The Impact of Exercise on Improving Body Composition and PSA in High-Risk Prostate Cancer Patients on Androgen-Deprivation Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients Enrollment
2.2. Androgen Deprivation Therapy Protocol
2.3. Exercise Program
2.4. Clinicopathological Data
2.5. Body Composition Measurement
2.6. Handgrip Strength Assessment
2.7. Laboratory Examinations
2.8. Statistical Analysis
3. Results
3.1. Baseline Demographics
3.2. Comparison between the Exercise and Usual Care Group before, during, and after Intervention
3.2.1. At Six Months Follow-Up
3.2.2. At Twelve Months Follow-Up
3.3. The Association between Body Composition and Laboratory Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Crawford, E.D.; Higano, C.S.; Shore, N.D.; Hussain, M.; Petrylak, D.P. Treating Patients with Metastatic Castration Resistant Prostate Cancer: A Comprehensive Review of Available Therapies. J. Urol. 2015, 194, 1537–1547. [Google Scholar] [CrossRef] [PubMed]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II-2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2021, 79, 263–282. [Google Scholar] [CrossRef]
- Eastham, J.A.; Boorjian, S.A.; Kirkby, E. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline. J. Urol. 2022, 208, 505–507. [Google Scholar] [CrossRef]
- Cormie, P.; Galvao, D.A.; Spry, N.; Joseph, D.; Chee, R.; Taaffe, D.R.; Chambers, S.K.; Newton, R.U. Can supervised exercise prevent treatment toxicity in patients with prostate cancer initiating androgen-deprivation therapy: A randomised controlled trial. BJU Int. 2015, 115, 256–266. [Google Scholar] [CrossRef]
- Owen, P.J.; Daly, R.M.; Livingston, P.M.; Fraser, S.F. Lifestyle guidelines for managing adverse effects on bone health and body composition in men treated with androgen deprivation therapy for prostate cancer: An update. Prostate Cancer Prostatic Dis. 2017, 20, 137–145. [Google Scholar] [CrossRef]
- Bienz, M.; Saad, F. Androgen-deprivation therapy and bone loss in prostate cancer patients: A clinical review. Bonekey Rep. 2015, 4, 716. [Google Scholar] [CrossRef]
- Kiwata, J.L.; Dorff, T.B.; Todd Schroeder, E.; Salem, G.J.; Lane, C.J.; Rice, J.C.; Gross, M.E.; Dieli-Conwright, C.M. A pilot randomised controlled trial of a periodised resistance training and protein supplementation intervention in prostate cancer survivors on androgen deprivation therapy. BMJ Open 2017, 7, e016910. [Google Scholar] [CrossRef]
- Cushen, S.J.; Power, D.G.; Murphy, K.P.; McDermott, R.; Griffin, B.T.; Lim, M.; Daly, L.; MacEneaney, P.; Sullivan, K.O.; Prado, C.M.; et al. Impact of body composition parameters on clinical outcomes in patients with metastatic castrate-resistant prostate cancer treated with docetaxel. Clin. Nutr. ESPEN 2016, 13, e39–e45. [Google Scholar] [CrossRef]
