A Magtein®, Magnesium L-Threonate, -Based Formula Improves Brain Cognitive Functions in Healthy Chinese Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Subject Selection
2.2. Testing Methods
2.3. Data Analysis
2.4. Instruments
2.5. Safety Parameters
3. Results
3.1. Subject Characteristics
3.2. Safety Parameters between Experimental Group and Control Group
3.3. Cognitive Parameter between Experimental Group and Control Group
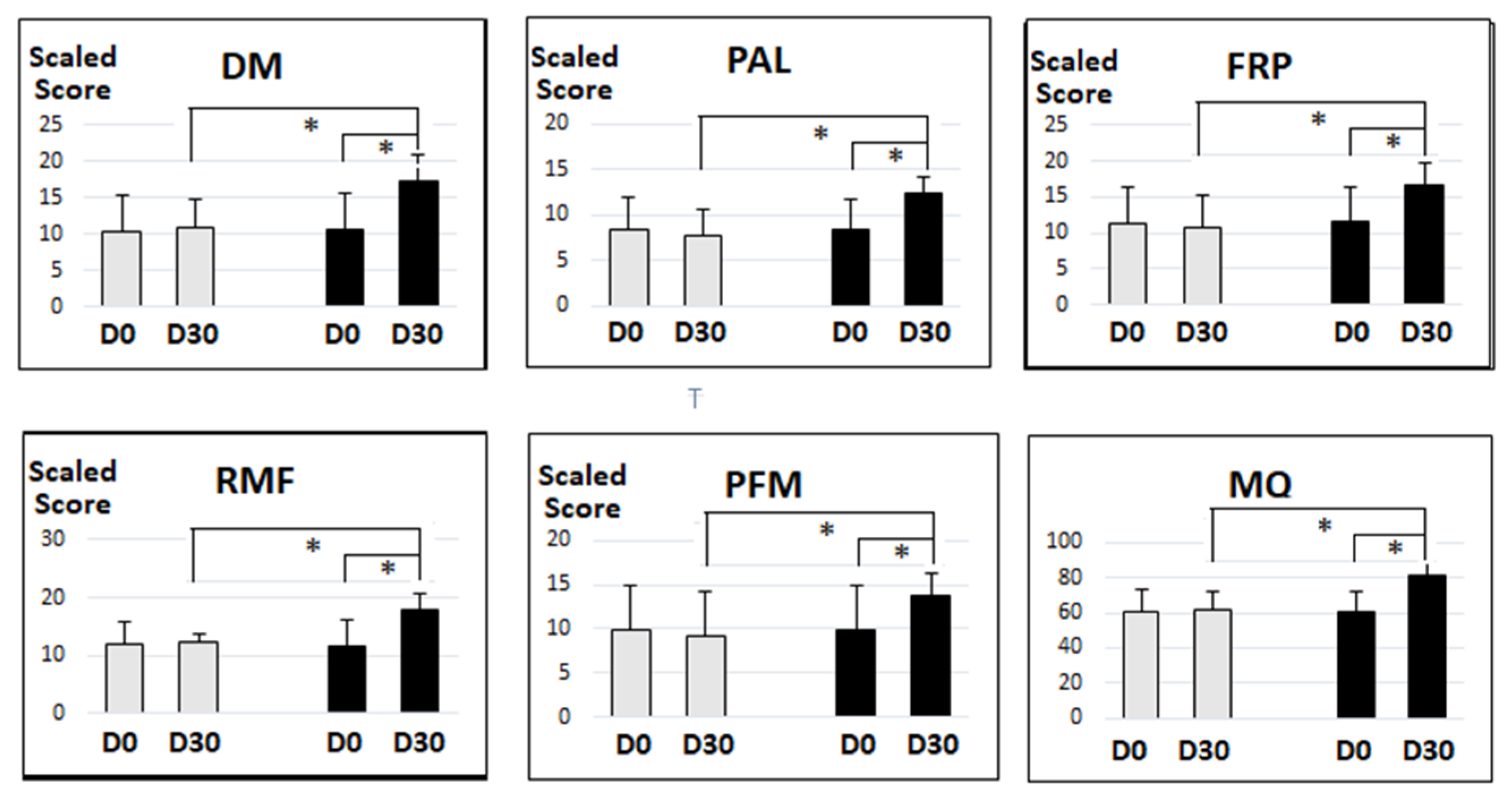
3.3.1. Between-Group Analysis
3.3.2. Within Group Analysis
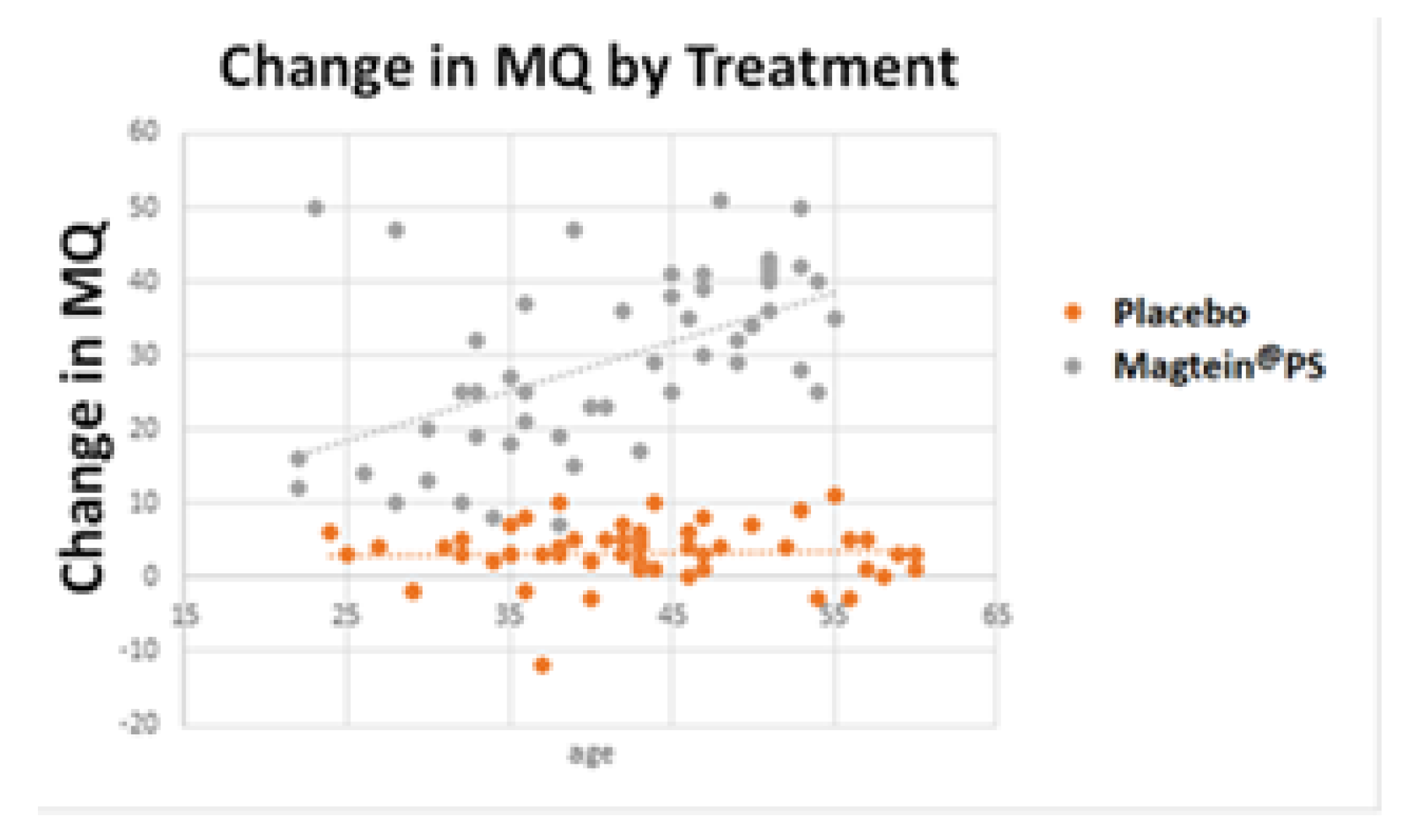
3.4. Benefit of Magtein®PS on the Cognitive Function Increases with Age
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Medeiros, D.M.; Wildman, R.E. Advanced Human Nutrition; Jones & Bartlett Learning: Burlington, MA, USA, 2018. [Google Scholar]
- Fardet, A.; Aubrun, K.; Rock, E. Nutrition transition and chronic diseases in China (1990–2019): Industrially processed and animal calories rather than nutrients and total calories as potential determinants of the health impact. Public Health Nutr. 2021, 24, 5561–5575. [Google Scholar] [CrossRef]
- Xu, M.; Cai, J.; Mo, X.; Liu, Q.; Zhang, J.; Wei, Y.; Liu, S.; Lin, Y.; Huang, S.; Mo, C.; et al. Association of Dietary and Plasma Magnesium with Glycaemic Markers in a Chinese Population. Biol. Trace Elem. Res. 2022. [Google Scholar] [CrossRef]
- Volpe, S.L. Magnesium in disease prevention and overall health. Adv. Nutr. 2013, 4, 378s–383s. [Google Scholar] [CrossRef]
- Boyle, N.B.; Lawton, C.L.; Dye, L. The effects of magnesium supplementation on subjective anxiety. Magnes. Res. 2016, 29, 120–125. [Google Scholar] [CrossRef]
- Derom, M.L.; Sayón-Orea, C.; Martínez-Ortega, J.M.; Martínez-González, M.A. Magnesium and depression: A systematic review. Nutr. Neurosci. 2013, 16, 191–206. [Google Scholar] [CrossRef]
- Grases, G.; Pérez-Castelló, J.A.; Sanchis, P.; Casero, A.; Perelló, J.; Isern, B.; Rigo, E.; Grases, F. Anxiety and stress among science students. Study of calcium and magnesium alterations. Magnes. Res. 2006, 19, 102–106. [Google Scholar]
- Tao, M.H.; Liu, J.; Cervantes, D. Association between magnesium intake and cognition in US older adults: National Health and Nutrition Examination Survey (NHANES) 2011 to 2014. Alzheimer’s Dement. 2022, 8, e12250. [Google Scholar] [CrossRef]
