Role of Residence Area on Diet Diversity and Micronutrient Intake Adequacy in Urban and Rural Costa Rican Adolescents
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Setting
2.2. Sociodemographic Variables
2.3. Anthropometric Assessment
2.4. Dietary Intake Assessment
2.5. Diet Diversity Score
2.6. Nutrient Adequacy
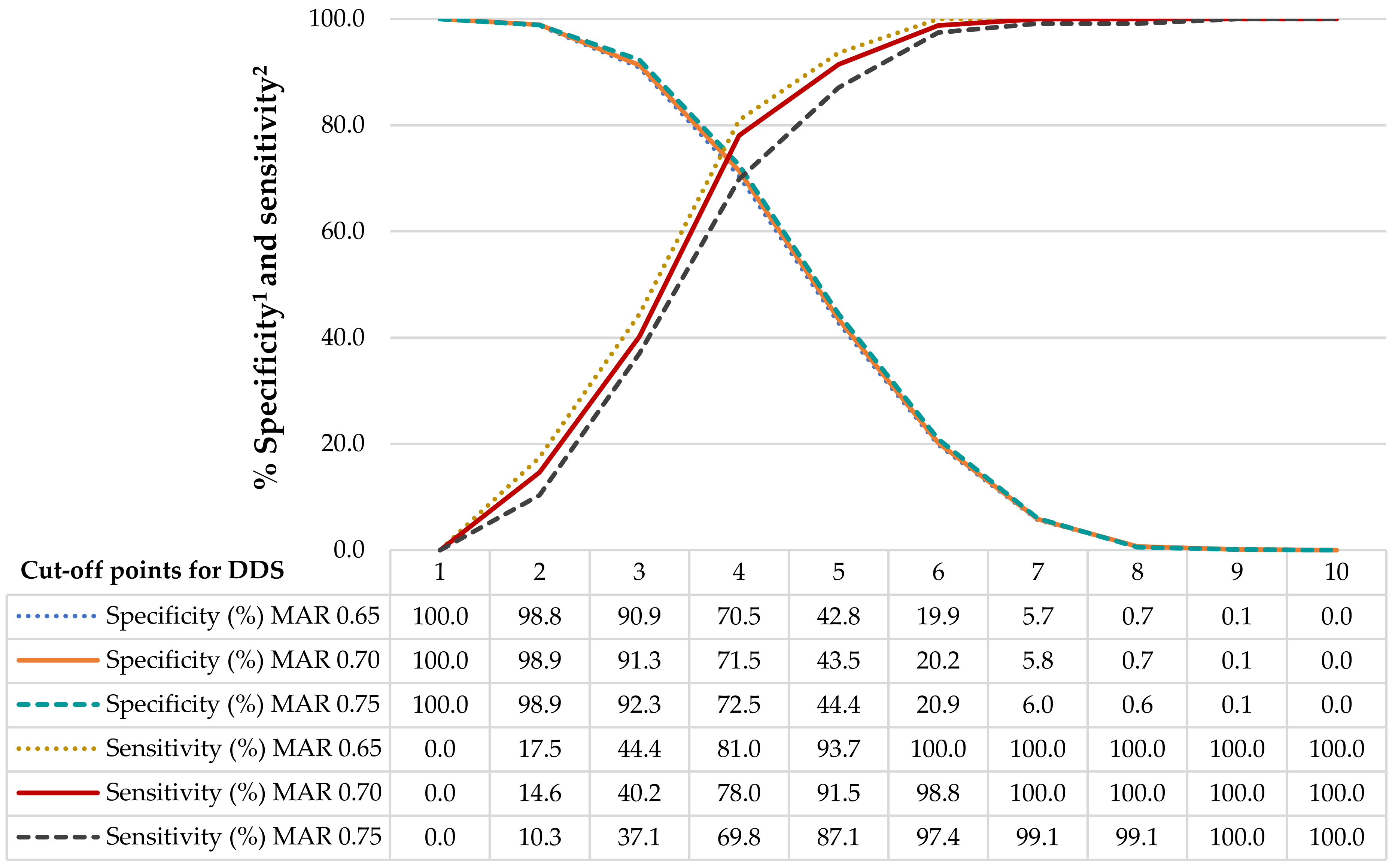
2.7. Diet Diversity Score Cut-Off
2.8. Statistical Analyses
3. Results
3.1. General Characteristics According to Residence Area
3.2. Diet Diversity Score According to General Characteristics
3.3. Consumption of Food Groups
3.4. Nutrient Adequacy Ratio and Correlation with Diet Diversity Score
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Norris, S.A.; Frongillo, E.A.; Black, M.M.; Dong, Y.; Fall, C.; Lampl, M.; Liese, A.D.; Naguib, M.; Prentice, A.; Rochat, T.; et al. Nutrition in Adolescent Growth and Development. Lancet 2022, 399, 172–184. [Google Scholar] [CrossRef]
- Demory-Luce, D.; Morales, M.; Nicklas, T.; Baranowski, T.; Zakeri, I.; Berenson, G. Changes in Food Group Consumption Patterns from Childhood to Young Adulthood: The Bogalusa Heart Study. J. Am. Diet. Assoc. 2004, 104, 1684–1691. [Google Scholar] [CrossRef]
- Story, M.; Neumark-Sztainer, D.; French, S. Individual and Environmental Influences on Adolescent Eating Behaviors. J. Am. Diet. Assoc. 2002, 102, S40–S51. [Google Scholar] [CrossRef] [PubMed]
- Birch, L.; Savage, J.S.; Ventura, A. Influences on the Development of Children’s Eating Behaviours: From Infancy to Adolescence. Can. J. Diet. Pr. Res. 2007, 68, s1–s56. [Google Scholar]
- Jenkins, S.; Horner, S.D. Barriers That Influence Eating Behaviors in Adolescents. J. Pediatr. Nurs. 2005, 20, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Cruz, F.; Ramos, E.; Lopes, C.; Araújo, J. Tracking of Food and Nutrient Intake from Adolescence into Early Adulthood. Nutrition 2018, 55–56, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Movassagh, E.; Baxter-Jones, A.; Kontulainen, S.; Whiting, S.; Vatanparast, H. Tracking Dietary Patterns over 20 Years from Childhood through Adolescence into Young Adulthood: The Saskatchewan Pediatric Bone Mineral Accrual Study. Nutrients 2017, 9, 990. [Google Scholar] [CrossRef] [PubMed]
- Craigie, A.M.; Lake, A.A.; Kelly, S.A.; Adamson, A.J.; Mathers, J.C. Tracking of Obesity-Related Behaviours from Childhood to Adulthood: A Systematic Review. Maturitas 2011, 70, 266–284. [Google Scholar] [CrossRef]
