cancer: Recent Evidence and Future Directions. Front. Oncol. 2020, 10, 914. [Google Scholar] [CrossRef]
- Tommasino, M.; Accardi, R.; Caldeira, S.; Dong, W.; Malanchi, I.; Smet, A.; Zehbe, I. The Role of TP53 in Cervical Carcinogenesis. Hum. Mutat. 2003, 21, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.H.; Opipari, M.I.; Wilson, H.; Bottomley, R.; Coltman, C.A. Mitomycin C, Vincristine, and Bleomycin Therapy for Advanced Cervical Cancer. Obstet. Gynecol. 1978, 52, 146–150. [Google Scholar] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, D.; Petrelli, F.; Coinu, A.; Raspagliesi, F.; Barni, S. A Systematic Review Comparing Cisplatin and Carboplatin plus Paclitaxel-Based Chemotherapy for Recurrent or Metastatic Cervical Cancer. Gynecol. Oncol. 2014, 133, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H. Long-Term Survival Rates of Cancer Patients Achieved by the End of the 20th Century: A Period Analysis. Lancet Lond. Engl. 2002, 360, 1131–1135. [Google Scholar] [CrossRef]
- Osei Appiah, E.; Amertil, N.P.; Oti-Boadi Ezekiel, E.; Lavoe, H.; Siedu, D.J. Impact of Cervical Cancer on the Sexual and Physical Health of Women Diagnosed with Cervical Cancer in Ghana: A Qualitative Phenomenological Study. Womens Health 2021, 17, 17455065211066076. [Google Scholar] [CrossRef]
- Weaver, B.A. How Taxol/Paclitaxel Kills Cancer Cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef]
- Nonomura, S.; Kanagawa, H.; Makimoto, A. Chemical constituents of polygonaceous plants. I. Studies on the components of ko-j o-kon. (polygonum cuspidatum sieb. Et zucc.). Yakugaku Zasshi 1963, 83, 988–990. [Google Scholar] [CrossRef]
- Takaoka, M. Of the Phenolic Substrate of Hellebore (Veratrum Grandiflorum Loes. Fil.). J. Fac. Sci. Hokkaido Imper Univ. 1940, 3, 1–16. [Google Scholar]
- Koushki, M.; Amiri-Dashatan, N.; Ahmadi, N.; Abbaszadeh, H.-A.; Rezaei-Tavirani, M. Resveratrol: A Miraculous Natural Compound for Diseases Treatment. Food Sci. Nutr. 2018, 6, 2473–2490. [Google Scholar] [CrossRef]
- Takaoka, M. Resveratrol, a New Phenolic Compound, from Veratrum Grandiflorum. Nippon Kagaku Kaishi 1939, 60, 1090–1100. [Google Scholar] [CrossRef]
- Malaguarnera, L. Influence of Resveratrol on the Immune Response. Nutrients 2019, 11, 946. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef] [PubMed]
- Bhat, K.P.L.; Kosmeder, J.W.; Pezzuto, J.M. Biological Effects of Resveratrol. Antioxid. Redox Signal. 2001, 3, 1041–1064. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T.; Szkudelska, K. Anti-Diabetic Effects of Resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 34–39. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic Potential of Resveratrol: The in Vivo Evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef]
- Bhat, K.P.L.; Pezzuto, J.M. Cancer Chemopreventive Activity of Resveratrol. Ann. N. Y. Acad. Sci. 2002, 957, 210–229. [Google Scholar] [CrossRef]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer Chemopreventive Activity of Resveratrol, a Natural Product Derived from Grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef]
- Yousef, M.; Vlachogiannis, I.A.; Tsiani, E. Effects of Resveratrol against Lung Cancer: In Vitro and In Vivo Studies. Nutrients 2017, 9, 1231. [Google Scholar] [CrossRef]
