Longitudinal Associations of Adherence to the Dietary World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) and Dutch Healthy Diet (DHD) Recommendations with Plasma Kynurenines in Colorectal Cancer Survivors after Treatment
Abstract
1. Introduction
2. Materials and Methods
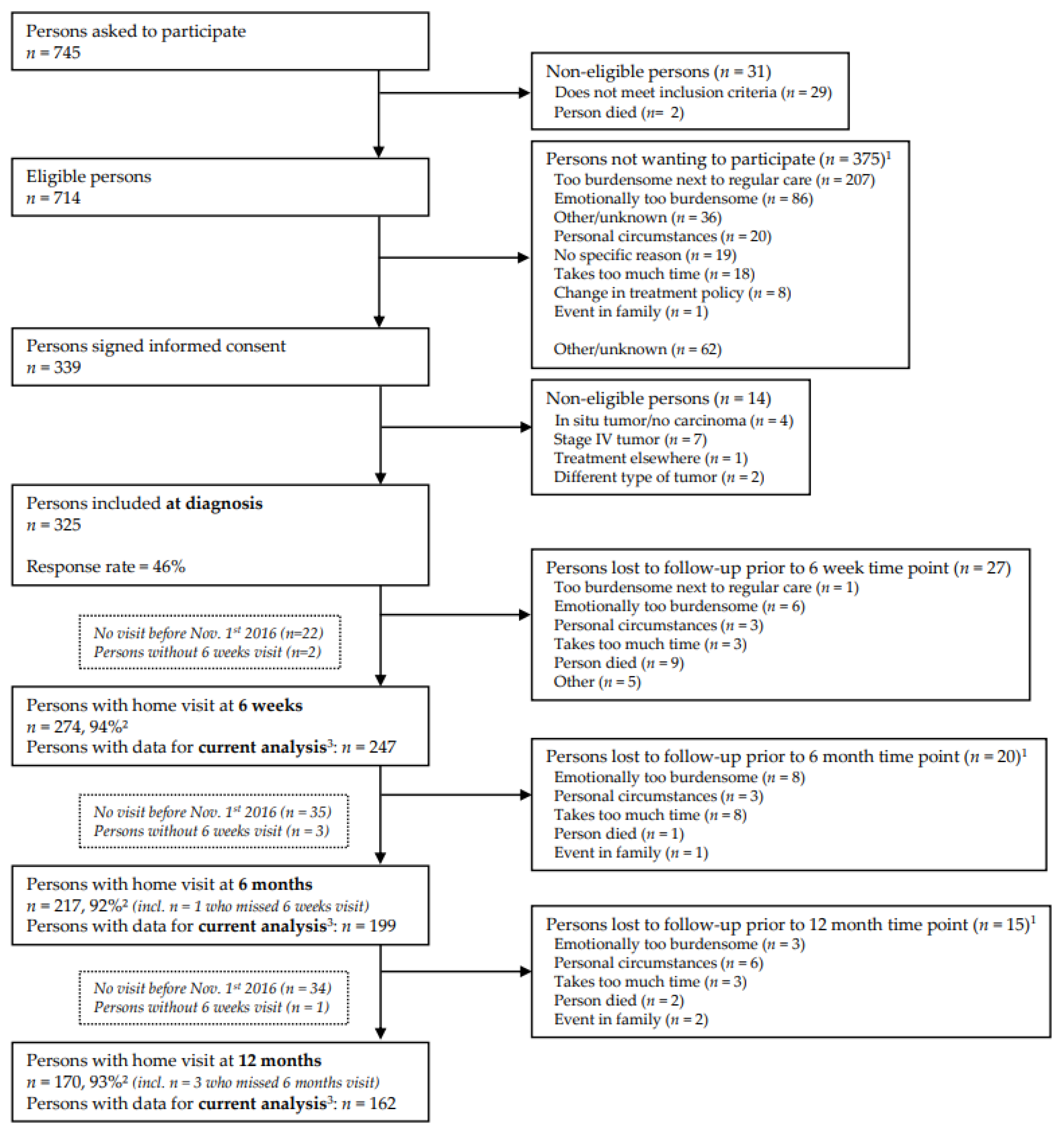
2.1. Study Design and Population
2.2. Data Collection
2.3. Dietary Intake
2.4. Blood Collection
2.5. Sociodemographic, Clinical and Lifestyle Variables
2.6. Statistical Analyses
3. Results
3.1. Participant Characteristics
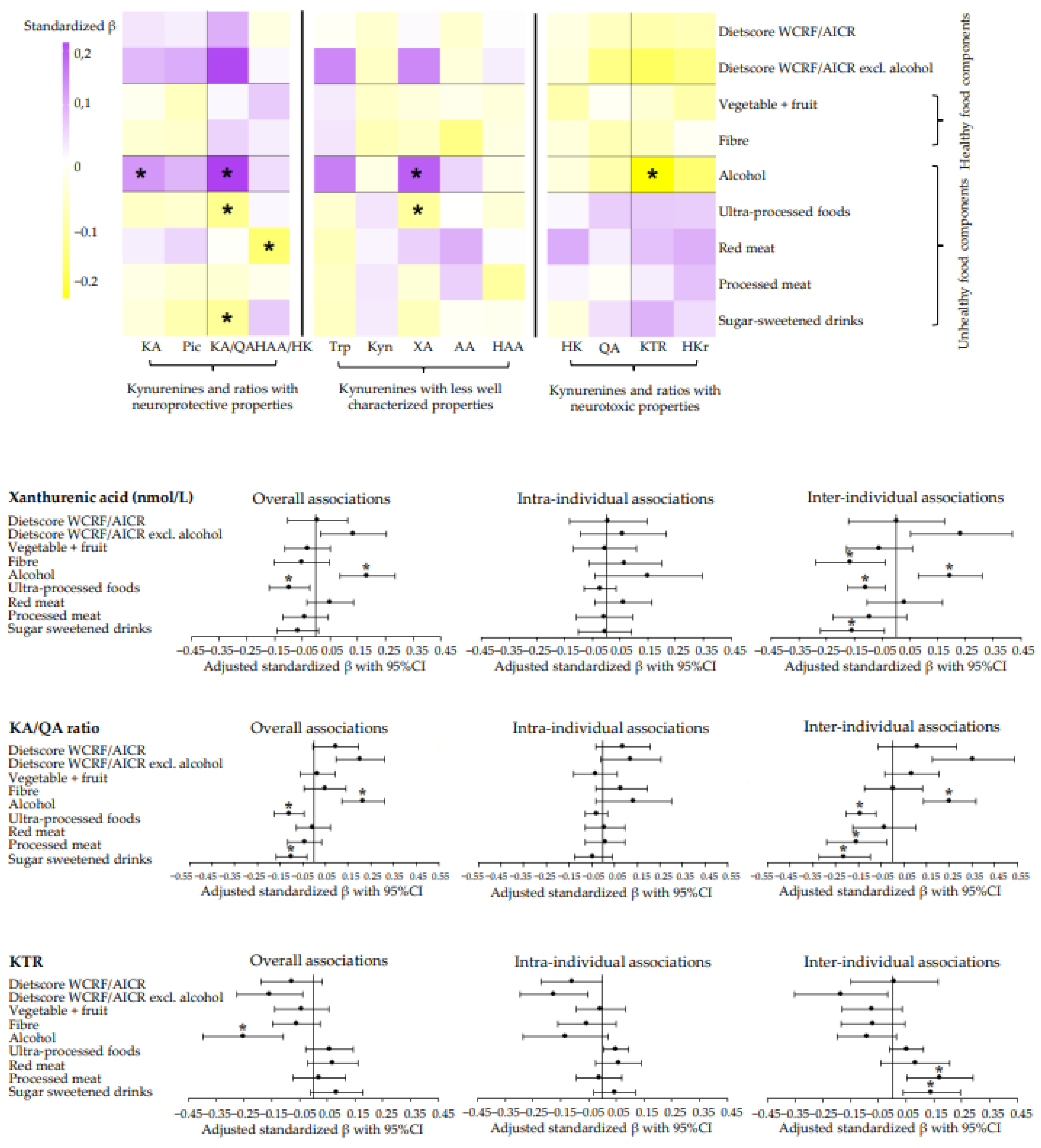
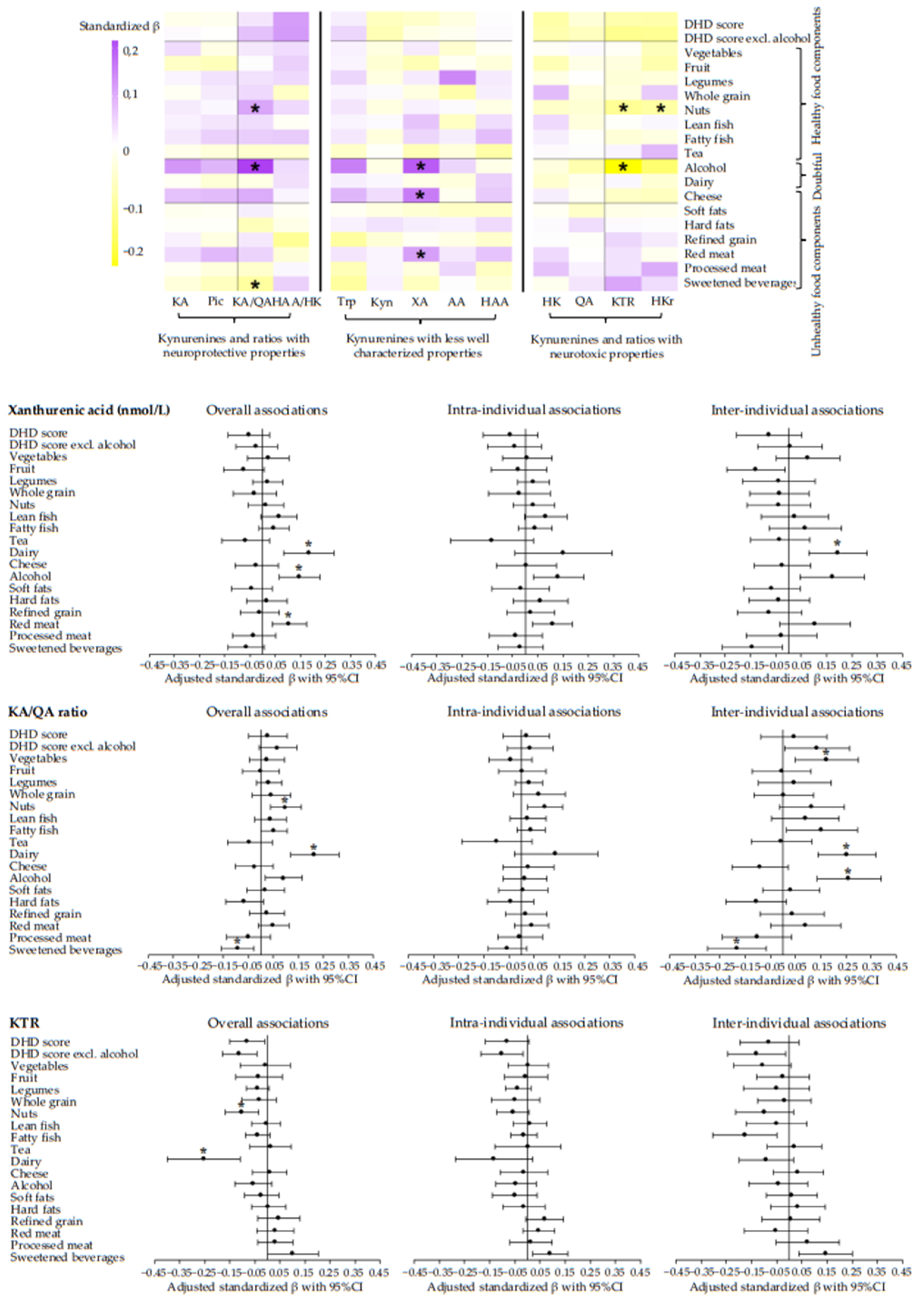
3.2. Dietary Intake and Metabolites and Ratios of the Kynurenine Pathway
3.3. Longitudinal Associations of Adherence to Dietary WCRF/AICR and DHD Recommendations with Metabolites of the Kynurenine Pathway and Metabolite Ratios
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Parry, C.; Kent, E.E.; Mariotto, A.B.; Alfano, C.M.; Rowland, J.H. Cancer survivors: A booming population. Cancer Epidemiol. Biomarkers Prev. 2011, 20, 1996–2005. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Jansen, L.; Herrmann, A.; Stegmaier, C.; Singer, S.; Brenner, H.; Arndt, V. Health-related quality of life during the 10 years after diagnosis of colorectal cancer: A population-based study. J. Clin. Oncol. 2011, 29, 3263–3269. [Google Scholar] [CrossRef] [PubMed]
- Jansen, L.; Koch, L.; Brenner, H.; Arndt, V. Quality of life among long-term (⩾5 years) colorectal cancer survivors–systematic review. Eur. J. Cancer 2010, 46, 2879–2888. [Google Scholar] [CrossRef]
