The Role of Partial Enteral Nutrition for Induction of Remission in Crohn’s Disease: A Systematic Review of Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Type of Intervention
2.4. Types of Outcome Measures
- -
- Clinical response or remission: assessed with some index of disease activity such as the Crohn’s Disease Activity Index (CDAI), the Harvey–Bradshaw Index (HBI), the Short Inflammatory Bowel Disease Questionnaire (SIBDQ), and the Lehmann score [5] for adults or the Pediatric Crohn’s Disease Activity Index (PCDAI) or Weighted Pediatric Crohn’s Disease Activity Index (wPCDAI) [28] in pediatric patients.
- -
- Endoscopic remission or mucosal healing: assessed by endoscopic study and using endoscopic scoring indices such as the Crohn’s Disease Endoscopic Index of Severity (CDEIS) or the Simple Endoscopic Score for Crohn’s Disease (SES-CD) [29].
- -
- Mucosal healing: visual or according to the SES-CD index (SES-CD = 0).
- -
- Analytical response of remission: with inflammatory markers such as C-reactive protein (CRP), erythrocyte sedimentation rate (ESR) or fecal calprotectin [16].
- -
- Anthropometric assessment: weight, height, body mass index (BMI).
- -
- Nutritional status and analytical parameters: insulin-like growth factor type 1 (IGF-1), albumin, and prealbumin.
- -
- Adherence and tolerance to treatment.
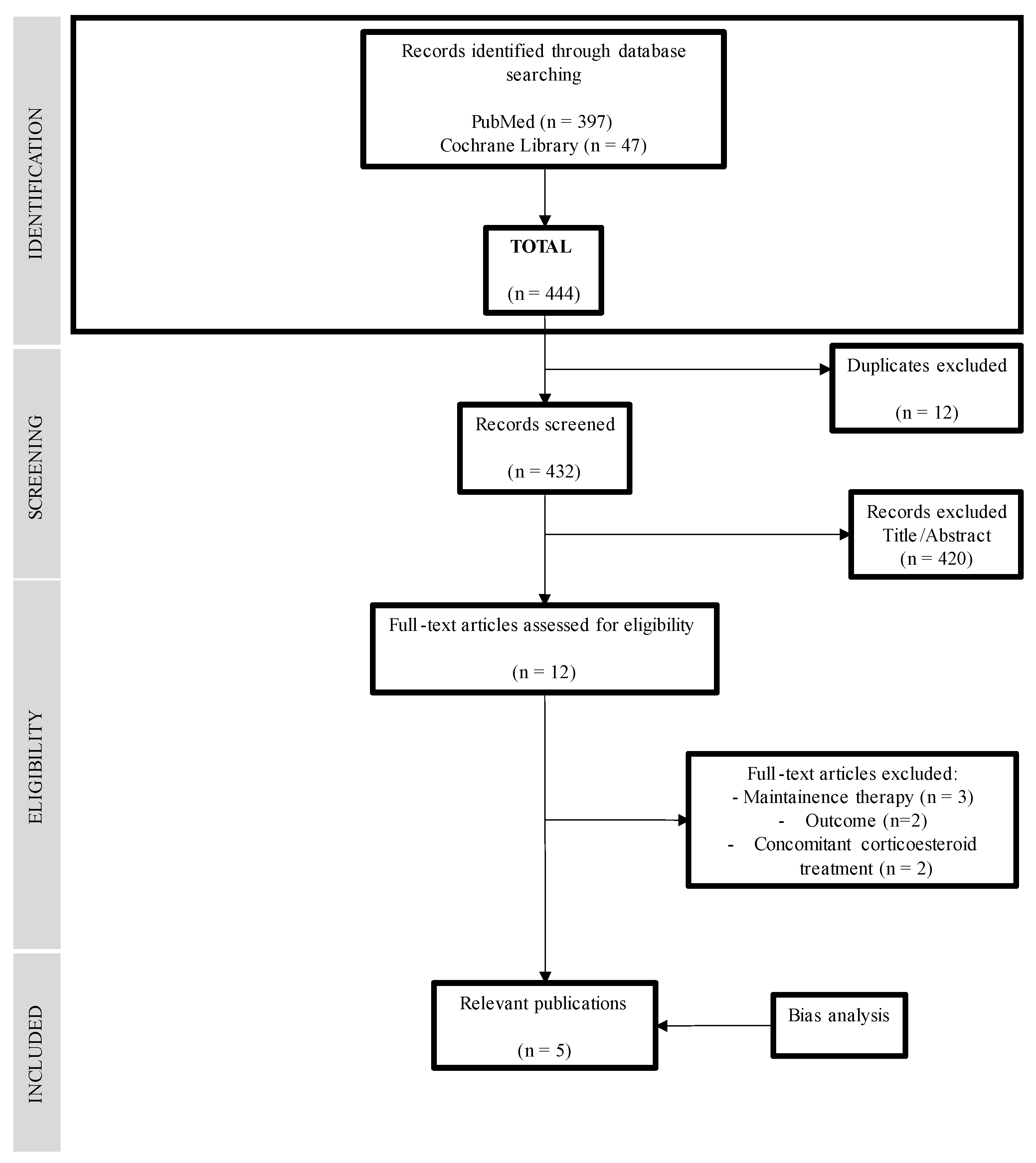
2.5. Selection of Studies
2.6. Data Extraction
2.7. Quality Assessment and Bias Analysis
3. Results
3.1. Risk of Bias
3.2. Study Characteristics and Type of Intervention
3.3. Clinical and Analytical Response
3.4. Fecal Calprotectin and Mucosal Healing
3.5. Tolerance, Adherence and Nutritional Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef] [PubMed]
- Ballester-Ferré, M.P.; Boscá-Watts, M.M.; Mínguez-Pérez, M. Crohn’s disease. Med. Clin. 2018, 151, 26–33. [Google Scholar] [CrossRef]
- Veauthier, B.; Hornecker, J.R. Crohn’s Disease: Diagnosis and Management. Am. Fam. Physician 2018, 98, 661–669. [Google Scholar] [PubMed]
- Torres, J.; Bonovas, S.; Doherty, G.; Kucharzik, T.; Gisbert, J.P.; Raine, T.; Adamina, M.; Armuzzi, M.; Bachmann, O.; Bageret, P.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohns Colitis 2020, 14, 4–22. [Google Scholar] [CrossRef]
- Olén, O.; Askling, J.; Sachs, M.C.; Frumento, P.; Neovius, M.; Smedby, K.E.; Ekbom, A.; Malmborg, P.; Ludvigsson, J.F. Increased Mortality of Patients with Childhood-Onset Inflammatory Bowel Diseases, Compared With the General Population. Gastroenterology 2019, 156, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.; Duerksen, D. Enteral Nutrition in the Management of Pediatric and Adult Crohn’s Disease. Nutrients 2018, 10, 537. [Google Scholar] [CrossRef]
- Herrador-López, M.; Martín-Masot, R.; Navas-López, V.M. EEN Yesterday and Today … CDED Today and Tomorrow. Nutrients 2020, 12, 3793. [Google Scholar] [CrossRef]