- Mikkilä, V.; Räsänen, L.; Raitakari, O.T.; Pietinen, P.; Viikari, J. Consistent Dietary Patterns Identified from Childhood to Adulthood: The Cardiovascular Risk in Young Finns Study. Br. J. Nutr. 2005, 93, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.C.; Dumith, S.D.C.; Lopes, C.; Severo, M.; Assunção, M.C.F. How Do Tracking and Changes in Dietary Pattern during Adolescence Relate to the Amount of Body Fat in Early Adulthood? PLoS ONE 2016, 11, e0149299. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.; Burrows, T.; Collins, C.E. Systematic Review of Diet Quality Indices and Their Associations with Health-Related Outcomes in Children and Adolescents. J. Hum. Nutr. Diet. 2014, 27, 577–598. [Google Scholar] [CrossRef] [PubMed]
- Verger, E.O.; Le Port, A.; Borderon, A.; Bourbon, G.; Moursi, M.; Savy, M.; Mariotti, F.; Martin-Prevel, Y. Dietary Diversity Indicators and Their Associations with Dietary Adequacy and Health Outcomes: A Systematic Scoping Review. Adv. Nutr. 2021, 12, 1659–1672. [Google Scholar] [CrossRef]
- Herforth, A.; Arimond, M.; Álvarez-Sánchez, C.; Coates, J.; Christianson, K.; Muehlhoff, E. A Global Review of Food-Based Dietary Guidelines. Adv. Nutr. 2019, 10, 590–605. [Google Scholar] [CrossRef] [PubMed]
- Baxter, J.B.; Wasan, Y.; Islam, M.; Cousens, S.; Soofi, S.B.; Ahmed, I.; Sellen, D.W.; Bhutta, Z.A. Dietary Diversity and Social Determinants of Nutrition among Late Adolescent Girls in Rural Pakistan. Matern. Child Nutr. 2022, 18, e13265. [Google Scholar] [CrossRef] [PubMed]
- Isabirye, N.; Bukenya, J.N.; Nakafeero, M.; Ssekamatte, T.; Guwatudde, D.; Fawzi, W. Dietary Diversity and Associated Factors among Adolescents in Eastern Uganda: A Cross-Sectional Study. BMC Public Health 2020, 20, 534. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Rahman, S.M.; Tarafder, C.; Rahman, M.M.; Rahman, A.; Ekström, E.-C. Exploring Rural Adolescents’ Dietary Diversity and Its Socioeconomic Correlates: A Cross-Sectional Study from Matlab, Bangladesh. Nutrients 2020, 12, 2230. [Google Scholar] [CrossRef] [PubMed]
- Golden, C.D.; Gephart, J.A.; Eurich, J.G.; McCauley, D.J.; Sharp, M.K.; Andrew, N.L.; Seto, K.L. Social-Ecological Traps Link Food Systems to Nutritional Outcomes. Glob. Food Secur. 2021, 30, 100561. [Google Scholar] [CrossRef]
- Dornelles, A.Z.; Boonstra, W.J.; Delabre, I.; Denney, J.M.; Nunes, R.J.; Jentsch, A.; Nicholas, K.A.; Schröter, M.; Seppelt, R.; Settele, J.; et al. Transformation Archetypes in Global Food Systems. Sustain. Sci. 2022, 17, 1827–1840. [Google Scholar] [CrossRef]
- Mirmiran, P.; Azadbakht, L.; Esmaillzadeh, A.; Azizi, F. Dietary Diversity Score in Adolescents—A Good Indicator of the Nutritional Adequacy of Diets: Tehran Lipid and Glucose Study. Asia Pac. J. Clin. Nutr. 2004, 13, 56–60. [Google Scholar]
