Neuroprotective and Anti-Neuroinflammatory Properties of Vignae Radiatae Semen in Neuronal HT22 and Microglial BV2 Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of VRSE
2.3. Cell Culture
2.4. Cell Viability
2.5. Intracellular ROS Determination
2.6. Western Blotting
2.7. Preparation of Cytosolic and Nuclear Fractions
2.8. Cytokine Determination
2.9. VRSE and Standard Solution Preparation
2.10. HPLC Conditions
2.11. Statistical Analysis
3. Results
3.1. Effects of VRSE on H2O2-Induced Neurotoxicity in HT22 Cells
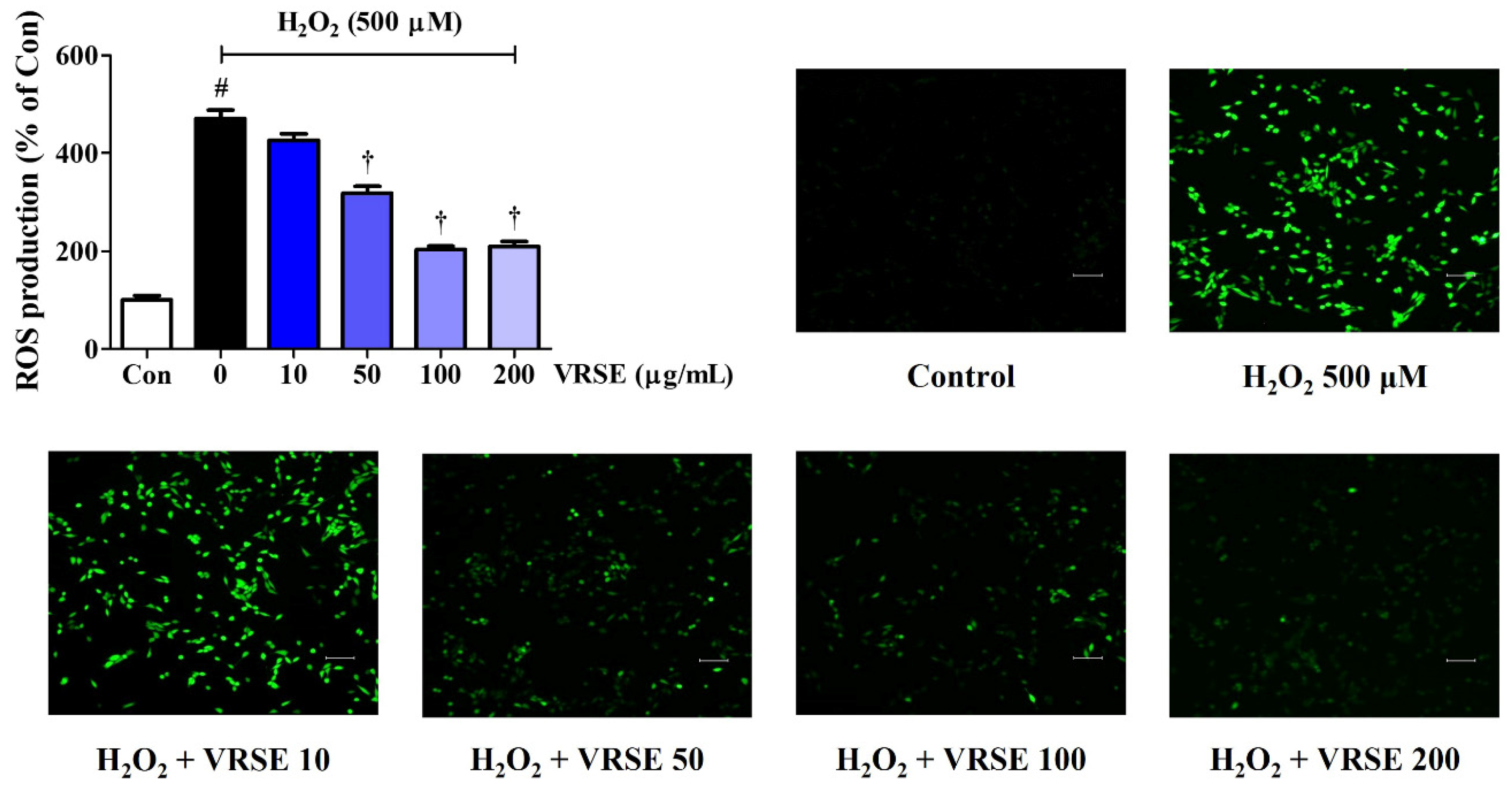
3.2. VRSE Reduced Intracellular ROS Generation by H2O2
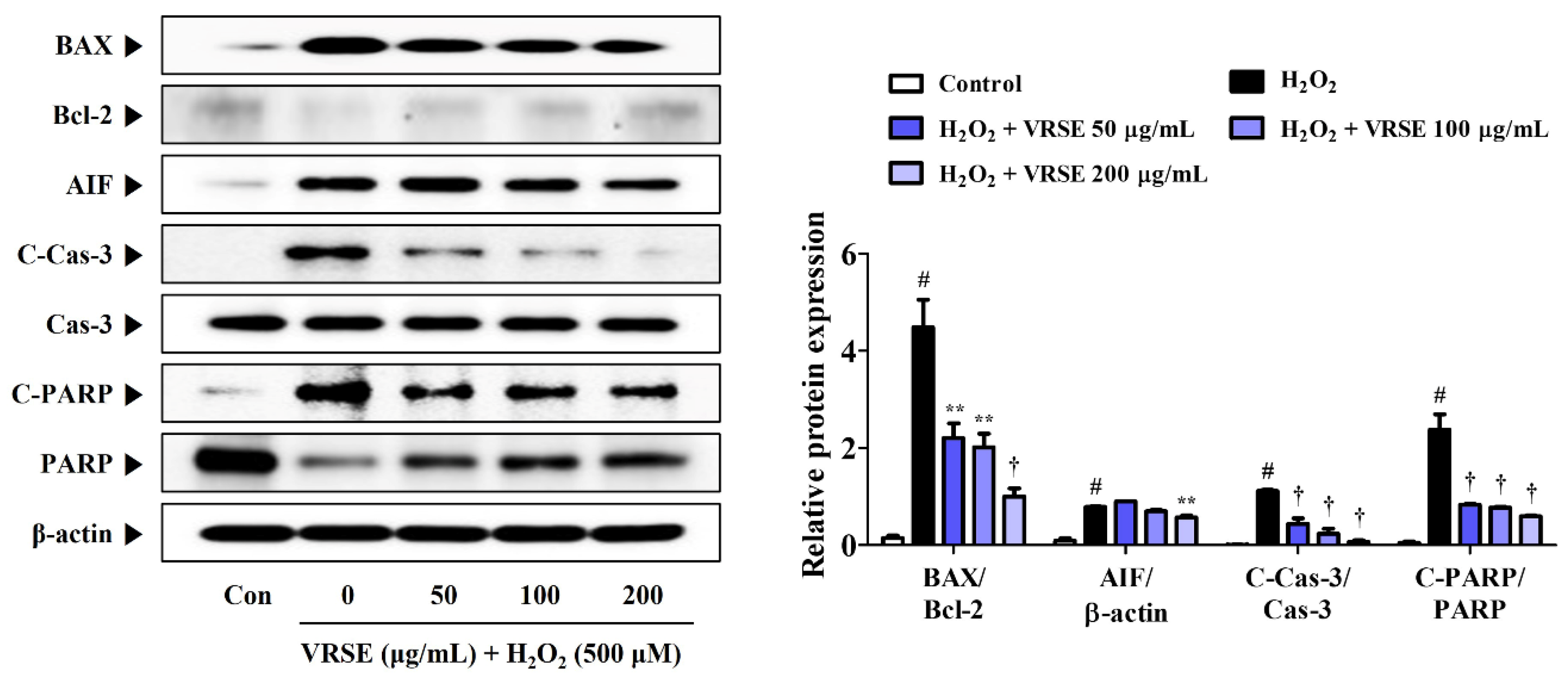
3.3. VRSE Suppress H2O2-Induced Apoptosis in HT22 Cells
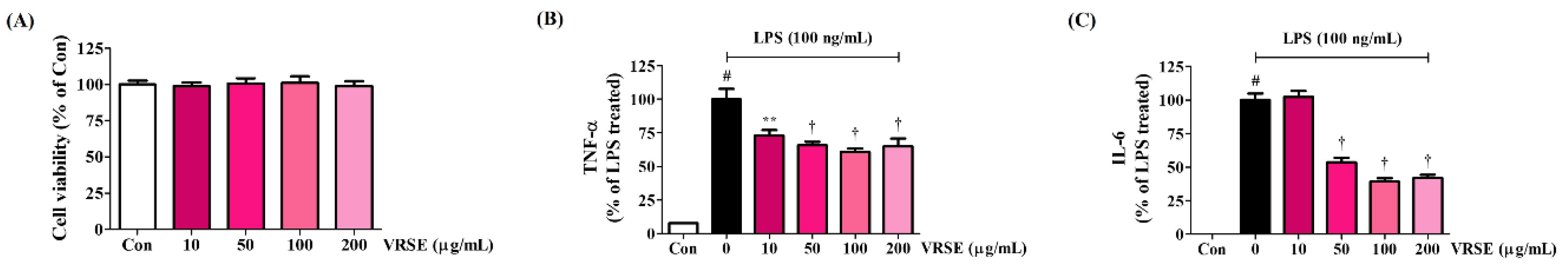
3.4. Effects of VRSE on the Production of Inflammatory Cytokines in LPS-Stimulated BV2 Cells
3.5. VRSE Ameliorate the Transcriptional Activity of NF-κB and Phosphorylation of MAPK in LPS-Stimulated BV2 Microglia
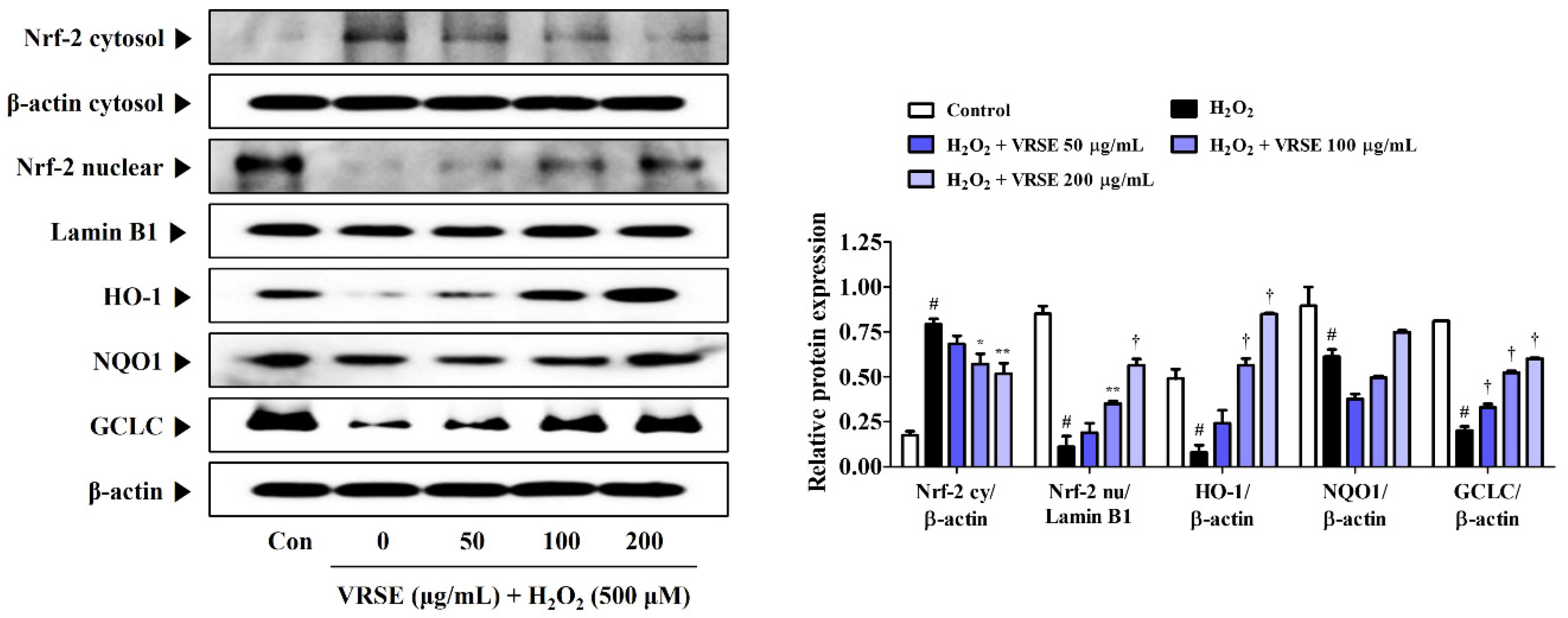
3.6. Effects of VRSE on Nrf-2-Mediated Antioxidant Enzyme Expression in H2O2-Exposed HT22 Hippocampal Cells
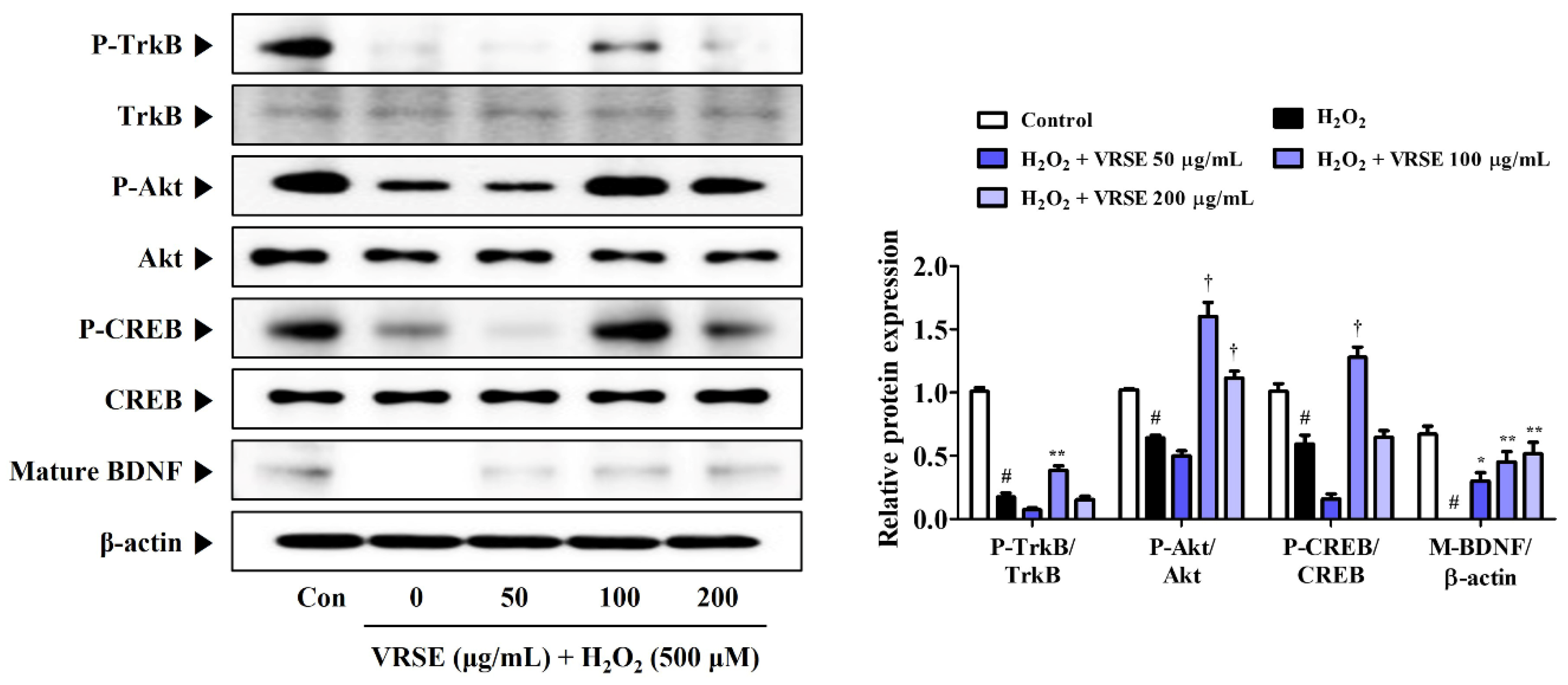
3.7. VRSE Enhances Mature BDNF Expression via TrkB/Akt/CREB Pathway Activation
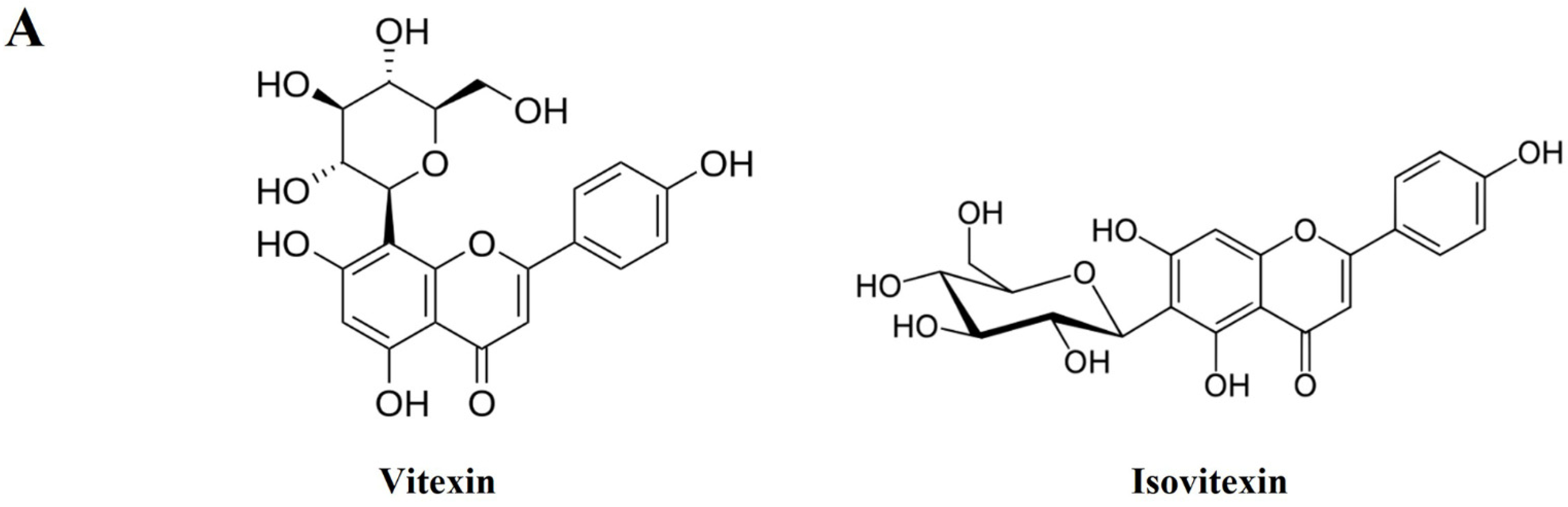
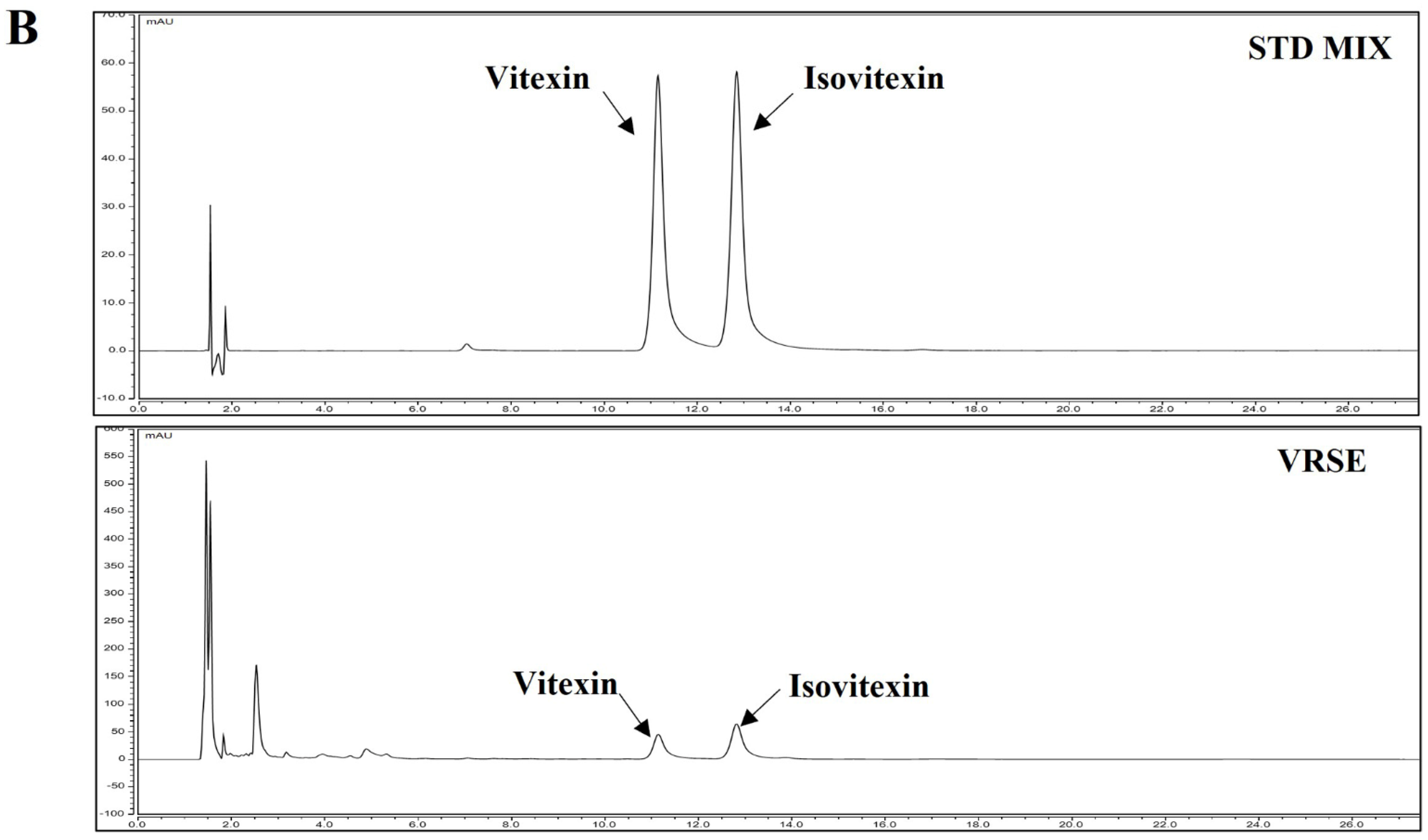
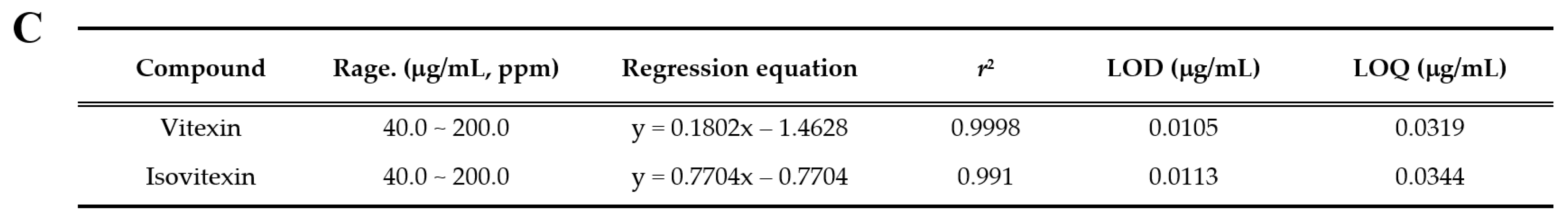
3.8. Identification and Quantitative Analysis of the Chemical Constituents of VRSE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swerdlow, R.H.; Burns, J.M.; Khan, S.M. The Alzheimer’s Disease Mitochondrial Cascade Hypothesis. J. Alzheimers Dis. 2010, 20, S265–S279. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.E.; Zhang, H. Abnormalities in oxidative processes in non-neuronal tissues from patients with Alzheimer’s disease. J. Alzheimers Dis. 2001, 3, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W.; Kim, H.P.; Hoetzel, A.; Park, J.W.; Nakahira, K.; Wang, X.; Choi, A.M.K. Mechanisms of cell death in oxidative stress. Antioxid. Redox Signal. 2007, 9, 49–89. [Google Scholar] [CrossRef] [PubMed]