- McKee, J.A.; Brewer, R.P.; Macy, G.E.; Phillips-Bute, B.; Campbell, K.A.; Borel, C.O.; Reynolds, J.D.; Warner, D.S. Analysis of the brain bioavailability of peripherally administered magnesium sulfate: A study in humans with acute brain injury undergoing prolonged induced hypermagnesemia. Crit. Care Med. 2005, 33, 661–666. [Google Scholar] [CrossRef]
- Fuchs-Buder, T.; Tramèr, M.R.; Tassonyi, E. Cerebrospinal fluid passage of intravenous magnesium sulfate in neurosurgical patients. J. Neurosurg. Anesthesiol. 1997, 9, 324–328. [Google Scholar] [CrossRef]
- Slutsky, I.; Abumaria, N.; Wu, L.J.; Huang, C.; Zhang, L.; Li, B.; Zhao, X.; Govindarajan, A.; Zhao, M.G.; Zhuo, M.; et al. Enhancement of learning and memory by elevating brain magnesium. Neuron 2010, 65, 165–177. [Google Scholar] [CrossRef]
- Sadir, S.; Tabassum, S.; Emad, S.; Liaquat, L.; Batool, Z.; Madiha, S.; Shehzad, S.; Sajid, I.; Haider, S. Neurobehavioral and biochemical effects of magnesium chloride (MgCl2), magnesium sulphate (MgSO4) and magnesium-L-threonate (MgT) supplementation in rats: A dose dependent comparative study. Pak. J. Pharm. Sci. 2019, 32, 277–283. [Google Scholar] [PubMed]
- Sun, Q.; Weinger, J.G.; Mao, F.; Liu, G. Regulation of structural and functional synapse density by L-threonate through modulation of intraneuronal magnesium concentration. Neuropharmacology 2016, 108, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Fay, M.J.; Verlangieri, A.J. Stimulatory action of calcium L-threonate on ascorbic acid uptake by a human T-lymphoma cell line. Life Sci. 1991, 49, 1377–1381. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, J.C.; Butler, J.A.; Marsh, A.M.; Ross, C.A.; Norris, J.M. Rapid mass spectrometric analysis for ascorbate and related organic acids in small volumes of plasma for use in pediatric subjects. J. Chromatogr. B Biomed. Sci. Appl. 1999, 726, 79–84. [Google Scholar] [CrossRef]
- Harding, J.J.; Hassett, P.C.; Rixon, K.C.; Bron, A.J.; Harvey, D.J. Sugars including erythronic and threonic acids in human aqueous humour. Curr. Eye Res. 1999, 19, 131–136. [Google Scholar] [CrossRef]