- Dziechciarz, P.; Horvath, A.; Shamir, R.; Szajewska, H. Meta-analysis: Enteral nutrition in active Crohn’s disease in children. Aliment. Pharmacol. Ther. 2007, 26, 795–806. [Google Scholar] [CrossRef]
- Narula, N.; Dhillon, A.; Zhang, D.; Sherlock, M.E.; Tondeur, M.; Zachos, M. Enteral nutritional therapy for induction of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2018, 4, CD000542. [Google Scholar] [CrossRef]
- McVeigh, L.; Payne, A. Inducing remission in paediatric Crohn’s disease using nutritional therapies—A systematic review. J. Hum. Nutr. Diet 2020, 33, 170–186. [Google Scholar] [CrossRef]
- Pigneur, B.; Lepage, P.; Mondot, S.; Schmitz, J.; Goulet, O.; Doré, J.; Ruemmele, F.M. Mucosal Healing and Bacterial Composition in Response to Enteral Nutrition Vs Steroid-based Induction Therapy—A Randomised Prospective Clinical Trial in Children With Crohn’s Disease. J. Crohns Colitis 2019, 13, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Turner, D.; Pfeffer-Gik, T.; Amil-Dias, J.; Veres, G.; Shaoul, R.; Staiano, A.; Escher, J.; Kolho, K.L.; Paerregaard, A.; et al. Comparison of outcomes parameters for induction of remission in new onset pediatric Crohn’s disease: Evaluation of the porto IBD group “growth relapse and outcomes with therapy” (GROWTH CD) study. Inflamm. Bowel Dis. 2014, 20, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Werkstetter, K.J.; Schatz, S.B.; Alberer, M.; Filipiak-Pittroff, B.; Koletzko, S. Influence of exclusive enteral nutrition therapy on bone density and geometry in newly diagnosed pediatric Crohn’s disease patients. Ann. Nutr. Metab. 2013, 63, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Verburgt, C.M.; Ghiboub, M.; Benninga, M.A.; de Jonge, W.J.; Van Limbergen, J.E. Nutritional Therapy Strategies in Pediatric Crohn’s Disease. Nutrients 2021, 13, 212. [Google Scholar] [CrossRef]
- Moriczi, M.; Pujol-Muncunill, G.; Martín-Masot, R.; Jiménez-Treviño, S.; Segarra-Cantón, O.; Ochoa-Sangrador, C.; Peña-Quintana, L.; González-Santana, D.; Rodríguez-Martínez, A.; Rosell-Camps, A.; et al. Predictors of Response to Exclusive Enteral Nutrition in Newly Diagnosed Crohn’s Disease in Children: PRESENCE Studyfrom SEGHNP. Nutrients 2020, 12, 1012. [Google Scholar] [CrossRef]
- Van Rheenen, P.F.; Aloi, M.; Assa, A.; Bronsky, J.; Escher, J.C.; Fagerberg, U.L.; Gasparetto, M.; Gerasimidis, K.; Griffiths, A.; Henderson, P.; et al. The Medical Management of Paediatric Crohn’s Disease: An ECCO-ESPGHAN Guideline Update. J. Crohns Colitis 2020, 15, 171–194. [Google Scholar] [CrossRef]
- Miele, E.; Shamir, R.; Aloi, M.; Assa, A.; Braegger, C.; Bronsky, J.; de Ridder, L.; Escher, J.C.; Hojsak, I.; Kolaček, S.; et al. Nutrition in Pediatric Inflammatory Bowel Disease: A Position Paper on Behalf of the Porto Inflammatory Bowel Disease Group of the European Society of Pediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 687–708. [Google Scholar] [CrossRef]