- Storr, S.J.; Woolston, C.M.; Zhang, Y.M.; Martin, S.G. Redox Environment, Free Radical, and Oxidative DNA Damage. Antioxid. Redox Signal. 2013, 18, 2399–2408. [Google Scholar] [CrossRef] [PubMed]
- Baillet, A.; Chanteperdrix, V.; Trocme, C.; Casez, P.; Garrel, C.; Besson, G. The Role of Oxidative Stress in Amyotrophic Lateral Sclerosis and Parkinson’s Disease. Neurochem. Res. 2010, 35, 1530–1537. [Google Scholar] [CrossRef]
- A Ma, Z. The role of peroxidation of mitochondrial membrane phospholipids in pancreatic β-cell failure. Curr. Diabetes Rev. 2012, 8, 69–75. [Google Scholar] [CrossRef]
- Walker, D.G.; Lue, L.F.; Beach, T.G. Gene expression profiling of amyloid beta peptide-stimulated human post-mortem brain microglia. Neurobiol. Aging 2001, 22, 957–966. [Google Scholar] [CrossRef]
- Glezer, I.; Simard, A.R.; Rivest, S. Neuroprotective role of the innate immune system by microglia. Neuroscience 2007, 147, 867–883. [Google Scholar] [CrossRef]
- Rossler, O.G.; Giehl, K.M.; Thiel, G. Neuroprotection of immortalized hippocampal neurones by brain-derived neurotrophic factor and raf-1 protein kinase: Role of extracellular signal-regulated protein kinase and phosphatidylinositol 3-kinase. J. Neurochem. 2004, 88, 1240–1252. [Google Scholar] [CrossRef]
- Baek, S.Y.; Kim, M.R. Neuroprotective Effect of Carotenoid-Rich Enteromorpha prolifera Extract via TrkB/Akt Pathway against Oxidative Stress in Hippocampal Neuronal Cells. Mar. Drugs 2020, 18, 372. [Google Scholar] [CrossRef]
- Johnson, J.A.; Johnson, D.A.; Kraft, A.D.; Calkins, M.J.; Jakel, R.J.; Vargas, M.R.; Chen, P.C. The Nrf2-ARE pathway: An indicator and modulator of oxidative stress in neurodegeneration. Ann. N. Y. Acad. Sci. 2008, 1147, 61–69. [Google Scholar] [CrossRef]