- Thompson, J.A.; Markey, S.P.; Fennessey, P.V. Gas-chromatographic/mass-spectrometric identification and quantitation of tetronic and deoxytetronic acids in urine from normal adults and neonates. Clin. Chem. 1975, 21, 1892–1898. [Google Scholar] [CrossRef]
- Lawson, A.M.; Chalmers, R.A.; Watts, R.W. Urinary organic acids in man. I. Normal patterns. Clin. Chem. 1976, 22, 1283–1287. [Google Scholar] [CrossRef]
- Azzam, M.M.; Zou, X.T.; Dong, X.Y.; Xie, P. Effect of supplemental L-threonine on mucin 2 gene expression and intestine mucosal immune and digestive enzymes activities of laying hens in environments with high temperature and humidity. Poult. Sci. 2011, 90, 2251–2256. [Google Scholar] [CrossRef]
- Liu, G.; Weinger, J.G.; Lu, Z.L.; Xue, F.; Sadeghpour, S. Efficacy and Safety of MMFS-01, a Synapse Density Enhancer, for Treating Cognitive Impairment in Older Adults: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Alzheimer’s Dis. JAD 2016, 49, 971–990. [Google Scholar] [CrossRef]
- ter Borg, S.; Verlaan, S.; Hemsworth, J.; Mijnarends, D.M.; Schols, J.M.; Luiking, Y.C.; de Groot, L.C. Micronutrient intakes and potential inadequacies of community-dwelling older adults: A systematic review. Br. J. Nutr. 2015, 113, 1195–1206. [Google Scholar] [CrossRef]
- Glade, M.J.; Smith, K. Phosphatidylserine and the human brain. Nutrition 2015, 31, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Turnlund, J.R.; Betschart, A.A.; Liebman, M.; Kretsch, M.J.; Sauberlich, H.E. Vitamin B-6 depletion followed by repletion with animal- or plant-source diets and calcium and magnesium metabolism in young women. Am. J. Clin. Nutr. 1992, 56, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Guillard, O.; Piriou, A.; Fauconneau, B.; Mauco, G.; Mettey, R. Unexpected toxicity induced by magnesium orotate treatment in congenital hypomagnesemia. J. Intern. Med. 2002, 252, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Spasov, A.A.; Petrov, V.I.; Iezhitsa, I.N.; Kravchenko, M.S.; Kharitonova, M.V.; Ozerov, A.A. Comparative study of magnesium salts bioavailability in rats fed a magnesium-deficient diet. Vestn. Ross. Akad. Meditsinskikh Nauk 2010, 29–37. [Google Scholar]