- Ko, J.-H.; Sethi, G.; Um, J.-Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Lertpiriyapong, K.; Steelman, L.S.; Abrams, S.L.; Yang, L.V.; Murata, R.M.; Rosalen, P.L.; Scalisi, A.; Neri, L.M.; Cocco, L.; et al. Effects of Resveratrol, Curcumin, Berberine and Other Nutraceuticals on Aging, Cancer Development, Cancer Stem Cells and MicroRNAs. Aging 2017, 9, 1477–1536. [Google Scholar] [CrossRef] [PubMed]
- Pezzuto, J.M. The Phenomenon of Resveratrol: Redefining the Virtues of Promiscuity. Ann. N. Y. Acad. Sci. 2011, 1215, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Zaffaroni, N.; Beretta, G.L. Resveratrol and Prostate Cancer: The Power of Phytochemicals. Curr. Med. Chem. 2021, 28, 4845–4862. [Google Scholar] [CrossRef] [PubMed]
- Zoberi, I.; Bradbury, C.M.; Curry, H.A.; Bisht, K.S.; Goswami, P.C.; Roti Roti, J.L.; Gius, D. Radiosensitizing and Anti-Proliferative Effects of Resveratrol in Two Human Cervical Tumor Cell Lines. Cancer Lett. 2002, 175, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Lim, J.; Kim, Y.; Suh, S.; Min, D.; Chang, D.; Lee, Y.; Park, Y.; Kwon, T. Resveratrol Inhibits Phorbol Myristate Acetate-Induced Matrix Metalloproteinase-9 Expression by Inhibiting JNK and PKC Delta Signal Transduction. Oncogene 2004, 23, 1845–1853. [Google Scholar] [CrossRef]
- Kramer, M.P.; Wesierska-Gadek, J. Monitoring of Long-Term Effects of Resveratrol on Cell Cycle Progression of Human HeLa Cells after Administration of a Single Dose. Ann. N. Y. Acad. Sci. 2009, 1171, 257–263. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, Q.; Nishitani, J.; Brown, J.; Shi, S.; Le, A.D. Overexpression of Human Papillomavirus Type 16 Oncoproteins Enhances Hypoxia-Inducible Factor 1 Alpha Protein Accumulation and Vascular Endothelial Growth Factor Expression in Human Cervical Carcinoma Cells. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007, 13, 2568–2576. [Google Scholar] [CrossRef]
- Hsu, K.-F.; Wu, C.-L.; Huang, S.-C.; Wu, C.-M.; Hsiao, J.-R.; Yo, Y.-T.; Chen, Y.-H.; Shiau, A.-L.; Chou, C.-Y. Cathepsin L Mediates Resveratrol-Induced Autophagy and Apoptotic Cell Death in Cervical Cancer Cells. Autophagy 2009, 5, 451–460. [Google Scholar] [CrossRef]
- Chen, T.-C.; Hung, Y.-C.; Lin, T.-Y.; Chang, H.-W.; Chiang, I.-P.; Chen, Y.-Y.; Chow, K.-C. Human Papillomavirus Infection and Expression of ATPase Family AAA Domain Containing 3A, a Novel Anti-Autophagy Factor, in Uterine Cervical Cancer. Int. J. Mol. Med. 2011, 28, 689–696. [Google Scholar] [CrossRef]
- Kim, Y.S.; Sull, J.W.; Sung, H.J. Suppressing Effect of Resveratrol on the Migration and Invasion of Human Metastatic Lung and Cervical Cancer Cells. Mol. Biol. Rep. 2012, 39, 8709–8716. [Google Scholar] [CrossRef]
- Dhandayuthapani, S.; Marimuthu, P.; Hörmann, V.; Kumi-Diaka, J.; Rathinavelu, A. Induction of Apoptosis in HeLa Cells via Caspase Activation by Resveratrol and Genistein. J. Med. Food 2013, 16, 139–146. [Google Scholar] [CrossRef]
- García-Zepeda, S.P.; García-Villa, E.; Díaz-Chávez, J.; Hernández-Pando, R.; Gariglio, P. Resveratrol Induces Cell Death in Cervical Cancer Cells through Apoptosis and Autophagy. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. ECP 2013, 22, 577–584. [Google Scholar] [CrossRef]
- Zhang, P.; Li, H.; Yang, B.; Yang, F.; Zhang, L.-L.; Kong, Q.-Y.; Chen, X.-Y.; Wu, M.-L.; Liu, J. Biological Significance and Therapeutic Implication of Resveratrol-Inhibited Wnt, Notch and STAT3 Signaling in Cervical Cancer Cells. Genes Cancer 2014, 5, 154–164. [Google Scholar] [CrossRef]