- McDonald, A.M.; Swain, T.A.; Mayhew, D.L.; Cardan, R.A.; Baker, C.B.; Harris, D.M.; Yang, E.S.; Fiveash, J.B. CT Measures of Bone Mineral Density and Muscle Mass Can Be Used to Predict Noncancer Death in Men with Prostate Cancer. Radiology 2017, 282, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.W.; Fairey, A.S.; Boule, N.G.; Field, C.J.; Wharton, S.A.; Courneya, K.S. Effects of Exercise on Cardiorespiratory Fitness and Biochemical Progression in Men With Localized Prostate Cancer Under Active Surveillance: The ERASE Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Keilani, M.; Hasenoehrl, T.; Baumann, L.; Ristl, R.; Schwarz, M.; Marhold, M.; Sedghi Komandj, T.; Crevenna, R. Effects of resistance exercise in prostate cancer patients: A meta-analysis. Support. Care Cancer 2017, 25, 2953–2968. [Google Scholar] [CrossRef] [PubMed]
- Wall, B.A.; DA, G.A.; Fatehee, N.; Taaffe, D.R.; Spry, N.; Joseph, D.; Hebert, J.J.; Newton, R.U. Exercise Improves V O2max and Body Composition in Androgen Deprivation Therapy-treated Prostate Cancer Patients. Med. Sci. Sports Exerc. 2017, 49, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Galvao, D.A.; Nosaka, K.; Taaffe, D.R.; Spry, N.; Kristjanson, L.J.; McGuigan, M.R.; Suzuki, K.; Yamaya, K.; Newton, R.U. Resistance training and reduction of treatment side effects in prostate cancer patients. Med. Sci. Sports Exerc. 2006, 38, 2045–2052. [Google Scholar] [CrossRef]
- Chang, A.J.; Autio, K.A.; Roach, M., 3rd; Scher, H.I. High-risk prostate cancer-classification and therapy. Nat. Rev. Clin. Oncol. 2014, 11, 308–323. [Google Scholar] [CrossRef]
- Cheng, K.H.; Wu, N.K.; Chen, C.T.; Hsu, C.Y.; Lin, Y.A.; Luo, J.J.; Lee, L.A.; Chuang, H.H. Effectiveness and response differences of a multidisciplinary workplace health promotion program for healthcare workers. Front. Med. 2022, 9, 930165. [Google Scholar] [CrossRef]
- Chuang, H.H.; Lin, R.H.; Chen, J.Y.; Yeh, W.C.; Lin, H.F.; Ueng, S.W.; Hsu, K.H. Effectiveness of a multi-faceted intervention among elementary school children. Medicine 2019, 98, e15079. [Google Scholar] [CrossRef]
- Hangartner, T.N.; Warner, S.; Braillon, P.; Jankowski, L.; Shepherd, J. The Official Positions of the International Society for Clinical Densitometry: Acquisition of dual-energy X-ray absorptiometry body composition and considerations regarding analysis and repeatability of measures. J. Clin. Densitom. 2013, 16, 520–536. [Google Scholar] [CrossRef]
- Lin, Y.C.; Lin, G.; Yeh, T.S. Visceral-to-subcutaneous fat ratio independently predicts the prognosis of locally advanced gastric cancer-----highlighting the role of adiponectin receptors and PPARalpha, beta/ delta. Eur. J. Surg. Oncol. 2021, 47, 3064–3073. [Google Scholar] [CrossRef]