- Wall, C.L.; McCombie, A.; Mulder, R.; Day, A.S.; Gearry, R.B. Adherence to exclusive enteral nutrition by adults with active Crohn’s disease is associated with conscientiousness personality trait: A sub-study. J. Hum. Nutr. Diet 2020, 33, 752–757. [Google Scholar] [CrossRef]
- Rodrigues, A.F.; Johnson, T.; Davies, P.; Murphy, M.S. Does polymeric formula improve adherence to liquid diet therapy in children with active Crohn’s disease? Arch. Dis. Child 2007, 92, 767–770. [Google Scholar] [CrossRef][Green Version]
- Gray, W.N.; Denson, L.A.; Baldassano, R.N.; Hommel, K.A. Treatment adherence in adolescents with inflammatory bowel disease: The collective impact of barriers to adherence and anxiety/depressive symptoms. J. Pediatr. Psychol. 2012, 37, 282–291. [Google Scholar] [CrossRef]
- Pigneur, B.; Ruemmele, F.M. Nutritional interventions for the treatment of IBD: Current evidence and controversies. Ther. Adv. Gastroenterol. 2019, 12, 1756284819890534. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.; Macdonald, S.; Hill, S.M.; Thomas, A.; Murphy, M.S. Treatment of active Crohn’s disease in children using partial enteral nutrition with liquid formula: A randomised controlled trial. Gut 2006, 55, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Sigall-Boneh, R.; Wine, E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut 2018, 67, 1726–1738. [Google Scholar] [CrossRef]
- Sigall-Boneh, R.; Pfeffer-Gik, T.; Segal, I.; Zangen, T.; Boaz, M.; Levine, A. Partial enteral nutrition with a Crohn’s disease exclusion diet is effective for induction of remission in children and young adults with Crohn’s disease. Inflamm. Bowel Dis. 2014, 20, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Svolos, V.; Hansen, R.; Nichols, B.; Quince, C.; Ijaz, U.Z.; Papadopoulou, R.T.; Edwards, C.A.; Watson, D.; Alghamdi, A.; Brejnrod, A.; et al. Treatment of Active Crohn’s Disease With an Ordinary Food-based Diet That Replicates Exclusive Enteral Nutrition. Gastroenterology 2019, 156, 1354–1367. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef]
- Turner, D.; Griffiths, A.M.; Walters, T.D.; Seah, T.; Markowitz, J.; Pfefferkorn, M.; Keljo, D.; Waxman, J.; Otley, A.; LeLeiko, N.S.; et al. Mathematical weighting of the pediatric Crohn’s disease activity index (PCDAI) and comparison with its other short versions. Inflamm. Bowel Dis. 2012, 18, 55–62. [Google Scholar] [CrossRef]