- Li, Y.-G.; Xia, H.-J.; Tao, J.-P.; Xin, P.; Liu, M.-Y.; Li, J.-B.; Zhu, W.; Wei, M. GRIM-19-mediated Stat3 Activation Is a Determinant for Resveratrol-induced Proliferation and Cytotoxicity in Cervical Tumor-derived Cell Lines. Mol. Med. Rep. 2015, 11, 1272–1277. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, P.; Yang, B.; Yao, Y.-Y.; Zhong, L.-X.; Chen, X.-Y.; Kong, Q.-Y.; Wu, M.-L.; Li, C.; Li, H.; Liu, J. PIAS3, SHP2 and SOCS3 Expression Patterns in Cervical Cancers: Relevance with Activation and Resveratrol-Caused Inactivation of STAT3 Signaling. Gynecol. Oncol. 2015, 139, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Ruíz, G.; Valencia-González, H.A.; León-Galicia, I.; García-Villa, E.; García-Carrancá, A.; Gariglio, P. Inhibition of RAD51 by SiRNA and Resveratrol Sensitizes Cancer Stem Cells Derived from HeLa Cell Cultures to Apoptosis. Stem Cells Int. 2018, 2018, 2493869. [Google Scholar] [CrossRef]
- Chatterjee, K.; AlSharif, D.; Mazza, C.; Syar, P.; Al Sharif, M.; Fata, J.E. Resveratrol and Pterostilbene Exhibit Anticancer Properties Involving the Downregulation of HPV Oncoprotein E6 in Cervical Cancer Cells. Nutrients 2018, 10, 243. [Google Scholar] [CrossRef] [PubMed]
- Flores-Pérez, A.; Elizondo, G. Apoptosis Induction and Inhibition of HeLa Cell Proliferation by Alpha-Naphthoflavone and Resveratrol Are Aryl Hydrocarbon Receptor-Independent. Chem. Biol. Interact. 2018, 281, 98–105. [Google Scholar] [CrossRef]
- Li, L.; Qiu, R.-L.; Lin, Y.; Cai, Y.; Bian, Y.; Fan, Y.; Gao, X.-J. Resveratrol Suppresses Human Cervical Carcinoma Cell Proliferation and Elevates Apoptosis via the Mitochondrial and P53 Signaling Pathways. Oncol. Lett. 2018, 15, 9845–9851. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, X.; Li, X.; Zhang, Y. Resveratrol Significantly Inhibits the Occurrence and Development of Cervical Cancer by Regulating Phospholipid Scramblase 1. J. Cell. Biochem. 2018, 120, 1527–1531. [Google Scholar] [CrossRef]
- Nakamura, H.; Taguchi, A.; Kawana, K.; Baba, S.; Kawata, A.; Yoshida, M.; Fujimoto, A.; Ogishima, J.; Sato, M.; Inoue, T.; et al. Therapeutic Significance of Targeting Survivin in Cervical Cancer and Possibility of Combination Therapy with TRAIL. Oncotarget 2018, 9, 13451–13461. [Google Scholar] [CrossRef][Green Version]
- Assad, D.X.; Borges, G.A.; Avelino, S.R.; Guerra, E.N.S. Additive Cytotoxic Effects of Radiation and MTOR Inhibitors in a Cervical Cancer Cell Line. Pathol.-Res. Pract. 2018, 214, 259–262. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; She, G.; Zheng, X.; Shao, L.; Wang, P.; Pang, M.; Xie, S.; Sun, Y. Resveratrol Induces Cervical Cancer HeLa Cell Apoptosis through the Activation and Nuclear Translocation Promotion of FOXO3a. Pharmazie 2020, 75, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Xu, Q.; Zeng, L.; Xie, L.; Zhao, Q.; Xu, H.; Wang, X.; Jiang, N.; Fu, P.; Sang, M. Resveratrol Suppresses the Growth and Metastatic Potential of Cervical Cancer by Inhibiting STAT3Tyr705 Phosphorylation. Cancer Med. 2020, 9, 8685–8700. [Google Scholar] [CrossRef]
- Pani, S.; Sahoo, A.; Patra, A.; Debata, P.R. Phytocompounds Curcumin, Quercetin, Indole-3-Carbinol, and Resveratrol Modulate Lactate–Pyruvate Level along with Cytotoxic Activity in HeLa Cervical Cancer Cells. Biotechnol. Appl. Biochem. 2021, 68, 1396–1402. [Google Scholar] [CrossRef] [PubMed]