- Newton, R.U.; Jeffery, E.; Galvao, D.A.; Peddle-McIntyre, C.J.; Spry, N.; Joseph, D.; Denham, J.W.; Taaffe, D.R. Body composition, fatigue and exercise in patients with prostate cancer undergoing androgen-deprivation therapy. BJU Int. 2018, 122, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Logan, L.; Toohey, K.; Kavanagh, P.S.; Paterson, C.; McKune, A.J. The Effect of Exercise on Body Composition and Physical Performance in Prostate Cancer Patients Undergoing Androgen Deprivation Therapy (ADT): A Narrative Synthesis. Semin. Oncol. Nurs. 2020, 36, 151067. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.K.; Dorff, T.B.; Todd Schroeder, E.; Lane, C.J.; Gross, M.E.; Dieli-Conwright, C.M. Impact of resistance training on body composition and metabolic syndrome variables during androgen deprivation therapy for prostate cancer: A pilot randomized controlled trial. BMC Cancer 2018, 18, 368. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.W.; Antonelli, J.; Masko, E.M.; Broadwater, G.; Lascola, C.D.; Fels, D.; Dewhirst, M.W.; Dyck, J.R.; Nagendran, J.; Flores, C.T.; et al. Exercise modulation of the host-tumor interaction in an orthotopic model of murine prostate cancer. J. Appl. Physiol. 2012, 113, 263–272. [Google Scholar] [CrossRef]
- Segal, R.J.; Reid, R.D.; Courneya, K.S.; Sigal, R.J.; Kenny, G.P.; Prud’Homme, D.G.; Malone, S.C.; Wells, G.A.; Scott, C.G.; Slovinec D’Angelo, M.E. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J. Clin. Oncol. 2009, 27, 344–351. [Google Scholar] [CrossRef]
- Hvid, T.; Lindegaard, B.; Winding, K.; Iversen, P.; Brasso, K.; Solomon, T.P.; Pedersen, B.K.; Hojman, P. Effect of a 2-year home-based endurance training intervention on physiological function and PSA doubling time in prostate cancer patients. Cancer Causes Control 2016, 27, 165–174. [Google Scholar] [CrossRef]
- Toliusiene, J.; Lesauskaite, V. The nutritional status of older men with advanced prostate cancer and factors affecting it. Support. Care Cancer 2004, 12, 716–719. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Feng, L.J.; Zhang, K.P.; Tang, M.; Song, M.M.; Ruan, G.T.; Zhang, X.W.; Li, W.; Zhou, F.X.; et al. The Application of Fat-Free Mass Index for Survival Prediction in Cancer Patients With Normal and High Body Mass Index. Front. Nutr. 2021, 8, 714051. [Google Scholar] [CrossRef]
- Lopez, P.; Newton, R.U.; Taaffe, D.R.; Singh, F.; Buffart, L.M.; Spry, N.; Tang, C.; Saad, F.; Galvao, D.A. Associations of fat and muscle mass with overall survival in men with prostate cancer: A systematic review with meta-analysis. Prostate Cancer Prostatic Dis. 2021. Online ahead of print. [Google Scholar] [CrossRef]