- Worku, M.; Hailemicael, G.; Asegedech, W. Dietary Diversity Score and Associated Factors among High School Adolescent Girls in Gurage Zone, Southwest Ethiopia. World J. Nutr. Health 2017, 5, 41–45. [Google Scholar] [CrossRef]
- Gyimah, L.A.; Annan, R.A.; Apprey, C.; Edusei, A.; Aduku, L.N.E.; Asamoah-Boakye, O.; Azanu, W.; Lutterodt, H. Dietary Diversity and Its Correlates among Pregnant Adolescent Girls in Ghana. PLoS ONE 2021, 16, e0247979. [Google Scholar] [CrossRef]
- Nithya, D.J.; Bhavani, R.V. Dietary Diversity and Its Relationship with Nutritional Status among Adolescents and Adults in Rural India. J. Biosoc. Sci. 2018, 50, 397–413. [Google Scholar] [CrossRef] [PubMed]
- FAO, FHI 360. Minimum Dietary Diversity for Women: A Guide for Measurement; FAO: Rome, Italy, 2016; ISBN 978-92-5-109153-1. [Google Scholar]
- Diop, L.; Becquey, E.; Turowska, Z.; Huybregts, L.; Ruel, M.T.; Gelli, A. Standard Minimum Dietary Diversity Indicators for Women or Infants and Young Children Are Good Predictors of Adequate Micronutrient Intakes in 24–59-Month-Old Children and Their Nonpregnant Nonbreastfeeding Mothers in Rural Burkina Faso. J. Nutr. 2021, 151, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ramírez, S.; Sánchez-Pimienta, T.G.; Batis, C.; Cediel, G.; Marrón-Ponce, J.A. Minimum Dietary Diversity in Mexico: Establishment of Cutoff Point to Predict Micronutrients Adequacy. Eur. J. Clin. Nutr. 2022, 76, 739–745. [Google Scholar] [CrossRef]
- Gómez, G.; Fisberg, R.; Nogueira Previdelli, Á.; Hermes Sales, C.; Kovalskys, I.; Fisberg, M.; Herrera-Cuenca, M.; Cortés Sanabria, L.; García, M.; Pareja Torres, R.; et al. Diet Quality and Diet Diversity in Eight Latin American Countries: Results from the Latin American Study of Nutrition and Health (ELANS). Nutrients 2019, 11, 1605. [Google Scholar] [CrossRef] [PubMed]
- Steyn, N.; Nel, J.; Nantel, G.; Kennedy, G.; Labadarios, D. Food Variety and Dietary Diversity Scores in Children: Are They Good Indicators of Dietary Adequacy? Public Health Nutr. 2006, 9, 644–650. [Google Scholar] [CrossRef]
- Hatløy, A.; Torheim, L.E.; Oshaug, A. Food Variety—A Good Indicator of Nutritional Adequacy of the Diet? A Case Study from an Urban Area in Mali, West Africa. Eur. J. Clin. Nutr. 1998, 52, 891–898. [Google Scholar] [CrossRef]
- Sistema de Información Estadística de Derechos de la Niñez y Adolescencia (SIEDNA). Personas Menores de Edad a La Luz Del Censo 2011/Underage Persons in Light of 2011 Census; UCR: San José, Costa Rica, 2013; Available online: https://accionsocial.ucr.ac.cr/sites/default/files/documentos/personas_menores_de_edad_a_la_luz_del_censo_2011.pdf (accessed on 10 October 2022).