- Einbond, L.S.; Zhou, J.; Wu, H.-A.; Mbazor, E.; Song, G.; Balick, M.; DeVoti, J.A.; Redenti, S.; Castellanos, M.R. A Novel Cancer Preventative Botanical Mixture, TriCurin, Inhibits Viral Transcripts and the Growth of W12 Cervical Cells Harbouring Extrachromosomal or Integrated HPV16 DNA. Br. J. Cancer 2021, 124, 901–913. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Fu, P.; Xie, L.; Chai, S.; Xu, Q.; Zeng, L.; Wang, X.; Jiang, N.; Sang, M. Resveratrol Inhibits the Progression of Cervical Cancer by Suppressing the Transcription and Expression of HPV E6 and E7 Genes. Int. J. Mol. Med. 2021, 47, 335–345. [Google Scholar] [CrossRef]
- Alharbi, H.; Alshehri, A.S.; Ahmad, M.; Guo, W.W. Promising Anti- Cervical Carcinoma and Inflammatory Agent, Resveratrol Targets Poly (ADP-Ribose) Polymerase 1 (PARP-1) Induced Premature Ovarian Failure with a Potent Enzymatic Modulatory Activity. J. Reprod. Immunol. 2021, 144, 103272. [Google Scholar] [CrossRef]
- Devi, R.V.; Raj, D.; Doble, M. Lockdown of Mitochondrial Ca2+ Extrusion and Subsequent Resveratrol Treatment Kill HeLa Cells by Ca2+ Overload. Int. J. Biochem. Cell Biol. 2021, 139, 106071. [Google Scholar] [CrossRef]
- Pani, S.; Mohapatra, S.; Sahoo, A.; Baral, B.; Debata, P.R. Shifting of Cell Cycle Arrest from the S-Phase to G2/M Phase and Downregulation of EGFR Expression by Phytochemical Combinations in HeLa Cervical Cancer Cells. J. Biochem. Mol. Toxicol. 2022, 36, e22947. [Google Scholar] [CrossRef]
- Rashid, A.; Liu, C.; Sanli, T.; Tsiani, E.; Singh, G.; Bristow, R.G.; Dayes, I.; Lukka, H.; Wright, J.; Tsakiridis, T. Resveratrol Enhances Prostate Cancer Cell Response to Ionizing Radiation. Modulation of the AMPK, Akt and MTOR Pathways. Radiat. Oncol. Lond. Engl. 2011, 6, 144. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Choi, H.-E.; Lee, H.-H.; Shin, J.-S.; Shin, D.-H.; Choi, J.-H.; Lee, Y.S.; Lee, K.-T. Resveratrol Analogue (E)-8-Acetoxy-2-[2-(3,4-Diacetoxyphenyl)Ethenyl]-Quinazoline Induces G₂/M Cell Cycle Arrest through the Activation of ATM/ATR in Human Cervical Carcinoma HeLa Cells. Oncol. Rep. 2015, 33, 2639–2647. [Google Scholar] [CrossRef]
- Hong Bin, W.; Da, L.H.; Xue, Y.; Jing, B. Pterostilbene (3′,5′-Dimethoxy-Resveratrol) Exerts Potent Antitumor Effects in HeLa Human Cervical Cancer Cells via Disruption of Mitochondrial Membrane Potential, Apoptosis Induction and Targeting m-TOR/PI3K/Akt Signalling Pathway. J. BUON Off. J. Balk. Union Oncol. 2018, 23, 1384–1389. [Google Scholar]
- Lee, K.-W.; Chung, K.-S.; Lee, J.-H.; Choi, J.-H.; Choi, S.Y.; Kim, S.; Lee, J.Y.; Lee, K.-T. Resveratrol Analog, N-(4-Methoxyphenyl)-3,5-Dimethoxybenzamide Induces G2/M Phase Cell Cycle Arrest and Apoptosis in HeLa Human Cervical Cancer Cells. Food Chem. Toxicol. 2019, 124, 101–111. [Google Scholar] [CrossRef]
- Chatterjee, K.; Mukherjee, S.; Vanmanen, J.; Banerjee, P.; Fata, J.E. Dietary Polyphenols, Resveratrol and Pterostilbene Exhibit Antitumor Activity on an HPV E6-Positive Cervical Cancer Model: An in Vitro and in Vivo Analysis. Front. Oncol. 2019, 9, 352. [Google Scholar] [CrossRef]
- Shin, H.J.; Han, J.M.; Choi, Y.S.; Jung, H.J. Pterostilbene Suppresses Both Cancer Cells and Cancer Stem-Like Cells in Cervical Cancer with Superior Bioavailability to Resveratrol. Molecules 2020, 25, 228. [Google Scholar] [CrossRef]
- Hao, X.; Sun, X.; Zhu, H.; Xie, L.; Wang, X.; Jiang, N.; Fu, P.; Sang, M. Hydroxypropyl-β-Cyclodextrin-Complexed Resveratrol Enhanced Antitumor Activity in a Cervical Cancer Model: In Vivo Analysis. Front. Pharmacol. 2021, 12, 573909. [Google Scholar] [CrossRef]