- Khanna, R.; Nelson, S.A.; Feagan, B.G.; D’Haens, G.; Sandborn, W.J.; Zou, G.Y.; MacDonald, J.K.; Parker, C.E.; Jairath, V.; Levesque, B.G. Endoscopic scoring indices for evaluation of disease activity in Crohn’s disease. Cochrane Database Syst. Rev. 2016, 8, CD010642. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Levine, A.; Wine, E.; Assa, A.; Sigall-Boneh, R.; Shaoul, R.; Kori, M.; Cohen, S.; Peleg, S.; Shamaly, H.; Onet, A.; et al. Crohn’s Disease Exclusion Diet Plus Partial Enteral Nutrition Induces Sustained Remission in a Randomized Controlled Trial. Gastroenterology 2019, 157, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Sigall-Boneh, R.; Van Limbergen, J.; Wine, E.; Assa, A.; Shaoul, R.; Milman, P.; Cohen, S.; Kori, M.; Peleg, S.; On, A.; et al. Dietary Therapies Induce Rapid Response and Remission in Pediatric Patients With Active Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2021, 19, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H.; Levine, A.; Hirsch, A.; Boneh, R.S.; Kopylov, U.; Eran, H.B.; Cohen, N.A.; Ron, Y.; Goren, I.; Leibovitzh, H.; et al. The Crohn’s disease exclusion diet for induction and maintenance of remission in adults with mild-to-moderate Crohn’s disease (CDED-AD): An open-label, pilot, randomised trial. Lancet Gastroenterol. Hepatol. 2022, 7, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Urlep, D.; Benedik, E.; Brecelj, J.; Orel, R. Partial enteral nutrition induces clinical and endoscopic remission in active pediatric Crohn’s disease: Results of a prospective cohort study. Eur. J. Pediatr. 2020, 179, 431–438. [Google Scholar] [CrossRef]
- Wall, C.L.; Gearry, R.B.; Day, A.S. Treatment of Active Crohn’s Disease with Exclusive and Partial Enteral Nutrition: A Pilot Study in Adults. Inflamm. Intest. Dis. 2018, 2, 219–227. [Google Scholar] [CrossRef]
- Kang, Y.; Kim, S.; Kim, S.Y.; Koh, H. Effect of short-term partial enteral nutrition on the treatment of younger patients with severe Crohn’s disease. Gut Liver 2015, 9, 87–93. [Google Scholar] [CrossRef][Green Version]
- Sökülmez, P.; Demirbağ, A.E.; Arslan, P.; Dişibeyaz, S. Effects of enteral nutritional support on malnourished patients with inflammatory bowel disease by subjective global assessment. Turk. J. Gastroenterol. 2014, 25, 493–507. [Google Scholar] [CrossRef]
- Lev-Tzion, R.; Ben-Moshe, T.; Abitbol, G.; Ledder, O.; Peleg, S.; Millman, P.; Shaoul, R.; Kori, M.; Assa, A.; Cohen, S.; et al. The Effect of Nutritional Therapy on Bone Mineral Density and Bone Metabolism in Pediatric Crohn Disease. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 877–882. [Google Scholar] [CrossRef]
- Marques, J.G.; Shokry, E.; Frivolt, K.; Werkstetter, K.J.; Brückner, A.; Schwerd, T.; Koletzko, S.; Koletzko, B. Metabolomic Signatures in Pediatric Crohn’s Disease Patients with Mild or Quiescent Disease Treated with Partial Enteral Nutrition: A Feasibility Study. SLAS Technol. 2021, 26, 165–177. [Google Scholar] [CrossRef]
- Brückner, A.; Werkstetter, K.J.; Frivolt, K.; Shokry, E.; Ahmed, M.; Metwaly, A.; Marques, J.G.; Uhl, O.; Krohn, K.; Hajji, M.; et al. Partial enteral nutrition has no benefit on bone health but improves growth in paediatric patients with quiescent or mild Crohn’s disease. Clin. Nutr. 2020, 39, 3786–3796. [Google Scholar] [CrossRef]
