Abstract
Obesity is a chronic and relapsing public health problem with an extensive list of associated comorbidities. The worldwide prevalence of obesity has nearly tripled over the last five decades and continues to pose a serious threat to wider society and the wellbeing of future generations. The pathogenesis of obesity is complex but diet plays a key role in the onset and progression of the disease. The human diet has changed drastically across the globe, with an estimate that approximately 72% of the calories consumed today come from foods that were not part of our ancestral diets and are not compatible with our metabolism. Additionally, multiple nutrient-independent factors, e.g., cost, accessibility, behaviours, culture, education, work commitments, knowledge and societal set-up, influence our food choices and eating patterns. Much research has been focused on ‘what to eat’ or ‘how much to eat’ to reduce the obesity burden, but increasingly evidence indicates that ‘when to eat’ is fundamental to human metabolism. Aligning feeding patterns to the 24-h circadian clock that regulates a wide range of physiological and behavioural processes has multiple health-promoting effects with anti-obesity being a major part. This article explores the current understanding of the interactions between the body clocks, bioactive dietary components and the less appreciated role of meal timings in energy homeostasis and obesity.
1. Introduction
Obesity is defined as a BMI (kg/m2) of ≥30 (further classified into class I (BMI 30.0 to 34.9), class II (BMI 35 to 39.9) and class III (BMI > 40.0) and a BMI between 25.0–29.9 is classified as overweight [1,2]. The World Health Organisation defines obesity as a disease and not just the biggest risk factor for the development of multiple non-communicable chronic diseases, such as metabolic syndrome, diabetes, hypertension, cardiovascular diseases and cancer [3]. The cost to treat obesity and associated diseases puts an immense pressure not only on the healthcare sector but also wider society. The rate of obesity is rising globally inexorably and most projections indicate that without a concerted action, by 2035 over 45% of the global population will be either overweight or obese [4].
The pathogenesis of obesity is complex, with multifaceted interactions between an individuals’ genetics and the environment. Although evidence suggests that there is 40–70% heritability for obesity, environmental factors, particularly diet and physical activity, are critical to its onset and progression [5]. At its simplest, obesity is a result of positive energy balance and the relatively lower cost and abundance of energy-dense foods occurring concomitantly with an increasingly sedentary lifestyle have driven the obesity pandemic. In many modern societies, a 24/7 work and social lifestyle has become the norm, leading to erratic sleep and food-consumption patterns, disrupting the harmony between the biological day/active phase and metabolic processes and further fuelling the crisis [6,7].
There is no simple solution to address the escalating obesity epidemic. Lifestyle interventions have been the focus of weight-loss strategies with limited success and issues with long-term compliance. Body clocks, present in virtually each cell in the body, synchronise all physiological and biochemical processes to the external environment of light/dark cycles, temperature and food availability. The internal body clocks also coordinate the metabolic processes in a way that light/dark cycles are aligned with active/rest and anabolic/catabolic reaction phases and the loss of this harmony leads to adverse metabolic outcomes. The relatively novel concept of chrononutrition, the interactions between the food components and timing with circadian mechanisms, offers a promising target for designing sustainable weight-management strategies.
2. Chronobiology
Life evolved and adapted to the light/dark cycles that are a result of the earth’s rotation on its own axis. Organisms have an internal 24-hour circadian (from the Latin words circa diem, about a day) clock that adapts their daily activities to their external environment. Zeitgebers (external cues that synchronise the biological rhythms) such as light/dark cycles entrain (synchronise) the circadian system to generate rhythms in bodily processes, including the sleep/wake cycle, immune activity, metabolism, body temperature and blood pressure.
At the molecular level, the circadian clock in mammals is composed of two sub-systems: the core and the peripheral clocks. The core circadian clock is situated in the anterior hypothalamus and includes the suprachiasmatic nucleus (SCN), composed of about 20,000 neurons, for which light is the primary zeitgeber. Light enters the eye and the photic signal is conveyed to the SCN, where it is integrated with non-photic signals that include food and external temperature [8]. An endogenous rhythm is generated and communicated to other parts of the brain and to peripheral organs via direct neuronal synaptic connections and endocrine signals, aligning the whole-body circadian clock to light. In addition to the core pacemaker, each cell in the body has its own local clock with its autonomous daily rhythmicity. These peripheral clock systems are influenced by the SCN but are also entrained by SCN-independent zeitgebers such as meal timing, locomotor activity and body temperature [9,10]. During the night, the SCN also regulates the synthesis and release of melatonin by the pineal gland. Melatonin is a sleep-inducing hormone with 24-hr rhythmicity; its production is inhibited by light, hence its low circulatory levels during the day [11]. The levels of melatonin start to rise approximately 2–3 h prior to the habitual nocturnal sleep time that also coincides with the onset of dim light conditions in the evening. This is defined as dim-light melatonin onset (DLMO), and is a reliable marker for the circadian entrainment [12,13]. Melatonin exerts its biological effects by binding to melatonin receptors, human melatonin receptor 1 A and 1 B (MTNR1A and MTNR1B). Sleep is an important modulator for multiple metabolic and endocrine pathways, linking melatonin levels, sleep duration and quality with obesity [14,15]. Melatonin also inhibits glucose-mediated insulin secretion and effects free radical scavenging, thus contributing directly to regulation of metabolic and immune function [16].
Genomics and Epigenomics of Chronobiology
An intricate programme of transcription-translation-posttranslational feedback loops controls the complex patterns of circadian rhythmicity in physiology and behaviour (Figure 1). During the day, the key players responsible for the oscillations of circadian rhythms include the circadian locomotor output cycles kaput (CLOCK) and brain and muscle Arnt-like protein-1 (BMAL1). These heterodimerise and CLOCK-BMAL1 binds to the E-box regulatory element in the promoter regions of multiple circadian genes. Primarily, these activate the expression of PER (Period PER1, PER2 and PER3) and CRY (Cryptochrome CRY1 and CRY2) genes. Upon translation and a time lag (by the end of the day), these PER and CRY proteins accumulate in the cytoplasm and once they reach certain level, these heterodimerize to form a repressor complex and translocate to nucleus where they inhibit CLOCK-BMAL1-mediated transcription. Towards the end of the night, there is a gradual degradation of PER and CRY proteins that leads to the release of the CLOCK-BMAL1 dimer from PER/CRY suppression, and this re-initiates the clock cycle by inducing the transcription of PER and CRY. This cycle of activation and repression results in a feedback loop that generates an oscillation pattern of PER and CRY proteins over a 24-h period [9,17,18].
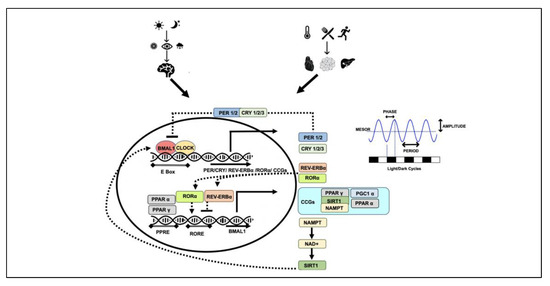
Figure 1.
Circadian clock mechanisms: different zeitgebers lead to intricate transcription-translation feedback loop. circadian locomotor output cycles kaput (CLOCK) and brain and muscle Arnt-like protein-1 (BMAL1) heterodimerise and regulate the transcription of multiple clock-dependent genes (solid arrows). PER, CRY, REV-ERBα, RORα, PPARα, PPARγ, SIRT1 all lead to regulation of CLOCK and BMAL1 and contribute to their own regulation (dotted arrows). Amplitude, period, phase and MESOR of the oscillations produced determine the rhythmicity and robustness of the circadian clock. PER (Period), CRY (Cryptochrome), RORα (receptor-related orphan receptor α), REV-ERBα (NR1D1 gene producing a protein called REV-ERBα), PPARα (peroxisome proliferator-activated receptor α), PPARγ (peroxisome proliferator-activated receptor γ), NAMPT (nicotinamide phosphoribosyltransferase), NAD+ (nicotinamide adenine dinucleotide), AMPK (AMP-activated protein kinase), CCGs (clock-controlled genes), SIRT1 (sirtuin 1), PGC1α (PPARγ coactivator 1α), MESOR (midline estimating statistic of rhythm).
A parallel secondary loop exists that improves the robustness of the primary loop, consisting of the transcriptional factors nuclear receptor subfamily 1, group D, member 1 (NR1D1 gene producing a protein called REV-ERBα) and retinoic-acid-receptor-related orphan receptor α (RORα). The transcription of these genes is also activated by CLOCK-BMAL1 through binding to the E-box element in their promoters. REV-ERBα and RORα then compete to bind to the ROR element (RORE) in the BMAL1 promoter and regulate BMAL1 transcription; REV-ERBα suppresses while RORα stimulates BMAL1 transcription. Two other members of the nuclear receptor family, peroxisome proliferator-activated receptor α (PPARα), and PPARγ coactivator 1α (PGC1α) are activated by CLOCK-BMAL1. Interestingly, PPARα and PGC1α also activate BMAL1 transcription (Figure 1) [19,20,21,22].
The clock components are also under the control of various post-translational modifications including phosphorylation, ubiquitination, acetylation, poly-ADP-ribosylation and proteasomal degradation. This additional layer of regulation allows plasticity in the circadian system, making them highly responsive to an organism’s environment [23]. PER, CRY and PGC1α are all modified in response to the nutritional status of the cell, have an impact on the inhibitory period on CLOCK-BMAL1 and contribute to the changes in rhythmic patterns. CLOCK is an acetyltransferase and acetylates its partner BMAL1 and regulates the transcriptional activity of the heterodimer. CLOCK itself can be modified by poly-ADP-ribosylation leading to the transcriptional inhibition of CLOCK-BMAL1 target genes and altering the circadian cycle [24,25,26].
Circadian regulation is not limited to the core clock-network genes mentioned above; approximately 10–40% of the rodent genes in a tissue and >80% of the protein coding genes in baboons have been found to exhibit 24-hr rhythmic oscillations, resulting in identification of these genes as clock-controlled genes, “CCGs” [27]. These CCGs regulate various biological processes including apoptosis, metabolism, detoxification, cell-cycle regulation and immune function [28,29,30]. Some genes are under the control of SCN-generated rhythms, but a large proportion of genes are influenced by tissue-specific peripheral clocks. It has been shown that in mouse liver, 90% of the transcripts showing circadian oscillations are under the influence of local clock machinery [31].
The oscillations produced are characterised by their amplitude (magnitude of cycle reflecting the strength of the rhythm), period (the time interval between two recurring waves within a rhythm), phase (any time point on a rhythmic cycle, e.g., peak relative to an external reference such as midnight) and MESOR (midline estimating statistic of rhythm). These features indicate the rhythmicity and robustness of the circadian clock and any changes in these parameters can be a predictor of health-related outcomes (Figure 1) [32,33,34]. Secondly, the rhythms generated by the core and peripheral clock systems need to be aligned given that misalignment between the clocks can potentially disrupt the body’s physiological patterns and metabolism.