| Variable | Total (n = 45) | Exercise (n = 31) | Usual (n = 14) | p-Value |
|---|---|---|---|---|
| Age, years | 67.4 ± 8.0 | 66.2 ± 7.2 | 70.1 ± 9.3 | 0.128 |
| Body mass index, kg/m2 | 25.5 ± 3.6 | 24.8 ± 3.7 | 27.1 ± 2.8 | 0.052 |
| Smoking | 5 (11.1) | 2 (6.5) | 3 (21.4) | 0.166 |
| Alcohol | 7 (15.6) | 3 (9.7) | 4 (28.6) | 0.180 |
| Betel nut | 1 (2.2) | 0 (0.0) | 1 (7.1) | 0.311 |
| Hypertension | 21 (46.7) | 13 (41.9) | 8 (57.1) | 0.520 |
| Cerebrovascular accident | 1 (2.2) | 0 (0.0) | 1 (7.1) | 0.311 |
| Diabetes mellitus | 10 (22.2) | 7 (22.6) | 3 (21.4) | 1.000 |
| Surgery | 20 (44.4) | 15 (48.4) | 5 (35.7) | 0.525 |
| Gleason Score | 8.51 ± 0.99 | 8.61 ± 0.88 | 8.29 ± 1.20 | 0.311 |
| Stage | 0.677 | |||
| 3B | 13 (28.9) | 9 (29.0) | 4 (28.6) | |
| 3C | 4 (8.9) | 3 (9.7) | 1 (7.1) | |
| 4A | 8 (17.8) | 4 (12.9) | 4 (28.6) | |
| 4B | 20 (44.4) | 15 (48.4) | 5 (35.7) | |
| Body composition | ||||
| Total fat, g | 23,005.4 ± 6661.6 | 22,002.2 ± 7125.3 | 25,155.1 ± 5121.5 | 0.146 |
| Total lean, g | 43,552.6 ± 4881.8 | 42,721.3 ± 5301.4 | 45,333.9 ± 3334.8 | 0.099 |
| Parameter/Time | Exercise (n = 31) | Usual (n = 14) | Mean Difference † (95% CI) | p for Interaction † | ||
|---|---|---|---|---|---|---|
| Mean (SE) | Change % | Mean (SE) | Change % | |||
| BMI, kg/m2 | ||||||
| Baseline | 25.5 (0.1) | - | 25.8 (0.1) | - | - | - |
| 6th month | 26.3 (0.3) | 3.19 | 25.2 (0.4) | −2.26 | 1.40 (0.44, 2.35) | 0.004 * |
| 12th month | 26.5 (0.3) | 4.18 | 25.9 (0.3) | 0.72 | 0.88 (−0.01, 1.76) | 0.052 |
| Fat at arm, g | ||||||
| Baseline | 2493.6 (70.1) | - | 2590.8 (80.3) | - | - | - |
| 6th month | 2756.4 (94.6) | 10.54 | 2765.2 (81.6) | 6.73 | 88.3 (−96.7, 273.4) | 0.349 |
| 12th month | 2868.3 (101.7) | 15.02 | 2770.3 (82.4) | 6.93 | 195.1 (−1.1, 391.4) | 0.051 |
| Fat at trunk, g | ||||||
| Baseline | 12,313.8 (357.2) | - | 13,390.9 (496.5) | - | - | - |
| 6th month | 13,462.4 (437.9) | 9.33 | 14,010.4 (548.3) | 4.63 | 529.1 (−285.0, 1343.1) | 0.203 |
| 12th month | 13,885.0 (454.0) | 12.76 | 14,163.1 (448.6) | 5.77 | 799.0 (−159.8, 1757.7) | 0.102 |
| Fat at leg, g | ||||||
| Baseline | 6088.7 (218.1) | - | 6198.0 (234.4) | - | - | - |
| 6th month | 6853.1 (253.1) | 12.55 | 6727.3 (256.5) | 8.54 | 235.0 (−236.2, 706.2) | 0.328 |
| 12th month | 7140.8 (264.7) | 17.28 | 6948.0 (234.8) | 12.10 | 302.0 (−171.5, 775.5) | 0.211 |
| Total fat, g | ||||||
| Baseline | 22,698.7 (878.7) | - | 23,827.7 (711.3) | - | - | - |
| 6th month | 24,917.3 (840.2) | 9.77 | 32,404.1 (3799.4) | 35.99 | −6357.7 (−13,912.3, 1196.9) | 0.099 |
