The Trajectory of Nutritional Status and Physical Activity before and after Transcatheter Aortic Valve Implantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection
2.2.1. Nutritional Status and Physical Activity
2.2.2. Measures of Patient Characteristics
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Nutritional Status and Physical Activity Level before and after the TAVI
3.3. Patient Characteristics Associated with Nutritional Status and Physical Activity Level
3.4. Baseline Characteristics Associated with Change in Nutritional Status and Physical Activity Level
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef] [PubMed]
- Aarden, J.J.; van der Schaaf, M.; van der Esch, M.; Reichardt, L.A.; van Seben, R.; Bosch, J.A.; Twisk, J.W.R.; Buurman, B.M.; Engelbert, R.H.H. Muscle Strength Is Longitudinally Associated with Mobility among Older Adults after Acute Hospitalization: The Hospital-ADL Study. PLoS ONE 2019, 14, e0219041. [Google Scholar] [CrossRef] [PubMed]
- Tsekoura, M.; Kastrinis, A.; Katsoulaki, M.; Billis, E.; Gliatis, J. Sarcopenia and Its Impact on Quality of Life. Adv. Exp. Med. Biol. 2017, 987, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Aarden, J.J.; Reijnierse, E.M.; van der Schaaf, M.; van der Esch, M.; Reichardt, L.A.; van Seben, R.; Bosch, J.A.; Twisk, J.W.R.; Maier, A.B.; Engelbert, R.H.H.; et al. Longitudinal Changes in Muscle Mass, Muscle Strength, and Physical Performance in Acutely Hospitalized Older Adults. J. Am. Med. Dir. Assoc. 2021, 22, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Steffl, M.; Bohannon, R.W.; Sontakova, L.; Tufano, J.J.; Shiells, K.; Holmerova, I. Relationship between Sarcopenia and Physical Activity in Older People: A Systematic Review and Meta-Analysis. Clin. Interv. Aging 2017, 12, 835–845. [Google Scholar] [CrossRef]
- Sawatzky, J.A.V.; Kehler, D.S.; Ready, A.E.; Lerner, N.; Boreskie, S.; Lamont, D.; Luchik, D.; Arora, R.C.; Duhamel, T.A. Prehabilitation Program for Elective Coronary Artery Bypass Graft Surgery Patients: A Pilot Randomized Controlled Study. Clin. Rehabil. 2014, 28, 648–657. [Google Scholar] [CrossRef]
- Mooney, M.; Fitzsimons, D.; Richardson, G. “No More Couch-Potato!” Patients’ Experiences of a Pre-Operative Programme of Cardiac Rehabilitation for Those Awaiting Coronary Artery Bypass Surgery. Eur. J. Cardiovasc. Nurs. 2007, 6, 77–83. [Google Scholar] [CrossRef]
- Van Erck, D.; Tieland, M.; Adriaens, N.W.; Weijs, P.J.M.; Scholte op Reimer, W.J.M.; Henriques, J.P.; Schoufour, J.D. GLIM-Based Malnutrition, Protein Intake and Diet Quality in Preprocedural Transcatheter Aortic Valve Implantation (TAVI) Patients. Clin. Nutr. ESPEN 2022, 51, 481–485. [Google Scholar] [CrossRef]