- Kenkhuis, M.-F.; Mols, F.; van Roekel, E.H.; Breedveld-Peters, J.J.; Breukink, S.O.; Janssen-Heijnen, M.L.; Keulen, E.T.; van Duijnhoven, F.J.; Weijenberg, M.P.; Bours, M.J. Longitudinal Associations of Adherence to the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) Lifestyle Recommendations with Quality of Life and Symptoms in Colorectal Cancer Survivors up to 24 Months Post-Treatment. Cancers 2022, 14, 417. [Google Scholar] [CrossRef]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef]
- Kim, S.; Miller, B.J.; Stefanek, M.E.; Miller, A.H. Inflammation-induced activation of the indoleamine 2, 3-dioxygenase pathway: Relevance to cancer-related fatigue. Cancer 2015, 121, 2129–2136. [Google Scholar] [CrossRef]
- Sforzini, L.; Nettis, M.A.; Mondelli, V.; Pariante, C.M. Inflammation in cancer and depression: A starring role for the kynurenine pathway. Psychopharmacology 2019, 236, 2997–3011. [Google Scholar] [CrossRef]
- Li, H.; Liu, T.; Heinsberg, L.W.; Lockwood, M.B.; Wainwright, D.A.; Jang, M.K.; Doorenbos, A.Z. Systematic review of the kynurenine pathway and psychoneurological symptoms among adult cancer survivors. Biol. Res. Nurs. 2020, 22, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.W.; Feng, G. Relationship between interferon-γ, indoleamine 2, 3-dioxygenase, and tryptophan catabolism. FASEB J. 1991, 5, 2516–2522. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guillemin, G.J. Kynurenine pathway metabolites in humans: Disease and healthy states. Int. J. Tryptophan Res. 2009, 2, IJTR.S2097. [Google Scholar] [CrossRef]