3. Chronotypes, Chronodisruption and Energy Homeostasis
The circadian cycle for humans has an average period of 24.2 h, but this period varies considerably between individuals and is defined as their chronotype [35]. Chronotypes range between early birds (morning people/advanced sleep phase (ASP)/early chronotypes, people going to bed and waking up early, circadian period shorter than 24.2 h) to night owls (evening people/delayed sleep phase (DSP)/late chronotypes, people preferring late bedtime and waking up late, a circadian period longer than 24.2 h). It is estimated that about 40% of the population can be classified into either of these extremes [35,36]. There is a continuum between these two extreme phenotypes, and individuals within this category are referred to as belonging to the intermediate or neutral chronotype; about 60% of the population falls into this category [37]. Melatonin rhythms and DLMO vary by as much as 2 h between the chronotypes [38]. Certain rare and genetic forms of extreme chronotypes have also been recognised. A type of insomnia where sleep patterns are shifted to a delayed onset and offset times compared to the societal norm is known as delayed sleep phase disorder (DSPD), while a phenotype associated with habitual sleep times that are earlier than the solar morning or societal norm is termed familial advanced sleep phase disorder (FASPD) [39]. In addition to the age, gender and societal set-up, an individual’s chronotype is also influenced by their genetic makeup and various genome-wide association studies (GWAS) and candidate-gene approaches have associated more than 350 loci with the morning chronotype and, not surprisingly, these include the components from the clock machinery [40]. Genetic variants within the clock machinery have been associated with sleep patterns, variation in energy intake, waist circumference, obesity and metabolic diseases (Table 1) [41,42,43]. Both morning and evening chronotypes are multigenic and are influenced by the environment, whereas non-genetic factors such as artificial light and social pressures contribute more to the evening chronotype [44]. Although these chronotypes result in a preferred choice of sleep and activity patterns, these do not directly contribute to the pathogenesis of metabolic diseases. However, some recent studies have suggested a link with these morningness or eveningness tendencies and metabolic health. The evening chronotype has been associated with unhealthy food choices, binge eating, night snacks and multiple metabolic disorders, including obesity, while morning individuals are associated with lower rates of depression and improved mental health [40,44,45,46,47,48,49,50]. The chronotype of an individual determines their sleep, dietary and activity patterns and although they indirectly influence the sleep duration and quality, these are distinct from sleep duration [51]. Any forced disruption to the normal sleep patterns, e.g., shift work or frequent traveling over two or more time zones (jetlag), can lead to circadian misalignment and have been associated with various metabolic diseases. An individual with the evening chronotype tends to go to bed late but due to external demands (occupational commitments such as working hours or school start time in case of children) would result in waking up to an alarm clock that is out of phase from their biological circadian cycle, resulting in shorter sleep duration. There can also be variation in bedtime within the week, e.g., weekdays vs. the weekends, leading to the concept of social jet-lag. These societal activities indirectly contribute to metabolic health (Table 1) [16,44].

Table 1.
A summary of genetic variants in clock components and their associations with chronotypes, eating behaviours and health parameters.
There is an intricate and bidirectional relationship between the circadian clock and metabolism that contributes to the overall metabolic homeostasis. There needs to be an optimal alignment between central and peripheral clock requiring energy intake to be aligned with the active phase/biological day for diurnal organisms such as humans (and night-time for nocturnal animals such as rodents). Mice consume about 80% and humans approximately 100% of the nutrients during the wake/active phase [75]. This pattern is in-tune with the oscillations in metabolic pathways of primitive hunter-gatherer humans, who were exposed to feast/famine cycles that coincided with active/rest phases. During the active phase, when humans could forage and hunt for food, the energy intake was higher and the metabolic pathways were geared towards replenishing the energy stores. While famine, generally associated with rest meant that the body had to adapt to starvation or restriction in food intake and the metabolism would switch to catabolic processes and mobilisation of energy stores. Humans are genetically programmed to these oscillations in energy stores, which is incompatible with a modern lifestyle with the constant availability of high-energy foods.
These results, when combined with a sedentary lifestyle, blunt the oscillations and lead to metabolic disturbances with a plethora of associated diseases [76,77,78].
Misalignment in the active/rest and feast/famine phases can be due to endogenous factors, e.g., genetic variants in the core clock machinery or due to external lifestyle factors such as extended exposure to artificial light, increased shift work, sedentarism, untimely and frequent snacking and jetlag, and leads to chronobiological vulnerabilities to various diseases (Table 2) [46,79,80,81,82]. An umbrella term used for circadian disruptions is ‘chronodisruption’. The term has evolved since it was first coined in 2003, with short-term disturbance being called circadian disruption. In contrast, long-term disturbances leading to adaptations without a negative impact on health are termed chronodisturbance, and long-term desynchronisation contributing to disease is called chronodisruption [83]. Artificial light exposure, even at low levels such as from electronic devices including phones, also interferes with the DLMO and melatonin levels, sleep onset and duration [16,44,84]. Additionally, night eating, irregular eating patterns or feeding over the resting periods even in the absence of evening chronotype lead to misalignment and impact the robustness of the oscillations, compromising the metabolic homeostasis and leading to higher BMI and disease development (Figure 2) [76,85,86,87,88,89,90,91,92,93,94,95].

Table 2.
A summary of evidence establishing the role of chronodisruption by mistimed eating habits and associated BMI and metabolic health outcomes.
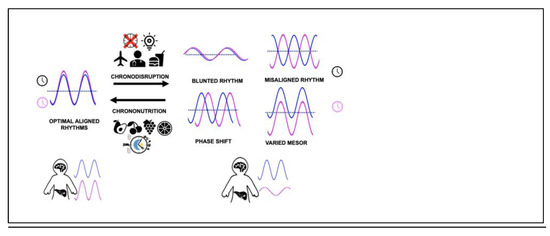
Figure 2.
Master (blue) and peripheral clocks (pink) alignment is key to optimal metabolic health. Chronodisruption by various means leads to misalignment of circadian rhythms and has health consequences. Chrononutritional approaches have the ability to reverse deleterious chronodisruptive rhythms. The dotted line represents MESOR (midline estimating statistic of rhythm).
4. Chrononutrition
Understanding the molecular basis of chronodisruption can potentially allow us to develop practical strategies to improve circadian alignment to mitigate the burden of metabolic diseases. One such relatively novel approach is termed ‘chrononutrition’, encompassing two elements: dietary components that regulate circadian system and meal timings to synchronise misaligned molecular clocks, which can act either positively or negatively on metabolic activity. These interventions (including physical activity) can improve the blunted rhythmic oscillations; even if these are not as robust as those accompanying feast/famine cycles, they can potentially be associated with positive metabolic health outcomes (Figure 2) [111].
4.1. What to Eat
It is well-established that nutritional components, including macronutrients and natural bioactive compounds, have the ability to (directly or indirectly) regulate the expression of various genes, and clock-network genes are no exception [112,113,114,115]. While feeding/fasting patterns mainly act as a potent zeitgeber for peripheral clocks and minimally impact the master clock, nutritional components are also able to influence the master clock in the SCN [116,117,118]. High-fat diets are the best-known circadian rhythm disruptors and can lead to the reversal of feeding patterns and perturbed metabolic parameters [117,119,120,121,122]. The relative distribution of macronutrients in diet can also contribute to central and peripheral clock modulation in humans [115,123]. Other nutrients have been investigated for their role in circadian remodelling; a ketogenic diet increases the activation of CCGs via CLOCK-BMAL1 activation, high sodium and high salt intake causes a phase delay in BMAL1 and CRY1 and PER2 peak expression and caffeine and theophylline lengthen the period of the cellular circadian clock [124,125,126,127].
A growing body of evidence is emerging that links the use of natural bioactive compounds to health via synchronising or improving circadian rhythmic patterns and potentially acting as a natural chronobiotic—an agent with the ability to adjust the timing of one’s internal biological rhythm. The best studied chronobiotic is melatonin, which when administered exogenously can shift the circadian clock phase and alter circadian rhythms in endogenous hormones, body temperature and behaviour [128]. There is also evidence that melatonin supplementation not only modulates body weight and metabolic parameters but also has the ability to reverse the metabolic perturbations caused by chronodisruption [129,130]. Most of the melatonin supplementation has been in a synthetic form through capsules, but melatonin also exists in natural food sources such as fish, eggs, poultry, milk, nuts, fruits and seeds [131]. Natural plant derivatives such as phytochemicals, plant bioactives and nutraceuticals have gained significant attention for their health-promoting properties. Plant polyphenols are one of the most abundant and widely distributed group of secondary metabolites driven from plants. A diverse range of polyphenolic compounds, including phenolic acids, flavanones, flavonoids, tannins, lignans, stilbenes and curcuminoids, have been associated with anti-obesity activities via a variety of mechanisms [132,133,134,135]. Although the exact mechanisms of their actions remain unclear and there are issues around their absorption, bioavailability and bio-accessibility, evidence suggests that some of the beneficial effects of these compounds are mediated by their ability to interact with circadian clocks via genetic/epigenetic mechanisms or indirectly via altering the gut microbiota (Table 3) [136,137]. These interactions are complex; phytochemical content from the plant source depends on various agricultural factors such as soil, light, season, temperature and even the endogenous circadian clock of the plant [138]. There are also seasonal factors, e.g., availability, polyphenolic composition from the same source and human-consumption patterns, which can add another layer of complexity to the seasonal biological oscillations over the period of 12 months, called circannual rhythms [138,139,140]. The timing of consumption of these compounds is also critical, as demonstrated by a study using tryptophan-enriched milk formula in infants. Infants taking tryptophan-enriched formula during the night had improved sleep parameters as compared to those who consumed it during the day [141]. Table 3 presents some of the direct interactions observed between the polyphenolic compounds, circadian mechanisms and health outcomes.

Table 3.
A summary of polyphenols with their interactions with circadian system and role in health.
4.2. When to Eat
Interestingly, other than what goes on your plate, chrononutrition also highlights the significance of aligning the meal timing, frequency and the patterns of energy intake with the circadian rhythm [167]. The concept of ‘when you eat’ was first proposed in 1967 as a link between the meal timing, energy metabolism and chronic diseases by Franz Halberg [168,169]. Food consumption is a strong entrainer for peripheral circadian clocks. Optimal health requires an alignment of energy intake with the biological day and active phase and to generate feed/fast cycle that human physiology is adapted to. The transition between the feed/fast cycles requires a different set of transcription factors and associated proteins, which display diurnal variation. Genes that are active and peak during the day are mainly associated with glycogenesis and lipogenesis, with an overall aim of replenishing the energy stores, while the fasting phase is enriched with genes responsible for growth, repair, glycogenolysis and lipolysis. Any perturbations in the availability of the key players in either of these phases and dietary intake could lead to the dysregulation of energy metabolism [21,170,171]. The same meal consumed at different times during the circadian cycle could have a varied impact on energy metabolism. The current 24/7 lifestyle and a constant supply of nutrients interrupts human circadian physiology. Emerging data suggest that the eating window for more than 50% of the population (non-shift-work) is approximately 15 h a day, with less than 30% of energy consumption happening before noon and 30–45% of daily energy consumed during dinner and post-dinner snacks and part of it spanning over the circadian rest period [172,173,174]. The increased eating window and shorter overnight fast contributes to increased energy intake. Mistimed eating accompanied by erratic sleep patterns leads to dampened circadian rhythms and increases the risk of metabolic disorders (Table 2). Interestingly, the dampening of circadian rhythms by a high-fat diet can be recovered from by just limiting food intake during the biological active phase, highlighting the importance of “when to eat” and aligning meals with our biological clocks [175].
Time-restricted feeding (TRF) in animals (time-restricted eating (TRE) in humans) is an approach that aims to align meal times with the circadian rhythm and has gained significant attention in recent years. Multiple animal and human studies have been conducted and some human feasibility studies and clinical trials are summarised in Table 4.

Table 4.
A summary of human studies investigating the impact of time-restricted eating in humans and metabolic health outcomes.
5. Time-Restricted Eating—Just Another Approach to Reduce Caloric Intake or a Circadian Alignment Tool
The role of calorie consumption in energy homeostasis is not disputed, and creating a negative energy balance is a logical approach to tilt the scales. Calorie restriction (CR) refers to a consistent dietary regimen low in calories, generally a daily 20–40% reduction as compared with ad libitum feeding, without malnutrition (Figure 3). This is not simply another term for fasting, which is commonly defined as the total abstinence from energy-containing foods and beverages for periods ranging from 12 h to 3 weeks, although some protocols employ modified fasting in which a minimal number of calories may be consumed [207]. CR is one of the most effective interventions for weight loss, improving health parameters in animals including primates and is a highly successful strategy for reducing age-related diseases and extending the mean and maximum lifespan in multiple species [208,209,210]. In addition to animal studies including mice and monkeys, CR over a 6-year period in a cohort of 18 participants showed improved BMI, glucose homeostasis and lipid profile and reduced inflammatory markers and blood pressure [211,212,213]. Although short-term caloric restriction is associated with 5–10% weight loss, long-term compliance is a massive challenge and there is a tendency to regain the lost weight [214,215]. To overcome these challenges, alternative dietary strategies such as intermittent caloric restriction have gained attention.
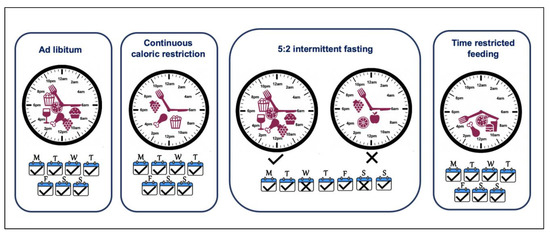
Figure 3.