- Walle, T. Bioavailability of Resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef]
- Azorín-Ortuño, M.; Yáñez-Gascón, M.J.; Vallejo, F.; Pallarés, F.J.; Larrosa, M.; Lucas, R.; Morales, J.C.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Espín, J.C. Metabolites and Tissue Distribution of Resveratrol in the Pig. Mol. Nutr. Food Res. 2011, 55, 1154–1168. [Google Scholar] [CrossRef]
- Li, F.; Han, Y.; Wu, X.; Cao, X.; Gao, Z.; Sun, Y.; Wang, M.; Xiao, H. Gut Microbiota-Derived Resveratrol Metabolites, Dihydroresveratrol and Lunularin, Significantly Contribute to the Biological Activities of Resveratrol. Front. Nutr. 2022, 9, 912591. [Google Scholar] [CrossRef]
- Almeida, L.; Vaz-da-Silva, M.; Falcão, A.; Soares, E.; Costa, R.; Loureiro, A.I.; Fernandes-Lopes, C.; Rocha, J.-F.; Nunes, T.; Wright, L.; et al. Pharmacokinetic and Safety Profile of Trans-Resveratrol in a Rising Multiple-Dose Study in Healthy Volunteers. Mol. Nutr. Food Res. 2009, 53 (Suppl. S1), S7–S15. [Google Scholar] [CrossRef]
- Boocock, D.J.; Faust, G.E.S.; Patel, K.R.; Schinas, A.M.; Brown, V.A.; Ducharme, M.P.; Booth, T.D.; Crowell, J.A.; Perloff, M.; Gescher, A.J.; et al. Phase I Dose Escalation Pharmacokinetic Study in Healthy Volunteers of Resveratrol, a Potential Cancer Chemopreventive Agent. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2007, 16, 1246–1252. [Google Scholar] [CrossRef]
- Asghar, W.; El Assal, R.; Shafiee, H.; Pitteri, S.; Paulmurugan, R.; Demirci, U. Engineering Cancer Microenvironments for in Vitro 3-D Tumor Models. Mater. Today Kidlington Engl. 2015, 18, 539–553. [Google Scholar] [CrossRef]
- Murakami, T.; Murata, T.; Kawaguchi, K.; Kiyuna, T.; Igarashi, K.; Hwang, H.K.; Hiroshima, Y.; Hozumi, C.; Komatsu, S.; Kikuchi, T.; et al. Cervical Cancer Patient-Derived Orthotopic Xenograft (PDOX) Is Sensitive to Cisplatinum and Resistant to Nab-Paclitaxel. Anticancer Res. 2017, 37, 61–65. [Google Scholar] [CrossRef]
- Poulsen, M.M.; Vestergaard, P.F.; Clasen, B.F.; Radko, Y.; Christensen, L.P.; Stødkilde-Jørgensen, H.; Møller, N.; Jessen, N.; Pedersen, S.B.; Jørgensen, J.O.L. High-Dose Resveratrol Supplementation in Obese Men: An Investigator-Initiated, Randomized, Placebo-Controlled Clinical Trial of Substrate Metabolism, Insulin Sensitivity, and Body Composition. Diabetes 2013, 62, 1186–1195. [Google Scholar] [CrossRef]
- Timmers, S.; de Ligt, M.; Phielix, E.; van de Weijer, T.; Hansen, J.; Moonen-Kornips, E.; Schaart, G.; Kunz, I.; Hesselink, M.K.C.; Schrauwen-Hinderling, V.B.; et al. Resveratrol as Add-on Therapy in Subjects With Well-Controlled Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Care 2016, 39, 2211–2217. [Google Scholar] [CrossRef]
- Sinha, D.; Sarkar, N.; Biswas, J.; Bishayee, A. Resveratrol for Breast Cancer Prevention and Therapy: Preclinical Evidence and Molecular Mechanisms. Semin. Cancer Biol. 2016, 40–41, 209–232. [Google Scholar] [CrossRef]
- Dandawate, P.R.; Subramaniam, D.; Jensen, R.A.; Anant, S. Targeting Cancer Stem Cells and Signaling Pathways by Phytochemicals: Novel Approach for Breast Cancer Therapy. Semin. Cancer Biol. 2016, 40–41, 192–208. [Google Scholar] [CrossRef]
- Gehm, B.D.; McAndrews, J.M.; Chien, P.Y.; Jameson, J.L. Resveratrol, a Polyphenolic Compound Found in Grapes and Wine, Is an Agonist for the Estrogen Receptor. Proc. Natl. Acad. Sci. USA 1997, 94, 14138–14143. [Google Scholar] [CrossRef]