- Shin, S.K.; Yoo, J.M.; Li, F.Y.; Baek, S.Y.; Kim, M.R. Mulberry fruit improves memory in scopolamine-treated mice: Role of cholinergic function, antioxidant system, and TrkB/Akt signaling. Nutr. Neurosci. 2021, 24, 940–950. [Google Scholar] [CrossRef]
- Ji, J.F.; Ji, S.J.; Sun, R.; Li, K.; Zhang, Y.; Zhang, L.Y.; Tian, Y. Forced running exercise attenuates hippocampal neurogenesis impairment and the neurocognitive deficits induced by whole-brain irradiation via the BDNF-mediated pathway. Biochem. Biophys. Res. Commun. 2014, 443, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Gong, J.; Yoshida, T.; Eberhart, C.G.; Xu, Z.; Kombairaju, P.; Sporn, M.B.; Handa, J.T.; Duh, E.J. Nrf2 has a protective role against neuronal and capillary degeneration in retinal ischemia-reperfusion injury. Free Radic. Biol. Med. 2011, 51, 216–224. [Google Scholar] [CrossRef]
- Cheng, Z.G.; Zhang, G.D.; Shi, P.Q.; Du, B.S. Expression and antioxidation of Nrf2/ARE pathway in traumatic brain injury. Asian Pac. J. Trop. Med. 2013, 6, 305–310. [Google Scholar] [CrossRef]
- Trinh, K.; Andrews, L.; Krause, J.; Hanak, T.; Lee, D.; Gelb, M.; Pallanck, L. Decaffeinated coffee and nicotine-free tobacco provide neuroprotection in Drosophila models of Parkinson’s disease through an NRF2-dependent mechanism. J. Neurosci. 2010, 30, 5525–5532. [Google Scholar] [CrossRef]
- Houghton, C.A.; Fassett, R.G.; Coombes, J.S. Sulforaphane and other nutrigenomic Nrf2 activators: Can the Clinician’s expectation be matched by the reality? Oxid. Med. Cell. Longev. 2016, 2016, 7857186. [Google Scholar] [CrossRef]
- Crunkhorn, S. Deal watch: Abbott boosts investment in NRF2 activators for reducing oxidative stress. Nat. Rev. Drug Discov. 2012, 11, 96. [Google Scholar] [CrossRef]
- Niranjan, R. The role of inflammatory and oxidative stress mechanisms in the pathogenesis of Parkinson’s disease: Focus on astrocytes. Mol. Neurobiol. 2014, 49, 28–38. [Google Scholar] [CrossRef]