- McCann, M.; Stamp, N.; Ngui, A.; Litton, E. Cardiac Prehabilitation. J. Cardiothorac. Vasc. Anesth. 2019, 33, 2255–2265. [Google Scholar] [CrossRef]
- Marmelo, F.; Rocha, V.; Gonçalves, D. The Impact of Prehabilitation on Post-Surgical Complications in Patients Undergoing Non-Urgent Cardiovascular Surgical Intervention: Systematic Review and Meta-Analysis. Eur. J. Prev. Cardiol. 2018, 25, 404–417. [Google Scholar] [CrossRef]
- Boreskie, K.F.; Hay, J.L.; Kehler, D.S.; Johnston, N.M.; Rose, A.v.; Oldfield, C.J.; Kumar, K.; Toleva, O.; Arora, R.C.; Duhamel, T.A. Prehabilitation: The Right Medicine for Older Frail Adults Anticipating Transcatheter Aortic Valve Replacement, Coronary Artery Bypass Graft, and Other Cardiovascular Care. Clin. Geriatr. Med. 2019, 35, 571–585. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.C.; Brown, C.H.; Sanjanwala, R.M.; McKelvie, R. “NEW” Prehabilitation: A 3-Way Approach to Improve Postoperative Survival and Health-Related Quality of Life in Cardiac Surgery Patients. Can. J. Cardiol. 2018, 34, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Gielen, S.; Simm, A. Frailty and Cardiac Rehabilitation: A Long-Neglected Connection. Eur. J. Prev. Cardiol. 2017, 24, 1488–1489. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hansen, T.B.; Berg, S.K.; Sibilitz, K.L.; Søgaard, R.; Thygesen, L.C.; Yazbeck, A.M.; Zwisler, A.D. Availability of, Referral to and Participation in Exercise-Based Cardiac Rehabilitation after Heart Valve Surgery: Results from the National CopenHeart Survey. Eur. J. Prev. Cardiol. 2015, 22, 710–718. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; de Bonis, M.; de Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the Management Ofvalvular Heart Disease Developed. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Osnabrugge, R.L.J.; Mylotte, D.; Head, S.J.; van Mieghem, N.M.; Nkomo, V.T.; LeReun, C.M.; Bogers, A.J.J.C.; Piazza, N.; Kappetein, A.P. Aortic Stenosis in the Elderly. J. Am. Coll. Cardiol. 2013, 62, 1002–1012. [Google Scholar] [CrossRef]
- Olsson, K.; Nilsson, J.; Hörnsten, Å.; Näslund, U. Patients’ Self-Reported Function, Symptoms and Health-Related Quality of Life before and 6 Months after Transcatheter Aortic Valve Implantation and Surgical Aortic Valve Replacement. Eur. J. Cardiovasc. Nurs. 2017, 16, 213–221. [Google Scholar] [CrossRef]
