Dietary Characteristics of Elders with Frailty and with Mild Cognitive Impairment: Cross-Sectional Findings and Implications from the Nutrition and Health Survey in Taiwan 2014–2017
Abstract
1. Introduction
2. Methods
2.1. Study Population and Ethics
2.2. Dietary Assessment
2.3. Frailty Definition
2.4. Cognitive Function Assessment
2.5. Statistical Analysis
3. Results
3.1. Total Energy Intake Levels by Sex, Age, and Geriatric Syndrome Subgroups
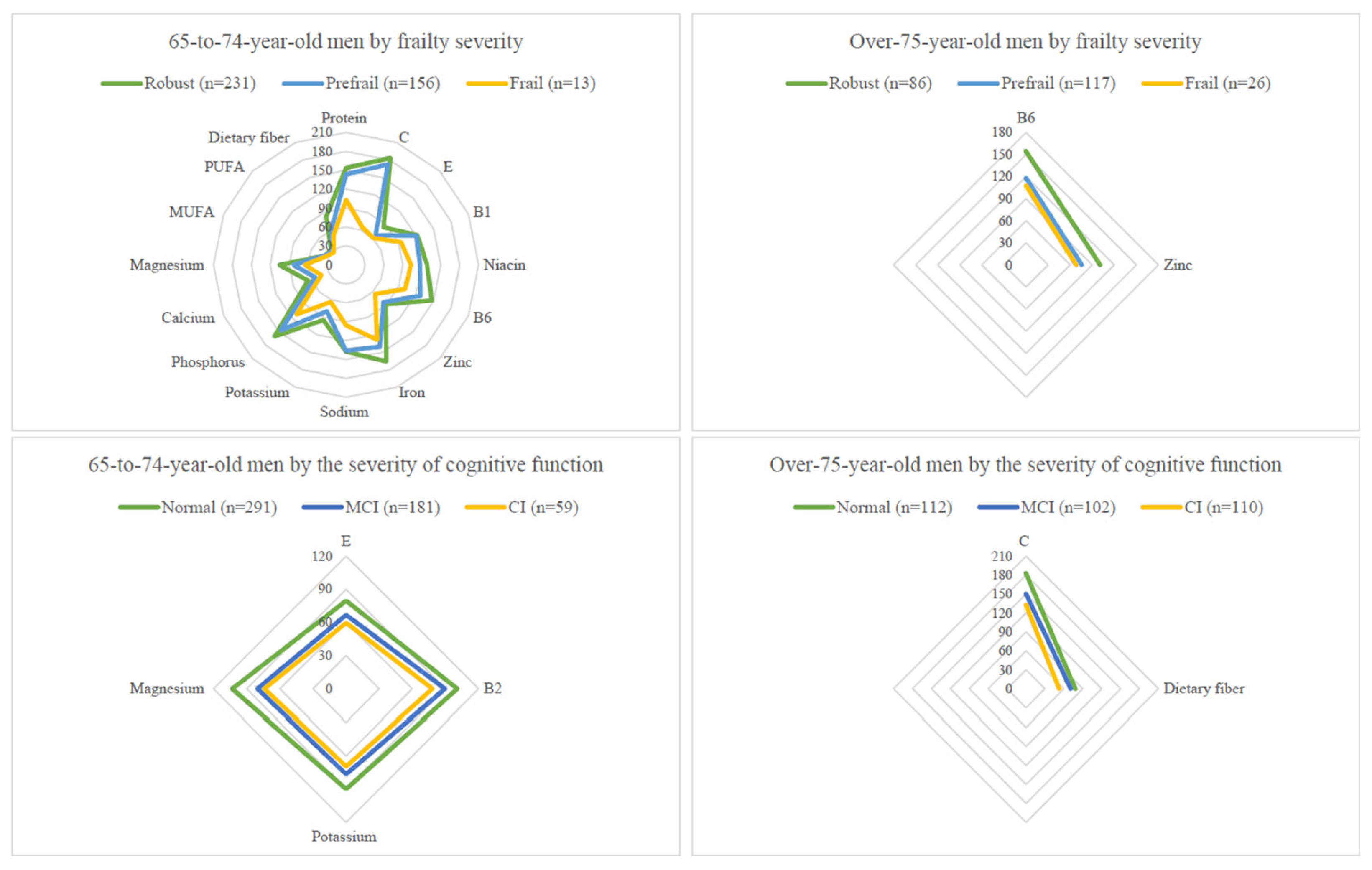
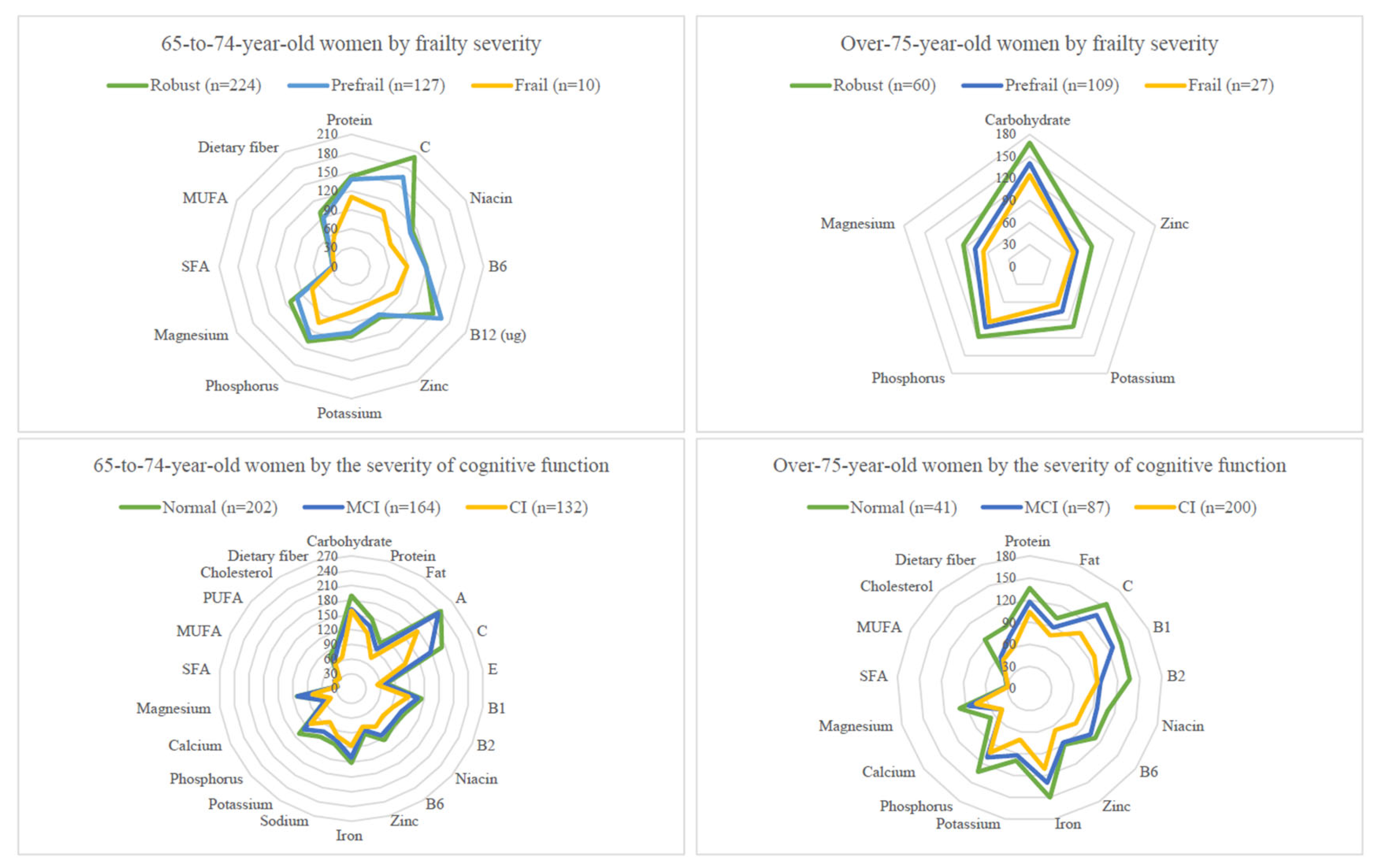
3.2. Nutrient Intake Levels by Sex, Age, and Geriatric Syndrome Subgroups
3.3. Frailty
3.4. Cognitive Impairment