- Gilad, E.; Wong, H.R.; Zingarelli, B.; Virág, L.; O’Connor, M.; Salzman, A.L.; Szabó, C. Melatonin inhibits expression of the inducible isoform of nitric oxide synthase in murine macrophages: Role of inhibition of NFkappaB activation. FASEB J. 1998, 12, 685–693. [Google Scholar] [CrossRef]
- Zucoloto, A.Z.; Manchope, M.F.; Staurengo-Ferrari, L.; Pinho-Ribeiro, F.A.; Zarpelon, A.C.; Saraiva, A.L.L.; Cecílio, N.T.; Alves-Filho, J.C.; Cunha, T.M.; Menezes, G.B.; et al. Probucol attenuates lipopolysaccharide-induced leukocyte recruitment and inflammatory hyperalgesia: Effect on NF-кB activation and cytokine production. Eur. J. Pharmacol. 2017, 809, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Karin, M. Mammalian MAP kinase signalling cascades. Nature 2001, 410, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Kim, D.C.; Yoon, C.S.; Ko, W.M.; Lee, S.J.; Sohn, J.H.; Jang, J.H.; Ahn, J.S.; Kim, Y.C.; Oh, H. Anti-neuroinflammatory effects of citreohybridonol involving TLR4-MyD88-mediated inhibition of NF-small ka, CyrillicB and MAPK signaling pathways in lipopolysaccharide-stimulated BV2 cells. Neurochem. Int. 2016, 95, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Gan, R.Y.; Lui, W.Y.; Wu, K.; Chan, C.L.; Dai, S.H.; Sui, Z.Q.; Corke, H. Bioactive compounds and bioactivities of germinated edible seeds and sprouts: An updated review. Trends Food Sci. Technol. 2017, 59, 1–14. [Google Scholar] [CrossRef]
- Liyanage, R.; Kiramage, C.; Visvanathan, R.; Jayathilake, C.; Weththasinghe, P.; Bangamuwage, R.; Chaminda Jayawardana, B.; Vidanarachchi, J. Hypolipidemic and hypoglycemic potential of raw, boiled, and sprouted mung beans (Vigna radiata L. Wilczek) in rats. J. Food Biochem. 2018, 42, e12457. [Google Scholar] [CrossRef]