- Bowers, J.L.; Tyulmenkov, V.V.; Jernigan, S.C.; Klinge, C.M. Resveratrol Acts as a Mixed Agonist/Antagonist for Estrogen Receptors Alpha and Beta. Endocrinology 2000, 141, 3657–3667. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, H.J.; Davis, F.B.; Tang, H.-Y.; Davis, P.J.; Lin, H.-Y. Oestrogen Inhibits Resveratrol-Induced Post-Translational Modification of P53 and Apoptosis in Breast Cancer Cells. Br. J. Cancer 2004, 91, 178–185. [Google Scholar] [CrossRef]
- Chow, H.-H.S.; Garland, L.L.; Heckman-Stoddard, B.M.; Hsu, C.-H.; Butler, V.D.; Cordova, C.A.; Chew, W.M.; Cornelison, T.L. A Pilot Clinical Study of Resveratrol in Postmenopausal Women with High Body Mass Index: Effects on Systemic Sex Steroid Hormones. J. Transl. Med. 2014, 12, 223. [Google Scholar] [CrossRef]
- Michan, S.; Sinclair, D. Sirtuins in Mammals: Insights into Their Biological Function. Biochem. J. 2007, 404, 1–13. [Google Scholar] [CrossRef]
- Buhrmann, C.; Shayan, P.; Popper, B.; Goel, A.; Shakibaei, M. Sirt1 Is Required for Resveratrol-Mediated Chemopreventive Effects in Colorectal Cancer Cells. Nutrients 2016, 8, 145. [Google Scholar] [CrossRef]
- Rawat, D.; Chhonker, S.K.; Naik, R.A.; Koiri, R.K. Modulation of Antioxidant Enzymes, SIRT1 and NF-ΚB by Resveratrol and Nicotinamide in Alcohol-Aflatoxin B1-Induced Hepatocellular Carcinoma. J. Biochem. Mol. Toxicol. 2021, 35, e22625. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Liu, C.; Li, M. Silent Information Regulator 1 Promotes Proliferation, Migration, and Invasion of Cervical Cancer Cells and Is Upregulated by Human Papillomavirus 16 E7 Oncoprotein. Gynecol. Obstet. Investig. 2022, 87, 22–29. [Google Scholar] [CrossRef]
- Velez-Perez, A.; Wang, X.I.; Li, M.; Zhang, S. SIRT1 Overexpression in Cervical Squamous Intraepithelial Lesions and Invasive Squamous Cell Carcinoma. Hum. Pathol. 2017, 59, 102–107. [Google Scholar] [CrossRef]
- Cottart, C.-H.; Nivet-Antoine, V.; Laguillier-Morizot, C.; Beaudeux, J.-L. Resveratrol Bioavailability and Toxicity in Humans. Mol. Nutr. Food Res. 2010, 54, 7–16. [Google Scholar] [CrossRef]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef]
- Ren, B.; Kwah, M.X.-Y.; Liu, C.; Ma, Z.; Shanmugam, M.K.; Ding, L.; Xiang, X.; Ho, P.C.-L.; Wang, L.; Ong, P.S.; et al. Resveratrol for Cancer Therapy: Challenges and Future Perspectives. Cancer Lett. 2021, 515, 63–72. [Google Scholar] [CrossRef]
- Brown, V.A.; Patel, K.R.; Viskaduraki, M.; Crowell, J.A.; Perloff, M.; Booth, T.D.; Vasilinin, G.; Sen, A.; Schinas, A.M.; Piccirilli, G.; et al. Repeat Dose Study of the Cancer Chemopreventive Agent Resveratrol in Healthy Volunteers: Safety, Pharmacokinetics, and Effect on the Insulin-like Growth Factor Axis. Cancer Res. 2010, 70, 9003–9011. [Google Scholar] [CrossRef]
- Pollack, R.M.; Barzilai, N.; Anghel, V.; Kulkarni, A.S.; Golden, A.; O’Broin, P.; Sinclair, D.A.; Bonkowski, M.S.; Coleville, A.J.; Powell, D.; et al. Resveratrol Improves Vascular Function and Mitochondrial Number but Not Glucose Metabolism in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1703–1709. [Google Scholar] [CrossRef]
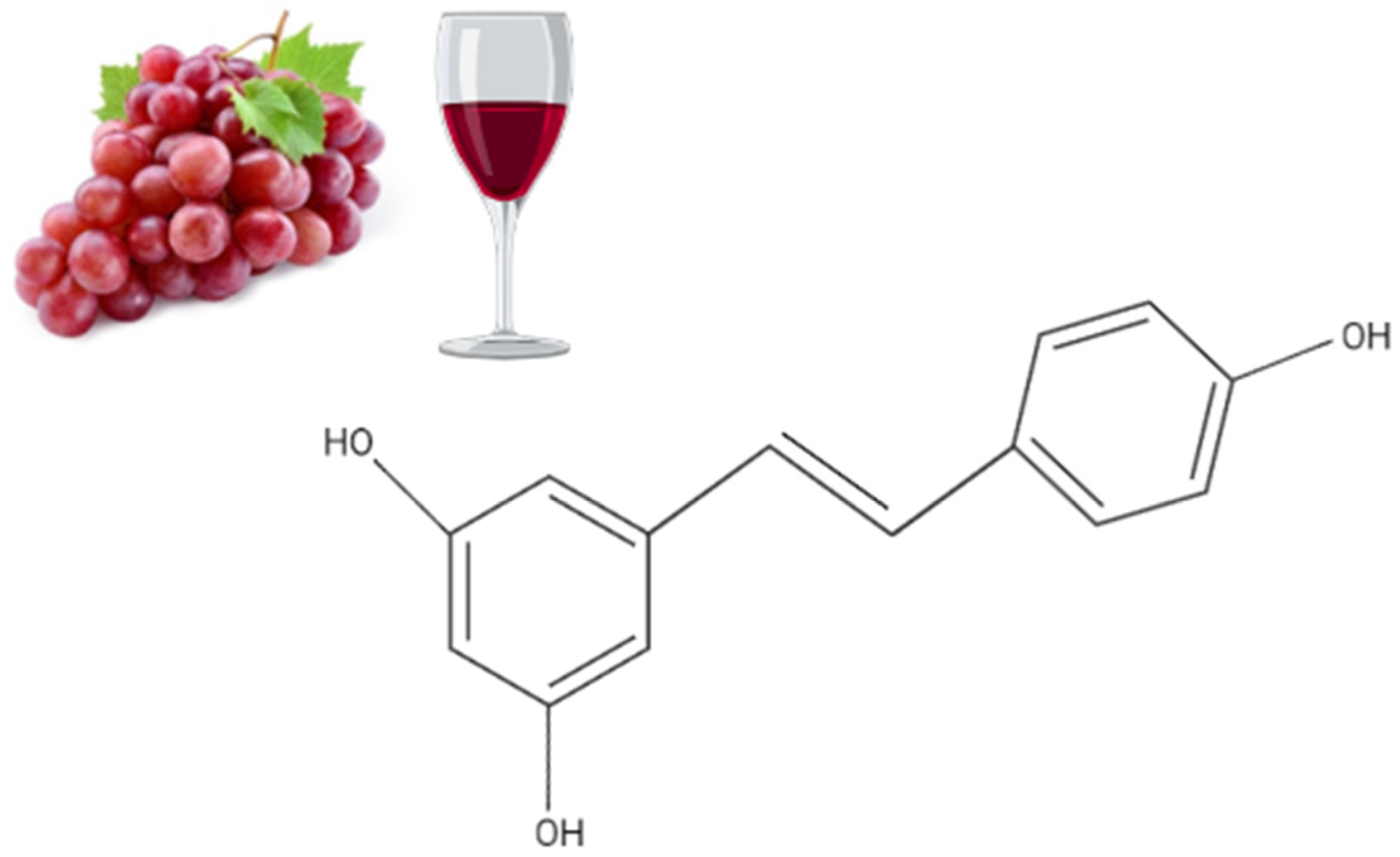
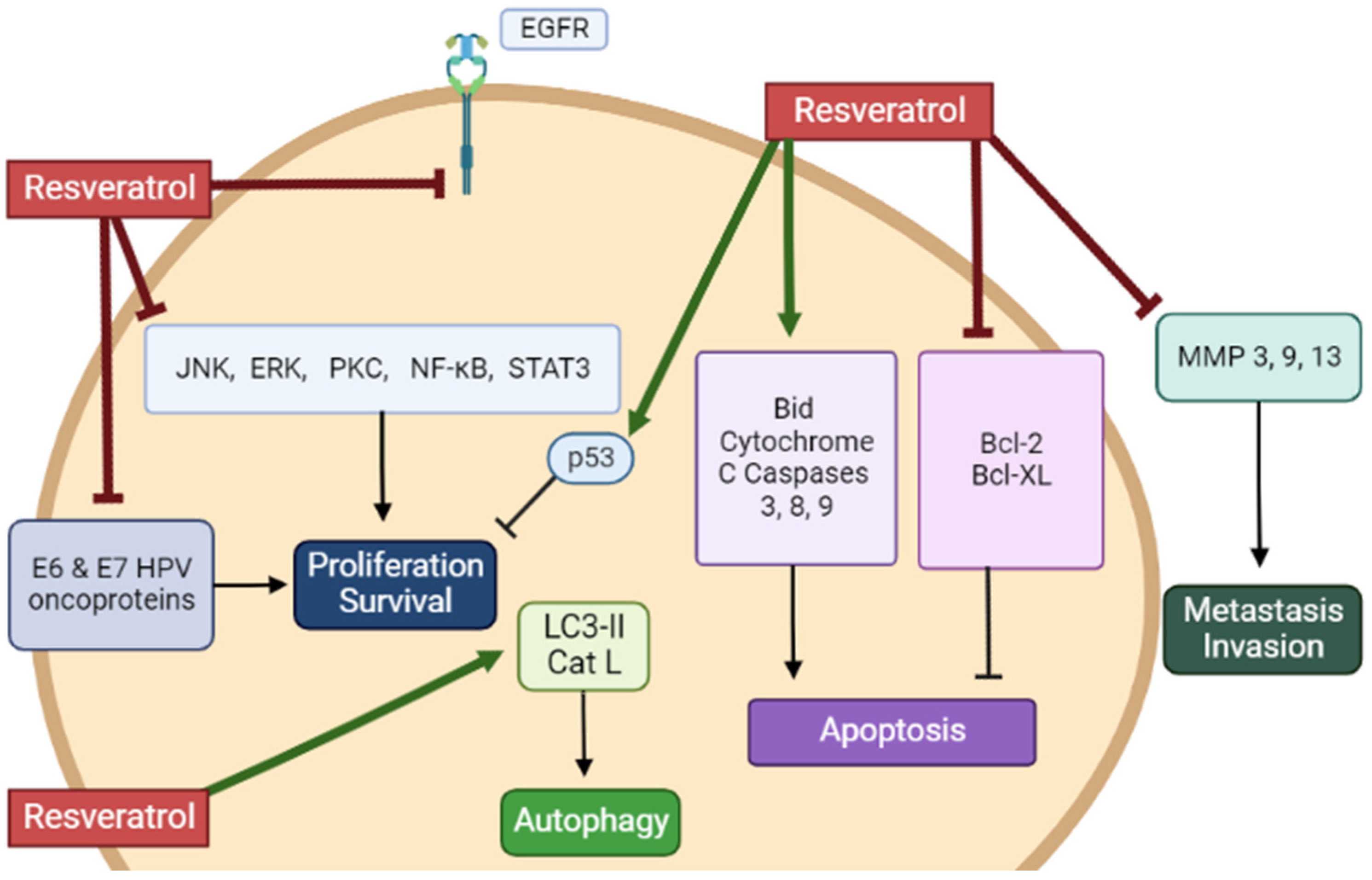

| Cell | Resveratrol Concentration/Duration | Effect | Reference |
|---|---|---|---|