Dietary weight-loss approaches. Continuous caloric restriction includes reducing caloric intake on each day of the week but does not restrict time of the day. Intermittent fasting, e.g., 5:2 diet, introduces two caloric restriction days with ad libitum eating for the rest of the week. Time-restricted feeding limits the eating window and extended fast period regularly.
The intermittent fasting (IF) approach involves introducing intermittent periods of eating deprivation, providing a less restrictive alternative to CR. The regimen includes periods of fasting where the energy restriction ranges from 60–100%, interrupted by periods of normal dietary intake (isocaloric/ad libitum). This approach is adopted in a variety of protocols; alternate day fasting (no calories on fasting day and ad libitum on feast days), alternate-day modified fasting (consuming <25% of usual caloric intake on fasting days and ad libitum on feast days), 5:2 diet (with 2 days of fasting with 60–100% energy restrictions and 5 days of isocaloric intake), 4:3 (with 3 days of fasting with 60–100% energy restrictions and 4 days of isocaloric intake) (Figure 3) [214,216,217,218]. Certain religious fasting practices observed including the Islamic month of Ramadan have been studied as part of IF approaches [219]. This approach, as compared to continuous CR, introduces periods of fasting when the metabolism shifts towards the catabolic state and mimics the feast/fast physiology of our hunter-gatherer past [220]. Even though the IF regimen suggests ad libitum feeding on non-fasting days, there is no full compensation for the fasting days/time, and overall there is an energy deficit or lack of calories. Various studies have compared IF approaches to continuous CR and reported comparable or better weight loss and improvement in metabolic health [189,221,222,223,224,225,226,227,228].
Time-restricted feeding (TRF) is considered a modified version of IF, where the energy intake is limited to a window of 4 to 12 h in order to extend the time spent in the fasted state regularly, without changes in caloric intake (Figure 3) [229]. Even though caloric restriction is not intentional in TRF, multiple studies have reported that restricting the feeding window to 8 h produces a mild caloric deficit [176,179]. In fact, any restriction to the eating window helps reduce the energy intake, e.g., just stopping night-time eating in healthy individuals leads to a reduction in energy intake [187]. Considering the deleterious effects of chronodisruption, extended eating duration and the imbalanced spread of energy intake during the day, it is clear that the timing of a meal is instrumental in fine tuning the energy balance. Thus, TRF is more than just a mode for caloric restriction or IF; it also synchronises the feeding time with the awake/active phase when the body is best able to metabolise it. More recently, the health-promoting role of caloric restriction has been shown to be partly due to TRF rather than just caloric intake and extended periods of fasting independent of caloric content share the same if not better health outcomes [230]. This leads to an alignment of the feeding-fasting cycle with circadian rhythms and offers a promising dietary strategy to mitigate the deleterious effect of chronodisruption [231]. Mice fed with a high-fat diet showed a dampened diurnal rhythm in physiology, which was recovered in the cohort on same diet but over a time-restricted period [232]. There is a plethora of research supporting TRF being beneficial in not only reducing body weight but also improving metabolic health in general (Table 4) [184,204,231,232,233,234]. Interestingly, as well as the length of the restriction window, the timing of the TRF within the 24-hour cycle is important and may provide slightly different outcomes. Restricting the feeding period to earlier in the day (eTRF) provides advantageous outcomes than mid-day TRF (mTRF) or later TRF (lTRF), as this aligns better with circadian biology, though larger studies and more data are required to fine-tune the interventions [204]. Attempts have also been made to have a pragmatic approach to adapt TRF approaches to life/work schedules. It has been shown that TRF for 8–9 h a day for 5 days and ad libitum for 2 days, still reverses or restricts diet-induced obesity [235,236].
Caloric restriction and intermittent fasting are not strictly part of the chrononutrition strategy, as the focus is not about aligning meal times with the biological clock. However, due to the common mechanisms of an overall negative energy balance, which is involuntary in IF and TRF, they share certain molecular mechanisms that contribute to the overall energy homeostasis. Each of the above-mentioned strategies have challenges and potential barriers to adherence for a long-term weight-loss strategy, though the current view supports TRF as a promising tool with greater-than-ordinary adherence, a good safety profile, and socially acceptable flexible implementation [237,238,239,240]
6. Mechanisms of Chrononutrition in Energy Homeostasis and Obesity
6.1. Appetite Control
Appetite, eating behaviour, hunger/satiety and energy homeostasis are controlled by the melanocortin system. This includes melanocortin receptor 4 (MC4R), which is present in the brain and is activated by its ligand, melanocyte-stimulating hormone (MSH). MSH is produced by the arcuate nucleus (ARC) in the hypothalamus that consists of two distinct types of neurons: anorexigenic neurons expressing proopiomelanocortin (POMC) the orexigenic neurons expressing NeuroPeptide-Y (NPY) and agouti-related protein (AgRP), having opposite effects on energy homeostasis. Leptin, an adipocyte-derived satiety hormone, activates POMC neurons, and its circulatory levels directly relate to adiposity. This results in the proteolytic conversion of POMC and the release of α-MSH, which activates MC4R, promoting satiety, reduced food intake and increased energy expenditure. Leptin also binds to AgRP neurons, resulting in the suppression of AgRP release, which is a potent antagonist for MC4R and increases food intake, energy conservation and weight [241,242]. An incretin hormone GLP1 (glucagon-like peptide-1) that stimulates insulin secretion, and PYY (peptide YY), both secreted from the gastrointestinal tract, are also anorexigenic and delay gastric emptying and promote satiety [243]. As opposed to leptin, ghrelin is an orexigenic hormone mainly derived from the stomach, which promotes hunger via activating AgRP neurons, which increases appetite and decreases energy expenditure (Figure 4) [244].

Figure 4.
Mechanisms of chrononutrition: the bidirectional interactions between clock machinery, dietary polyphenols and meal times lead to rhythmic expression of various genes involved in sleep, energy balance, metabolism and regulation of gut microbiota. NAD, AMPK, SIRT1, SCFA, all derived from gut microbiota via PGC1a, lead to mitochondrial biogenesis and browning of white adipose tissue. MC4R pathway, via leptin, ghrelin, GLP1 and PYY, controls energy expenditure and satiety. Gut microbiota influenced by chrononutritional approaches also contributes to the overall energy homeostasis and BMI regulation. UCP1 (uncoupling protein 1) SCFA (short chain fatty acids), GLP1 (glucagon-like peptide-1), PYY (peptide YY), AgRP (agouti-related protein), POMC 9 (proopiomelanocortin), α-MSH (melanocyte-stimulating hormone), MC4R (melanocortin receptor 4), AMPK (AMP-activated protein kinase), NAD+ (nicotinamide adenine dinucleotide), PPARγ (peroxisome proliferator-activated receptor γ), NAMPT (nicotinamide phosphoribosyltransferase), NAD+ (nicotinamide adenine dinucleotide), AMPK (AMP-activated protein kinase), CCGs (clock-controlled genes), SIRT1 (sirtuin 1), PGC1α (PPARγ coactivator 1α).
Leptin and ghrelin levels both exhibit diurnal oscillations and are influenced by obesity and food intake [245,246,247,248,249]. Circulating leptin levels peak at night and are lowest in the afternoon, but in obese subjects the amplitude in these oscillations is lost [249]. Circadian disruption abolishes circadian oscillation patterns of plasma leptin and induces leptin resistance [104,247,250]. Leptin is high in obese subjects, but, due to leptin resistance, the satiety signal is absent/compromised. Diets rich in fat inhibit the anorectic effects of leptin while sucrose- and fructose-rich diets promote leptin resistance [251,252]. Fasting leads to a drop in leptin levels and intermittent fasting improves leptin resistance. [253,254,255,256]. Leptin also regulates energy homeostasis through AMP-activated protein kinase (AMPK) by increasing fatty acid oxidation and reducing fatty acid biosynthesis [257,258]. Leptin also increases the expression of uncoupling protein-1(UCP1) and the browning of white adipose tissue and thermogenesis, discussed later in this section [259]. GLP1 and PYY are under the control of clock machinery and exhibit circadian patterns of rhythmicity [260,261].
Ghrelin levels increase during fasting or just before the habitual feeding time, dropping postprandially. Overweight and obese subjects lose this variation with a lesser drop in postprandial ghrelin levels leading to lower level of satiety after a meal, promoting snacking and overconsumption of food [262]. Ghrelin levels increase in response to caloric restriction and remain high for a considerable length of time, leading to increased food intake and regaining weight [263,264,265]. The response of plasma ghrelin levels to time-restricted feeding are inconsistent, with some studies reporting its reduction, while others show no effect [182,185,266,267].
Sleep duration also regulates ghrelin and leptin levels in circulation and contribute to energy homeostasis [268,269,270]. Insufficient sleep possibly works via altering the levels of melatonin, which plays a key role in food intake and energy expenditure. Melatonin reduces the expression of AgRP/NPY and increases the expression of POMC, hence regulating energy homeostasis via the MC4R pathway in the hypothalamus. Melatonin also inhibits leptin secretion and ameliorates leptin resistance, which accompanies obesity [249]. Lack of sleep also interferes with the weight loss achieved by caloric restriction, indicating the key role of sleep in terms of the efficacy of weight-loss strategies [269]. TRF and multiple natural bioactive compounds have been associated with an improvement in sleep [76,271,272].
6.2. Energy Sensors in the Body
6.2.1. AMP-Activated Protein Kinase (AMPK)
AMP-activated protein kinase (AMPK) is the key energy sensor in the cell and has the ability to regulate whole-body metabolism. AMPK is activated upon a fall in intracellular ATP levels and increases in ADP or AMP levels, which reflects the energy status of the cell.
Upon activation, AMPK switches on the catabolic pathways, leading to ATP generation and switching off the anabolic ATP-consuming pathways. ATP generation happens by promoting glycolysis and fatty acid oxidation and in the long term, by increasing mitochondrial content and the use of mitochondrial substrates as an energy source [273].
Fasting/intermittent fasting/nutritional deprivation activates AMPK, converting this nutritional signal to a circadian signal by phosphorylating CRY, resulting in its degradation. AMPK also phosphorylates casein kinase I epsilon, which in turn phosphorylates and degrades PER [274]. This removes repression on CLOCK-BMAL1, shortens the timing of the feedback loop and activates the transcription of the target genes including REV-ERBα, PER and CRY [275]. When a high-fat diet is administered ad libitum, this leads to a disturbed and dampened circadian rhythm of AMPK, while eTRF increases the amplitude of expression of AMPK [232,276].
One key target for activated AMPK is nicotinamide phosphoribosyltransferase (NAMPT), an enzyme that promotes an increase in intracellular levels of nicotinamide adenine dinucleotide (NAD+) levels (Figure 4). NAD+ is essential for cellular energy maintenance and central to cell health. NAD+ acts as a redox carrier that gets converted to NADH in various metabolic pathways including glycolysis, the TCA cycle and fatty acid oxidation. NADH serves as the hydride donor to the electron-transport chain for the production of ATP in mitochondria. Additionally, NAD+ acts as a cofactor or co-substrate to enzymes such as sirtuins and poly (ADP-ribose) polymerases (PARPs). All these processes continuously deplete the NAD+ pool in the cell, which can be replenished by de novo pathway from tryptophan and the predominant salvage pathway from the NAD+ degradation product nicotinamide (NAM) [277]. In the salvage pathway, NAM is converted by NAMPT, the rate-limiting enzyme in the pathway, to an intermediate product, nicotinamide mononucleotide (NMN). NMN adenyltransferase 1-3 (NMNAT1-3) then converts NMN into NAD+ [278]. The CLOCK-BMAL1 complex binds to the E-boxes in the NAMPT promoter and controls its transcription and the levels display robust circadian oscillations providing a 24-h rhythm to NAD+ levels in the cell. NAD+ levels peak approximately 4 h after the peak in NAMPT [279,280]. NAD+ levels depend on cellular energy levels. Glucose deprivation, fasting, caloric restriction and exercise lead to an increase, and high-fat diets decrease NAD+ levels [281,282,283]. Fasting or calorie restriction increases cellular NAD+ levels by activating NAMPT and feeding suppresses NAMPT, providing a link between metabolism and the circadian clock [21,280,283,284]. Sirtuins and PARPs (poly (ADP-ribose) polymerases) depend on and compete for the cellular pool of NAD+. Cellular NAD+ stores are important for cell health, delay the onset of multiple diseases and enhance longevity, and lifestyle interventions that lead to increased NAD+ bioavailability are recommended for positive health outcomes [285].