- Programa Estado de la Nación. Sétimo Informe Estado de La Educación/Seventh State of Education Report; Programa Estado de la Nación: San José, Costa Rica, 2019; Available online: https://estadonacion.or.cr/wp-content/uploads/2019/08/Estado-Educaci%C3%B3n-RESUMEN-2019-WEB.pdf (accessed on 15 October 2022).
- Ryan, T.P. Sample Size Determination and Power. In Wiley Series in Probability and Statistics, 1st ed.; Wiley: New York, NY, USA, 2013; ISBN 978-1-118-43760-5. [Google Scholar]
- Alam, M. Selection of the Samples with Probability Proportional to Size. Sci. J. Appl. Math. Stat. 2015, 3, 230. [Google Scholar] [CrossRef]
- Sánchez-Muñoz, C.; Zabala, M.; Williams, K. Handbook of Anthropometry: Physical Measures of Human Form in Health and Disease; Preedy, V.R., Ed.; Springer: New York, NY, USA, 2012; ISBN 978-1-4419-1787-4. [Google Scholar]
- World Health Organization. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development; De Onis, M., Ed.; WHO child growth standards; WHO Press: Geneva, Switzerland, 2006; ISBN 978-92-4-154693-5. [Google Scholar]
- Chinnock, A. Diario de Consumo de Alimentos/Food Consumption Diary; Instrumento para el Registro de Información/Instrument for Information Registration; UCR: San José, Costa Rica, 2007. [Google Scholar]
- Trevino, R.P.; Ravelo, A.V.; Senne-Duff, B.; Murad, M.; Diaz, J.F.; Birkenfeld, E. Poor Validity of Dietary Recall in Low-Income Hispanic Children Using Digital Food Imaging Analysis as the Reference. J. Food Nutr. Diet. 2016, 1, 107. [Google Scholar] [CrossRef][Green Version]
- Dutch, D.C.; Golley, R.K.; Johnson, B.J. Diet Quality of Australian Children and Adolescents on Weekdays versus Weekend Days: A Secondary Analysis of the National Nutrition and Physical Activity Survey 2011–2012. Nutrients 2021, 13, 4128. [Google Scholar] [CrossRef]
- Monge-Rojas, R.; Vargas-Quesada, R.; Colón-Ramos, U.; Chinnock, A. Dietary Intake and Sources of Added Sugars in Various Food Environments in Costa Rican Adolescents. Nutrients 2022, 14, 959. [Google Scholar] [CrossRef]
- Institute of Medicine of the National Academies. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; National Academies Press: Washington, DC, USA, 2006; p. 11537. ISBN 978-0-309-15742-1. [Google Scholar]
- Institute of Medicine of the National Academies. Dietary Reference Intakes: Applications in Dietary Assessment; National Academies Press: Washington, DC, USA, 2000; p. 9956. ISBN 978-0-309-07183-3. [Google Scholar]
- Oldewage-Theron, W.H.; Kruger, R. Food Variety and Dietary Diversity as Indicators of the Dietary Adequacy and Health Status of an Elderly Population in Sharpeville, South Africa. J. Nutr. Elder. 2008, 27, 101–133. [Google Scholar] [CrossRef] [PubMed]
- Madden, J.P.; Goodman, S.J.; Guthrie, H.A. Validity of the 24-Hr. Recall. Analysis of Data Obtained from Elderly Subjects. J. Am. Diet. Assoc. 1976, 68, 143–147. [Google Scholar] [PubMed]
- McCarthy, H.; Dixon, M.; Crabtree, I.; Eaton-Evans, M.J.; McNulty, H. The Development and Evaluation of the Screening Tool for the Assessment of Malnutrition in Paediatrics (STAMP©) for Use by Healthcare Staff: Development and Evaluation of STAMP©. J. Hum. Nutr. Diet. 2012, 25, 311–318. [Google Scholar] [CrossRef]
- ValorNut UCR: Programa Para El Cálculo de Valor Nutritivo de Los Alimentos. Available online: https://www.nutricion.ucr.ac.cr/index.php/es/valornut (accessed on 2 October 2022).