| HeLa, SiHa cervical cancer cells | 10, 25 µM 1–8 days | Increased effects of IR ↓cell growth ↓cell survival ↑cell cycle arrest (S phase) ↓COX-1 activity | [36] |
| HeLa cells | 50 & 75 µM 24 h | ↓PMA effects ↓MMP-9 mRNA, protein & activity ↓JNK ↓PKC δ ↓AP-1 ↓NFkB | [37] |
| HeLa cervical cancer cells | 5, 25 & 50 µM 24–48 h | ↓cell growth Accumulation in the S phase of the cell cycle | [38] |
| C-33A and HeLa Expressing HPV E6, E7 | 25, 50 &100 µM 16 h | ↓HIF-1a ↓VEGF | [39] |
| hHeLa, Cx, SiHa and SKGIIIb cervical cancer cells | 100–400 µM 24–72 h | ↓cell growth ↑autophagy ↑apoptosis ↑LC3-II ↑LMP ↑Cat L ↑Cytochrome C ↑caspase-3 | [40] |
| SKG-I SKG-II SKG-IIIa Nuz HeLa Cervical cancer cells | 10, 30 & 100 μΜ | ↑autophagy ↑apoptosis ↓drug resistance ↓ATAD3A ↑abrasion of the mitochondrial outer membrane ↑autophagosomes | [41] |
| HeLa cervical cancer cells | 10, 30 & 100 μΜ 24 h | ↓invasion ↓metastasis ↓MMP9 levels-activity ↓NF κΒ ↓AP-1 | [42] |
| HeLa cervical cancer cells | 25 μΜ 24, 48 & 72 h | ↓cell proliferation ↑apoptosis ↑caspase-9 ↑caspase-3 ↓mitochondrial membrane potential -JC-1 in monomeric form ↓HDM2 | [43] |
| C33A (with mutated p53) HeLa(HPV18positive) CaLo(HPV18positive) CaSki(HPV16positive) SiHa(HPV16 positive) | 150–250 µM 48 h | ↓proliferation ↑apoptosis ↓mitochondrial membrane potential ↑mitochondrial and lysosomal permeability ↑p53 levels ↓p65 NF κB levels | [44] |
| HeLa SiHa cervical cancer cells | 100 μΜ 12–48 h | ↑S-phase cell cycle arrest ↑apoptosis ↓p-STAT3 ↓Notch1/2 ↓Hes1 ↓Wnt2/5a ↓β-catenin ↑PIAS3 | [45] |
| HeLa cervical cancer cells | 10 & 100 μΜ 24 h | ↓cell viability ↓cell proliferation ↓cell survival ↑GRIM-19 ↓p-STAT3 ↓cyclin B1 ↓VEGF ↓Bcl-2 | [46] |
| HeLa SiHa C33A cervical cancer cells | 100 μΜ 12, 24, 36 & 48 h | ↓cell growth ↓proliferation ↑apoptosis ↓p-STAT3 ↓survivin ↓c-Myc ↓cyclin D1 ↓VEGF ↑SOCS3 ↑PIAS3 | [47] |
| cancer stem cells (CSC) from HeLa cultures (HeLa SP) | 137 μM 48–72 h | ↓cell viability ↑apoptosis ↓RAD51 | [48] |
| HeLa cervical cancer cells | 5–40 µM 24–48 h | ↓cell viability ↓cell migration ↑cell cycle arrest (S phase) ↓viral oncogene E6 ↑p53 levels | [49] |
| HeLa | 10–80 µM 12–36 h | ↓cell proliferation ↑cell cycle arrest at G1/S phase ↑p53 levels ↑apoptosis | [50] |
| HeLa | 10–40 µM 24–48 h | ↓cell viability ↓cell proliferation ↑apoptosis ↑caspase-3 ↑caspase-9 ↑Bax ↓Bcl-2 ↓Bcl-XL ↑p53 ↓Cyclin B1 | [51] |
| HeLa | 0–100 µM 24–96 h | ↓Cell growth ↓Cell viability ↓Proliferation ↓Phospholipid scramblase 1 | [52] |
| SiHa | 100 µM 24 h | ↓Cell viability ↑Cell cycle arrest in G2/M ↑Apoptosis ↓Survivin mRNA levels ↓Survivin protein levels ↑E-cadherin | [53] |
| HeLa | 2.5–150 µM 24–48 h | ↓Cell viability ↑Cytotoxicity ↑necrosis | [54] |
| HeLa | 0–80 µM 48 h | ↓Proliferation ↑Apoptosis ↓p-FOXO3a ↑FOXO3a ↑Bim ↓p-ERK | [55] |
| HeLa SiHa | 0–40 µM 24 h | ↓Proliferation ↓Wound healing ↓Migration/invasion ↓Metastasis ↑E-cadherin ↓N-cadherin ↓vimentin ↓MMP-3/13 protein levels ↓STAT3 protein levels | [56] |
| HeLa | 20 µM 24 h | ↓Cell viability ↑Cytotoxicity ↓Glucose uptake ↓NADH/NAD+ ratio ↓Lactate ↑Pyruvate | [57] |
| W12 | 0–100 µM | ↓Proliferation | [58] |
| HeLa Ca Ski | 5–40 µM 24 h | ↓Proliferation ↑Cell cycle arrest in S phase ↑Apoptosis ↑p16/21/27 ↓CDK4 ↓E2F1 ↓p-pRb1 ↓Bcl-2 mRNA & protein levels ↓Bcl-xL mRNA levels ↑Bax protein levels ↑E6/7 ↑p53 | [59] |
| HT-3 | 0.16–1.25 µM 0–48 h | ↓Cell viability ↓Cell growth ↓Proliferation ↑Apoptosis | [60] |