| 12th month | 26,526.2 (1237.9) | 16.86 | 27,518.3 (2044.6) | 15.49 | 137.0 (−4517.4, 4791.3) | 0.954 |
| Lean at arm, g | ||||||
| Baseline | 4799.9 (72.9) | - | 4691.9 (108.4) | - | - | - |
| 6th month | 4709.8 (82.5) | −1.88 | 4369.7 (129.3) | −6.87 | 232.03 (1.97, 462.10) | 0.048 * |
| 12th month | 4632.8 (85.3) | −3.48 | 4430.6 (103.1) | −5.57 | 94.23 (−104.29, 292.75) | 0.352 |
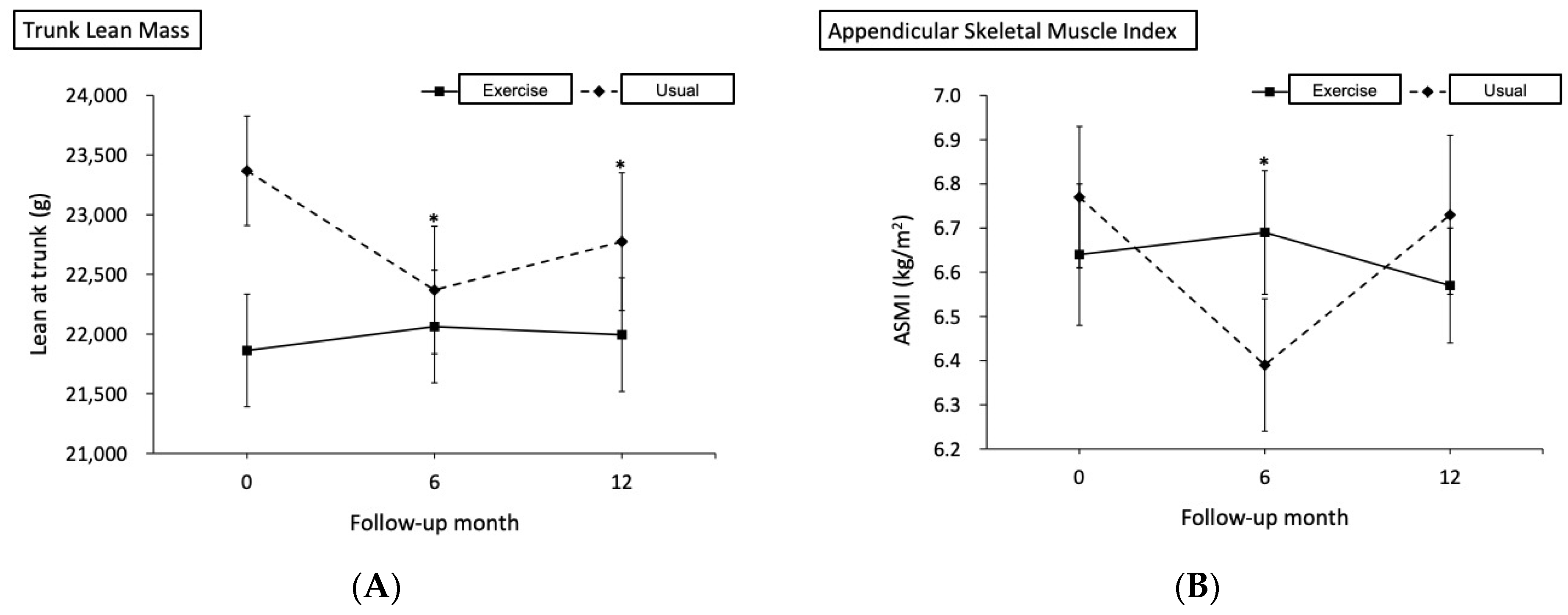
| Lean at trunk, g | ||||||
| Baseline | 22,226.7 (258.5) | - | 22,583.2 (268.5) | - | - | - |
| 6th month | 22,427.2 (352.2) | 0.90 | 21,584.9 (407.3) | −4.42 | 1198.8 (418.2, 1979.3) | 0.003 * |
| 12th month | 22,363.1 (380.7) | 0.61 | 21,971.2 (433.3) | −2.71 | 748.3 (34.1, 1462.6) | 0.040 * |
| Lean at leg, g | ||||||
| Baseline | 13,858.1 (170.0) | - | 13,360.6 (297.3) | - | - | - |
| 6th month | 14,118.2 (215.7) | 1.88 | 12,626.9 (377.6) | −5.49 | 993.8 (380.2, 1607.4) | 0.002 * |
| 12th month | 13,848.3 (237.6) | −0.07 | 13,277.2 (361.7) | −0.62 | 73.7 (−687.6, 835.0) | 0.850 |
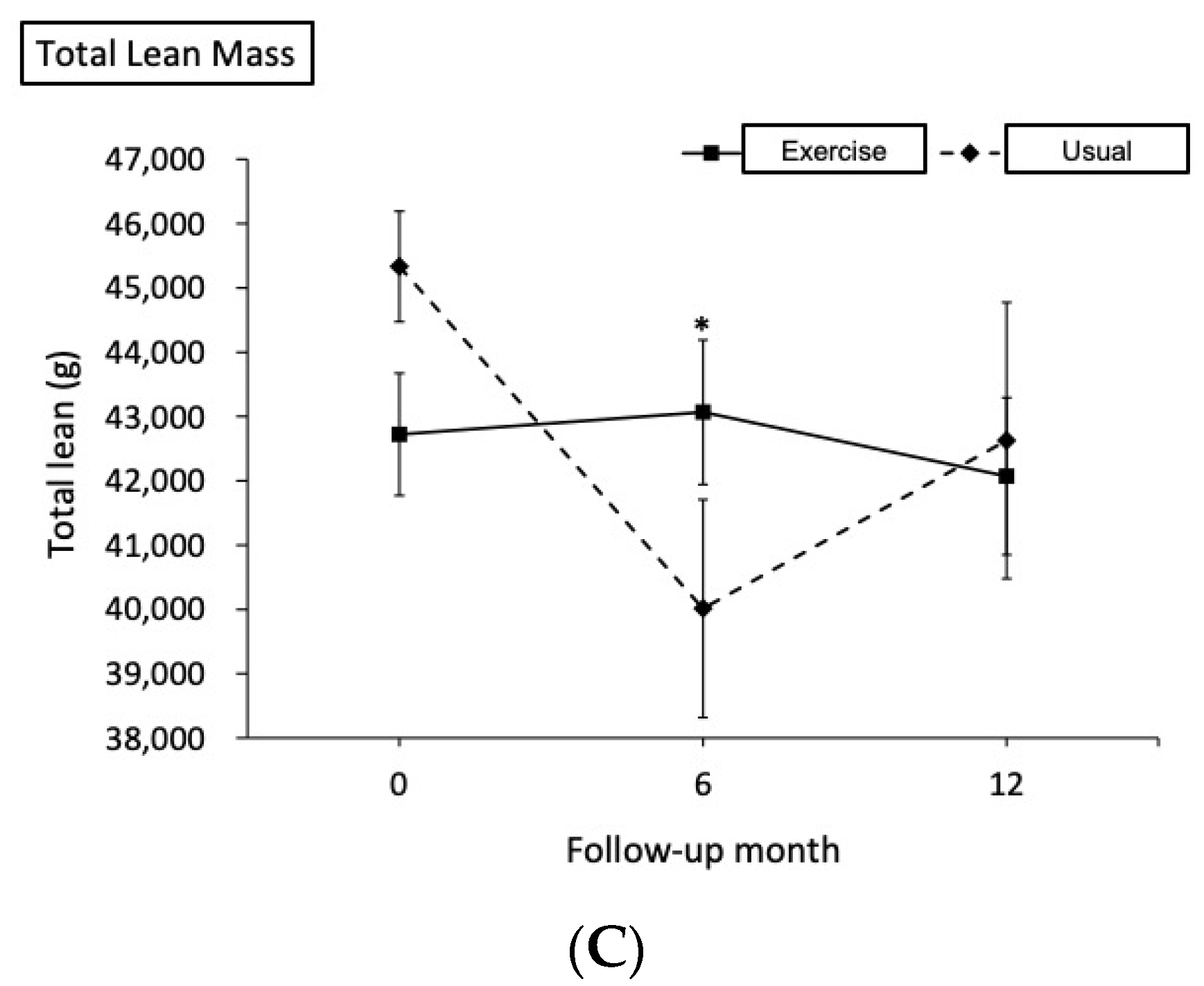
| Total lean, g | ||||||
| Baseline | 43,530.3 (265.1) | - | 43,573.2 (436.6) | - | - | - |
| 6th month | 43,875.1 (930.1) | 0.79 | 38,252.4 (1782.8) | −12.21 | 5666 (1547, 9785) | 0.007 * |
| 12th month | 42,867.1 (1224.4) | −1.52 | 40,395.6 (2289.7) | −7.29 | 2514 (−2916, 7945) | 0.364 |
| Android, %Fat | ||||||
| Baseline | 37.6 (0.8) | - | 39.2 (1.0) | - | - | - |
| 6th month | 39.3 (0.7) | 4.73 | 41.2 (1.0) | 5.16 | −0.24 (−2.27, 1.78) | 0.813 |
| 12th month | 40.6 (0.7) | 7.94 | 41.0 (1.1) | 4.63 | 1.17 (−1.23, 3.56) | 0.339 |
| Gynoid, %Fat | ||||||
| Baseline | 30.9 (0.6) | - | 31.8 (0.7) | - | - | - |
| 6th month | 33.3 (0.7) | 7.75 | 34.5 (0.5) | 8.30 | −0.25 (−1.91, 1.41) | 0.769 |
| 12th month | 34.7 (0.7) | 12.22 | 33.9 (0.5) | 6.34 | 1.76 (0.002, 3.51) | 0.050 |
| ASMI, kg/m2 | ||||||
| Baseline | 6.78 (0.07) | - | 6.48 (0.11) | - | - | - |
| 6th month | 6.83 (0.07) | 0.78 | 6.10 (0.12) | −5.88 | 0.43 (0.16, 0.71) | 0.002 * |
| 12th month | 6.70 (0.09) | −1.10 | 6.43 (0.15) | −0.88 | −0.02 (−0.35, 0.31) | 0.915 |
| Parameter/Time | Exercise (n = 31) | Usual (n = 14) | Mean Difference † (95% CI) | p for Interaction † | ||
|---|---|---|---|---|---|---|
| Mean (SE) | Change % | Mean (SE) | Change % | |||
| Handgrip strength, kg | ||||||
| Baseline | 34.64 (1.04) | - | 32.08 (1.73) | - | - | - |
| 6th month | 35.12 (0.94) | 1.41 | 29.11 (2.14) | −9.25 | 3.45 (0.20, 6.71) | 0.038 * |
| 12th month | 34.95 (1.05) | 0.91 | 31.88 (1.70) | −0.61 | 0.51 (−1.78, 2.80) | 0.662 |
| Para. area, cm2 | ||||||
| Baseline | 53.26 (0.95) | - | 49.75 (1.87) | - | - | - |
| 6th month | 52.57 (1.20) | −1.29 | 46.96 (1.72) | −5.62 | 2.11 (−0.61, 4.83) | 0.128 |