- Arce, R. Lo Rural es Diverso: Evidencia para el Caso de Costa Rica; Rodríguez, A., Saborío, M., Eds.; Instituto Interamericano de Cooperación para la Agricultura (IICA): San José, Costa Rica, 2008; ISBN 978-92-9039-855-4. [Google Scholar]
- Valenciano Salazar, J.A.; Pagani Centeno, L.; Álvarez Madrigal, N. Seguridad y Soberanía Alimentaria en Costa Rica; Editorial UNA: Heredia, Costa Rica, 2020; ISBN 978-9930-588-01-7. [Google Scholar]
- Singh, N. Pulses: An Overview. J. Food Sci. 2017, 54, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Salud de Costa Rica. Reforma Reglamento para el Enriquecimiento del Arroz. Decreto Ejecutivo N° 39985-S 2016. Available online: https://www.fao.org/faolex/results/details/es/c/LEX-FAOC162902 (accessed on 1 October 2022).
- Research Institute (IFPRI), I.F.P. Urbanization and the Nutrition Transition; International Food Policy Research Institute: Washington, DC, USA, 2017. [Google Scholar]
- Barría, R.M.; Amigo, H. Nutrition transition: A review of Latin American profile. Arch. Latinoam. Nutr. 2006, 56, 3–11. [Google Scholar] [PubMed]
- Popkin, B.M. Urbanization, Lifestyle Changes and the Nutrition Transition. World Dev. 1999, 27, 1905–1916. [Google Scholar] [CrossRef]
- Doak, C.M.; Adair, L.S.; Bentley, M.; Monteiro, C.; Popkin, B.M. The Dual Burden Household and the Nutrition Transition Paradox. Int. J. Obes. 2005, 29, 129–136. [Google Scholar] [CrossRef]
- Popkin, B.M. Nutritional Patterns and Transitions. Popul. Dev. Rev. 1993, 19, 138. [Google Scholar] [CrossRef]
- Willett, W. Nutritional Epidemiology. In Monographs in Epidemiology and Biostatistics, 3rd ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 2013; ISBN 978-0-19-975403-8. [Google Scholar]
| Characteristics | Overall 1 (n = 818) | Residence Area | ||
|---|---|---|---|---|
| Urban (n = 411) | Rural (n = 407) | p-Value 2 | ||
| Age (y) | 15.0 ± 1.7 | 14.9 ± 1.7 | 15.1 ± 1.7 | 0.208 |
| Sex | ||||
| Female | 520 (63.6) | 259 (49.8) a | 261 (50.2) a | 0.741 |
| Male | 298 (36.4) | 152 (51.0) a | 146 (49.0) a | |
| Socioeconomic status | ||||
| Low | 263 (32.2) | 87 (33.1) a | 179 (66.9) b | <0.0001 |