- Abdul-Jawad Altisent, O.; Puri, R.; Regueiro, A.; Chamandi, C.; Rodriguez-Gabella, T.; del Trigo, M.; Campelo-Parada, F.; Couture, T.; Marsal, J.; Cote, M.; et al. Predictors and Association With Clinical Outcomes of the Changes in Exercise Capacity After Transcatheter Aortic Valve Replacement. Circulation 2017, 136, 632–643. [Google Scholar] [CrossRef]
- Eichler, S.; Salzwedel, A.; Harnath, A.; Butter, C.; Wegscheider, K.; Chiorean, M.; Völler, H.; Reibis, R. Nutrition and Mobility Predict All-Cause Mortality in Patients 12 Months after Transcatheter Aortic Valve Implantation. Clin. Res. Cardiol. 2018, 107, 304–311. [Google Scholar] [CrossRef]
- Sathananthan, J.; Lauck, S.; Piazza, N.; Martucci, G.; Kim, D.H.; Popma, J.J.; Asgar, A.W.; Perrault, L.P.; Lefèvre, T.; Labinaz, M.; et al. Habitual Physical Activity in Older Adults Undergoing TAVR. JACC Cardiovasc. Interv. 2019, 12, 781–789. [Google Scholar] [CrossRef]
- Van Erck, D.; Dolan, C.D.; Limpens, J.; Scholte op Reimer, W.J.M.; Henriques, J.; Delewi, R.; Schoufour, J.D. Preprocedural Muscle Strength and Physical Performance Are Associated with Mortality in Frail Older Patients Undergoing Transcatheter Aortic Valve Implementation: A Systematic Review and Meta-Analysis. Age Ageing 2022, 51, afac211. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Green, P.; Maurer, M.; Lazarte, R.; Kuzniecky, J.R.; Hung, M.Y.; Garcia, M.; Kodali, S.; Harris, T. Relationship between Accelerometer-Measured Activity and Self-Reported or Performance-Based Function in Older Adults with Severe Aortic Stenosis. Curr. Geriatr. Rep. 2015, 4, 377–384. [Google Scholar] [CrossRef]
- Bertschi, D.; Kiss, C.M.; Schoenenberger, A.W.; Stuck, A.E.; Kressig, R.W. Sarcopenia in Patients Undergoing Transcatheter Aortic Valve Implantation (TAVI): A Systematic Review of the Literature. J. Nutr. Health Aging 2021, 25, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, L.Z.; Harker, J.O.; Salva, A.; Guigoz, Y.; Vellas, B. Screening for Undernutrition in Geriatric Practice: Developing the Short-Form Mini-Nutritional Assessment (MNA-SF). J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M366–M372. [Google Scholar] [CrossRef]
- Hergenroeder, A.L.; Barone Gibbs, B.; Kotlarczyk, M.P.; Kowalsky, R.J.; Perera, S.; Brach, J.S. Accuracy of Objective Physical Activity Monitors in Measuring Steps in Older Adults. Gerontol. Geriatr. Med. 2018, 4, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Matthews, C.E.; Chen, K.Y.; Freedson, P.S.; Buchowski, M.S.; Beech, B.M.; Pate, R.R.; Troiano, R.P. Amount of Time Spent in Sedentary Behaviors in the United States, 2003–2004. Am. J. Epidemiol. 2008, 167, 875–881. [Google Scholar] [CrossRef]