- Ali, N.M.; Mohd, Y.H.; Yeap, S.K.; Ho, W.Y.; Beh, B.K.; Long, K.; Koh, S.P.; Abdullah, M.P.; Alitheen, N.B. Anti-inflammatory and antinociceptive activities of untreated, germinated, and fermented mung bean aqueous extract. Evid. Based Complement. Altern. Med. 2014, 2014, 350507. [Google Scholar] [CrossRef]
- Gupta, N.; Srivastava, N.; Bhagyawant, S.S. Vicilin—A major storage protein of mungbean exhibits antioxidative potential, antiproliferative effects and ace inhibitory activity. PLoS ONE 2018, 13, e0191265. [Google Scholar] [CrossRef]
- Chai, W.M.; Wei, Q.M.; Deng, W.L.; Zheng, Y.L.; Chen, X.Y.; Huang, Q.; Ou-Yang, C.; Peng, Y.Y. Anti-melanogenesis properties of condensed tannins from Vigna angularis seeds with potent antioxidant and DNA damage protection activities. Food Funct. 2019, 10, 99–111. [Google Scholar] [CrossRef]
- Xie, J.; Du, M.; Shen, M.; Wu, T.; Lin, L. Physico-chemical properties, antioxidant activities and angiotensin-i converting enzyme inhibitory of protein hydrolysates from mung bean (Vigna radiate). Food Chem. 2019, 270, 243–250. [Google Scholar] [CrossRef]
- Lopes, L.A.R.; Martins, M.D.C.C.E.; Farias, L.M.; Brito, A.K.D.S.; Lima, G.M.; Carvalho, V.B.L.; Pereira, C.F.C.; Conde Júnior, A.M.; Saldanha, T.; Arêas, J.A.G.; et al. Cholesterol-lowering and liver-protective effects of cooked and germinated mung beans (Vigna radiata L.). Nutrients 2018, 10, 821. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Kim, T.I.; Oh, Y.C.; Ma, J.Y. Selaginella tamariscina Inhibits Glutamate-Induced Autophagic Cell Death by Activating the PI3K/AKT/mTOR Signaling Pathways. Int. J. Mol. Sci. 2022, 23, 11445. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Oh, Y.C.; Cho, W.K.; Yim, N.H.; Ma, J.Y. Anti-inflammatory effect of Rhapontici Radix ethanol extract via inhibition of NF-κB and MAPK and induction of HO-1 in macrophages. Mediat. Inflamm. 2016, 2016, 7216912. [Google Scholar] [CrossRef]