Sirtuins (silencing information regulator) are a seven-member superfamily of proteins, which deacetylases histones and non-histone proteins and use NAD+ as a co-substrate, converting it into nicotinamide (NAM). The dependence of sirtuins on NAD+ suggests their role as energy sensors of the cell [286]. Studies over the past two decades have provided strong evidence that sirtuins are the key mediators of the beneficial effects of caloric restriction [287,288,289,290,291]. NAD+ levels are increased in fasting, intermittent fasting or caloric restriction, leading to the activation of sirtuins. Activated sirtuins deacetylate a number of proteins playing key roles in metabolism, inflammation, autophagy, aging, apoptosis, oxidative stress, neurodegeneration and cancer [286,292]. SIRT1, the most extensively studied sirtuin, interacts with the clock machinery contributing to the circadian rhythms. SIRT1 expression follows circadian patterns mainly due to circadian regulation of NAMPT and NAD+ levels. In turn, SIRT1 binds to the CLOCK-BMAL1 complex in a circadian manner and regulates clock-dependent gene expression and oscillations, NAMPT being one of them (Figure 1) [293,294]. CLOCK is an acetyltransferase and acetylates BMAL1, which results in the activation of the dimer while SIRT1 counterbalances CLOCK activity and deacetylates BMAL1. Additionally, SIRT1 also deacetylates PER2 and enhances its degradation. The absence of SIRT1 leads to PER2 stabilisation and the inhibition of CLOCK-BMAL1 activity, impacting the expression of various clock-dependent genes while PER2 negatively regulates SIRT1 [293,294,295,296]. SIRT1, playing a dual role as an energy sensor and a regulator of clock components, couples metabolism and circadian mechanisms. SIRT1 activation also leads to deacetylation and enhanced PGC1α transcriptional activation [273,297,298,299].
In addition to caloric restriction, multiple dietary polyphenols have been investigated for their health-promoting properties via activating sirtuins—resveratrol being the most extensively researched (Table 3) [300,301,302]. In fact, a new term has emerged for food components that modulate sirtuin activities mainly by increasing the bioavailability of NAD+: ‘Sirtfood” [303,304,305,306].
Although a vast amount of data is available connecting sirtuins and caloric restriction, fasting and intermittent fasting, the data on the link between the time-restricted feeding and sirtuins have only started to emerge. In mice, TRF has been shown to increase SIRT1 expression and reverse the loss of circadian rhythm in SIRT1 expression caused by a high-fat ad libitum diet [200,307]. Human studies indicate that restricting eating time to the earlier part of the day upregulates SIRT1 expression and the amplitude of SIRT1 circadian oscillations and associated health benefits [184,200].
PARPs (poly (ADP-ribose) polymerases) catalyse the transfer of poly (ADP-ribose) from NAD+ to acceptor proteins to modulate their activity and deplete the cellular NAD+ stores. PARP1 is the best characterised member and responsible for about 90% of the total cellular poly-ADP-ribosylation activity. PARP1 plays a critical active role in DNA repair, metabolism, inflammation and cell death [285,308,309]. PARP1 expression exhibits circadian patterns and poly-ADP-ribosylates and modulates the activities of CLOCK-protein-inhibiting CLOCK-BMAL1 binding activity in a circadian manner [24]. Fasting reduces while a high-fat diet increases PARP1 protein and its activity [310]. The knockdown or inhibition of PARP1 in mice leads to leaner phenotype, increased availability of NAD+, activation of SIRT1 and related metabolic effects, while the reverse was observed in the case of a PARP1 inducer [310]. Loss of PARP1 contributes to the browning of white adipose tissue, partly by activating SIRT1 and PPARγ [311,312,313].
6.2.2. Mitochondrial Dynamics
Mitochondria play a pivotal role in cellular metabolism by generating the basic unit of energy, ATP. Metabolic diseases such as obesity, a high-fat diet or excessive caloric intake all lead to mitochondrial dysfunction, which leads to energy deficiency and an increase in the production of reactive oxygen species, causing cell damage [314,315]. Cellular quality control processes including mitophagy and mitochondrial biogenesis ensure that mitochondrial capacity, function and integrity are preserved. Mitochondrial biogenesis is regulated by many transcriptional regulators present in the cell, PGC1α being the key one. PGC1α is an inducible transcriptional coactivator that regulates multiple transcription factors involved in energy metabolism and exhibits strong circadian rhythms in multiple tissues [316,317,318,319]. The activity of PGC1α is regulated by posttranslational modifications; the deacetylation and phosphorylation of PGC1α lead to its activation (Figure 4) [320,321].
Caloric restriction via fasting, intermittent fasting or time-restricted feeding leads to SIRT1 and AMPK activation, which leads to the deacetylation and phosphorylation of PGC1α, respectively; both these modifications activate PGC1α and an increased mitochondrial biogenesis [298,299,321,322,323]. The process of mitochondrial biogenesis is generally associated with improved cell health and has been reported in various cell types [324,325,326]. Inactive phase feeding in rats leads to an altered regulation of mitochondrial biogenesis [327]. Dietary interventions such as whole-grain bioactive compounds and various polyphenolic compounds also contribute to mitochondrial biogenesis, mainly via the SIRT- PGC1α pathway [328,329,330]. One of the tissues where mitochondrial biogenesis contributes to obesity and energy homeostasis is adipocytes.
6.2.3. Adipose Tissue
There are two distinct categories of adipose tissue, white adipose tissue (WAT) and brown adipose tissue (BAT). WAT stores energy in the form of triglycerides in times of caloric excess and is associated with metabolic disease states. BAT is rich in mitochondria and has a high expression of uncoupling protein-1 (UCP1), can uncouple fatty acid oxidation from ATP production and is specialized for energy expenditure via thermogenesis, modulating the energy homeostasis. It has a protective role in metabolic health. BAT can also protect against diet-induced obesity, and genetic variants in key genes involved in the process have been recognised to contribute to impaired thermogenesis [331,332]. In addition to classical WAT and BAT, trans-differentiation of WAT can lead to a beige or brite adipose tissue via a process called ‘browning’, with positive health outcomes. These beige cells start to express UCP1 accompanied by mitochondrial biogenesis leading to an increased mass of mitochondria (Figure 4). PGC1α, PPARγ and PRDM16 are the three key modulators of browning of WAT and all have been associated with circadian clocks [333,334,335]. PPARγ is a nuclear transcription factor and a nutrient sensor that plays critical roles in adipocyte differentiation. PPARγ exhibits circadian rhythmicity at mRNA and protein level. Nocturin, a circadian-regulated gene also positively regulates PPARγ activity. PPARγ induces REV-ERBα expression and PER2 directly inhibits the expression of PPARγ [336]. PPARγ activation by synthetic ligands can upregulate browning in WAT and it requires the recruitment of PR (PRD1-BF1-RIZ1 homologous)-domain-containing 15 (PRDM16) to form the transcription complex. PRDM16 also induces the expression of PGC1α and UCP1 [337,338,339]. Additionally, the circadian components BMAL1, RORα and REV-ERBα also regulate BAT differentiation [335,340,341,342]. Thus, the modulation of the adipocyte phenotype to BAT contributes to enhanced energy homeostasis, and any strategy mediating this effect can positively contribute to health.
Caloric restriction and exercise contribute to PGC1α activation and associated health outcomes [343,344,345,346,347,348]. Aligning meal times with the circadian rhythms by time-restricted feeding in animal models can also modulate PGC1α activity and mitochondrial biogenesis. [323,349,350,351,352,353]. Additionally, various polyphenolic compounds contribute to mitochondrial biogenesis via PGC1α activation, which is mediated via AMPK-SIRT1 activation [354,355]. Sleep, melatonin and leptin pathways also contribute to the browning of WAT and energy homeostasis via the induction of UCP1 expression and mitochondrial biogenesis (Figure 4) [249,259].
6.2.4. Gut Microbiota
One of the mechanisms for health benefits from chrononutrition is via modulation of the gut microbial community. The gut is not just the site of nutrient absorption, it is also the home to a vast, complex, diverse microbial community. The delicate balance in gut flora diversity and its species/phyla distribution is critical for host health. In addition to dietary components, circadian oscillations also influence the microbial composition and functional profile during the 24-h period. Chronodisruption, including CLOCK mutant models, leads to dysbiosis and promotes obesity, while fasting, intermittent fasting or time-restricted feeding help re-set the balance. Multiple mechanisms are involved between these complex interactions [356,357,358,359]. Firstly, the species distribution of the microbes varies significantly between obese and lean individuals. The two dominant divisions of bacteria in human gut are Firmicutes and Bacteroidetes, and an increased Firmicutes:Bacteroidetes ratio has been associated with obesity [360]. High-fat diets and feeding during the biological night led to an increase in Firmicutes, while caloric restrictions, intermittent fasting, time-restricted feeding, plant-based, high-fibre diets, green tea, cranberry extracts, quercetin, resveratrol and persimmon tannins normalise this ratio and contribute to weight loss (Figure 4) [307,359,361,362,363,364,365,366,367].
Secondly, the gut flora is not a passive and silent community with actions limited to the gut; they are metabolically active and release metabolites into the gut, which are absorbed, enter host circulation and contribute to host physiology, including nutrient sensing. The dietary components and dietary patterns both define the microbial diversity, species distribution and microbial metabolites and have been linked to BMI, glucose homeostasis, metabolic disorders, several inflammatory and cardiovascular diseases, intestinal health, bio-activation of nutrients and vitamins, sleep disorders, neurodegenerative diseases and malignancy [368,369]. Plant-based diets, rich in fruit, vegetables, whole grains and polyphenols, promote microbial diversity, while Western diets, rich in fats and low in fibre, reduce gut microbial diversity [367]. One class of metabolites, called short-chain fatty acids (SCFAs), play multiple roles, including the regulation of energy derived from food, reducing inflammation, preventing pathogen invasion and maintaining barrier integrity. These also act as ligands to G-protein coupled receptors (GPCRs), which upon activation can lead to a variety of metabolic effects, including the stimulation of the secretion of insulin, glucagon-like peptide 1 (GLP-1) and peptide YY (PYY), which in turn, act to increase satiety and increase transit time [368,369,370,371]. Butyrate, a SCFA, also leads to AMPK activation and accompanying beneficial effects discussed above. Butyrate, a type of SCFA in the cecum of mice fed with regular chow, showed diurnal patterns with the ability to enhance the circadian gene amplitude in the liver, which was absent in high-fat-diet fed mice [372]. TRF and intermittent energy restriction caused the enrichment of species that upregulated the generation of SCFA [373]. The gut microbiota also contributes to the adipocyte phenotype and the expression of UCP1. Mice fed with a high-fat diet but over a limited feeding period showed an improved rhythmic expression of UCP1 and improved night-time energy consumption and oscillations in PPARα expression [232]. There is a reciprocal relationship between dietary polyphenols and the gut microbiota. Gut microbiota, as demonstrated by germ-free models, influence the metabolism and bioavailability of these compounds, while the health-promoting properties of polyphenols are partly mediated by their ability to act as a prebiotic to promote growth, reshape the microbial composition and reverse the disturbances in microflora caused by chronodisruption [137,163].
7. Conclusions
Obesity is a critical global public health threat that without urgent and multifaceted approaches will continue its inexorable rise. Diet and energy balance remain key targets for any weight-regulatory intervention, but their application to manage long-term body weight have yielded limited success. The key to a successful dietary intervention requires sustained adherence with pragmatic implementation, such that there is minimal interference to day-to-day activities. Optimising circadian mechanisms via nutritional means is a valid and innovative approach to address energy balance. Additionally, educating and empowering the public to make informed lifestyle choices is key for sustainable behavioural changes. These modifications based on the appreciation of interactions between ‘what we eat’ and ‘when we eat’ can potentially prevent, delay the onset of and manage obesity. However, larger and longer human studies with clearly defined bioactive dietary components, timing of consumption, eating windows and energy content in genetically diverse populations are required before translating chrononutritional strategies into effective interventions for the general population.