| Middle | 325 (39.7) | 158 (48.6) a | 167 (51.4) a | |
| High | 230 (28.1) | 166 (72.2) a | 64 (27.8) b | |
| Nutritional status | ||||
| Non-overweight | 551 (67.4) | 279 (50.6) a | 272 (49.4) a | 0.748 |
| Overweight/obesity | 267 (32.6) | 132 (49.4) a | 135 (50.6) a | |
| Characteristic | Diet Diversity Score (DDS) | Participants Reaching the Minimum DDS | |||
|---|---|---|---|---|---|
| Mean | SD | p-Value 2 | n (%) | p-Value 2 | |
| Overall | 4.17 | 1.43 | 544 (66.5) | ||
| Sex | |||||
| Female | 4.18 | 1.43 | 0.679 | 349 (67.1) | 0.624 |
| Male | 4.14 | 1.44 | 195 (65.4) | ||
| Residence area | |||||
| Urban | 4.00 | 1.42 | 0.001 | 251 (61.1) | 0.001 |
| Rural | 4.33 | 1.43 | 293 (72.0) | ||
| Socioeconomic status | |||||
| Low | 4.28 | 1.44 | 0.303 | 183 (69.6) | 0.420 |
| Middle | 4.11 | 1.39 | 213 (65.5) | ||
| High | 4.11 | 1.48 | 148 (64.4) | ||
| Nutritional status | |||||
| Non overweight | 4.15 | 1.43 | 0.680 | 367 (66.6) | 0.929 |
| Overweight/obesity | 4.19 | 1.43 | 177 (66.3) | ||
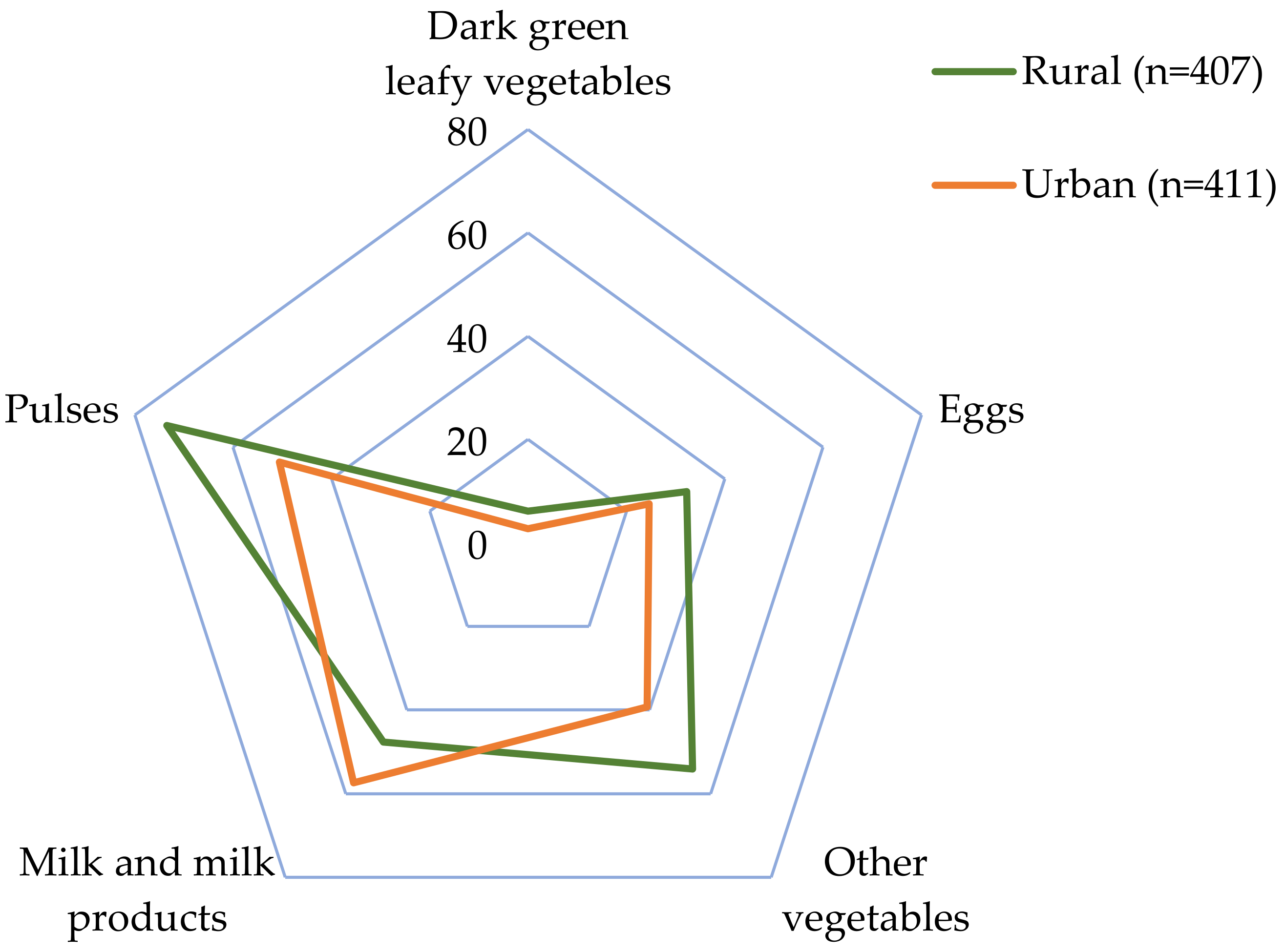
| Food Group 1 (g/d) | Diet Diversity | Residence Area | ||||
|---|---|---|---|---|---|---|
| DDS < 4 (n = 274) | DDS ≥ 4 (n = 544) | p-Value 2 | Urban (n = 411) | Rural (n = 407) | p-Value 2 | |
| Starchy staples | 357.1 ± 184.3 | 387.7 ± 197.9 | 0.031 | 353.1 ± 190.0 | 402.1 ± 196.8 | <0.0001 |
| Milk and milk products | 91.1 ± 204.0 | 166.5 ± 207.1 | <0.0001 | 155.1 ± 220.7 | 127.4 ± 195.8 | 0.010 |
| Pulses | 55.1 ± 108.2 | 106.9 ± 112.2 | <0.0001 | 74.1 ± 105.9 | 105.1 ± 118.8 | <0.0001 |
| Flesh foods | 56.5 ± 84.7 | 102.3 ± 94.5 | <0.0001 | 91.3 ± 98.6 | 82.6 ± 88.6 | 0.245 |
| Other fruits | 25.4 ± 78.1 | 91.8 ± 145.0 | <0.0001 | 73.4 ± 145.5 | 65.7 ± 109.2 | 0.816 |
| Other vegetables | 11.8 ± 36.3 | 62.3 ± 86.8 | <0.0001 | 35.8 ± 69.4 | 55.1 ± 84.0 | <0.0001 |
| Eggs | 7.0 ± 21.9 | 23.4 ± 37.9 | <0.0001 | 15.9 ± 32.7 | 20.1 ± 35.7 | 0.029 |
| Other vitamin A-rich F&V | 5.9 ± 45.6 | 15.0 ± 57.3 | <0.0001 | 14.3 ± 64.1 | 9.7 ± 40.9 | 0.405 |
| Dark green leafy vegetables | 0.9 ± 14.5 | 6.7 ± 31.4 | <0.0001 | 2.9 ± 21.3 | 6.6 ± 31.8 | 0.016 |
| Nuts and seeds | 0.3 ± 3.4 | 2.8 ± 20.0 | 0.003 | 1.8 ± 14.2 | 2.1 ± 18.5 | 0.467 |