- Tan, S.; Sagara, Y.; Liu, Y.; Maher, P.; Schubert, D. The regulation of reactive oxygen species production during programmed cell death. J. Cell Biol. 1998, 141, 1423–1432. [Google Scholar] [CrossRef]
- Seiler, M.; Schneider, H.; Forster, S.; Roth, E.K.; Wirth, C.; Culmsee, N.; Plesnila, E.; Kremmer, O.; Radmark, W.; Wurst, G.W.; et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 2008, 8, 237–248. [Google Scholar] [CrossRef]
- Schipper, H.M.; Bennett, D.A.; Liberman, A.; Bienias, J.L.; Schneider, J.A.; Kelly, J.; Arvanitakis, Z. Glial heme oxygenase-1 expression in Alzheimer disease and mild cognitive impairment. Neurobiol. Aging 2006, 27, 252–261. [Google Scholar] [CrossRef]
- Wang, Y.; Santa–Cruz, K.; DeCarli, C.; Johnson, J.A. NAD (P) H: Quinone oxidoreductase activity is increased in hippocampal pyramidal neurons of patients with Alzheimer’s disease. Neurobiol. Aging 2000, 21, 525–531. [Google Scholar] [CrossRef]
- Brown, J.; Wang, H.; Hajishengallis, G.N.; Martin, M. TLR-signaling networks: An integration of adaptor molecules, kinases, and cross-talk. J. Dent. Res. 2011, 90, 417–427. [Google Scholar] [CrossRef]
- Kim, B.W.; Koppula, S.; Hong, S.S.; Jeon, S.B.; Kwon, J.H.; Hwang, B.Y.; Park, E.J.; Choi, D.K. Regulation of microglia activity by glaucocalyxin-A: Attenuation of lipopolysaccharide-stimulated neuroinflammation through NF-κB and p38 MAPK signaling pathways. PLoS ONE 2013, 8, e55792. [Google Scholar] [CrossRef]
- Kim, A.R.; Lee, M.S.; Choi, J.W.; Utsuki, T.; Kim, J.I.; Jang, B.C.; Kim, H.R. Phlorofucofuroeckol A suppresses expression of inducible nitric oxide synthase, cyclooxygenase-2, and proinflammatory cytokines via inhibition of nuclear factor-κB, c-Jun NH2-terminal kinases, and Akt in microglial cells. Inflammation 2013, 36, 259–271. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, B.; Shan, S.; Zhao, X. Neuroprotective effects of vitexin against isoflurane-induced neurotoxicity by targeting the TRPV1 and NR2B signaling pathways. Mol. Med. Rep. 2016, 14, 5607–5613. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, T.; Liu, J.; Gu, L. Vitexin attenuates lipopolysaccharide-induced acute lung injury by controlling the Nrf2 pathway. PLoS ONE 2018, 13, e0196405. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, K.C.; Borges, T.V.; Olescowicz, G.; Ludka, F.K.; Santos, C.A.; Molz, S. Passiflora actinia hydroalcoholic extract and its major constituent, isovitexin, are neuroprotective against glutamate-induced cell damage in mice hippocampal slices. J. Pharm. Pharmacol. 2016, 68, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Yu, Z.; Zheng, Y.; Wang, L.; Qin, X.; Cheng, G.; Ci, X. Isovitexin Exerts Anti-Inflammatory and Anti-Oxidant Activities on Lipopolysaccharide-Induced Acute Lung Injury by Inhibiting MAPK and NF-κB and Activating HO-1/Nrf2 Pathways. Int. J. Biol. Sci. 2016, 12, 72–86. [Google Scholar] [CrossRef] [PubMed]


| Antibody | Corporation | Product No. | RRID | Dilution Rate |
|---|---|---|---|---|
| BAX | Cell Signaling | #2772 | AB_10695870 | 1:1000 |
| Bcl-2 | Cell Signaling | #3498 | AB_1903907 | 1:1000 |
| AIF | Cell Signaling | #4642 | AB_2224542 | 1:1000 |
| C-CAS-3 | Cell Signaling | #9664 | AB_2070042 | 1:1000 |
| CAS-3 | Cell Signaling | #9662 | AB_331439 | 1:1000 |
| C-PARP | Cell Signaling | #9548 | AB_2160592 | 1:1000 |
| PARP | Cell Signaling | #9532 | AB_659884 | 1:1000 |
| β-actin | Cell Signaling | #4970 | AB_2223172 | 1:1000 |
| NF-κB p65 | Cell Signaling | #8242 | AB_10859369 | 1:1000 |
| Lamin B1 | Cell Signaling | #13435 | AB_2737428 | 1:1000 |
| P-ERK | Cell Signaling | #4377 | AB_331775 | 1:1000 |
| ERK | Cell Signaling | #9102 | AB_330744 | 1:1000 |
| P-p38 | Cell Signaling | #9211 | AB_331641 | 1:1000 |
| P38 | Cell Signaling | #9212 | AB_330713 | 1:1000 |