- Wroolie, T.E.; Chen, K.; Watson, K.T.; Iagaru, A.; Sonni, I.; Snyder, N.; Lee, W.; Reiman, E.M.; Rasgon, N.L. An 8-week open label trial of l-Threonic Acid Magnesium Salt in patients with mild to moderate dementia. Pers. Med. Psychiatry 2017, 4, 7–12. [Google Scholar] [CrossRef][Green Version]
- Ritchie, G.; Kerstan, D.; Dai, L.-J.; Kang, H.S.; Canaff, L.; Hendy, G.N.; Quamme, G.A. 1,25(OH)2D3 stimulates Mg2+ uptake into MDCT cells: Modulation by extracellular Ca2+ and Mg2+. Am. J. Physiol.-Ren. Physiol. 2001, 280, F868–F878. [Google Scholar] [CrossRef]
- Cascella, M.; Vaqar, S. Hypermagnesemia. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Chai, B.; Gao, F.; Wu, R.; Dong, T.; Gu, C.; Lin, Q.; Zhang, Y. Vitamin D deficiency as a risk factor for dementia and Alzheimer’s disease: An updated meta-analysis. BMC Neurol. 2019, 19, 284. [Google Scholar] [CrossRef]
- Jayedi, A.; Rashidy-Pour, A.; Shab-Bidar, S. Vitamin D status and risk of dementia and Alzheimer’s disease: A meta-analysis of dose-response†. Nutr. Neurosci. 2019, 22, 750–759. [Google Scholar] [CrossRef]
- Eby, G.A., 3rd; Eby, K.L. Magnesium for treatment-resistant depression: A review and hypothesis. Med. Hypotheses 2010, 74, 649–660. [Google Scholar] [CrossRef]
- Hynd, M.R.; Scott, H.L.; Dodd, P.R. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer’s disease. Neurochem. Int. 2004, 45, 583–595. [Google Scholar] [CrossRef]
- Yu, X.; Guan, P.P.; Zhu, D.; Liang, Y.Y.; Wang, T.; Wang, Z.Y.; Wang, P. Magnesium Ions Inhibit the Expression of Tumor Necrosis Factor α and the Activity of γ-Secretase in a β-Amyloid Protein-Dependent Mechanism in APP/PS1 Transgenic Mice. Front. Mol. Neurosci. 2018, 11, 172. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Pizzol, D.; Smith, L.; Dominguez, L.J.; Barbagallo, M. Effect of Magnesium Supplementation on Inflammatory Parameters: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 679. [Google Scholar] [CrossRef] [PubMed]
- Picón-Pagès, P.; Garcia-Buendia, J.; Muñoz, F.J. Functions and dysfunctions of nitric oxide in brain. Biochim. Biophys. Acta. Mol. Basis Dis. 2019, 1865, 1949–1967. [Google Scholar] [CrossRef] [PubMed]
| Index | Experimental Group | Control Group | p * |
|---|---|---|---|
| MQ | 60.31 ± 11.35 | 60.75 ± 12.31 | 0.854 |
| Age (years) | 41.04 ± 9.41 | 42.47 ± 9.40 | 0.444 |
| Male/Female | 24/27 | 19/32 | 0.423 |