Funding
This research received no external funding. The APC was funded by the Cardiff School of Sport and Health Sciences.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. Obesity and Overweight. World Health Organization. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 16 September 2022).
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H. Obesity and Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 968–976. [Google Scholar] [CrossRef] [PubMed]
- New WHO Report: Europe Can Reverse Its Obesity “Epidemic”. 2022. Available online: https://www.who.int/europe/news/item/03-05-2022-new-who-report--europe-can-reverse-its-obesity--epidemic (accessed on 16 September 2022.).
- Loos, R.J.F.; Yeo, G.S.H. The genetics of obesity: From discovery to biology. Nat. Rev. Genet. 2021, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Sandholt, C.H.; Hansen, T.; Pedersen, O. Beyond the fourth wave of genome-wide obesity association studies. Nutr. Diabetes 2012, 2, e37. [Google Scholar] [CrossRef]
- Jiang, P.; Turek, F.W. The endogenous circadian clock programs animals to eat at certain times of the 24-hour day: What if we ignore the clock? Physiol. Behav. 2018, 193, 211–217. [Google Scholar] [CrossRef]
- Stenvers, D.J.; Jongejan, A.; Atiqi, S.; Vreijling, J.P.; Limonard, E.J.; Endert, E.; Baas, F.; Moerland, P.D.; Fliers, E.; Kalsbeek, A.; et al. Diurnal rhythms in the white adipose tissue transcriptome are disturbed in obese individuals with type 2 diabetes compared with lean control individuals. Diabetologia 2019, 62, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Challet, E. Interactions between photic and nonphotic stimuli to synchronize the master circadian clock in mammals. Front. Biosci. 2003, 8, s246–s257. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2016, 18, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Lazar, M.A. Transcriptional Control of Circadian Rhythms and Metabolism: A Matter of Time and Space. Endocr. Rev. 2020, 41, 707–732. [Google Scholar] [CrossRef]
- Grivas, T.B.; Savvidou, O.D. Melatonin the “light of night” in human biology and adolescent idiopathic scoliosis. Scoliosis 2007, 2, 6–14. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; Smits, M.; Spence, W.; Srinivasan, V.; Cardinali, D.P.; Lowe, A.D.; Kayumov, L. Dim light melatonin onset (DLMO): A tool for the analysis of circadian phase in human sleep and chronobiological disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2007, 31, 1–11. [Google Scholar] [CrossRef]
- Zisapel, N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharmacol. 2018, 175, 3190–3199. [Google Scholar] [CrossRef]
- Beccuti, G.; Pannain, S. Sleep and obesity. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Ciccone, G.; Durazzo, M.; Ghinamo, L.; Villois, P.; Canil, S.; Gambino, R.; Cassader, M.; Gentile, L.; Cavallo-Perin, P. Contributors to the obesity and hyperglycemia epidemics. A prospective study in a population-based cohort. Int. J. Obes. 2011, 35, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Potter, G.D.M.; Skene, D.J.; Arendt, J.; Cade, J.E.; Grant, P.J.; Hardie, L.J. Circadian Rhythm and Sleep Disruption: Causes, Metabolic Consequences, and Countermeasures. Endocr. Rev. 2016, 37, 584–608. [Google Scholar] [CrossRef] [PubMed]
- Buhr, E.D.; Takahashi, J.S. Molecular components of the Mammalian circadian clock. Handb. Exp. Pharmacol. 2013, 217, 3–27. [Google Scholar]
- Mohawk, J.A.; Green, C.B.; Takahashi, J.S. Central and Peripheral Circadian Clocks in Mammals. Annu. Rev. Neurosci. 2012, 35, 445–462. [Google Scholar] [CrossRef]
- Liu, A.C.; Tran, H.G.; Zhang, E.E.; Priest, A.A.; Welsh, D.K.; Kay, S.A. Redundant Function of REV-ERBα and β and Non-Essential Role for Bmal1 Cycling in Transcriptional Regulation of Intracellular Circadian Rhythms. PLoS Genet. 2008, 4, e1000023. [Google Scholar] [CrossRef]
- Liu, C.; Li, S.; Liu, T.; Borjigin, J.; Lin, J.D. Transcriptional coactivator PGC-1α integrates the mammalian clock and energy metabolism. Nature 2007, 447, 477–481. [Google Scholar] [CrossRef]
- Bass, J.; Takahashi, J.S. Circadian Integration of Metabolism and Energetics. Science 2010, 330, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Canaple, L.; Rambaud, J.; Dkhissi-Benyahya, O.; Rayet, B.; Tan, N.S.; Michalik, L.; Delaunay, F.; Wahli, W.; Laudet, V. Reciprocal Regulation of Brain and Muscle Arnt-Like Protein 1 and Peroxisome Proliferator-Activated Receptor α Defines a Novel Positive Feedback Loop in the Rodent Liver Circadian Clock. Mol. Endocrinol. 2006, 20, 1715–1727. [Google Scholar] [CrossRef] [PubMed]
- Masri, S.; Sassone-Corsi, P. Plasticity and specificity of the circadian epigenome. Nat. Neurosci. 2010, 13, 1324–1329. [Google Scholar] [CrossRef] [PubMed]
- Asher, G.; Reinke, H.; Altmeyer, M.; Gutierrez-Arcelus, M.; Hottiger, M.O.; Schibler, U. Poly(ADP-Ribose) Polymerase 1 Participates in the Phase Entrainment of Circadian Clocks to Feeding. Cell 2010, 142, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, J.; Sahar, S.; Grimaldi, B.; Tamaru, T.; Takamatsu, K.; Nakahata, Y.; Sassone-Corsi, P. CLOCK-mediated acetylation of BMAL1 controls circadian function. Nature 2007, 450, 1086–1090. [Google Scholar] [CrossRef] [PubMed]
- Doi, M.; Hirayama, J.; Sassone-Corsi, P. Circadian Regulator CLOCK Is a Histone Acetyltransferase. Cell 2006, 125, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Mure, L.S.; Le, H.D.; Benegiamo, G.; Chang, M.W.; Rios, L.; Jillani, N.; Ngotho, M.; Kariuki, T.; Dkhissi-Benyahya, O.; Cooper, H.M.; et al. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science 2018, 359, 6381. [Google Scholar] [CrossRef]
- Fuhr, L.; Abreu, M.; Pett, P.; Relógio, A. Circadian systems biology: When time matters. Comput. Struct. Biotechnol. J. 2015, 13, 417–426. [Google Scholar] [CrossRef]
- Li, J.; Nie, P.; Turck, C.W.; Wang, G.-Z. Gene networks under circadian control exhibit diurnal organization in primate organs. Commun. Biol. 2022, 5, 1–14. [Google Scholar] [CrossRef]
- Eckel-Mahan, K.; Sassone-Corsi, P. Metabolism and the Circadian Clock Converge. Physiol. Rev. 2013, 93, 107–135. [Google Scholar] [CrossRef]
- Kornmann, B.; Schaad, O.; Bujard, H.; Takahashi, J.S.; Schibler, U. System-Driven and Oscillator-Dependent Circadian Transcription in Mice with a Conditionally Active Liver Clock. O’Rahilly, S., editor. PLoS Biol. 2007, 5, e34. [Google Scholar] [CrossRef]
- Peng, X.; Fan, R.; Xie, L.; Shi, X.; Dong, K.; Zhang, S.; Tao, J.; Xu, W.; Ma, D.; Chen, J.; et al. A Growing Link between Circadian Rhythms, Type 2 Diabetes Mellitus and Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 504. [Google Scholar] [CrossRef]
- Ki, Y.; Ri, H.; Lee, H.; Yoo, E.; Choe, J.; Lim, C. Warming Up Your Tick-Tock: Temperature-Dependent Regulation of Circadian Clocks. The Neuroscientist: A Review. J. Bringing Neurobiol. Neurol. Psychiatry 2015, 21, 503–518. [Google Scholar]
- Saini, R.; Jaskolski, M.; Davis, S.J. Circadian oscillator proteins across the kingdoms of life: Structural aspects. BMC Biol. 2019, 17, 13. [Google Scholar]
- Kalmbach, D.A.; Schneider, L.D.; Cheung, J.; Bertrand, S.J.; Kariharan, T.; Pack, A.I.; Gehrman, P.R. Genetic Basis of Chronotype in Humans: Insights from Three Landmark GWAS. Sleep 2016, 40, zsw048. [Google Scholar] [CrossRef] [PubMed]
- Sack, R.L.; Auckley, D.; Auger, R.R.; Carskadon, M.A.; Wright, J.K.P.; Vitiello, M.V.; Zhdanova, I.V. Circadian Rhythm Sleep Disorders: Part II, Advanced Sleep Phase Disorder, Delayed Sleep Phase Disorder, Free-Running Disorder, and Irregular Sleep-Wake Rhythm. Sleep 2007, 30, 1484–1501. [Google Scholar] [CrossRef]
- Adan, A.; Archer, S.N.; Hidalgo, M.P.; Di Milia, L.; Natale, V.; Randler, C. Circadian Typology: A Comprehensive Review. Chronobiol. Int. 2012, 29, 1153–1175. [Google Scholar] [CrossRef] [PubMed]
- Taillard, J.; Philip, P.; Claustrat, B.; Capelli, A.; Coste, O.; Chaumet, G.; Sagaspe, P. Time course of neurobehavioral alertness during extended wakefulness in morning- and evening-type healthy sleepers. Chronobiol. Int. 2011, 28, 520–527. [Google Scholar] [CrossRef]
- Jones, C.R.; Huang, A.L.; Ptáček, L.J.; Fu, Y.-H. Genetic basis of human circadian rhythm disorders. Exp. Neurol. 2013, 243, 28–33. [Google Scholar] [CrossRef]
- Jones, S.E.; Lane, J.M.; Wood, A.R.; van Hees, V.T.; Tyrrell, J.; Beaumont, R.N.; Jeffries, A.R.; Dashti, H.S.; Hillsdon, M.; Ruth, K.S.; et al. Genome-wide association analyses of chronotype in 697,828 individuals provides insights into circadian rhythms. Nat. Commun. 2019, 10, 343. [Google Scholar] [CrossRef]
- Valladares, M.; Obregón, A.M.; Chaput, J.-P. Association between genetic variants of the clock gene and obesity and sleep duration. J. Physiol. Biochem. 2015, 71, 855–860. [Google Scholar] [CrossRef]
- Rijo-Ferreira, F.; Takahashi, J.S. Genomics of circadian rhythms in health and disease. Genome Med. 2019, 11, 82. [Google Scholar] [CrossRef]
- Molina-Montes, E.; Rodríguez-Barranco, M.; Ching-López, A.; Artacho, R.; Huerta, J.M.; Amiano, P.; Lasheras, C.; Moreno-Iribas, C.; Jimenez-Zabala, A.; Chirlaque, M.D.; et al. Circadian clock gene variants and their link with chronotype, chrononutrition, sleeping patterns and obesity in the European prospective investigation into cancer and nutrition (EPIC) study. Clin. Nutr. 2022, 41, 1977–1990. [Google Scholar] [CrossRef] [PubMed]
- Ashbrook, L.H.; Krystal, A.D.; Fu, Y.-H.; Ptáček, L.J. Genetics of the human circadian clock and sleep homeostat. Neuropsychopharmacology 2019, 45, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Reutrakul, S.; Hood, M.M.; Crowley, S.J.; Morgan, M.K.; Teodori, M.; Knutson, K.L.; Van Cauter, E. Chronotype Is Independently Associated with Glycemic Control in Type 2 Diabetes. Diabetes Care 2013, 36, 2523–2529. [Google Scholar] [CrossRef] [PubMed]
- Patterson, F.; Malone, S.K.; Grandner, M.A.; Lozano, A.; Perkett, M.; Hanlon, A. Interactive effects of sleep duration and morning/evening preference on cardiovascular risk factors. Eur. J. Public Health 2017, 28, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wu, M.; Yuan, F.; Zhang, H. Sugary beverage consumption mediates the relationship between late chronotype, sleep duration, and weight increase among undergraduates: A cross-sectional study. Environ. Health Prev. Med. 2018, 23, 63. [Google Scholar] [CrossRef]