| Variable | Bivariate Analysis | Multivariate Analysis 1 | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | Adj OR | 95% CI | p-Value | |
| Sex | ||||||
| Women | 1.08 | 0.80–1.46 | 0.624 | 1.08 | 0.80–1.46 | 0.622 |
| Age | 1.09 | 1.00–1.19 | 0.047 | 1.08 | 0.99–1.18 | 0.067 |
| Residence area | ||||||
| Rural | 1.64 | 1.22–2.20 | 0.001 | 1.62 | 1.19–2.20 | 0.002 |
| Socioeconomic status | ||||||
| Middle | 0.83 | 0.59–1.18 | 0.299 | 0.91 | 0.64–1.30 | 0.617 |
| High | 0.79 | 0.54–1.15 | 0.217 | 0.98 | 0.66–1.46 | 0.922 |
| Nutritional status | ||||||
| Overweight/obesity | 0.99 | 0.72–1.34 | 0.929 | 0.99 | 0.73–1.36 | 0.989 |
| Constant | - | - | - | 0.46 | 0.12–1.84 | 0.275 |
| Nutrients | Overall (n = 818) | DDS < 4 (n = 274) | DDS ≥ 4 (n = 544) | r1 | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | p-Value 2 | |||
| Calcium | 0.492 | 0.292 | 0.368 | 0.271 | 0.555 | 0.283 | <0.0001 | 0.383 | <0.0001 |
| Iron | 0.968 | 0.131 | 0.938 | 0.190 | 0.983 | 0.084 | 0.001 | 0.171 | <0.0001 |
| Zinc | 0.788 | 0.249 | 0.666 | 0.284 | 0.849 | 0.204 | <0.0001 | 0.354 | <0.0001 |
| Vitamin C | 0.885 | 0.268 | 0.778 | 0.357 | 0.939 | 0.187 | <0.0001 | 0.265 | <0.0001 |
| Thiamin | 0.965 | 0.132 | 0.923 | 0.197 | 0.985 | 0.073 | <0.0001 | 0.205 | <0.0001 |
| Riboflavin | 0.940 | 0.173 | 0.869 | 0.248 | 0.976 | 0.102 | <0.0001 | 0.276 | <0.0001 |
| Niacin | 0.954 | 0.151 | 0.900 | 0.223 | 0.981 | 0.084 | <0.0001 | 0.232 | <0.0001 |
| Vitamin B6 | 0.898 | 0.213 | 0.802 | 0.284 | 0.947 | 0.143 | <0.0001 | 0.311 | <0.0001 |
| Folate equivalents | 0.969 | 0.128 | 0.934 | 0.189 | 0.987 | 0.075 | <0.0001 | 0.163 | <0.0001 |
| Cobalamin | 0.957 | 0.159 | 0.908 | 0.234 | 0.982 | 0.093 | <0.0001 | 0.215 | <0.0001 |
| Vitamin A | 0.686 | 0.310 | 0.578 | 0.338 | 0.740 | 0.280 | <0.0001 | 0.309 | <0.0001 |
| MAR | 0.864 | 0.135 | 0.788 | 0.176 | 0.902 | 0.087 | <0.0001 | 0.433 | <0.0001 |
| Nutrient | Overall 1 (n = 818) | DDS < 4 (n = 274) | DDS ≥ 4 (n = 544) | Urban (n = 411) | Rural (n = 407) | ||
|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | p-Value 2 | n (%) | n (%) | p-Value 2 | |