| HeLa | 262.87 µM 24 h | ↓Cell viability ↑Apoptosis ↑mRNA caspases-3/-8/-9 levels ↑NCLX | [61] |
| HeLa | 20 µM 24 h | ↑Cell cycle arrest in S phase ↓Colony formation ↓EGFR | [62] |
| Cell | Analog Name | Resveratrol Concentration/Duration | Effect | Reference |
|---|---|---|---|---|
| HeLa | 8-ADEQ | 8 µM 25 h | ↓proliferation ↑Cell cycle arrest at G2/M phase ↑cyclin B1 levels ↑Cdk1, Cdc25C phosphorylation ↑Chk1, Chk2 activation ↑ATM/ATR activation | [64] |
| HeLa | Pterostilbene | 0–400 µM 24–48 h | ↑Cell morphology ↓Cell growth ↑DNA fragmentation ↓Proliferation ↑Apoptosis ↓p-mTOR ↓p-PI3K ↓p-Akt | [65] |
| HeLa | N-(4-methoxyphenyl)-3,5-dimethoxybenamide (MPDB) | 35 µM 15 h | ↓Cell growth ↓Survival ↑Cell cycle arrest at G2/M phase ↓Proliferation ↑DNA fragmentation ↑Apoptosis ↑Cdc2 ↑Cdc25c ↑Chk1/2 ↑p53 ↓Bcl-xL ↑Fas ↑Caspases-3/-8/-9 ↑Cleaved PARP | [66] |
| TC1 | Pterostilbene | 20, 30 µM 48 h | ↓Cell viability ↑Cytotoxicity ↑Apoptosis ↓E6 | [67] |
| HeLa | Pterostilbene | 20 µM 24–48 h | ↓Cell growth ↓Survival ↓Metastasis ↑Cell cycle arrest at S and G2/M phase ↑p21/53 protein levels ↓Cyclin E1/B1 ↓Bcl-2 protein levels ↓Bcl-xL protein levels ↑Cleaved caspases-3/-9 ↓MMPs-2/-9 | [68] |
| Cell | Resveratrol Concentration/Duration | Effect | Reference |
|---|---|---|---|
| Female BALB/C nude mice subcutaneously injected 2 × 106 HeLa cells/mL (100 µL/mouse) | 10 mg/kg RSV orally, daily for 28 days | ↓Tumor weight ↑PLSCR1 | [52] |
| C57BL/6 female mice injected with TC-1 (HPV oncogene E6, E7 positive) cells subcutaneously | Injection of RSV intralesionally administrated for 5 days | ↓Tumor size ↑cell cycle arrest ↓Tumor E6 levels ↓Tumor VEGF levels ↓Tumor PCNA levels | [67] |
| Athymic BALB/C nude mice subcutaneously injected 5 × 106 HeLa cells/mouse | 30 mg/kg RSV intragastrically, 3 times/week for 2 weeks (pre-treatment) | ↓Tumor volume ↓Tumor weight ↓STAT3 protein levels ↓MMP-3/13 protein levels ↑E-cadherin ↓N-cadherin protein levels ↓Vimentin protein levels | [56] |
| Female BALB/C nude mice subcutaneously injected 2 × 106 HeLa cells/mouse | 30 mg/kg RSV orally, 3 times/week for 3 weeks | ↓Tumor volume ↓Tumor weight ↓E6/7 mRNA levels ↓E6/7 protein levels ↑p53 expression ↑Rb1 expression | [69] |
| Female BALB/C nude mice subcutaneously injected 2 × 106 HeLa cells/mL (100 µL/mouse) | 15 mg/kg RSV intragastrically, 3 times/week for 5 weeks | ↓Tumor volume ↓Tumor weight ↓E6/7 mRNA levels ↓E6/7 protein levels ↓p-pRb1 ↑p53 expression | [59] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nadile, M.; Retsidou, M.I.; Gioti, K.; Beloukas, A.; Tsiani, E. Resveratrol against Cervical Cancer: Evidence from In Vitro and In Vivo Studies. Nutrients 2022, 14, 5273. https://doi.org/10.3390/nu14245273
Nadile M, Retsidou MI, Gioti K, Beloukas A, Tsiani E. Resveratrol against Cervical Cancer: Evidence from In Vitro and In Vivo Studies. Nutrients. 2022; 14(24):5273. https://doi.org/10.3390/nu14245273
Chicago/Turabian StyleNadile, Matteo, Maria Ilektra Retsidou, Katerina Gioti, Apostolos Beloukas, and Evangelia Tsiani. 2022. "Resveratrol against Cervical Cancer: Evidence from In Vitro and In Vivo Studies" Nutrients 14, no. 24: 5273. https://doi.org/10.3390/nu14245273
APA StyleNadile, M., Retsidou, M. I., Gioti, K., Beloukas, A., & Tsiani, E. (2022). Resveratrol against Cervical Cancer: Evidence from In Vitro and In Vivo Studies. Nutrients, 14(24), 5273. https://doi.org/10.3390/nu14245273