- Joisten, N.; Ruas, J.L.; Braidy, N.; Guillemin, G.J.; Zimmer, P. The kynurenine pathway in chronic diseases: A compensatory mechanism or a driving force? Trends Mol. Med. 2021, 27, 946–954. [Google Scholar] [CrossRef]
- Baumgartner, R.; Forteza, M.J.; Ketelhuth, D.F. The interplay between cytokines and the Kynurenine pathway in inflammation and atherosclerosis. Cytokine 2019, 122, 154148. [Google Scholar] [CrossRef] [PubMed]
- Vécsei, L.; Szalárdy, L.; Fülöp, F.; Toldi, J. Kynurenines in the CNS: Recent advances and new questions. Nat. Rev. Drug Discov. 2013, 12, 64–82. [Google Scholar] [CrossRef]
- Mor, A.; Tankiewicz-Kwedlo, A.; Krupa, A.; Pawlak, D. Role of Kynurenine Pathway in Oxidative Stress during Neurodegenerative Disorders. Cells 2021, 10, 1603. [Google Scholar] [CrossRef]
- Takikawa, O.; Kuroiwa, T.; Yamazaki, F.; Kido, R. Mechanism of interferon-gamma action. Characterization of indoleamine 2, 3-dioxygenase in cultured human cells induced by interferon-gamma and evaluation of the enzyme-mediated tryptophan degradation in its anticellular activity. J. Biol. Chem. 1988, 263, 2041–2048. [Google Scholar] [CrossRef]
- Badawy, A.A.; Guillemin, G. The plasma [kynurenine]/[tryptophan] ratio and indoleamine 2, 3-dioxygenase: Time for appraisal. Int. J. Tryptophan Res. 2019, 12, 1178646919868978. [Google Scholar] [CrossRef]
- Stone, T.W. Kynurenic acid antagonists and kynurenine pathway inhibitors. Expert Opin. Investig. Drugs 2001, 10, 633–645. [Google Scholar] [CrossRef]