- Yamamoto, T.; Shiraki, M.; Nakahigashi, M.; Umegae, S.; Matsumoto, K. Enteral nutrition to suppress postoperative Crohn’s disease recurrence: A five-year prospective cohort study. Int. J. Color. Dis. 2013, 28, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Noble, A.; Kachelries, K.E.; Albenberg, L.; Kelsen, J.R.; Grossman, A.B.; Baldassano, R.N. A novel enteral nutrition protocol for the treatment of pediatric Crohn’s disease. Inflamm. Bowel Dis. 2013, 19, 1374–1378. [Google Scholar] [CrossRef] [PubMed]
- Szczubełek, M.; Pomorska, K.; Korólczyk-Kowalczyk, M.; Lewandowski, K.; Kaniewska, M.; Rydzewska, G. Effectiveness of Crohn’s Disease Exclusion Diet for Induction of Remission in Crohn’s Disease Adult Patients. Nutrients 2021, 13, 4112. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Wine, E. Effects of enteral nutrition on Crohn’s disease: Clues to the impact of diet on disease pathogenesis. Inflamm. Bowel Dis. 2013, 19, 1322–1329. [Google Scholar] [CrossRef]
- Ghiboub, M.; Penny, S.; Verburgt, C.M.; Boneh, R.S.; Wine, E.; Cohen, A.; Dunn, K.A.; Pinto, D.M.; Benninga, M.A.; de Jonge, W.J.; et al. Metabolome Changes with Diet-Induced Remission in Pediatric Crohn’s Disease. Gastroenterology 2022, 163, 922–936. [Google Scholar] [CrossRef] [PubMed]
- Niseteo, T.; Sila, S.; Trivić, I.; Mišak, Z.; Kolaček, S.; Hojsak, I. Modified Crohn’s disease exclusion diet is equally effective as exclusive enteral nutrition: Real-world data. Nutr. Clin. Pract. 2022, 37, 435–441. [Google Scholar] [CrossRef]
- Logan, M.; Clark, C.M.; Ijaz, U.Z.; Gervais, L.; Duncan, H.; Garrick, V.; Curtis, L.; Buchanan, E.; Cardigan, T.; Armstrong, L.; et al. The reduction of fecal calprotectin during exclusive enteral nutrition is lost rapidly after food re-introduction. Aliment. Pharmacol. Ther. 2019, 50, 664–674. [Google Scholar] [CrossRef]
- Lee, D.; Baldassano, R.N.; Otley, A.R.; Albenberg, L.; Griffiths, A.M.; Compher, C.; Chen, E.Z.; Li, H.; Gilroy, E.; Nessel, L.; et al. Comparative Effectiveness of Nutritional and Biological Therapy in North American Children with Active Crohn’s Disease. Inflamm. Bowel Dis. 2015, 21, 1786–1793. [Google Scholar] [CrossRef]
- Frivolt, K.; Schwerd, T.; Werkstetter, K.J.; Schwarzer, A.; Schatz, S.B.; Bufler, P.; Koletzko, S. Repeated exclusive enteral nutrition in the treatment of paediatric Crohn’s disease: Predictors of efficacy and outcome. Aliment. Pharmacol. Ther. 2014, 39, 1398–1407. [Google Scholar] [CrossRef]
- Ricciuto, A.; Aardoom, M.; Orlanski-Meyer, E.; Navon, D.; Carman, N.; Aloi, M.; Bronsky, J.; Däbritz, J.; Dubinsky, M.; Hussey, S.; et al. Predicting Outcomes in Pediatric Crohn’s Disease for Management Optimization: Systematic Review and Consensus Statements from the Pediatric Inflammatory Bowel Disease—Ahead Program. Gastroenterology 2021, 160, 403–436. [Google Scholar] [CrossRef]