| Experimental Group | Control Group | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before Clinical Trial | After Clinical Trial | Before Clinical Trial | After Clinical Trial | |||||||||||
| Good | Normal | Bad | Good | Normal | Bad | p1 | Good | Normal | Bad | Good | Normal | Bad | p1 | |
| Mental condition | 50 | 1 | 0 | 51 | 0 | 0 | 1.000 | 51 | 0 | 0 | 51 | 0 | 0 | 1.000 |
| Sleep condition | 50 | 1 | 0 | 51 | 0 | 0 | 1.000 | 51 | 0 | 0 | 51 | 0 | 0 | 1.000 |
| Appetite | 50 | 1 | 0 | 51 | 0 | 0 | 1.000 | 51 | 0 | 0 | 51 | 0 | 0 | 1.000 |
| Exercise | 50 | 1 | 0 | 51 | 0 | 0 | 1.000 | 51 | 0 | 0 | 51 | 0 | 0 | 1.000 |
| Systolic blood pressure (mmHg) | 120.00 ± 8.60 | 119.31 ± 7.36 | 0.184 | 119.65 ± 7.40 | 119.65 ± 7.40 | 0.124 | ||||||||
| Diastolic blood pressure (mmHg) | 76.24 ± 3.94 | 76.25 ± 3.82 | 0.957 | 76.55 ± 3.60 | 76.55 ± 3.60 | 0.203 | ||||||||
| Heart rate | 69.39 ± 9.69 | 70.37 ± 7.56 | 0.070 | 71.20 ± 9.87 | 70.82 ± 9.87 | 0.583 | ||||||||
| Index | Before Clinical Trial | After Clinical Trial | ||||
|---|---|---|---|---|---|---|
| Experimental Group | Control Group | p | Experimental Group | Control Group | p | |
| Leukocyte (109/L) | 6.15 ± 1.73 | 5.90 ± 1.17 | 0.389 | 6.25 ± 1.42 | 5.87 ± 1.46 | 0.180 |
| RBC (1012/L) | 4.56 ± 0.6 | 4.38 ± 0.44 | 0.084 | 4.62 ± 0.53 | 4.46 ± 0.46 | 0.110 |
| Platelet (109/L) | 176.2 ± 48.65 | 176.2 ± 48.21 | 1.000 | 188.61 ± 50.6 | 200.22 ± 46.34 | 0.230 |
| Hemoglobin (g/L) | 136.27 ± 17.79 | 130.9 ± 17.93 | 0.132 | 135.94 ± 16.57 | 130.59 ± 19.36 | 0.137 |
| Total protein (g/L) | 71.70 ± 3.82 | 71.05 ± 3.09 | 0.352 | 74.54 ± 5.38 | 75.35 ± 3.78 | 0.384 |
| Albumin (U/L) | 46.38 ± 2.46 | 45.39 ± 2.29 | 0.067 | 48.15 ± 4.67 | 48.51 ± 2.53 | 0.627 |
| Alanine Aminotransferase (U/L) | 20.68 ± 15.00 | 23.7 ± 25.11 | 0.464 | 20.1 ± 12.92 | 24.00 ± 27.38 | 0.359 |
| Aspartate transaminase (U/L) | 19.39 ± 5.61 | 20.2 ± 12.23 | 0.671 | 19.04 ± 4.97 | 20.88 ± 12.66 | 0.335 |
| Urea (mmol/L) | 5.6 ± 1.6 | 5.31 ± 1.32 | 0.325 | 5.03 ± 1.36 | 4.90 ± 1.38 | 0.624 |
| Creatinine (umol/L) | 62.73 ± 11.16 | 59.61 ± 14.62 | 0.229 | 72.78 ± 13.80 | 68.8 ± 15.9 | 0.180 |
| Blood sugar (mmol/L) | 5.68 ± 0.5 | 6.08 ± 1.68 | 0.101 | 4.72 ± 0.57 | 5.17 ± 1.23 | 0.071 |