- Turski, M.P.; Turska, M.; Zgrajka, W.; Kuc, D.; Turski, W.A. Presence of kynurenic acid in food and honeybee products. Amino Acids 2009, 36, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, C.; Gökmen, V. Determination of tryptophan derivatives in kynurenine pathway in fermented foods using liquid chromatography tandem mass spectrometry. Food Chem. 2018, 243, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Bertazzo, A.; Ragazzi, E.; Visioli, F. Evolution of tryptophan and its foremost metabolites’ concentrations in milk and fermented dairy products. Pharmanutrition 2016, 4, 62–67. [Google Scholar] [CrossRef]
- Majewski, M.; Kozlowska, A.; Thoene, M.; Lepiarczyk, E.; Grzegorzewski, W. Overview of the role of vitamins and minerals on the kynurenine pathway in health and disease. J. Physiol. Pharmacol. 2016, 67, 3–19. [Google Scholar] [PubMed]
- Yu, E.; Ruiz-Canela, M.; Guasch-Ferré, M.; Zheng, Y.; Toledo, E.; Clish, C.B.; Salas-Salvadó, J.; Liang, L.; Wang, D.D.; Corella, D. Increases in plasma tryptophan are inversely associated with incident cardiovascular disease in the Prevencion con Dieta Mediterranea (PREDIMED) Study. J. Nutr. 2017, 147, 314–322. [Google Scholar] [CrossRef]
- Li, S.X.; Hodge, A.M.; MacInnis, R.J.; Bassett, J.K.; Ueland, P.M.; Midttun, Ø.; Ulvik, A.; Rinaldi, S.; Meyer, K.; Navionis, A.-S. Inflammation-Related Marker Profiling of Dietary Patterns and All-cause Mortality in the Melbourne Collaborative Cohort Study. J. Nutr. 2021, 151, 2908–2916. [Google Scholar] [CrossRef]
- WCRF/AICR. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective—Continuous Update Project Export Report. 2018. Available online: https://www.wcrf.org/dietandcancer (accessed on 20 July 2022).