- Sabino, J.; Lewis, J.D.; Colombel, J.F. Treating Inflammatory Bowel Disease with Diet: A Taste Test. Gastroenterology 2019, 157, 295–297. [Google Scholar] [CrossRef] [PubMed]
| Inclusion Criteria | |
|---|---|
| Population | Patients of any age with a diagnosis of active CD, as defined by an index of clinical disease activity. |
| Intervention | The administration of PEN as the main therapy for induction of remission of active CD. |
| Comparison | EEN or any other control group. |
| Outcome |
|
| Reference (Year, Country) | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| A. Levine et al., 2019 [31] Canada, Israel |
|
|
| D. Urlep et al., 2019 [34] Slovenia |
|
|
| C. Wall et al., 2018 [35] New Zeland |
|
|
| R. Sigall-Boneh et al., 2020 [32] Israel, Canada |
|
|
| Yanai H. et al., 2022 [33] Israel |
|
|
| Reference (Year, Country) | Population n (Age) | Intervention (Total Weeks) | Formula Employed | Diet | Gender 1 and Age 2 | Location 1 (Paris) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| A. Levine et al., 2019 [31] Canada, Israel | 78 (4–18 years) |
| Modulen® | CDED | EEN group (n = 40) | PEN group (n = 34) | EEN group (n = 40) | PEN group (n = 34) | ||
| Female, n (%) | 14 (41%) | 14 (35%) | ||||||||
| L1 | 14 (41%) | 18 (41%) | ||||||||
| Age, (years) | 14.5 ± 2.6 | 13.8 ±2.8 | L2 | 1 (2.9%) | 2 (5%) | |||||
| L3 | 15 (34%) | 19 (47%) | ||||||||
| L4a | 13 (38%) | 14 (35%) | ||||||||
| L4b | 3(8.8%) | 2 (5%) | ||||||||
| D. Urlep et al., 2019 [34] Slovenia | 22 (<25 years) |
| Alicam® (Nutricia) | AID-CD | EEN group (n = 11) | PEN group (n = 11) | EEN group (n = 11) | PEN group (n = 11) | ||
| Female, n (%) | 8 (73%) | 5 (46%) | L1 | 0 | 1 (9%) | |||||
| L2 | 4 (36%) | 4 (36%) | ||||||||
| Age, (years) | 13.8 (3.6–18) | 13.4 (9.8–17.9) | L3 | 7 (64%) | 6 (55%) | |||||
| L4a | 9 (82%) | 10 (91%) | ||||||||
| L4b | 1 (9%) | 3 (27%) | ||||||||
| L4ab | 1 (9%) | 2 (27%) | ||||||||
| C. Wall et al., 2018 [35] New Zealand | 38 (16–40 years) |
| Ensure Plus® | Free diet | EEN group (n = 25) | PEN group (n = 13) | EEN group (n = 25) | PEN group (n = 13) | ||
| Female, n (%) | 18 (72%) | 12 (92%) | L1 | 12 (48%) | 9 (69%) | |||||
| L3 | 13 (52%) | 4 (31%) | ||||||||
| Age, (years) | 23.3 (15.8 to 38.4) | 19.2 (16.5–38.2) | L4 | 3 (12%) | 3 (23%) | |||||
| P | 2 (8%) | 1 (8%) | ||||||||
| R. Sigall-Boneh et al., 2020 [32] Israel, Canada | 78 (4–18 years) |
| Modulén® | CDED | EEN group (n = 34) | PEN group (n = 40) | EEN group (n = 34) | PEN group (n = 40) | ||
| Female, n (%) | 14 (41%) | 14 (35%) | L1 | 14 (41%) | 18 (41%) | |||||
| L2 | 1 (3%) | 2 (5%) | ||||||||
| Age, (years) | 14.5 ± 2.6 | 13.8 ±2.8 | L3 | 15 (34%) | 19 (47%) | |||||
| L4a | 13 (38%) | 14 (35%) | ||||||||
| L4b | 3 (8.8%) | 2 (5%) | ||||||||
| Yanai H. et al., 2022 [33] Israel | 91 (18–55) |