3.5. Nutrient Intake Levels Per Kilogram of Body Weight by Sex, Age, and Geriatric Syndrome Subgroups
3.6. Nutrients Not Reaching 75% DRIs in Elders with Pre-Frailty/Frailty or with MCI/CI
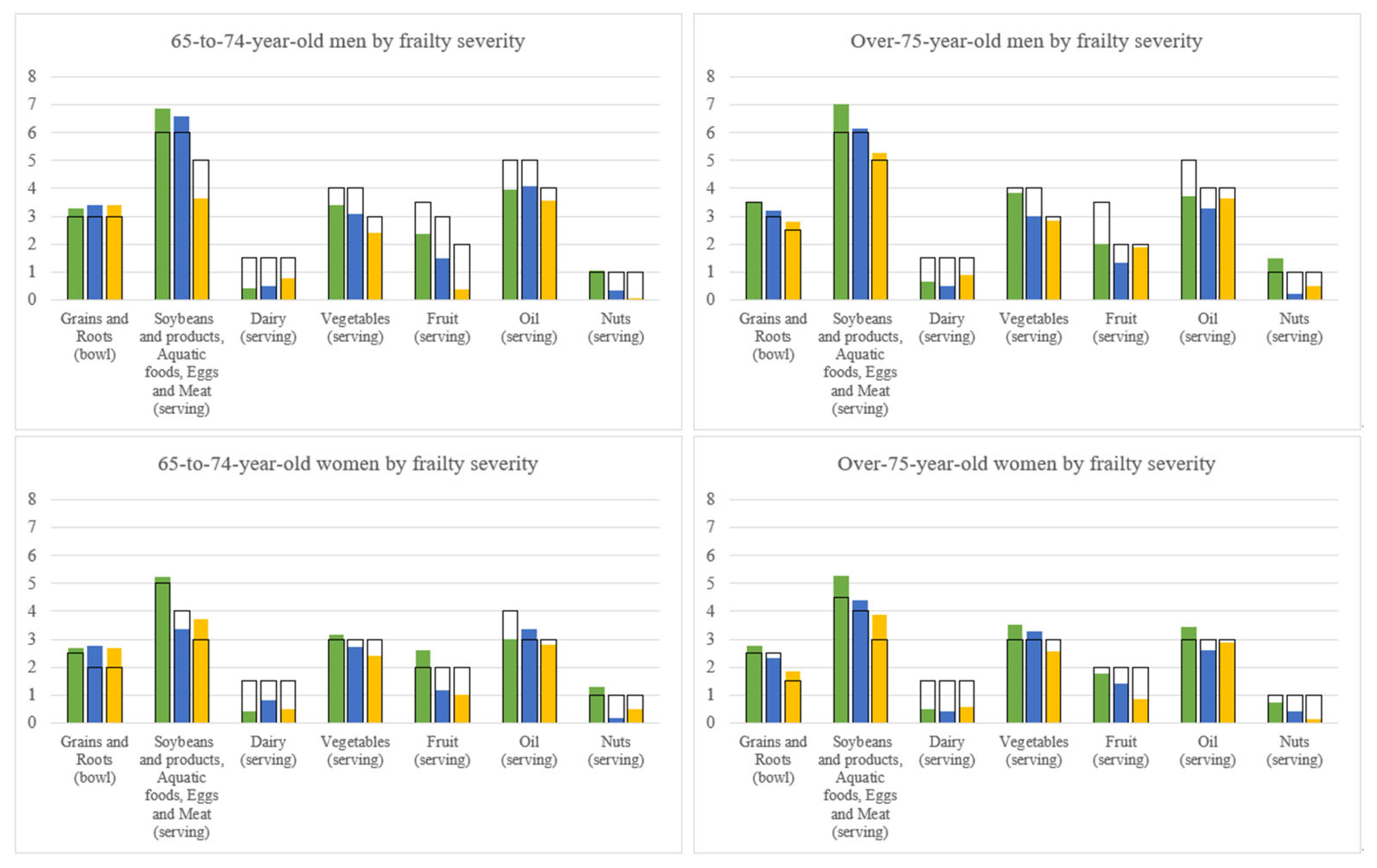
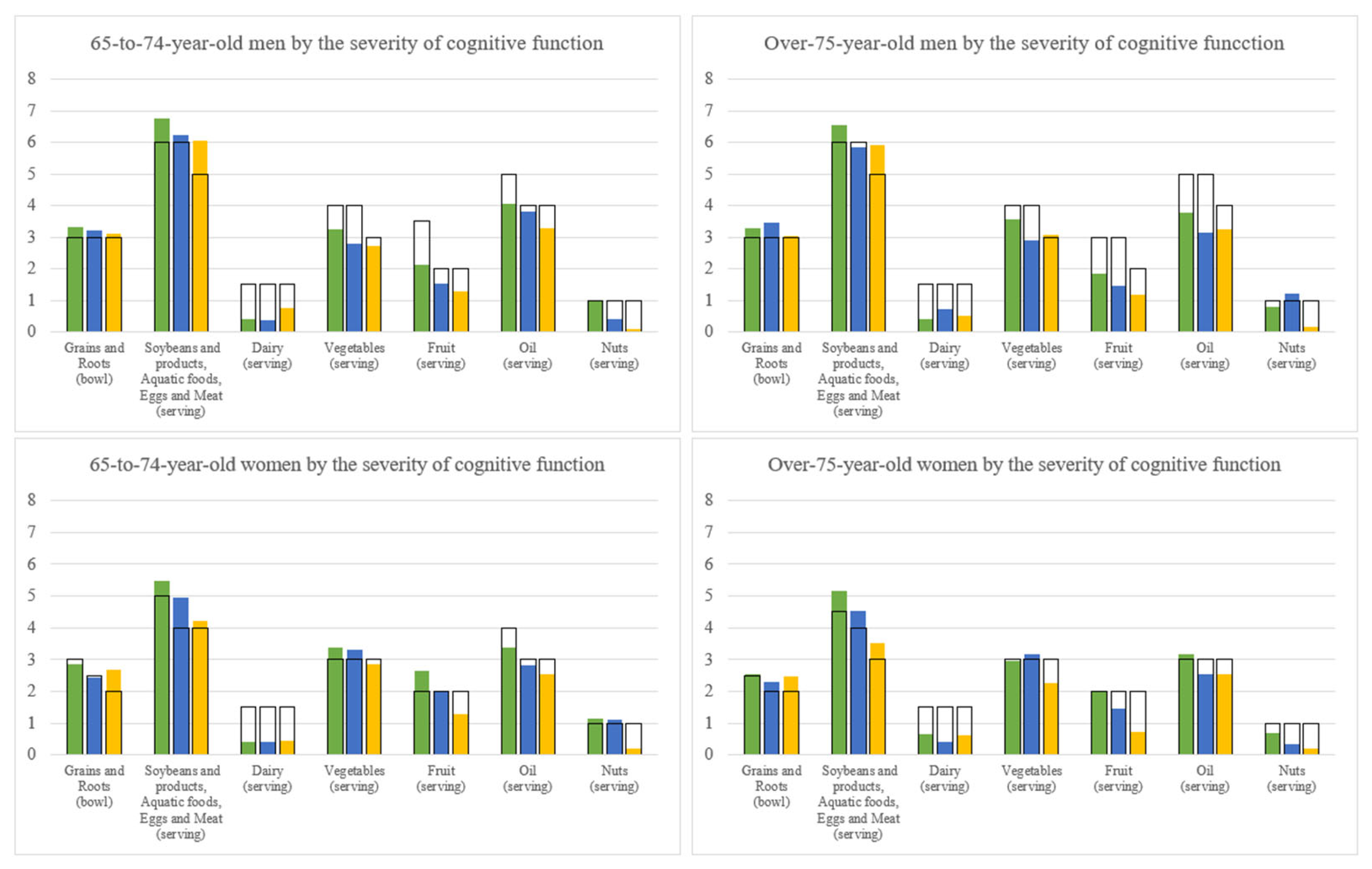
3.7. Dietary Pattern by Geriatric Syndrome Subgroups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fabrício, D.M.; Chagas, M.H.N.; Diniz, B.S. Frailty and cognitive decline. Transl. Res. 2020, 221, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.A.; Savva, G.M.; Kenny, R.A. Frailty and cognitive impairment—A review of the evidence and causal mechanisms. Ageing Res. Rev. 2013, 12, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Panza, F.; Seripa, D.; Solfrizzi, V.; Tortelli, R.; Greco, A.; Pilotto, A.; Logroscino, G. Targeting Cognitive Frailty: Clinical and Neurobiological Roadmap for a Single Complex Phenotype. J. Alzheimer’s Dis. JAD 2015, 47, 793–813. [Google Scholar] [CrossRef] [PubMed]
- Panza, F.; Solfrizzi, V.; Seripa, D.; Imbimbo, B.P.; Capozzo, R.; Quaranta, N.; Pilotto, A.; Logroscino, G. Age-related hearing impairment and frailty in Alzheimer’s disease: Interconnected associations and mechanisms. Front. Aging Neurosci. 2015, 7, 113. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Gomez, M.E.; Zapico, S.C. Frailty, Cognitive Decline, Neurodegenerative Diseases and Nutrition Interventions. Int. J. Mol. Sci. 2019, 20, 2842. [Google Scholar] [CrossRef]
- Kelaiditi, E.; Cesari, M.; Canevelli, M.; van Kan, G.A.; Ousset, P.J.; Gillette-Guyonnet, S.; Ritz, P.; Duveau, F.; Soto, M.E.; Provencher, V.; et al. Cognitive frailty: Rational and definition from an (I.A.N.A./I.A.G.G.) international consensus group. J. Nutr. Health Aging 2013, 17, 726–734. [Google Scholar] [CrossRef]
- Pilgrim, A.L.; Robinson, S.M.; Sayer, A.A.; Roberts, H.C. An overview of appetite decline in older people. Nurs. Older People 2015, 27, 29–35. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Wu, S.Y.; Yeh, N.H.; Chang, H.Y.; Wang, C.F.; Hung, S.Y.; Wu, S.J.; Pan, W.H. Adequate protein intake in older adults in the context of frailty: Cross-sectional results of the Nutrition and Health Survey in Taiwan 2014–2017. Am. J. Clin. Nutr. 2021, 114, 649–660. [Google Scholar] [CrossRef]
- Tu, S.H.; Chen, C.; Hsieh, Y.T.; Chang, H.Y.; Yeh, C.J.; Lin, Y.C.; Pan, W.H. Design and sample characteristics of the 2005–2008 Nutrition and Health Survey in Taiwan. Asia Pac. J. Clin. Nutr. 2011, 20, 225–237. [Google Scholar]
- Health Promotion Administration; Ministry of Health and Welfare. Taiwan. Daily Food Guide; Health Promotion Administration; Ministry of Health and Welfare: Taipei, Taiwan, 2011.