- Gezondheidsraad—Dutch Health Council. Dutch Dietary Guidelines 2015 (Richtlijnen Goede Voeding 2015); Gezondheidsraad: The Hague, The Netherlands, 2015; Volume 2015/24. [Google Scholar]
- van Roekel, E.H.; Bours, M.J.; de Brouwer, C.P.; Ten Napel, H.; Sanduleanu, S.; Beets, G.L.; Kant, I.; Weijenberg, M.P. The applicability of the international classification of functioning, disability, and health to study lifestyle and quality of life of colorectal cancer survivors. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1394–1405. [Google Scholar] [CrossRef]
- Koole, J.L.; Bours, M.J.; Breedveld-Peters, J.J.; van Roekel, E.H.; Breukink, S.O.; Janssen-Heijnen, M.L.; Vogelaar, F.J.; Aquarius, M.; Keulen, E.; Stoot, J. Is dietary supplement use longitudinally associated with fatigue in stage I-III colorectal cancer survivors? Clin. Nutr. 2020, 39, 234–241. [Google Scholar] [CrossRef]
- Shams-White, M.M.; Brockton, N.T.; Mitrou, P.; Romaguera, D.; Brown, S.; Bender, A.; Kahle, L.L.; Reedy, J. Operationalizing the 2018 World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) cancer prevention recommendations: A standardized scoring system. Nutrients 2019, 11, 1572. [Google Scholar] [CrossRef]
- Shams-White, M.M.; Romaguera, D.; Mitrou, P.; Reedy, J.; Bender, A.; Brockton, N.T. Further Guidance in Implementing the Standardized 2018 World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) ScoreFurther Guidance in Implementing the 2018 WCRF/AICR Score. Cancer Epidemiol. Biomark. Prev. 2020, 29, 889–894. [Google Scholar] [CrossRef]
- Kenkhuis, M.-F.; van der Linden, B.W.; Breedveld-Peters, J.J.; Koole, J.L.; van Roekel, E.H.; Breukink, S.O.; Mols, F.; Weijenberg, M.P.; Bours, M.J. Associations of the dietary World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) recommendations with patient-reported outcomes in colorectal cancer survivors 2–10 years post-diagnosis: A cross-sectional analysis. Br. J. Nutr. 2021, 125, 1188–1200. [Google Scholar] [CrossRef] [PubMed]
- Looman, M.; Feskens, E.J.; de Rijk, M.; Meijboom, S.; Biesbroek, S.; Temme, E.H.; de Vries, J.; Geelen, A. Development and evaluation of the Dutch Healthy Diet index 2015. Public Health Nutr. 2017, 20, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Midttun, Ø.; Hustad, S.; Ueland, P.M. Quantitative profiling of biomarkers related to B-vitamin status, tryptophan metabolism and inflammation in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Midttun, Ø.; Kvalheim, G.; Ueland, P.M. High-throughput, low-volume, multianalyte quantification of plasma metabolites related to one-carbon metabolism using HPLC-MS/MS. Anal. Bioanal. Chem. 2013, 405, 2009–2017. [Google Scholar] [CrossRef]
- Ulvik, A.; Theofylaktopoulou, D.; Midttun, Ø.; Nygård, O.; Eussen, S.J.; Ueland, P.M. Substrate product ratios of enzymes in the kynurenine pathway measured in plasma as indicators of functional vitamin B-6 status. Am. J. Clin. Nutr. 2013, 98, 934–940. [Google Scholar] [CrossRef]
- Sangha, O.; Stucki, G.; Liang, M.H.; Fossel, A.H.; Katz, J.N. The Self-Administered Comorbidity Questionnaire: A new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003, 49, 156–163. [Google Scholar] [CrossRef]
- Wendel-Vos, G.W.; Schuit, A.J.; Saris, W.H.; Kromhout, D. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J. Clin. Epidemiol. 2003, 56, 1163–1169. [Google Scholar] [CrossRef]