- Tudor-Locke, C.; Han, H.; Aguiar, E.J.; Barreira, T.v.; Schuna, J.M.; Kang, M.; Rowe, D.A. How Fast Is Fast Enough? Walking Cadence (Steps/Min) as a Practical Estimate of Intensity in Adults: A Narrative Review. Br. J. Sports Med. 2018, 52, 776–788. [Google Scholar] [CrossRef]
- Tudor-Locke, C.; Craig, C.L.; Aoyagi, Y.; Bell, R.C.; Croteau, K.A.; de Bourdeaudhuij, I.; Ewald, B.; Gardner, A.W.; Hatano, Y.; Lutes, L.D.; et al. How Many Steps/Day Are Enough? For Older Adults and Special Populations. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 80. [Google Scholar] [CrossRef]
- Adams, B.; Fidler, K.; Demoes, N.; Aguiar, E.J.; Ducharme, S.W.; McCullough, A.K.; Moore, C.C.; Tudor-Locke, C.; Thomas, D. Cardiometabolic Thresholds for Peak 30-Min Cadence and Steps/Day. PLoS ONE 2019, 14, e0219933. [Google Scholar] [CrossRef]
- Friedman, B.; Heisel, M.J.; Delavan, R.L. Psychometric Properties of the 15-Item Geriatric Depression Scale in Functionally Impaired, Cognitively Intact, Community-Dwelling Elderly Primary Care Patients. J. Am. Geriatr. Soc. 2005, 53, 1570–1576. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. Mini-Mental State” a Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Eifert, G.H.; Thompson, R.N.; Zvolensky, M.J.; Edwards, K.; Frazer, N.L.; Haddad, J.W.; Davig, J. The Cardiac Anxiety Questionnaire: Development and Preliminary Validity. Behav. Res. Ther. 2000, 38, 1039–1053. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A Short Physical Performance Battery Assessing Lower Extremity Function: Association With Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W.; Crouch, R.H. Two-Minute Step Test of Exercise Capacity: Systematic Review of Procedures, Performance, and Clinimetric Properties. J. Geriatr. Phys. Ther. 2019, 42, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.E.; Kon, S.S.C.; Canavan, J.L.; Patel, M.S.; Clark, A.L.; Nolan, C.M.; Polkey, M.I.; Man, W.D.C. The Five-Repetition Sit-to-Stand Test as a Functional Outcome Measure in COPD. Thorax 2013, 68, 1015–1020. [Google Scholar] [CrossRef]
- Hamirudin, A.H.; Charlton, K.; Walton, K. Outcomes Related to Nutrition Screening in Community Living Older Adults: A Systematic Literature Review. Arch. Gerontol. Geriatr. 2016, 62, 9–25. [Google Scholar] [CrossRef]
- Norman, K.; Stobäus, N.; Gonzalez, M.C.; Schulzke, J.D.; Pirlich, M. Hand Grip Strength: Outcome Predictor and Marker of Nutritional Status. Clin. Nutr. 2011, 30, 135–142. [Google Scholar] [CrossRef]