| P-JNK | Cell Signaling | #9251 | AB_331659 | 1:1000 |
| JNK | Cell Signaling | #9252 | AB_2250373 | 1:1000 |
| Nrf-2 | Cell Signaling | #12721 | AB_2715528 | 1:1000 |
| HO-1 | Cell Signaling | #82206 | AB_2799989 | 1:1000 |
| NQO1 | Santa Cruz | #SC-32793 | AB_628036 | 1:1000 |
| GCLC | Thermo Fisher | #PA5-87854 | AB_2804457 | 1:1000 |
| P-TrkB | Cell Signaling | #4621 | AB_916186 | 1:1000 |
| TrkB | Cell Signaling | #4603 | AB_2155125 | 1:1000 |
| P-Akt | Cell Signaling | #4060 | AB_2315049 | 1:1000 |
| Akt | Cell Signaling | #4691 | AB_915783 | 1:1000 |
| P-CREB | Cell Signaling | #9191 | AB_331606 | 1:1000 |
| CREB | Cell Signaling | #9197 | AB_331277 | 1:1000 |
| BDNF | Cell Signaling | #47808 | AB_2894709 | 1:1000 |
| 2nd anti-mouse | Cell Signaling | #7076 | AB_330924 | 1:5000 |
| 2nd anti-rabbit | Cell Signaling | #7074 | AB_2099233 | 1:5000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, Y.H.; Oh, Y.-C.; Kim, T.I.; Ma, J.Y. Neuroprotective and Anti-Neuroinflammatory Properties of Vignae Radiatae Semen in Neuronal HT22 and Microglial BV2 Cell Lines. Nutrients 2022, 14, 5265. https://doi.org/10.3390/nu14245265
Jeong YH, Oh Y-C, Kim TI, Ma JY. Neuroprotective and Anti-Neuroinflammatory Properties of Vignae Radiatae Semen in Neuronal HT22 and Microglial BV2 Cell Lines. Nutrients. 2022; 14(24):5265. https://doi.org/10.3390/nu14245265
Chicago/Turabian StyleJeong, Yun Hee, You-Chang Oh, Tae In Kim, and Jin Yeul Ma. 2022. "Neuroprotective and Anti-Neuroinflammatory Properties of Vignae Radiatae Semen in Neuronal HT22 and Microglial BV2 Cell Lines" Nutrients 14, no. 24: 5265. https://doi.org/10.3390/nu14245265
APA StyleJeong, Y. H., Oh, Y.-C., Kim, T. I., & Ma, J. Y. (2022). Neuroprotective and Anti-Neuroinflammatory Properties of Vignae Radiatae Semen in Neuronal HT22 and Microglial BV2 Cell Lines. Nutrients, 14(24), 5265. https://doi.org/10.3390/nu14245265