- Anothaisintawee, T.; Lertrattananon, D.; Thamakaison, S.; Thakkinstian, A.; Reutrakul, S. The Relationship Among Morningness-Eveningness, Sleep Duration, Social Jetlag, and Body Mass Index in Asian Patients with Prediabetes. Front. Endocrinol. 2018, 9, 435. [Google Scholar] [CrossRef]
- Culnan, E.; Kloss, J.D.; Grandner, M. A prospective study of weight gain associated with chronotype among college freshmen. Chronobiol. Int. 2013, 30, 682–690. [Google Scholar] [CrossRef]
- Sun, X.; Gustat, J.; Bertisch, S.; Redline, S.; Bazzano, L. The association between sleep chronotype and obesity among black and white participants of the Bogalusa Heart Study. Chronobiol. Int. 2019, 37, 123–134. [Google Scholar] [CrossRef]
- Roenneberg, T.; Wirz-Justice, A.; Merrow, M. Life between Clocks: Daily Temporal Patterns of Human Chronotypes. J. Biol. Rhythm. 2003, 18, 80–90. [Google Scholar] [CrossRef]
- Mishima, K.; Tozawa, T.; Satoh, K.; Saitoh, H.; Mishima, Y. The 3111T/C polymorphism ofhClock is associated with evening preference and delayed sleep timing in a Japanese population sample. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2005, 133, 101–104. [Google Scholar] [CrossRef]
- Garaulet, M.; Corbalán, M.D.; Madrid, J.A.; Morales, E.; Baraza, J.C.; Lee, Y.C.; Ordovas, J.M. CLOCK gene is implicated in weight reduction in obese patients participating in a dietary programme based on the Mediterranean diet. Int. J. Obes. 2010, 34, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, P.; Tortorella, A.; Docimo, L.; Maldonato, M.N.; Canestrelli, B.; De Luca, L.; Maj, M. Investigation of 3111T/C polymorphism of the CLOCK gene in obese individuals with or without binge eating disorder: Association with higher body mass index. Neurosci. Lett. 2008, 435, 30–33. [Google Scholar] [CrossRef]
- López-Guimerà, G.; Dashti, H.S.; Smith, C.E.; Sánchez-Carracedo, D.; Ordovas, J.M.; Garaulet, M. CLOCK 3111 T/C SNP Interacts with Emotional Eating Behavior for Weight-Loss in a Mediterranean Population. Rosenfeld CS, editor. PLoS ONE 2014, 9, e99152. [Google Scholar] [CrossRef] [PubMed]
- Giovaninni, N.P.; Fuly, J.T.; Moraes, L.I.; Coutinho, T.N.; Trarbach, E.B.; de LJorge, A.A.; Costalonga, E.F. Study of the association between 3111T/C polymorphism of the CLOCK gene and the presence of overweight in schoolchildren. J. Pediatr. 2014, 90, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Bandín, C.; Martinez-Nicolas, A.; Ordovás, J.M.; Lucas, J.A.R.; Castell, P.; Silvente, T.; Madrid, J.A.; Garaulet, M. Differences in circadian rhythmicity in CLOCK 3111T/C genetic variants in moderate obese women as assessed by thermometry, actimetry and body position. Int. J. Obes. 2012, 37, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, M.; Sánchez-Moreno, C.; Smith, C.E.; Lee, Y.-C.; Nicolás, F.; Ordovás, J.M. Ghrelin, Sleep Reduction and Evening Preference: Relationships to CLOCK 3111 T/C SNP and Weight Loss. Tomé D, editor. PLoS ONE 2011, 6, e17435. [Google Scholar] [CrossRef]
- Garcia-Rios, A.; Gomez-Delgado, F.J.; Garaulet, M.; Alcala-Diaz, J.F.; Delgado-Lista, F.J.; Marin, C.; Rangel-Zuñiga, O.A.; Rodriguez-Cantalejo, F.; Gomez-Luna, P.; Ordovas, J.M.; et al. Beneficial effect of CLOCKgene polymorphism rs1801260 in combination with low-fat diet on insulin metabolism in the patients with metabolic syndrome. Chronobiol. Int. 2013, 31, 401–408. [Google Scholar] [CrossRef]
- Sookoian, S.; Gemma, C.; Gianotti, T.F.; Burgueño, A.; Castaño, G.; Pirola, C.J. Genetic variants of Clock transcription factor are associated with individual susceptibility to obesity. Am. J. Clin. Nutr. 2008, 87, 1606–1615. [Google Scholar] [CrossRef]
- Garaulet, M.; Lee, Y.-C.; Shen, J.; Parnell, L.D.; Arnett, D.K.; Tsai, M.Y.; Lai, C.-Q.; Ordovas, J.M. Genetic variants in human CLOCK associate with total energy intake and cytokine sleep factors in overweight subjects (GOLDN population). Eur. J. Hum. Genet. 2009, 18, 364–369. [Google Scholar] [CrossRef]
- Camblor Murube, M.; Borregon-Rivilla, E.; Colmenarejo, G.; Aguilar-Aguilar, E.; Martínez, J.A.; Ramírez De Molina, A.; Reglero, G.; Loria-Kohen, V. Polymorphism of CLOCK Gene rs3749474 as a Modulator of the Circadian Evening Carbohydrate Intake Impact on Nutritional Status in an Adult Sample. Nutrients 2020, 12, 1142. [Google Scholar] [CrossRef]
- Ye, D.; Cai, S.; Jiang, X.; Ding, Y.; Chen, K.; Fan, C.; Jin, M. Associations of polymorphisms in circadian genes with abdominal obesity in Chinese adult population. Obes. Res. Clin. Pract. 2016, 10 (Suppl. 1), S133–S141. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.M.; Carter, A.M.; Grant, P.J. Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. Int. J. Obes. 2008, 32, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Corella, D.; Asensio, E.M.; Coltell, O.; Sorlí, J.V.; Estruch, R.; Martínez-González, M.Á.; Salas-Salvadó, J.; Castañer, O.; Arós, F.; Lapetra, J.; et al. CLOCK gene variation is associated with incidence of type-2 diabetes and cardiovascular diseases in type-2 diabetic subjects: Dietary modulation in the PREDIMED randomized trial. Cardiovasc. Diabetol. 2016, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Delgado, F.; Garcia-Rios, A.; Alcala-Diaz, J.F.; Rangel-Zuñiga, O.; Delgado-Lista, J.; Yubero-Serrano, E.M.; Lopez-Moreno, J.; Tinahones, F.J.; Ordovas, J.M.; Garaulet, M.; et al. Chronic consumption of a low-fat diet improves cardiometabolic risk factors according to theCLOCKgene in patients with coronary heart disease. Mol. Nutr. Food Res. 2015, 59, 2556–2564. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, M.; Lee, Y.-C.; Shen, J.; Parnell, L.D.; Arnett, D.K.; Tsai, M.Y.; Lai, C.-Q.; Ordovas, J.M. CLOCK genetic variation and metabolic syndrome risk: Modulation by monounsaturated fatty acids. Am. J. Clin. Nutr. 2009, 90, 1466–1475. [Google Scholar] [CrossRef] [PubMed]
- Dashti, H.S.; Follis, J.L.; Smith, C.E.; Tanaka, T.; Cade, B.E.; Gottlieb, D.J.; Hruby, A.; Jacques, P.F.; Lamon-Fava, S.; Richardson, K.; et al. Habitual sleep duration is associated with BMI and macronutrient intake and may be modified by CLOCK genetic variants. Am. J. Clin. Nutr. 2015, 101, 135–143. [Google Scholar] [CrossRef]
- Allebrandt, K.V.; Teder-Laving, M.; Akyol, M.; Pichler, I.; Müller-Myhsok, B.; Pramstaller, P.; Merrow, M.; Meitinger, T.; Metspalu, A.; Roenneberg, T. CLOCK Gene Variants Associate with Sleep Duration in Two Independent Populations. Biol. Psychiatry 2010, 67, 1040–1047. [Google Scholar] [CrossRef]
- Kim, H.-I.; Lee, H.-J.; Cho, C.-H.; Kang, S.-G.; Yoon, H.-K.; Park, Y.-M.; Lee, S.H.; Moon, J.H.; Song, H.M.; Lee, E.; et al. Association of CLOCK, ARNTL, andNPAS2gene polymorphisms and seasonal variations in mood and behavior. Chronobiol. Int. 2015, 32, 785–791. [Google Scholar] [CrossRef]
- Garaulet, M.; Esteban Tardido, A.; Lee, Y.-C.; Smith, C.E.; Parnell, L.D.; Ordovás, J.M. SIRT1 and CLOCK 3111T>C combined genotype is associated with evening preference and weight loss resistance in a behavioral therapy treatment for obesity. Int. J. Obes. 2012, 36, 1436–1441. [Google Scholar] [CrossRef]
- Garaulet, M.; Corbalán-Tutau, M.D.; Madrid, J.A.; Baraza, J.C.; Parnell, L.D.; Lee, Y.-C.; Ordovas, J.M. PERIOD2 Variants Are Associated with Abdominal Obesity, Psycho-Behavioral Factors, and Attrition in the Dietary Treatment of Obesity. J. Am. Diet. Assoc. 2010, 110, 917–921. [Google Scholar] [CrossRef]
- Partonen, T.; Treutlein, J.; Alpman, A.; Frank, J.; Johansson, C.H.; Depner, M.; Aron, L.; Rietschel, M.; Wellek, S.; Soronen, P.; et al. Three circadian clock genes Per2, Arntl, and Npas2 contribute to winter depression. Ann. Med. 2007, 39, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, M.; Smith, C.E.; Gomez-Abellán, P.; Ordovás-Montañés, M.; Lee, Y.-C.; Parnell, L.D.; Arnett, D.K.; Ordovás, J.M. REV-ERB-ALPHA circadian gene variant associates with obesity in two independent populations: Mediterranean and North American. Mol. Nutr. Food Res. 2013, 58, 821–829. [Google Scholar] [CrossRef]
- Reinke, H.; Asher, G. Crosstalk between metabolism and circadian clocks. Nat. Rev. Mol. Cell Biol. 2019, 20, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Panda, S. Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab. 2016, 23, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, M.V.; Booth, F.W. Eating, exercise, and “thrifty” genotypes: Connecting the dots toward an evolutionary understanding of modern chronic diseases. J. Appl. Physiol. 2004, 96, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Challet, E. The circadian regulation of food intake. Nat. Rev. Endocrinol. 2019, 15, 393–405. [Google Scholar] [CrossRef]
- Lane, J.M.; Vlasac, I.; Anderson, S.G.; Kyle, S.D.; Dixon, W.G.; Bechtold, D.A.; Gill, S.; Little, M.A.; Luik, A.; Loudon, A.; et al. Genome-wide association analysis identifies novel loci for chronotype in 100,420 individuals from the UK Biobank. Nat. Commun. 2016, 7, 10889. [Google Scholar] [CrossRef]
- Jones, S.E.; Tyrrell, J.; Wood, A.R.; Beaumont, R.N.; Ruth, K.S.; Tuke, M.A.; Yaghootkar, H.; Hu, T.; Teder-Laving, M.; Hayward, C.; et al. Genome-Wide Association Analyses in 128,266 Individuals Identifies New Morningness and Sleep Duration Loci. Shi, J., editor. PLOS Genet. 2016, 12, e1006125. [Google Scholar] [CrossRef]
- Neves, A.R.; Albuquerque, T.; Quintela, T.; Costa, D. Circadian rhythm and disease: Relationship, new insights, and future perspectives. J. Cell Physiol. 2022, 237, 3239–3256. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, J.; Duan, P.; Liu, B.; Li, T.; Wang, C.; Li, H.; Yang, T.; Gan, Y.; Wang, X.; et al. Is shift work associated with a higher risk of overweight or obesity? A systematic review of observational studies with meta-analysis. Int. J. Epidemiol. 2018, 47, 1956–1971. [Google Scholar] [CrossRef]