- Pilgrim, A.L.; Robinson, S.M.; Sayer, A.A.; Roberts, H.C. An Overview of Appetite Decline in Older People. Nurs. Older People 2015, 27, 29–35. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Rwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, E72–E227. [Google Scholar] [CrossRef]
- Hansen, B.H.; Kolle, E.; Dyrstad, S.M.; Holme, I.; Anderssen, S.A. Accelerometer-Determined Physical Activity in Adults and Older People. Med. Sci. Sports Exerc. 2012, 44, 266–272. [Google Scholar] [CrossRef]
- Ungar, A.; Mannarino, G.; van der Velde, N.; Baan, J.; Thibodeau, M.P.; Masson, J.B.; Santoro, G.; van Mourik, M.; Jansen, S.; Deutsch, C.; et al. Comprehensive Geriatric Assessment in Patients Undergoing Transcatheter Aortic Valve Implantation—Results from the CGATAVI Multicentre Registry. BMC Cardiovasc. Disord. 2018, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Michie, S.; van Stralen, M.M.; West, R. The Behaviour Change Wheel: A New Method for Characterising and Designing Behaviour Change Interventions. Implement. Sci. 2011, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Azzolino, D.; Arosio, B.; Marzetti, E.; Calvani, R.; Cesari, M. Nutritional Status as a Mediator of Fatigue and Its Underlying Mechanisms in Older People. Nutrients 2020, 12, 444. [Google Scholar] [CrossRef] [PubMed]
- De Lima, T.R.; Silva, D.A.S.; de Castro, J.A.C.; Christofaro, D.G.D. Handgrip Strength and Associated Sociodemographic and Lifestyle Factors: A Systematic Review of the Adult Population. J. Bodyw. Mov. Ther. 2017, 21, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Bäz, L.; Puscholt, M.; Lasch, C.; Diab, M.; Möbius-winkler, S.; Schulze, P.C.; Dannberg, G.; Franz, M. Delayed Improvement of Depression and Anxiety after Transcatheter Aortic Valve Implantation (Tavi) in Stages of Extended Extra-valvular Cardiac Damage. J. Clin. Med. 2021, 10, 1579. [Google Scholar] [CrossRef] [PubMed]
- Bäz, L.; Wiesel, M.; Möbius-Winkler, S.; Westphal, J.G.; Schulze, P.C.; Franz, M.; Dannberg, G. Depression and Anxiety in Elderly Patients with Severe Symptomatic Aortic Stenosis Persistently Improves after Transcatheter Aortic Valve Replacement (TAVR). Int. J. Cardiol. 2020, 309, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.; Bordado, J.; Peralta, M.; Gouveia, E.R.; Tesler, R.; Demetriou, Y.; Gomez Baya, D. Cross-Sectional and Prospective Relationship between Physical Activity and Depression Symptoms. Sci. Rep. 2020, 10, 16114. [Google Scholar] [CrossRef]
- Deutz, N.E.P.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznariç, Z.; Nair, K.S.; et al. Protein Intake and Exercise for Optimal Muscle Function with Aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef]