| Calcium | 214 (26.2) | 38 (13.9) | 176 (32.4) | <0.0001 | 123 (29.9) | 91 (22.4) | 0.014 |
| Iron | 776 (94.9) | 249 (90.9) | 527 (96.9) | <0.0001 | 386 (93.9) | 390 (95.8) | 0.217 |
| Zinc | 534 (65.3) | 127 (46.4) | 407 (74.8) | <0.0001 | 253 (61.6) | 281 (69.0) | 0.025 |
| Vitamin C | 690 (84.4) | 195 (71.2) | 495 (91.0) | <0.0001 | 336 (81.8) | 354 (87.0) | 0.040 |
| Thiamin | 774 (94.6) | 240 (87.6) | 534 (98.2) | <0.0001 | 386 (93.9) | 388 (95.3) | 0.370 |
| Riboflavin | 743 (90.8) | 218 (79.6) | 525 (96.5) | <0.0001 | 368 (89.5) | 375 (92.1) | 0.198 |
| Niacin | 752 (91.9) | 228 (83.2) | 524 (96.3) | <0.0001 | 378 (92.0) | 374 (92.0) | 0.967 |
| Vitamin B6 | 680 (83.1) | 184 (67.2) | 496 (91.2) | <0.0001 | 326 (79.3) | 354 (87.0) | 0.003 |
| Folate equivalents | 779 (95.2) | 245 (89.4) | 534 (98.2) | <0.0001 | 389 (94.7) | 390 (95.8) | 0.430 |
| Cobalamin | 762 (93.2) | 236 (86.1) | 526 (96.7) | <0.0001 | 386 (93.9) | 376 (92.4) | 0.385 |
| Vitamin A | 430 (52.6) | 110 (40.2) | 320 (58.8) | <0.0001 | 215 (52.1) | 215 (52.8) | 0.883 |
| MAR | 736 (90.0) | 210 (76.6) | 526 (96.7) | <0.0001 | 368 (89.5) | 368 (90.4) | 0.675 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monge-Rojas, R.; Vargas-Quesada, R.; Gómez, G. Role of Residence Area on Diet Diversity and Micronutrient Intake Adequacy in Urban and Rural Costa Rican Adolescents. Nutrients 2022, 14, 5093. https://doi.org/10.3390/nu14235093
Monge-Rojas R, Vargas-Quesada R, Gómez G. Role of Residence Area on Diet Diversity and Micronutrient Intake Adequacy in Urban and Rural Costa Rican Adolescents. Nutrients. 2022; 14(23):5093. https://doi.org/10.3390/nu14235093
Chicago/Turabian StyleMonge-Rojas, Rafael, Rulamán Vargas-Quesada, and Georgina Gómez. 2022. "Role of Residence Area on Diet Diversity and Micronutrient Intake Adequacy in Urban and Rural Costa Rican Adolescents" Nutrients 14, no. 23: 5093. https://doi.org/10.3390/nu14235093
APA StyleMonge-Rojas, R., Vargas-Quesada, R., & Gómez, G. (2022). Role of Residence Area on Diet Diversity and Micronutrient Intake Adequacy in Urban and Rural Costa Rican Adolescents. Nutrients, 14(23), 5093. https://doi.org/10.3390/nu14235093