- Van Roekel, E.H.; Duchâteau, J.; Bours, M.; van Delden, L.; Breedveld-Peters, J.; Koole, J.; Kenkhuis, M.; van den Brandt, P.; Jansen, R.; Kant, I. Longitudinal associations of light-intensity physical activity with quality of life, functioning and fatigue after colorectal cancer. Qual. Life Res. 2020, 29, 2987–2998. [Google Scholar] [CrossRef]
- Theofylaktopoulou, D.; Midttun, Ø.; Ulvik, A.; Ueland, P.; Tell, G.; Vollset, S.; Nygård, O.; Eussen, S. A community-based study on determinants of circulating markers of cellular immune activation and kynurenines: The H ordaland H ealth S tudy. Clin. Exp. Immunol. 2013, 173, 121–130. [Google Scholar] [CrossRef]
- VanderWeele, T.J. Principles of confounder selection. Eur. J. Epidemiol. 2019, 34, 211–219. [Google Scholar] [CrossRef]
- Twisk, J.W.; de Vente, W. Hybrid models were found to be very elegant to disentangle longitudinal within-and between-subject relationships. J. Clin. Epidemiol. 2019, 107, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- World Health Organization. Global Status Report on Alcohol and Health 2018; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Vidal, R.; García-Marchena, N.; O’Shea, E.; Requena-Ocaña, N.; Flores-López, M.; Araos, P.; Serrano, A.; Suárez, J.; Rubio, G.; de Fonseca, F.R. Plasma tryptophan and kynurenine pathway metabolites in abstinent patients with alcohol use disorder and high prevalence of psychiatric comorbidity. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 102, 109958. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, S.; Schwarz, M.; Delzenne, N.M.; Stärkel, P.; de Timary, P. Alterations of kynurenine pathway in alcohol use disorder and abstinence: A link with gut microbiota, peripheral inflammation and psychological symptoms. Transl. Psychiatry. 2021, 11, 503. [Google Scholar] [CrossRef] [PubMed]
- Siegel, F.L.; Roach, M.K.; Pomeroy, L.R. Plasma amino acid patterns in alcoholism: The effects of ethanol loading. Proc. Natl. Acad. Sci. USA 1964, 51, 605. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.-B. Tryptophan metabolism in alcoholism. Nutr. Res. Rev. 2002, 15, 123–152. [Google Scholar] [CrossRef]
- Badawy, A.A.; Doughrty, D.M.; Marsh-Richard, D.M.; Steptoe, A. Activation of liver tryptophan pyrrolase mediates the decrease in tryptophan availability to the brain after acute alcohol consumption by normal subjects. Alcohol Alcohol. 2009, 44, 267–271. [Google Scholar] [CrossRef]
- Chan, A.M.; von Mühlen, D.; Kritz-Silverstein, D.; Barrett-Connor, E. Regular alcohol consumption is associated with increasing quality of life and mood in older men and women: The Rancho Bernardo Study. Maturitas 2009, 62, 294–300. [Google Scholar] [CrossRef]
- Karlsson, T.; Strand, E.; Dierkes, J.; Drevon, C.A.; Øyen, J.; Midttun, Ø.; Ueland, P.M.; Gudbrandsen, O.A.; Pedersen, E.R.; Nygård, O. Associations between intake of fish and n-3 long-chain polyunsaturated fatty acids and plasma metabolites related to the kynurenine pathway in patients with coronary artery disease. Eur. J. Nutr. 2017, 56, 261–272. [Google Scholar] [CrossRef]
- Arnone, D.; Saraykar, S.; Salem, H.; Teixeira, A.L.; Dantzer, R.; Selvaraj, S. Role of Kynurenine pathway and its metabolites in mood disorders: A systematic review and meta-analysis of clinical studies. Neurosci. Biobehav. Rev. 2018, 92, 477–485. [Google Scholar] [CrossRef]