- Guo, N.W.; Liu, H.C.; Wong, P.F.; Liao, K.K.; Yan, S.H.; Lin, K.P.; Chang, C.Y.; Hsu, T.C. Chinese Version and Norms of the Mini-Mental State Examination. J. Rehab. Med. 1988, 16, 52–59. [Google Scholar]
- Tsai, J.C.; Chen, C.W.; Chu, H.; Yang, H.L.; Chung, M.H.; Liao, Y.M.; Chou, K.R. Comparing the Sensitivity, Specificity, and Predictive Values of the Montreal Cognitive Assessment and Mini-Mental State Examination When Screening People for Mild Cognitive Impairment and Dementia in Chinese Population. Arch. Psychiatr. Nurs. 2016, 30, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Health Promotion Administration, Ministry of Health and Welfare, Taiwan. Taiwanese Dietary Reference Intakes: The 8th Version. Available online: https://www.hpa.gov.tw/Pages/Detail.aspx?nodeid=4248&pid=12285 (accessed on 14 September 2022).
- Health Promotion Administration, Ministry of Health and Welfare, Taiwan. Salt Reduction Tips Handbook. Available online: https://www.hpa.gov.tw/Pages/EBook.aspx?nodeid=1160 (accessed on 14 September 2022).
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Food and Nutrition Board; Committee to Review the Dietary Reference Intakes for Sodium and Potassium. Dietary Reference Intakes for Sodium and Potassium; Oria, M., Harrison, M., Stallings, V.A., Eds.; National Academies Press: Washington, DC, USA, 2019. [Google Scholar]
- Jahns, L.; Davis-Shaw, W.; Lichtenstein, A.H.; Murphy, S.P.; Conrad, Z.; Nielsen, F. The History and Future of Dietary Guidance in America. Adv. Nutr. 2018, 9, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Bartali, B.; Frongillo, E.A.; Bandinelli, S.; Lauretani, F.; Semba, R.D.; Fried, L.P.; Ferrucci, L. Low nutrient intake is an essential component of frailty in older persons. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Castaneda-Gameros, D.; Redwood, S.; Thompson, J.L. Low Nutrient Intake and Frailty Among Overweight and Obese Migrant Women From Ethnically Diverse Backgrounds Ages 60 Years and Older: A Mixed-Methods Study. J. Nutr. Educ. Behav. 2017, 49, 3–10.e11. [Google Scholar] [CrossRef]
- Schoufour, J.D.; Franco, O.H.; Kiefte-de Jong, J.C.; Trajanoska, K.; Stricker, B.; Brusselle, G.; Rivadeneira, F.; Lahousse, L.; Voortman, T. The association between dietary protein intake, energy intake and physical frailty: Results from the Rotterdam Study. Br. J. Nutr. 2019, 121, 393–401. [Google Scholar] [CrossRef]
- Watanabe, D.; Yoshida, T.; Nanri, H.; Watanabe, Y.; Date, H.; Itoi, A.; Goto, C.; Ishikawa-Takata, K.; Sagayama, H.; Ebine, N.; et al. Association Between the Prevalence of Frailty and Doubly Labeled Water-Calibrated Energy Intake Among Community-Dwelling Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 876–884. [Google Scholar] [CrossRef]
- Khor, P.Y.; Vearing, R.M.; Charlton, K.E. The effectiveness of nutrition interventions in improving frailty and its associated constructs related to malnutrition and functional decline among community-dwelling older adults: A systematic review. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2022, 35, 566–582. [Google Scholar] [CrossRef]
- Coelho-Junior, H.J.; Calvani, R.; Picca, A.; Tosato, M.; Landi, F.; Marzetti, E. Protein Intake and Frailty in Older Adults: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2022, 14, 2767. [Google Scholar] [CrossRef]