- Carriazo, S.; Ramos, A.M.; Sanz, A.B.; Sanchez-Niño, M.D.; Kanbay, M.; Ortiz, A. Chronodisruption: A Poorly Recognized Feature of CKD. Toxins 2020, 12, 151. [Google Scholar] [CrossRef] [PubMed]
- Zerbini, G.; Kantermann, T.; Merrow, M. Strategies to decrease social jetlag: Reducing evening blue light advances sleep and melatonin. Eur. J. Neurosci. 2018, 51, 2355–2366. [Google Scholar] [CrossRef] [PubMed]
- Bonham, M.P.; Kaias, E.; Zimberg, I.; Leung, G.K.W.; Davis, R.; Sletten, T.L.; Windsor-Aubrey, H.; Huggins, C.E. Effect of Night Time Eating on Postprandial Triglyceride Metabolism in Healthy Adults: A Systematic Literature Review. J. Biol. Rhythm. 2019, 34, 119–130. [Google Scholar] [CrossRef]
- Leung, G.K.W.; Huggins, C.E.; Bonham, M.P. Effect of meal timing on postprandial glucose responses to a low glycemic index meal: A crossover trial in healthy volunteers. Clin. Nutr. 2019, 38, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Colles, S.L.; Dixon, J.B.; O’Brien, P.E. Night eating syndrome and nocturnal snacking: Association with obesity, binge eating and psychological distress. Int. J. Obes. 2007, 31, 1722–1730. [Google Scholar] [CrossRef] [PubMed]
- Harb, A.; Levandovski, R.; Oliveira, C.; Caumo, W.; Allison, K.; Stunkard, A.; Hidalgo, M.P. Night eating patterns and chronotypes: A correlation with binge eating behaviors. Psychiatry Res. 2012, 200, 489–493. [Google Scholar] [CrossRef]
- McHill, A.W.; Melanson, E.L.; Higgins, J.; Connick, E.; Moehlman, T.M.; Stothard, E.R.; Wright, K.P., Jr. Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proc. Natl. Acad. Sci. USA 2014, 111, 17302–17307. [Google Scholar] [CrossRef] [PubMed]
- Cahill, L.E.; Chiuve, S.E.; Mekary, R.A.; Jensen, M.K.; Flint, A.J.; Hu, F.B.; Rimm, E.B. A prospective study of breakfast Eating and Incident Coronary Heart Disease in a Cohort of Male, US Health Professionals. Circulation 2013, 128, 337–343. [Google Scholar]
- Pickel, L.; Sung, H.-K. Feeding Rhythms and the Circadian Regulation of Metabolism. Front. Nutr. 2020, 7, 39. [Google Scholar] [CrossRef]
- Kräuchi, K.; Cajochen, C.; Werth, E.; Wirz-Justice, A. Alteration of Internal Circadian Phase Relationships after Morning versus Evening Carbohydrate-Rich Meals in Humans. J. Biol. Rhythm. 2002, 17, 364–376. [Google Scholar] [CrossRef]
- Wehrens, S.M.T.; Christou, S.; Isherwood, C.; Middleton, B.; Gibbs, M.A.; Archer, S.N.; Skene, D.J.; Johnston, J.D. Meal Timing Regulates the Human Circadian System. Curr. Biol. 2017, 27, 1768–1775.e3. [Google Scholar] [CrossRef] [PubMed]
- Damiola, F. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000, 14, 2950–2961. [Google Scholar] [CrossRef] [PubMed]
- Baron, K.G.; Reid, K.J.; Kern, A.S.; Zee, P.C. Role of Sleep Timing in Caloric Intake and BMI. Obesity 2011, 19, 1374–1381. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, J.; Eguchi, E.; Nagaoka, K.; Ito, T.; Ogino, K. Association of night eating habits with metabolic syndrome and its components: A longitudinal study. BMC Public Health 2018, 18, 1366. [Google Scholar] [CrossRef] [PubMed]
- Okada, C.; Imano, H.; Muraki, I.; Yamada, K.; Iso, H. The Association of Having a Late Dinner or Bedtime Snack and Skipping Breakfast with Overweight in Japanese Women. J. Obes. 2019, 2019, 2439571. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lozano, N.; Tvarijonaviciute, A.; Ríos, R.; Barón, I.; Scheer, F.A.J.L.; Garaulet, M. Late Eating Is Associated with Obesity, Inflammatory Markers and Circadian-Related Disturbances in School-Aged Children. Nutrients 2020, 12, 2881. [Google Scholar] [CrossRef]
- Adnan, D.; Trinh, J.; Bishehsari, F. Inconsistent eating time is associated with obesity: A prospective study. EXCLI J. 2022, 21, 300–306. [Google Scholar]
- Olds, T.S.; Maher, C.A.; Matricciani, L. Sleep Duration or Bedtime? Exploring the Relationship between Sleep Habits and Weight Status and Activity Patterns. Sleep 2011, 34, 1299–1307. [Google Scholar] [CrossRef]
- Arora, T.; Taheri, S. Associations among late chronotype, body mass index and dietary behaviors in young adolescents. Int. J. Obes. 2014, 39, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Garaulet, M.; Scheer, F.A.J.L. Meal timing and obesity: Interactions with macronutrient intake and chronotype. Int. J. Obes. 2019, 43, 1701–1711. [Google Scholar] [CrossRef]
- Morris, C.J.; Purvis, T.E.; Hu, K.; Scheer, F.A.J.L. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc. Natl. Acad. Sci. USA 2016, 113, E1402–E1411. [Google Scholar] [CrossRef] [PubMed]
- Scheer, F.A.J.L.; Hilton, M.F.; Mantzoros, C.S.; Shea, S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA 2009, 106, 4453–4458. [Google Scholar] [CrossRef] [PubMed]
- Zhao, I.; Bogossian, F.; Song, S.; Turner, C. The Association Between Shift Work and Unhealthy Weight. J. Occup. Environ. Med. 2011, 53, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Rong, Y.; Huang, X.; Lai, H.; Luo, X.; Zhang, Z.; Liu, Y.; He, M.; Wu, T.; Chen, W. Shift Work and the Relationship with Metabolic Syndrome in Chinese Aged Workers. PLoS ONE 2015, 10, e0120632. [Google Scholar] [CrossRef]
- Kubo, T.; Oyama, I.; Nakamura, T.; Shirane, K.; Otsuka, H.; Kunimoto, M.; Kadowaki, K.; Maruyama, T.; Otomo, H.; Fujino, Y.; et al. Retrospective cohort study of the risk of obesity among shift workers: Findings from the Industry-based Shift Workers’ Health study, Japan. Occup. Environ. Med. 2010, 68, 327–331. [Google Scholar] [CrossRef]
- Canuto, R.; Pattussi, M.P.; Macagnan, J.B.A.; Henn, R.L.; Olinto, M.T.A. Sleep deprivation and obesity in shift workers in southern Brazil. Public Health Nutr. 2013, 17, 2619–2623. [Google Scholar] [CrossRef] [PubMed]
- Brum, M.C.B.; Dantas Filho, F.F.; Schnorr, C.C.; Bertoletti, O.A.; Bottega, G.B.; da Costa Rodrigues, T. Night shift work, short sleep and obesity. Diabetol. Metab. Syndr. 2020, 12, 13. [Google Scholar] [CrossRef]
- Hulsegge, G.; Proper, K.I.; Loef, B.; Paagman, H.; Anema, J.R.; van Mechelen, W. The mediating role of lifestyle in the relationship between shift work, obesity and diabetes. Int. Arch. Occup. Environ. Health 2021, 94, 1287–1295. [Google Scholar] [CrossRef]
- Halberg, N.; Henriksen, M.; Söderhamn, N.; Stallknecht, B.; Ploug, T.; Schjerling, P.; Dela, F. Effect of intermittent fasting and refeeding on insulin action in healthy men. J. Appl. Physiol. 2005, 99, 2128–2136. [Google Scholar] [CrossRef]
- Cousins, R.J. Nutritional regulation of gene expression. Am. J. Med. 1999, 106, 20S–23S, discussion 50S–51S. [Google Scholar] [CrossRef] [PubMed]
- Haro, D.; Marrero, P.; Relat, J. Nutritional Regulation of Gene Expression: Carbohydrate-, Fat- and Amino Acid-Dependent Modulation of Transcriptional Activity. Int. J. Mol. Sci. 2019, 20, 1386. [Google Scholar] [CrossRef] [PubMed]
- Mierziak, J.; Kostyn, K.; Boba, A.; Czemplik, M.; Kulma, A.; Wojtasik, W. Influence of the Bioactive Diet Components on the Gene Expression Regulation. Nutrients 2021, 13, 3673. [Google Scholar] [CrossRef]
- Shon, J.; Han, Y.; Park, Y.J. Effects of Dietary Fat to Carbohydrate Ratio on Obesity Risk Depending on Genotypes of Circadian Genes. Nutrients 2022, 14, 478. [Google Scholar] [CrossRef] [PubMed]
- Tognini, P.; Samad, M.; Kinouchi, K.; Liu, Y.; Helbling, J.-C.; Moisan, M.-P.; Eckel-Mahan, K.L.; Baldi, P.; Sassone-Corsi, P. Reshaping circadian metabolism in the suprachiasmatic nucleus and prefrontal cortex by nutritional challenge. Proc. Natl. Acad. Sci. USA 2020, 117, 29904–29913. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, J.; Pévet, P.; Challet, E. High-fat feeding alters the clock synchronization to light. J. Physiol. 2008, 586, 5901–5910. [Google Scholar] [CrossRef]
- Petrus, P.; Cervantes, M.; Samad, M.; Sato, T.; Chao, A.; Sato, S.; Koronowski, K.B.; Park, G.; Alam, Y.; Mejhert, N.; et al. Tryptophan metabolism is a physiological integrator regulating circadian rhythms. Mol. Metab. 2022, 64, 101556. [Google Scholar] [CrossRef]
- Jeong, K.; He, B.; Nohara, K.; Park, N.; Shin, Y.; Kim, S.; Shimomura, K.; Koike, N.; Yoo, S.H.; Chen, Z. Dual attenuation of proteasomal and autophagic BMAL1 degradation in ClockΔ19/+ mice contributes to improved glucose homeostasis. Sci. Rep. 2015, 5, 12801. [Google Scholar] [CrossRef] [PubMed]
- Kohsaka, A.; Laposky, A.D.; Ramsey, K.M.; Estrada, C.; Joshu, C.; Kobayashi, Y.; Turek, F.W.; Bass, J. High-Fat Diet Disrupts Behavioral and Molecular Circadian Rhythms in Mice. Cell Metab. 2007, 6, 414–421. [Google Scholar] [CrossRef]
- Barnea, M.; Madar, Z.; Froy, O. High-Fat Diet Delays and Fasting Advances the Circadian Expression of Adiponectin Signaling Components in Mouse Liver. Endocrinology 2009, 150, 161–168. [Google Scholar] [CrossRef]
- Cha, M.C.; Chou, C.J.; Boozer, C.N. High-fat diet feeding reduces the diurnal variation of plasma leptin concentration in rats. Metabolism 2000, 49, 503–507. [Google Scholar] [CrossRef]
- Pivovarova, O.; Jürchott, K.; Rudovich, N.; Hornemann, S.; Ye, L.; Möckel, S.; Murahovschi, V.; Kessler, K.; Seltmann, A.C.; Maser-Gluth, C.; et al. Changes of Dietary Fat and Carbohydrate Content Alter Central and Peripheral Clock in Humans. J. Clin. Endocrinol. Metab. 2015, 100, 2291–2302. [Google Scholar] [CrossRef] [PubMed]
- Sherman, H.; Gutman, R.; Chapnik, N.; Meylan, J.; le Coutre, J.; Froy, O. Caffeine alters circadian rhythms and expression of disease and metabolic markers. Int. J. Biochem. Cell Biol. 2011, 43, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Burke, T.M.; Markwald, R.R.; McHill, A.W.; Chinoy, E.D.; Snider, J.A.; Bessman, S.C.; Jung, C.M.; O’Neill, J.S.; Wright, K.P. Effects of caffeine on the human circadian clock in vivo and in vitro. Sci. Transl. Med. 2015, 7, 305ra146. [Google Scholar] [CrossRef] [PubMed]
- Oishi, K.; Uchida, D.; Ohkura, N.; Doi, R.; Ishida, N.; Kadota, K.; Horie, S. Ketogenic Diet Disrupts the Circadian Clock and Increases Hypofibrinolytic Risk by Inducing Expression of Plasminogen Activator Inhibitor-1. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1571–1577. [Google Scholar] [CrossRef] [PubMed]
- Speed, J.S.; Hyndman, K.A.; Roth, K.J.; Heimlich, J.B.; Kasztan, M.; Fox, B.M.; Johnston, J.G.; Becker, B.K.; Jin, C.; Gamble, K.L.; et al. High dietary sodium causes dyssynchrony of the renal molecular clock in rats. Am. J. Physiol.-Ren. Physiol. 2018, 314, F89–F98. [Google Scholar] [CrossRef] [PubMed]