- Savitz, J. The kynurenine pathway: A finger in every pie. Mol. Psychiatry 2020, 25, 131–147. [Google Scholar] [CrossRef] [PubMed]
| Total, n = 247 * | |
|---|---|
| Sex (male), n (%) | 170 (68.8) |
| Age (years), mean (SD) | 66.7 (9.1) |
| Cancer location, n (%) | |
| Colon | 151 (61.1) |
| Rectum | 96 (38.9) |
| Cancer stage, n (%) | |
| Stage I | 80 (32.4) |
| Stage II | 58 (23.5) |
| Stage III | 109 (44.1) |
| Cancer treatment, n (%) | |
| Surgery (yes) | 221 (89.5) |
| Chemotherapy (yes) | 93 (37.7) |
| Radiotherapy (yes) | 68 (27.5) |
| Number of comorbidities, n (%) | |
| 0 comorbidities | 50 (20.2) |
| 1 comorbidity | 60 (24.3) |
| ≥2 comorbidities | 137 (55.5) |
| Stoma (yes), n (%) | 78 (31.7) |
| BMI (kg m²), mean (SD) | 27.8 (4.4) |
| Educational level, n (%) | |
| Low | 62 (25.2) |
| Medium | 98 (39.8) |
| High | 86 (35.0) |
| Smoking status, n (%) | |
| Never | 83 (33.9) |
| Former | 140 (57.1) |
| Current | 22 (8.9) |
| Physical activity, median (IQR) | |
| LPA (hours/week) | 7.0 (12.5) |
| MVPA (hours/week) | 7.2 (11.7) |
| Total PA (hours/week) | 18.3 (21.0) |
| Total energy intake (kcal/week), mean (SD) | 14,687.0 (3633.3) |
| Creatinine (µmol/L), mean (SD) | 83.6 (18.8) |
| Neopterin (nmol/L), median (IQR) | 15.9 (10.7) |
| 6 Weeks Post-Treatment (n = 247) | 6 Months Post-Treatment (n = 199) | 12 Months Post-Treatment (n = 162) | |
|---|---|---|---|
| WCRF/AICR diet score | 1.9 (0.7) | 2.1 (0.7) | 2.0 (0.7) |
| Fruit, vegetable and fibre intake | |||
| Fruit intake (g/day) | 119.1 (86.8) | 123.3 (93.0) | 108.8 (90.5) |
| Vegetable intake (g/day) | 133.7 (71.8) | 135.4 (73.5) | 130.8 (69.2) |
| Dietary fibre intake (g/day) | 21.0 (5.9) | 21.0 (6.4) | 21.1 (6.1) |
| Alcohol intake (g/day) | 13.6 (18.6) | 13.0 (19.2) | 14.5 (19.3) |
| Sugar-sweetened drinks (g/day) | 135.8 (147.4) | 107.0 (134.9) | 101.2 (114.9) |
| UPF (% energy) | 35.1 (10.9) | 33.2 (10.3) | 33.3 (9.8) |
| Meat intake | |||
| Red meat intake (g/week) | 614.0 (311.5) | 600.0 (278.8) | 611.8 (312.1) |
| Processed meat intake (g/week) | 326.7 (209.2) | 312.5 (190.1) | 323.8 (228.5) |
| DHD total score | 60.7 (14.8) | 62.2 (13.7) | 61.3 (15.4) |
| Fruit intake (g/day) | 117.5 (86.9) | 122.2 (92.8) | 107.0 (89.7) |
| Vegetable intake (g/day) | 135.2 (71.6) | 136.7 (72.4) | 131.2 (70.2) |
| Legumes intake (g/day) | 2.1 (8.0) | 5.0 (15.0) | 2.6 (8.2) |
| Nuts intake (g/day) | 3.0 (7.2) | 3.6 (7.8) | 3.0 (7.2) |
| Grain intake | |||
| Wholegrain intake (g/day) | 113.6 (63.3) | 120.6 (64.4) | 122.8 (62.0) |
| Refined grain intake (g/day) | 80.8 (52.2) | 71.0 (48.1) | 77.0 (51.3) |
| Alcohol intake (g/day) | 13.6 (18.6) | 13.0 (19.2) | 14.5 (19.3) |
| Dairy intake | |||
| Dairy intake (g/day) | 187.1 (160.2) | 165.9 (139.8) | 157.0 (128.3) |
| Cheese intake (g/day) | 28.4 (20.6) | 28.5 (22.2) | 28.0 (18.8) |
| Fish intake | |||
| Fatty fish intake (g/day) | 11.0 (20.4) | 9.3 (14.1) | 7.9 (11.6) |
| Lean fish intake (g/day) | 11.5 (14.7) | 9.8 (15.6) | 10.6 (18.2) |
| Tea intake (g/day) | 209.8 (255.3) | 209.4 (270.4) | 216.0 (265.2) |
| Fat intake | |||
| Hard fats intake (g/day) | 8.3 (11.1) | 9.6 (12.7) | 8.9 (12.3) |
| Soft fats intake (g/day) | 22.1 (14.9) | 19.2 (13.5) | 21.6 (13.9) |
| Meat intake | |||
| Red meat intake (g/day) | 35.2 (26.4) | 37.5 (27.0) | 35.7 (27.3) |