- Doorduijn, A.S.; van de Rest, O.; van der Flier, W.M.; Visser, M.; de van der Schueren, M.A.E. Energy and Protein Intake of Alzheimer’s Disease Patients Compared to Cognitively Normal Controls: Systematic Review. J. Am. Med. Dir. Assoc. 2019, 20, 14–21. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Veronese, N.; Vernuccio, L.; Catanese, G.; Inzerillo, F.; Salemi, G.; Barbagallo, M. Nutrition, Physical Activity, and Other Lifestyle Factors in the Prevention of Cognitive Decline and Dementia. Nutrients 2021, 13, 4080. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Júnior, H.J.; Trichopoulou, A.; Panza, F. Cross-sectional and longitudinal associations between adherence to Mediterranean diet with physical performance and cognitive function in older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2021, 70, 101395. [Google Scholar] [CrossRef] [PubMed]
- Ntanasi, E.; Charisis, S.; Yannakoulia, M.; Georgiadi, K.; Balomenos, V.; Kosmidis, M.H.; Dardiotis, Ε.; Hadjigeorgiou, G.; Sakka, P.; Maraki, M.; et al. Adherence to the Mediterranean diet and incident frailty: Results from a longitudinal study. Maturitas 2022, 162, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Shiau, M.H.; Lee, M.C.; Lin, F.L.; Hurng, B.S.; Yeh, C.J. Cross-Sectional, Short-, Medium-, and Long-Term Effects of Dietary Pattern on Frailty in Taiwan. Int. J. Environ. Res. Public Health 2021, 18, 9717. [Google Scholar] [CrossRef]
- Rashidi Pour Fard, N.; Amirabdollahian, F.; Haghighatdoost, F. Dietary patterns and frailty: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 498–513. [Google Scholar] [CrossRef]
- van den Brink, A.C.; Brouwer-Brolsma, E.M.; Berendsen, A.A.M.; van de Rest, O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diets Are Associated with Less Cognitive Decline and a Lower Risk of Alzheimer’s Disease-A Review. Adv. Nutr. 2019, 10, 1040–1065. [Google Scholar] [CrossRef]
- Ntanasi, E.; Yannakoulia, M.; Kosmidis, M.H.; Anastasiou, C.A.; Dardiotis, E.; Hadjigeorgiou, G.; Sakka, P.; Scarmeas, N. Adherence to Mediterranean Diet and Frailty. J. Am. Med. Dir. Assoc. 2018, 19, 315–322.e312. [Google Scholar] [CrossRef]
- Chuang, S.-Y.; Lo, Y.-L.; Wu, S.-Y.; Wang, P.-N.; Pan, W.-H. Dietary patterns and foods associated with cognitive function in Taiwanese elderly: The cross-sectional and longitudinal studies. J. Am. Med. Directors Assoc. 2019, 20, 544–550. [Google Scholar] [CrossRef]
- Lo, Y.L.; Hsieh, Y.T.; Hsu, L.L.; Chuang, S.Y.; Chang, H.Y.; Hsu, C.C.; Chen, C.Y.; Pan, W.H. Dietary Pattern Associated with Frailty: Results from Nutrition and Health Survey in Taiwan. J. Am. Geriatr. Soc. 2017, 65, 2009–2015. [Google Scholar] [CrossRef]
- Pan, W.-H.; Wu, S.-Y.; Yeh, N.-H.; Hung, S.-Y. Healthy Taiwanese Eating Approach (TEA) toward Total Wellbeing and Healthy Longevity. Nutrients 2022, 14, 2774. [Google Scholar] [CrossRef]
- Hsieh, T.J.; Su, S.C.; Chen, C.W.; Kang, Y.W.; Hu, M.H.; Hsu, L.L.; Wu, S.Y.; Chen, L.; Chang, H.Y.; Chuang, S.Y.; et al. Individualized home-based exercise and nutrition interventions improve frailty in older adults: A randomized controlled trial. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 119. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Hsu, L.L.; Hsu, C.C.; Hsieh, T.J.; Su, S.C.; Peng, Y.W.; Guo, T.M.; Kang, Y.W.; Pan, W.H. Dietary education with customised dishware and food supplements can reduce frailty and improve mental well-being in elderly people: A single-blind randomized controlled study. Asia Pac. J. Clin. Nutr. 2018, 27, 1018–1030. [Google Scholar] [CrossRef] [PubMed]