| Cholesterol (mmol/L) | 5.37 ± 0.69 | 5.18 ± 0.88 | 0.213 | 4.67 ± 0.85 | 4.53 ± 0.88 | 0.408 |
| Triglyceride (mmol/L) | 1.55 ± 1.83 | 1.31 ± 0.71 | 0.385 | 1.61 ± 1.45 | 1.63 ± 1.85 | 0.953 |
| Urine test | Normal | Normal | Normal | Normal | ||
| CMT Item | Period | Magtein® PS | Placebo | p-Value b | ||
|---|---|---|---|---|---|---|
| Mcan ± SD | p-Value a | Mcan ± SD | p-Value a | |||
| DM | Day 0 | 10.69 ± 4.98 | <0.001 | 10.47 ± 5.00 | 0.222 | 0.828 |
| Day 30 | 17.20 ± 4.26 | 10.98 ± 3.94 | <0.001 | |||
| PAL | Day 0 | 8.37 ± 3.26 | <0.001 | 8.27 ± 3.56 | 0.263 | 0.885 |
| Day 30 | 12.37 ± 2.61 | 7.7 ± 2.95 | <0.001 | |||
| FRP | Day 0 | 11.47 ± 5.04 | <0.001 | 11.39 ± 5.11 | 0.073 | 0.938 |
| Day 30 | 16.65 ± 3.07 | 10.75 ± 4.46 | <0.001 | |||
| RMF | Day 0 | 11.73 ± 4.61 | <0.001 | 12.16 ± 3.54 | 0.840 | 0.597 |
| Day 30 | 17.88 ± 2.73 | 12.24 ± 1.54 | <0.001 | |||
| PFM | Day 0 | 9.82 ± 5.08 | <0.001 | 9.76 ± 5.11 | 0.017 | 0.954 |
| Day 30 | 13.75 ± 2.42 | 9.20 ± 4.97 | <0.001 | |||
| MQ | Day 0 | 60.31 ± 11.35 | <0.001 | 60.75 ± 12.31 | 0.206 | 0.854 |
| Day 30 | 81.84 ± 7.18 | 61.73 ± 10.27 | <0.001 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Hu, Q.; Li, S.; Dai, F.; Qian, W.; Hewlings, S.; Yan, T.; Wang, Y. A Magtein®, Magnesium L-Threonate, -Based Formula Improves Brain Cognitive Functions in Healthy Chinese Adults. Nutrients 2022, 14, 5235. https://doi.org/10.3390/nu14245235
Zhang C, Hu Q, Li S, Dai F, Qian W, Hewlings S, Yan T, Wang Y. A Magtein®, Magnesium L-Threonate, -Based Formula Improves Brain Cognitive Functions in Healthy Chinese Adults. Nutrients. 2022; 14(24):5235. https://doi.org/10.3390/nu14245235
Chicago/Turabian StyleZhang, Chengxiang, Qi Hu, Shifen Li, Feifei Dai, Wen Qian, Susan Hewlings, Ting Yan, and Yubang Wang. 2022. "A Magtein®, Magnesium L-Threonate, -Based Formula Improves Brain Cognitive Functions in Healthy Chinese Adults" Nutrients 14, no. 24: 5235. https://doi.org/10.3390/nu14245235
APA StyleZhang, C., Hu, Q., Li, S., Dai, F., Qian, W., Hewlings, S., Yan, T., & Wang, Y. (2022). A Magtein®, Magnesium L-Threonate, -Based Formula Improves Brain Cognitive Functions in Healthy Chinese Adults. Nutrients, 14(24), 5235. https://doi.org/10.3390/nu14245235