- Bloom, I.; Shand, C.; Cooper, C.; Robinson, S.; Baird, J. Diet Quality and Sarcopenia in Older Adults: A Systematic Review. Nutrients 2018, 10, 308. [Google Scholar] [CrossRef]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on Sports Cardiology and Exercise in Patients with Cardiovascular Disease. Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef]
- English, K.L.; Paddon-Jones, D. Protecting Muscle Mass and Function in Older Adults during Bed Rest. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Van den Helder, J.; Verlaan, S.; Tieland, M.; Scholten, J.; Mehra, S.; Visser, B. Digitally Supported Dietary Protein Counseling Changes Dietary Protein Intake, Sources, and Distribution in Community-Dwelling Older Adults. Nutrients 2021, 13, 502. [Google Scholar] [CrossRef] [PubMed]
| N | 112 |
|---|---|
| Demographics Age (years), mean ± SD | 81 ± 5 |
| Sex, male, n (%) | 66 (58) |
| BMI, mean ± SD | 26.6 ± 4.4 |
| Living with partner (%) | 60 (54) |
| Clinical characteristics Euroscore II, median (IQR) | 2.2 (1.6–3.0) |
| NYHA III/IV, n (%) | 53 (47) |
| COPD, n (%) | 13 (11.6) |
| AVA, mean ± SD | 0.76 ± 0.17 |
| LVEF, n (%) | |
| Good | 75 (66.4) |
| Moderate | 34 (30.2) |
| Poor | 2 (1.7) |
| Transfemoral TAVI, n (%) | 102 (91.9) |
| History of coronary disease, n (%) | 32 (29) |
| History of peripheral arterial disease, n (%) | 11 (10) |
| MNA-SF malnutrition risk, n (%) | 30 (27) |
| Psychosocial factors | |
| Cardiac anxiety questionnaire, mean ± SD | 18 ± 9 |
| Geriatric depression score—15, median (IQR) | 3 (2–4) |
| Mini-mental state score, median (IQR) | 28 (27–29) |
| Dyspnea, n (%) | 51 (46) |
| Fatigue, median (IQR) | 5 (2–8) |
| Syncope, n (%) | 34 (30) |
| Pain, median (IQR) | 0 (0–5) |
| Fear of falling, median (IQR) | 2 (0–5) |
| Physical factors SPPB score, points, mean ± SD | 7.8 ± 2.8 |
| Handgrip strength, kg, mean ± SD | |
| Male | 36 ± 10 |
| Female | 24 ± 6 |
| TMST, steps, mean ± SD | 52 ± 15 |
| Chair stand test, seconds, mean ± SD | 15.7 ± 5.2 |
| Variable | Preprocedural | 30 Days | 6 Months | Adjusted Mixed Linear Model of Change per Month | ||
|---|---|---|---|---|---|---|
| β | 95% CI | p | ||||
| Nutritional status | ||||||
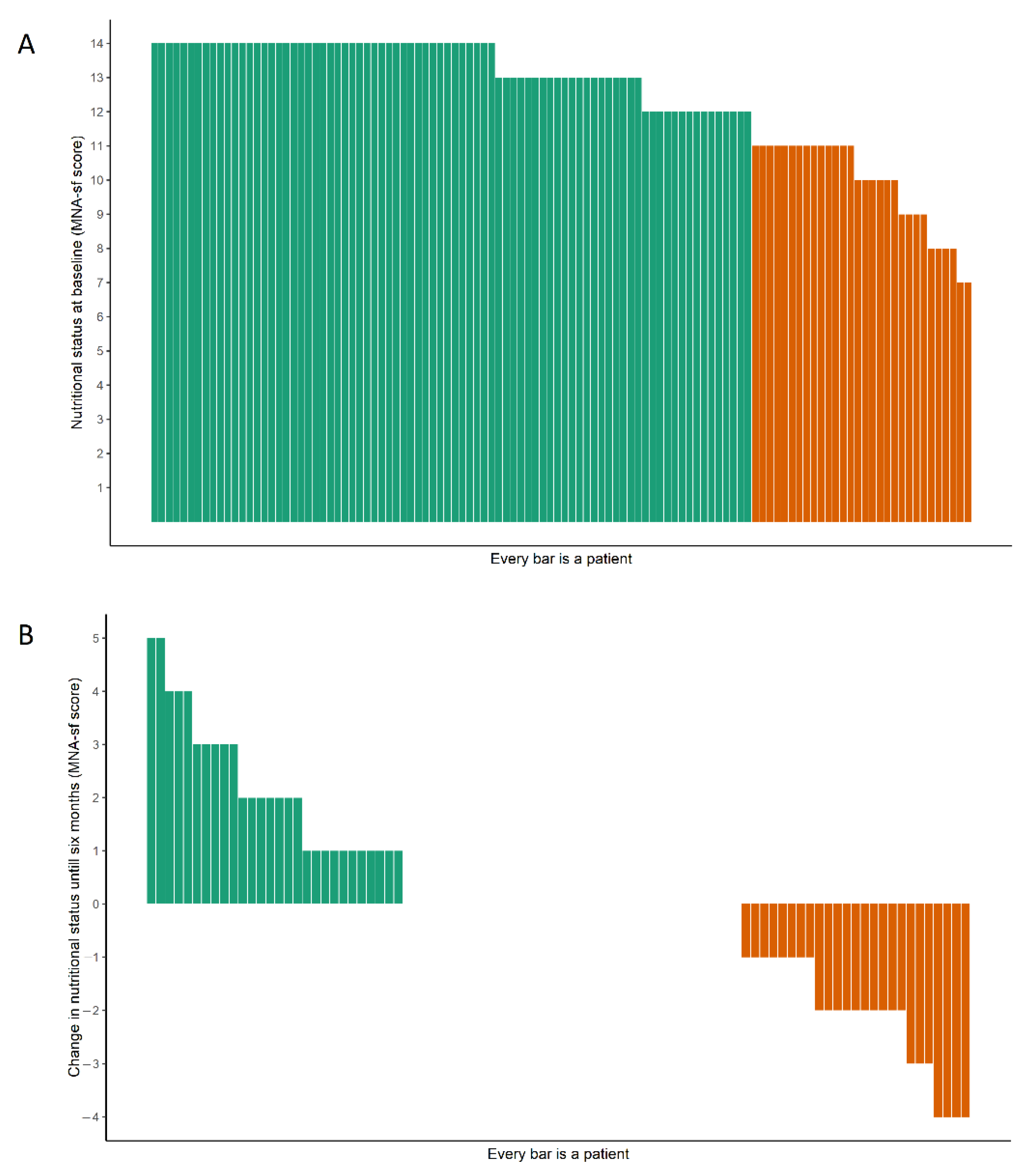
| MNA-SF score, median (IQR) | 13 (11–14) | 13 (12–14) | 13 (12–14) | 0.02 | −0.03, 0.07 | 0.50 |
| At risk of malnutrition 1, % | 27 | 24 | 22 | −0.81 | −3.38, 1.75 | 0.53 |
| Physical activity | ||||||
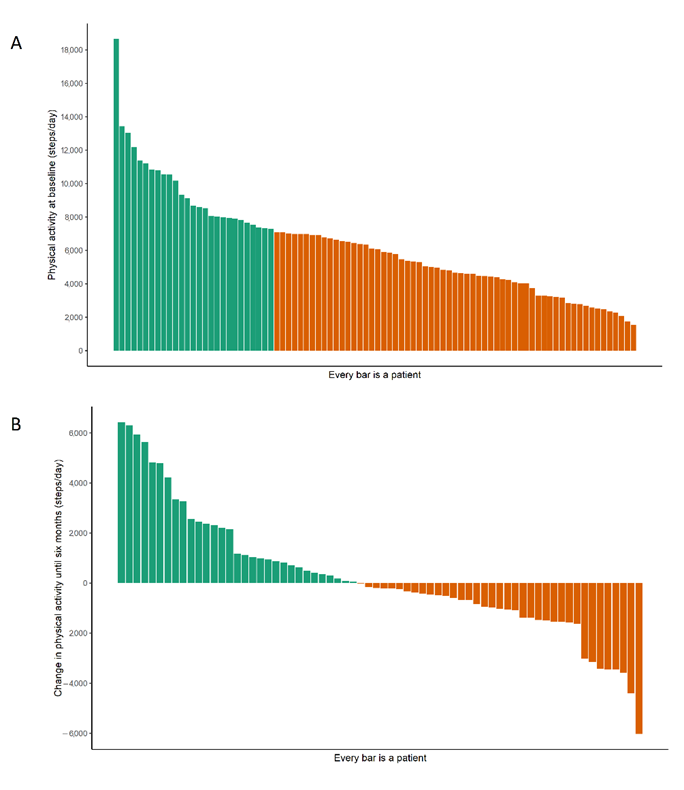
| Steps per day, mean ± SD | 6273 ± 3007 | 6708 ± 3113 | 6866 ± 3233 | 16 | –47, 79 | 0.62 |
| Low physical activity 2, % | 69 | 53 | 52 | −0.07 | −0.18, 0.03 | 0.16 |