- Ghoreishy, S.M.; Asoudeh, F.; Jayedi, A.; Mohammadi, H. Fruit and vegetable intake and risk of frailty: A systematic review and dose response meta-analysis. Ageing Res. Rev. 2021, 71, 101460. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, J.; Cao, L.; Shi, M.; Liu, H.; Zhao, Y.; Xia, Y. Fruit and Vegetable Consumption and Cognitive Disorders in Older Adults: A Meta-Analysis of Observational Studies. Front. Nutr. 2022, 9, 871061. [Google Scholar] [CrossRef]
| n | Geriatric Syndrome Groups | P Values | |||||
|---|---|---|---|---|---|---|---|
| Sex | Age | Trend b | |||||
| Robust | Prefrail | Frail | |||||
| All participants | 601/509/76 | 1869 ± 41.34 | 1693 ± 40.93 | 1537 ± 81.20 | <0.0001 | 0.103 | 0.001 |
| Men | |||||||
| 65~74-year-old | 231/156/13 | 2078 ± 49.12 | 1953 ± 74.79 | 1761 ± 192.7 | - | - | 0.114 |
| ≥75-year-old | 86/117/26 | 2164 ± 122.7 | 1888 ± 107.8 | 1840 ± 179.7 | - | - | 0.158 |
| Women | |||||||
| 65~74-year-old | 224/127/10 | 1697 ± 61.18 | 1629 ± 62.04 | 1426 ± 106.1 | - | - | 0.027 |
| ≥75-year-old | 60/109/27 | 1484 ± 66.16 | 1313 ± 66.19 | 1246 ± 117.4 | - | - | 0.085 |
| Normal | MCI | CI | |||||
| All participants | 646/534/501 | 1851 ± 40.22 | 1695 ± 38.64 | 1553 ± 44.45 | <0.0001 | 0.217 | <0.0001 |
| Men | |||||||
| 65~74-year-old | 291/181/59 | 2045 ± 43.04 | 1872 ± 65.68 | 1829 ± 171.9 | - | - | 0.254 |
| ≥75-year-old | 112/102/110 | 1945 ± 88.32 | 2045 ± 135.4 | 1770 ± 125.4 | - | - | 0.267 |
| Women | |||||||
| 65~74-year-old | 202/164/132 | 1761 ± 60.54 | 1530 ± 72.96 | 1409 ± 46.63 | - | - | <0.0001 |
| ≥75-year-old | 41/87/200 | 1594 ± 127.3 | 1383 ± 72.93 | 1314 ± 49.97 | - | - | 0.043 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.-Y.; Lee, S.-C.; Yeh, N.-H.; Wang, C.-F.; Hung, S.-Y.; Wu, S.-J.; Pan, W.-H. Dietary Characteristics of Elders with Frailty and with Mild Cognitive Impairment: Cross-Sectional Findings and Implications from the Nutrition and Health Survey in Taiwan 2014–2017. Nutrients 2022, 14, 5216. https://doi.org/10.3390/nu14245216
Wu S-Y, Lee S-C, Yeh N-H, Wang C-F, Hung S-Y, Wu S-J, Pan W-H. Dietary Characteristics of Elders with Frailty and with Mild Cognitive Impairment: Cross-Sectional Findings and Implications from the Nutrition and Health Survey in Taiwan 2014–2017. Nutrients. 2022; 14(24):5216. https://doi.org/10.3390/nu14245216
Chicago/Turabian StyleWu, Szu-Yun, Shu-Chen Lee, Nai-Hua Yeh, Chi-Fen Wang, Shu-Yi Hung, Shin-Jiuan Wu, and Wen-Harn Pan. 2022. "Dietary Characteristics of Elders with Frailty and with Mild Cognitive Impairment: Cross-Sectional Findings and Implications from the Nutrition and Health Survey in Taiwan 2014–2017" Nutrients 14, no. 24: 5216. https://doi.org/10.3390/nu14245216
APA StyleWu, S.-Y., Lee, S.-C., Yeh, N.-H., Wang, C.-F., Hung, S.-Y., Wu, S.-J., & Pan, W.-H. (2022). Dietary Characteristics of Elders with Frailty and with Mild Cognitive Impairment: Cross-Sectional Findings and Implications from the Nutrition and Health Survey in Taiwan 2014–2017. Nutrients, 14(24), 5216. https://doi.org/10.3390/nu14245216