| 12th month | 52.98 (1.18) | −0.53 | 47.31 (1.79) | −4.92 | 2.16 (−1.40, 5.73) | 0.235 |
| Para. density, HU | ||||||
| Baseline | 38.81 (1.44) | - | 37.33 (2.11) | - | - | - |
| 6th month | 38.00 (1.56) | −2.08 | 33.41 (2.13) | −10.51 | 3.12 (0.88, 5.35) | 0.006 * |
| 12th month | 35.40 (1.56) | −8.80 | 30.80 (2.90) | −17.49 | 3.12 (−1.01, 7.24) | 0.139 |
| Ps. area, cm2 | ||||||
| Baseline | 16.79 (0.67) | - | 14.42 (0.75) | - | - | - |
| 6th month | 15.17 (0.62) | −9.67 | 12.50 (0.95) | −13.35 | 0.30 (−1.40, 2.00) | 0.728 |
| 12th month | 14.87 (0.51) | −11.47 | 13.13 (0.81) | −8.98 | −0.63 (−2.47, 1.20) | 0.500 |
| Ps. density, HU | ||||||
| Baseline | 42.88 (0.94) | - | 43.39 (2.17) | - | - | - |
| 6th month | 42.45 (0.77) | −1.00 | 44.50 (3.78) | 2.56 | −1.54 (−7.80, 4.72) | 0.629 |
| 12th month | 41.54 (0.80) | −3.13 | 43.44 (3.71) | 0.13 | −1.40 (−8.86, 6.06) | 0.713 |
| SAT area, cm2 | ||||||
| Baseline | 116.77 (5.62) | - | 122.47 (6.75) | - | - | - |
| 6th month | 133.76 (8.09) | 14.56 | 131.35 (6.87) | 7.25 | 8.11 (−5.72, 21.95) | 0.250 |
| 12th month | 140.42 (7.86) | 20.26 | 132.96 (8.19) | 8.57 | 13.16 (−5.75, 32.08) | 0.173 |
| SAT density, HU | ||||||
| Baseline | −87.97 (2.14) | - | −90.28 (1.55) | - | - | - |
| 6th month | −91.86 (1.20) | 4.42 | −92.50 (1.38) | 2.46 | −1.67 (−6.17, 2.83) | 0.467 |
| 12th month | −93.45 (1.44) | 6.23 | −90.92 (1.70) | 0.72 | −4.83 (−11.09, 1.42) | 0.130 |
| VAT area, cm2 | ||||||
| Baseline | 172.23 (9.37) | - | 202.89 (17.66) | - | - | - |
| 6th month | 196.49 (10.87) | 14.09 | 202.42 (18.04) | −0.23 | 24.73 (3.75, 45.71) | 0.021 * |
| 12th month | 203.28 (12.13) | 18.03 | 206.02 (19.71) | 1.54 | 27.93 (3.26, 52.59) | 0.026 * |
| VAT density, HU | ||||||
| Baseline | −92.96 (1.72) | - | −95.04 (1.21) | - | - | - |
| 6th month | −94.62 (1.16) | 1.79 | −95.37 (1.55) | 0.35 | −1.34 (−4.71, 2.04) | 0.438 |
| 12th month | −96.77 (1.25) | 4.10 | −93.46 (1.74) | −1.66 | −5.39 (−10.36, −0.42) | 0.034 * |
| Parameter/Time | Exercise (n = 31) | Usual (n = 14) | Mean Difference (95% CI) † | p for Interaction † | ||
|---|---|---|---|---|---|---|
| Mean (SE) | Change % | Mean (SE) | Change % | |||
| Prognostic nutrition index | ||||||
| Baseline | 53.63 (0.78) | - | 55.65 (1.09) | - | - | - |
| 6th month | 51.70 (0.89) | −3.61 | 50.23 (1.54) | −9.74 | 3.48 (−0.12, 7.09) | 0.058 |
| 12th month | 50.58 (0.58) | −5.70 | 50.38 (1.61) | −9.47 | 2.21 (−0.93, 5.36) | 0.168 |
| Platelet lymphocyte ratio | ||||||
| Baseline | 150.15 (12.32) | - | 112.76 (11.13) | - | - | - |
| 6th month | 180.92 (14.73) | 20.49 | 217.18 (37.46) | 92.60 | −73.65 (−157.32, 10.02) | 0.084 |