| Peak 30 min cadence, mean | 66 ± 20 | 70 ± 22 | 70 ± 22 | 0.02 | −0.41, 0.45 | 0.94 |
| Low intensity (<70 steps/min), % | 61 | 51 | 52 | −0.01 | −0.11, 0.09 | 0.81 |
| Nutritional Status (MNA-SF Score) | Physical Activity (Steps per Day) | |||||
|---|---|---|---|---|---|---|
| β | 95% CI | p | β | 95% CI | p | |
| Body mass index, per point | 0.10 | 0.04, 0.15 | <0.01 | −162 | −265, −59 | <0.01 |
| COPD, yes | - | - | - | −1831 | −3318, −345 | 0.01 |
| Cognition, per point on the MMSE | 0.09 | 0.01, 0.17 | 0.03 | - | - | - |
| Fear of falling, per point on the NRS (1–10) | - | - | - | −130 | −240, −19 | 0.02 |
| Handgrip strength, per kg | 0.04 | 0.02, 0.07 | <0.01 | - | - | - |
| Pain, per point on the NRS (1–10) | −0.11 | −0.19, −0.04 | <0.01 | - | - | - |
| Physical performance, per point on the SPPB | - | - | - | 336 | 199, 476 | <0.01 |
| Δ Nutritional Status (MNA-SF Score) Preprocedural–6 Month | Δ Physical Activity (Steps per Day) Preprocedural–6 Months | |||||
|---|---|---|---|---|---|---|
| β | 95% CI | p | β | 95% CI | p | |
| Fatigue, per point on NRS (1–10) | −0.14 | −0.26, −0.03 | 0.01 | - | - | - |
| Depression, per point on GDS | −0.15 | −0.26, −0.03 | 0.01 | 276 | 96, 455 | <0.01 |
| Handgrip strength, per kg | - | - | - | 71 | 17, 124 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Erck, D.; Dolman, C.D.; Scholte op Reimer, W.J.M.; Henriques, J.P.; Weijs, P.J.M.; Delewi, R.; Schoufour, J.D. The Trajectory of Nutritional Status and Physical Activity before and after Transcatheter Aortic Valve Implantation. Nutrients 2022, 14, 5137. https://doi.org/10.3390/nu14235137
van Erck D, Dolman CD, Scholte op Reimer WJM, Henriques JP, Weijs PJM, Delewi R, Schoufour JD. The Trajectory of Nutritional Status and Physical Activity before and after Transcatheter Aortic Valve Implantation. Nutrients. 2022; 14(23):5137. https://doi.org/10.3390/nu14235137
Chicago/Turabian Stylevan Erck, Dennis, Christine D. Dolman, Wilma J. M. Scholte op Reimer, José P. Henriques, Peter J. M. Weijs, Ronak Delewi, and Josje D. Schoufour. 2022. "The Trajectory of Nutritional Status and Physical Activity before and after Transcatheter Aortic Valve Implantation" Nutrients 14, no. 23: 5137. https://doi.org/10.3390/nu14235137
APA Stylevan Erck, D., Dolman, C. D., Scholte op Reimer, W. J. M., Henriques, J. P., Weijs, P. J. M., Delewi, R., & Schoufour, J. D. (2022). The Trajectory of Nutritional Status and Physical Activity before and after Transcatheter Aortic Valve Implantation. Nutrients, 14(23), 5137. https://doi.org/10.3390/nu14235137