| Modulén® | CDED | CDED group (n = 21) | PEN group (n = 19) | * | CDED group (n = 21) | PEN group (n = 19) | |
| Female, n (%) | 13 (62%) | 9 (47%) | L1 | 16 (84%) | 19 (90%) | |||||
| L3 | 3 (16%) | 2 (10%) | ||||||||
| Age, (years) | 26 (33 to 38) | 34 (25 to 39) | L4 | 0 | 2 (10%) | |||||
| Reference (Year, Country) | Clinical Response 1 | Clinical Remission (Week 6) 1 | Sustained Remission (Week 12) 1 | Endoscopic Remission 1 | Analitical Response (CRP Improvement Week 6) 2 | Calprotectin Decreased (Week 6) 3 | Compliance/ Tolerance 1 |
|---|---|---|---|---|---|---|---|
| A. Levine et al., 2019 [31] Canada, Israel | (PCDAI < 10) | - | |||||
| PEN: 34/40 (85%) EEN: 29/34 (85.3%) | PEN: 30/40 (75%) EEN: 20/34 (58.8%) | PEN: 28/37 (76%) EEN: 14/31 (45%) | PEN: 23.6 mg/dL→5 mg/L EEN: 24 mg/dL→4.1 mg/dL | PEN: −1473 mcg/g (47%) EEN: −948 mcg/g (35.8%) | PEN: 39/40 (98%) EEN: 28/38 (73%) | ||
| D. Urlep et al., 2019 [34] Slovenia | - | (SES-CD ≤ 2, at week 6) | |||||
| PEN: 11/11 (100%) EEN: 10/11 (90.9%) | (PCDAI < 10) | PEN 5/11 (45.5%) EEN 5/11 (45.5%) | PEN 16.5→8.8 mg/dL EEN 18.4→7.9 mg/dL | PEN: −288.3 (67%) EEN: −174.2 (45.7%) | PEN 11/12 (91%) EEN 11/13 (85%) | ||
| C. Wall et al., 2018 [35] New Zealand | - | - | - | - | - | - | PEN 9/11 (81%) EEN: 14/21 (67%) |
| Yanai H. et al., 2021 [33] Israel | (HBI < 5) | SES-CD ≤ 3, at week 24) | |||||
| PEN 14/19 (74%) CDED 14/19 (67%) | PEN 13/19 (68%) CDED 12/21(57%) | PEN 12/19 (63%) CDED 10/21 (48%) | PEN 8/13 (42%) CDED 6/13 (29%) | PEN 15.8 mg/dL→8.8 mg/dL CDED 12.1 mg/dL→8.2 mg/dL | PEN: +39 mcg/g CDED: −78.5 mcg/g | PEN 16/19 (84%) CDED 13/21 (62%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Torres, L.; Moreno-Álvarez, A.; Fernández-Lorenzo, A.E.; Leis, R.; Solar-Boga, A. The Role of Partial Enteral Nutrition for Induction of Remission in Crohn’s Disease: A Systematic Review of Controlled Trials. Nutrients 2022, 14, 5263. https://doi.org/10.3390/nu14245263
González-Torres L, Moreno-Álvarez A, Fernández-Lorenzo AE, Leis R, Solar-Boga A. The Role of Partial Enteral Nutrition for Induction of Remission in Crohn’s Disease: A Systematic Review of Controlled Trials. Nutrients. 2022; 14(24):5263. https://doi.org/10.3390/nu14245263
Chicago/Turabian StyleGonzález-Torres, Lucía, Ana Moreno-Álvarez, Ana Estefanía Fernández-Lorenzo, Rosaura Leis, and Alfonso Solar-Boga. 2022. "The Role of Partial Enteral Nutrition for Induction of Remission in Crohn’s Disease: A Systematic Review of Controlled Trials" Nutrients 14, no. 24: 5263. https://doi.org/10.3390/nu14245263
APA StyleGonzález-Torres, L., Moreno-Álvarez, A., Fernández-Lorenzo, A. E., Leis, R., & Solar-Boga, A. (2022). The Role of Partial Enteral Nutrition for Induction of Remission in Crohn’s Disease: A Systematic Review of Controlled Trials. Nutrients, 14(24), 5263. https://doi.org/10.3390/nu14245263