| 12th month | 190.46 (19.60) | 26.85 | 169.12 (29.29) | 49.98 | −16.05 (−82.84, 50.74) | 0.638 |
| Total cholesterol, mg/dL | ||||||
| Baseline | 180.80 (7.10) | - | 210.85 (9.44) | - | - | - |
| 6th month | 193.94 (8.20) | 7.26 | 212.78 (15.44) | 0.91 | 11.20 (−16.44, 38.85) | 0.427 |
| 12th month | 189.53 (7.36) | 4.83 | 211.87 (15.92) | 0.48 | 7.71 (−24.61, 40.03) | 0.640 |
| PSA, ng/mL | ||||||
| Baseline | 247.86 (104.34) | - | 47.16 (13.98) | - | - | - |
| 6th month | −6.50 (8.10) | −102.62 | 16.77 (9.33) | −64.43 | −223.97 (−434.43, −13.51) | 0.037 * |
| 12th month | 7.51 (15.65) | −96.97 | 26.42 (11.36) | −43.99 | −219.60 (−437.25, −1.94) | 0.048 * |
| Parameters | 6th Month—Baseline | 12th Month—Baseline | ||
|---|---|---|---|---|
| B (95% CI) | p-Value | B (95% CI) | p-Value | |
| DXA parameters | ||||
| Fat, per 100 g | ||||
| Arm | 1.48 (0.41, 2.55) | 0.008 * | 0.47 (0.07, 0.88) | 0.023 * |
| Trunk | 0.18 (0.06, 0.29) | 0.004 * | 0.12 (0.04, 0.20) | 0.007 * |
| Leg | 0.76 (0.35, 1.18) | 0.001 * | 0.23 (0.08, 0.38) | 0.004 * |
| Total | 0.32 (−0.10, 0.75) | 0.129 | 0.10 (−0.17, 0.37) | 0.448 |
| Lean, per 100 g | ||||
| Arm | 1.79 (0.89, 2.69) | <0.001 * | 0.57 (0.19, 0.95) | 0.005 * |
| Trunk | 0.002 (−0.48, 0.48) | 0.994 | −0.31 (−0.65, 0.03) | 0.073 |
| Leg | 0.97 (0.61, 1.34) | <0.001 * | 0.21 (0.08, 0.34) | 0.002 * |
| Total | −0.08 (−0.31, 0.16) | 0.514 | −0.13 (−0.31, 0.05) | 0.137 |
| ASMI, kg/m² | 10.75 (6.93, 14.57) | <0.001 * | 4.44 (1.58, 7.29) | 0.004 * |
| CT parameters | ||||
| SAT | ||||
| Area, cm2 | 0.12 (0.06, 0.19) | 0.001 * | 0.08 (0.03, 0.13) | 0.004 * |
| Density, HU | −0.33 (−0.50, −0.15) | 0.001 * | −0.11 (−0.23, 0.01) | 0.081 |
| VAT | ||||
| Area, cm2 | 0.05 (−0.01, 0.12) | 0.095 | 0.05 (0.01, 0.09) | 0.015 * |
| Density, HU | −0.53 (−0.72, −0.33) | <0.001 * | −0.21 (−0.34, −0.07) | 0.004 * |
| Para. | ||||
| Area, cm2 | 0.78 (0.43, 1.13) | <0.001 * | 0.38 (0.09, 0.67) | 0.012 * |
| Density, HU | 0.21 (−0.35, 0.77) | 0.445 | 0.22 (−0.12, 0.56) | 0.189 |
| Ps. | ||||
| Area, cm2 | 0.88 (0.31, 1.44) | 0.004 * | 0.82 (0.31, 1.33) | 0.003 * |
| Density, HU | 0.49 (0.04, 0.93) | 0.035 * | 0.43 (0.15, 0.71) | 0.005 * |
| Muscle function | ||||
| Handgrip strength, kg | 0.74 (0.21, 1.26) | 0.008 * | 0.61 (0.30, 0.92) | <0.001 * |
| Parameters | 6th Month—Baseline | 12th Month—Baseline | ||
|---|---|---|---|---|
| B (95% CI) | p-Value | B (95% CI) | p-Value | |
| DXA parameters | ||||
| Fat, per 100 g | ||||
| Arm | −156.44 (−253.26, −59.61) | 0.003 * | −80.07 (−135.61, −24.53) | 0.007 * |
| Trunk | −9.38 (−21.31, 2.55) | 0.118 | −10.84 (−23.98, 2.30) | 0.101 |
| Leg | −57.56 (−99.92, −15.20) | 0.010 * | −33.97 (−55.69, −12.24) | 0.004 * |