| Processed meat intake (g/day) | 49.6 (32.7) | 48.0 (29.0) | 50.8 (36.0) |
| Sweetened beverages and fruit juices (g/day) | 106.8 (134.2) | 79.4 (112.2) | 72.4 (97.3) |
| 6 Weeks Post-Treatment (n = 247) | 6 Months Post-Treatment (n = 199) | 12 Months Post-Treatment (n = 162) | p-Value | |
|---|---|---|---|---|
| Tryptophan (µmol/L) | 65.7 (11.7) | 67.4 (13.1) | 68.1 (11.8) | 0.014 * |
| Kynurenine (µmol/L) | 2.0 (0.5) | 1.9 (0.5) | 1.9 (0.4) | 0.005 * |
| 3-Hydroxykynurenine (nmol/L) 1 | 58.2 (31.2) | 53.7 (26.3) | 51.5 (24.8) | 0.050 |
| Kynurenic acid (nmol/L) | 57.9 (26.5) | 63.9 (28.8) | 67.1 (32.9) | <0.001 * |
| Xanthurenic acid (nmol/L) | 14.4 (8.1) | 15.9 (7.5) | 16.9 (8.6) | <0.001 * |
| Anthranilic acid (nmol/L) 1 | 16.8 (5.9) | 17.5 (5.9) | 17.9 (8.4) | 0.084 |
| 3-Hydroxyanthranilic acid (nmol/L) 1 | 44.8 (14.4) | 43.9 (14.9) | 44.2 (14.5) | 0.321 |
| Picolinic acid (nmol/L) | 36.7 (15.6) | 37.5 (13.7) | 38.8 (15.8) | 0.010 * |
| Quinolinic acid (nmol/L) | 628.6 (389.9) | 609.8 (434.2) | 539.2 (274.5) | <0.001 * |
| KTR | 31.2 (10.1) | 29.8 (11.3) | 28.4 (8.2) | <0.001 * |
| HKr | 0.45 (0.23) | 0.39 (0.14) | 0.36 (0.13) | <0.001 * |
| HAA/HK ratio | 0.88 (0.33) | 0.93 (0.48) | 0.95 (0.35) | 0.001 * |
| KA/QA ratio | 0.11 (0.05) | 0.12 (0.05) | 0.14 (0.05) | <0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holthuijsen, D.D.B.; Bours, M.J.L.; Roekel, E.H.v.; Breukink, S.O.; Janssen-Heijnen, M.L.G.; Keulen, E.T.P.; Ueland, P.M.; Midttun, Ø.; Brezina, S.; Gigic, B.; et al. Longitudinal Associations of Adherence to the Dietary World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) and Dutch Healthy Diet (DHD) Recommendations with Plasma Kynurenines in Colorectal Cancer Survivors after Treatment. Nutrients 2022, 14, 5151. https://doi.org/10.3390/nu14235151
Holthuijsen DDB, Bours MJL, Roekel EHv, Breukink SO, Janssen-Heijnen MLG, Keulen ETP, Ueland PM, Midttun Ø, Brezina S, Gigic B, et al. Longitudinal Associations of Adherence to the Dietary World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) and Dutch Healthy Diet (DHD) Recommendations with Plasma Kynurenines in Colorectal Cancer Survivors after Treatment. Nutrients. 2022; 14(23):5151. https://doi.org/10.3390/nu14235151
Chicago/Turabian StyleHolthuijsen, Daniëlle D. B., Martijn J. L. Bours, Eline H. van Roekel, Stéphanie O. Breukink, Maryska L. G. Janssen-Heijnen, Eric T. P. Keulen, Per M. Ueland, Øivind Midttun, Stefanie Brezina, Biljana Gigic, and et al. 2022. "Longitudinal Associations of Adherence to the Dietary World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) and Dutch Healthy Diet (DHD) Recommendations with Plasma Kynurenines in Colorectal Cancer Survivors after Treatment" Nutrients 14, no. 23: 5151. https://doi.org/10.3390/nu14235151
APA StyleHolthuijsen, D. D. B., Bours, M. J. L., Roekel, E. H. v., Breukink, S. O., Janssen-Heijnen, M. L. G., Keulen, E. T. P., Ueland, P. M., Midttun, Ø., Brezina, S., Gigic, B., Gsur, A., Kok, D. E., Ose, J., Ulrich, C. M., Weijenberg, M. P., & Eussen, S. J. P. M. (2022). Longitudinal Associations of Adherence to the Dietary World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) and Dutch Healthy Diet (DHD) Recommendations with Plasma Kynurenines in Colorectal Cancer Survivors after Treatment. Nutrients, 14(23), 5151. https://doi.org/10.3390/nu14235151