| Total | −9.95 (−51.27, 31.38) | 0.626 | −7.03 (−46.33, 32.27) | 0.715 |
| Lean, per 100 g | ||||
| Arm | −107.64 (−205.94, −9.33) | 0.033 * | −79.41 (−134.81, −24.01) | 0.007 * |
| Trunk | 0.57 (−44.44, 45.58) | 0.980 | −0.16 (−52.49, 52.17) | 0.995 |
| Leg | −54.72 (−99.20, −10.24) | 0.018 * | −26.82 (−46.61, −7.03) | 0.010 * |
| Total | 3.80 (−18.63, 26.22) | 0.731 | 1.69 (−25.60, 28.98) | 0.899 |
| ASMI, kg/m2 | −555.51 (−1040.85, −70.18) | 0.026 * | −564.60 (−996.65, −132.54) | 0.013 * |
| CT parameters | ||||
| SAT | ||||
| Area, cm2 | −7.32 (−14.45, −0.20) | 0.044 * | −7.82 (−16.94, 1.29) | 0.089 |
| Density, HU | 6.59 (−13.64, 26.83) | 0.510 | 8.58 (−9.65, 26.80) | 0.340 |
| VAT | ||||
| Area, cm2 | −2.11 (−8.23, 4.02) | 0.487 | −3.18 (−9.81, 3.44) | 0.331 |
| Density, HU | 16.02 (−9.74, 41.77) | 0.213 | 14.42 (−7.97, 36.82) | 0.196 |
| Para. | ||||
| Area, cm2 | −54.96 (−93.00, −16.92) | 0.006 * | −60.98 (−102.85, −19.11) | 0.006 * |
| Density, HU | −5.58 (−58.26, 47.11) | 0.830 | −2.51 (−53.96, 48.94) | 0.920 |
| Ps. | ||||
| Area, cm2 | −63.06 (−119.90, −6.22) | 0.031 * | −86.10 (−168.13, −4.08) | 0.040 * |
| Density, HU | −18.63 (−63.79, 26.52) | 0.405 | −20.45 (−68.81, 27.90) | 0.391 |
| Muscle function | ||||
| Handgrip strength, kg | −27.52 (−82.94, 27.90) | 0.318 | −46.34 (−101.36, 8.68) | 0.095 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-C.; Shao, I.-H.; Juan, Y.-H.; Yeh, K.-Y.; Hou, C.-P.; Chen, C.-L.; Yu, K.-J.; Chen, L.-S.; Lin, C.-L.; Chuang, H.-H. The Impact of Exercise on Improving Body Composition and PSA in High-Risk Prostate Cancer Patients on Androgen-Deprivation Therapy. Nutrients 2022, 14, 5088. https://doi.org/10.3390/nu14235088
Lin Y-C, Shao I-H, Juan Y-H, Yeh K-Y, Hou C-P, Chen C-L, Yu K-J, Chen L-S, Lin C-L, Chuang H-H. The Impact of Exercise on Improving Body Composition and PSA in High-Risk Prostate Cancer Patients on Androgen-Deprivation Therapy. Nutrients. 2022; 14(23):5088. https://doi.org/10.3390/nu14235088
Chicago/Turabian StyleLin, Yu-Ching, I-Hung Shao, Yu-Hsiang Juan, Kun-Yun Yeh, Chen-Pang Hou, Chien-Lun Chen, Kai-Jie Yu, Liang-Sien Chen, Chin-Li Lin, and Hai-Hua Chuang. 2022. "The Impact of Exercise on Improving Body Composition and PSA in High-Risk Prostate Cancer Patients on Androgen-Deprivation Therapy" Nutrients 14, no. 23: 5088. https://doi.org/10.3390/nu14235088
APA StyleLin, Y.-C., Shao, I.-H., Juan, Y.-H., Yeh, K.-Y., Hou, C.-P., Chen, C.-L., Yu, K.-J., Chen, L.-S., Lin, C.-L., & Chuang, H.-H. (2022). The Impact of Exercise on Improving Body Composition and PSA in High-Risk Prostate Cancer Patients on Androgen-Deprivation Therapy. Nutrients, 14(23), 5088. https://doi.org/10.3390/nu14235088