- Arendt, J.; Skene, D.J. Melatonin as a chronobiotic. Sleep Med. Rev. 2005, 9, 25–39. [Google Scholar] [CrossRef]
- Guan, Q.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Mechanisms of Melatonin in Obesity: A Review. Int. J. Mol. Sci. 2021, 23, 218. [Google Scholar] [CrossRef]
- Delpino, F.M.; Figueiredo, L.M. Melatonin supplementation and anthropometric indicators of obesity: A systematic review and meta-analysis. Nutrition 2021, 91–92, 111399. [Google Scholar] [CrossRef]
- Gomes Domingos, A.L.; Hermsdorff, H.H.M.; Bressan, J. Melatonin intake and potential chronobiological effects on human health. Crit. Rev. Food Sci. Nutr. 2017, 59, 133–140. [Google Scholar] [CrossRef]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2016, 20, 1689–1699. [Google Scholar] [CrossRef]
- Huang, J.; Lu, M.; Ho, C.-T. Health benefits of dietary chronobiotics: Beyond resynchronizing internal clocks. Food Funct. 2021, 12, 6136–6156. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.W. Possible anti-obesity therapeutics from nature—A review. Phytochemistry 2010, 71, 1625–1641. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.; Shen, C.-L. Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 2014, 25, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Román, J.; Soliz-Rueda, J.R.; Bravo, F.I.; Aragonès, G.; Suárez, M.; Arola-Arnal, A.; Mulero, M.; Salvadó, M.-J.; Arola, L.; Torres-Fuentes, C.; et al. Phenolic compounds and biological rhythms: Who takes the lead? Trends Food Sci. Technol. 2021, 113, 77–85. [Google Scholar] [CrossRef]
- Man, A.W.C.; Xia, N.; Daiber, A.; Li, H. The roles of gut microbiota and circadian rhythm in the cardiovascular protective effects of polyphenols. Br. J. Pharmacol. 2020, 177, 1278–1293. [Google Scholar] [CrossRef]
- Arola-Arnal, A.; Cruz-Carrión, Á.; Torres-Fuentes, C.; Ávila-Román, J.; Aragonès, G.; Mulero, M.; Bravo, F.I.; Muguerza, B.; Arola, L.; Suárez, M. Chrononutrition and Polyphenols: Roles and Diseases. Nutrients 2019, 11, 2602. [Google Scholar] [CrossRef] [PubMed]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Ana, F. Analytical Methods for Determining Bioavailability and Bioaccessibility of Bioactive Compounds from Fruits and Vegetables: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, S.; Garau, C.; Esteban, S.; Nicolau, M.C.; Rivero, M.; Rial, R.V. Chrononutrition: Use of dissociated day/night infant milk formulas to improve the development of the wake–sleep rhythms. Eff. Tryptophan. Nutr. Neurosci. 2007, 10, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Li, L.; Wang, J.; Zhao, B.; Pan, J.; Wang, L.; Liu, X.; Liu, X.; Liu, Z. Resveratrol Prevents Acrylamide-Induced Mitochondrial Dysfunction and Inflammatory Responses via Targeting Circadian Regulator Bmal1 and Cry1 in Hepatocytes. J. Agric. Food Chem. 2019, 67, 8510–8519. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, Y.; Song, Y.; Cheng, X.-R.; Xia, S.; Rahman, M.R.T.; Shi, Y.; Le, G. Resveratrol restores the circadian rhythmic disorder of lipid metabolism induced by high-fat diet in mice. Biochem. Biophys. Res. Commun. 2015, 458, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Mi, Y.; Liu, Z.; Fan, R.; Qiao, Q.; Sun, Y.; Ren, B.; Liu, X. Dietary tea polyphenols ameliorate metabolic syndrome and memory impairment via circadian clock related mechanisms. J. Funct. Foods 2017, 34, 168–180. [Google Scholar] [CrossRef]
- Oike, H.; Kobori, M. Resveratrol Regulates Circadian Clock Genes in Rat-1 Fibroblast Cells. Biosci. Biotechnol. Biochem. 2008, 72, 3038–3040. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.; Portillo, M.P.; Madrid, J.A.; Arias, N.; Macarulla, M.T.; Garaulet, M. Effects of resveratrol on changes induced by high-fat feeding on clock genes in rats. Br. J. Nutr. 2013, 110, 1421–1428. [Google Scholar] [CrossRef]
- Li, J.; Wei, L.; Zhao, C.; Li, J.; Liu, Z.; Zhang, M.; Wang, Y. Resveratrol Maintains Lipid Metabolism Homeostasis via One of the Mechanisms Associated with the Key Circadian Regulator Bmal1. Molecules 2019, 24, 2916. [Google Scholar] [CrossRef]
- Okada, Y.; Okada, M. Quercetin, caffeic acid and resveratrol regulate circadian clock genes and aging-related genes in young and old human lung fibroblast cells. Mol. Biol. Rep. 2020, 47, 1021–1032. [Google Scholar] [CrossRef]
- Ribas-Latre, A.; Baselga-Escudero, L.; Casanova, E.; Arola-Arnal, A.; Salvadó, M.-J.; Bladé, C.; Arola, L. Dietary proanthocyanidins modulate BMAL1 acetylation, Nampt expression and NAD levels in rat liver. Sci. Rep. 2015, 5, 10954. [Google Scholar] [CrossRef]
- Jiang, P.; Xu, C.; Zhang, P.; Ren, J.; Mageed, F.; Wu, X.; Chen, L.; Zeb, F.; Feng, Q.; Li, S. Epigallocatechin-3-gallate inhibits self-renewal ability of lung cancer stem-like cells through inhibition of CLOCK. Int. J. Mol. Med. 2020, 46, 2216–2224. [Google Scholar] [CrossRef]
- Li, H.; Kek, H.C.; Lim, J.; Gelling, R.W.; Han, W. Green tea (-)-epigallocatechin-3-gallate counteracts daytime overeating induced by high-fat diet in mice. Mol. Nutr. Food Res. 2016, 60, 2565–2575. [Google Scholar] [CrossRef]
- Mi, Y.; Qi, G.; Fan, R.; Ji, X.; Liu, Z.; Liu, X. EGCG ameliorates diet-induced metabolic syndrome associating with the circadian clock. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 1575–1589. [Google Scholar] [CrossRef]
- He, B.; Nohara, K.; Park, N.; Park, Y.-S.; Guillory, B.; Zhao, Z.; Garcia, J.M.; Koike, N.; Lee, C.C.; Takahashi, J.S.; et al. The small molecule Nobiletin targets the molecular oscillator to enhance circadian rhythms and protect against metabolic syndrome. Cell Metab. 2016, 23, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Guo, R.; Tian, H.; Li, L.; Liu, H.; Mi, Y.; Liu, X. Nobiletin protects against insulin resistance and disorders of lipid metabolism by reprogramming of circadian clock in hepatocytes. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2018, 1863, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, A.; Misawa, K.; Ikeda, Y.; Haraguchi, A.; Kamagata, M.; Tahara, Y.; Shibata, S. Potent Effects of Flavonoid Nobiletin on Amplitude, Period, and Phase of the Circadian Clock Rhythm in PER2::LUCIFERASE Mouse Embryonic Fibroblasts. PLoS ONE 2017, 12, e0170904. [Google Scholar] [CrossRef] [PubMed]
- Lellupitiyage Don, S.S.; Robertson, K.L.; Lin, H.-H.; Labriola, C.; Harrington, M.E.; Taylor, S.R.; Farkas, M.E. Nobiletin affects circadian rhythms and oncogenic characteristics in a cell-dependent manner. PLoS ONE 2020, 15, e0236315. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Nohara, K.; Wirianto, M.; Escobedo, G.; Lim, J.; Morales, R.; Yoo, S.-H.; Chen, Z. Effects of the Clock Modulator Nobiletin on Circadian Rhythms and Pathophysiology in Female Mice of an Alzheimer’s Disease Model. Biomolecules 2021, 11, 1004. [Google Scholar] [CrossRef]
- Larion, S.; Padgett, C.A.; Butcher, J.T.; Mintz, J.D.; Fulton, D.J.; Stepp, D.W. The biological clock enhancer nobiletin ameliorates steatosis in genetically obese mice by restoring aberrant hepatic circadian rhythm. Am. J. Physiol.-Gastrointest. Liver Physiol. 2022, 323, G387–G400. [Google Scholar] [CrossRef]
- Wirianto, M.; Wang, C.; Kim, E.; Koike, N.; Gomez-Gutierrez, R.; Nohara, K.; Escobedo, G., Jr.; Choi, J.M.; Han, C.; Yagita, K.; et al. The clock modulator Nobiletin mitigates astrogliosis-associated neuroinflammation and disease hallmarks in an Alzheimer’s disease model. FASEB J. 2022, 36, e22186. [Google Scholar] [CrossRef]
- Oyama, Y.; Bartman, C.M.; Gile, J.; Sehrt, D.; Eckle, T. The Circadian PER2 Enhancer Nobiletin Reverses the Deleterious Effects of Midazolam in Myocardial Ischemia and Reperfusion Injury. Curr. Pharm. Des. 2018, 24, 3376–3383. [Google Scholar] [CrossRef]
- Nohara, K.; Mallampalli, V.; Nemkov, T.; Wirianto, M.; Yang, J.; Ye, Y.; Sun, Y.; Han, L.; Esser, K.A.; Mileykovskaya, E.; et al. Nobiletin fortifies mitochondrial respiration in skeletal muscle to promote healthy aging against metabolic challenge. Nat. Commun. 2019, 10, 3923. [Google Scholar] [CrossRef]
- Petrenko, V.; Gandasi, N.R.; Sage, D.; Tengholm, A.; Barg, S.; Dibner, C. In pancreatic islets from type 2 diabetes patients, the dampened circadian oscillators lead to reduced insulin and glucagon exocytosis. Proc. Natl. Acad. Sci. USA 2020, 117, 2484–2495. [Google Scholar] [CrossRef]
- Guo, T.; Ho, C.-T.; Zhang, X.; Cao, J.; Wang, H.; Shao, X.; Pan, D.; Wu, Z. Oolong Tea Polyphenols Ameliorate Circadian Rhythm of Intestinal Microbiome and Liver Clock Genes in Mouse Model. J. Agric. Food Chem. 2019, 67, 11969–11976. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yan, R.; Wu, Z. Metagenomics analysis of intestinal flora modulatory effect of green tea polyphenols by a circadian rhythm dysfunction mouse model. J. Food Biochem. 2020, 44, e13430. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Zhao, B.; Wang, Y.; Wu, D.; Wang, Y.; Yu, Y.; Yan, Y.; Zhang, W.; Liu, Z.; Liu, X. Cichoric Acid Prevents Free-Fatty-Acid-Induced Lipid Metabolism Disorders via Regulating Bmal1 in HepG2 Cells. J. Agric. Food Chem. 2018, 66, 9667–9678. [Google Scholar] [CrossRef]
- Numata, M.; Hirano, A.; Yamamoto, Y.; Yasuda, M.; Miura, N.; Sayama, K.; Shibata, M.A.; Asai, T.; Oku, N.; Miyoshi, N.; et al. Metastasis of Breast Cancer Promoted by Circadian Rhythm Disruption due to Light/Dark Shift and its Prevention by Dietary Quercetin in Mice. J. Circadian Rhythm. 2021, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Al Abdi, T.; Andreou, E.; Papageorgiou, A.; Heraclides, A.; Philippou, E. Personality, Chrono-nutrition and Cardiometabolic Health: A Narrative Review of the Evidence. Adv. Nutr. 2020, 11, 1201–1210. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, M.S.; Lee, J.S.; Halberg, F.E.; Halberg, J.; Schwartzkopff, O.; Cornélissen, G. A Tribute to Franz Halberg, MD. Hypertension 2015, 66, 1090–1092. [Google Scholar] [CrossRef]
- Cornelissen, G. When You Eat Matters: 60 Years of Franz Halberg’s Nutrition Chronomics. Open Nutraceuticals J. 2012, 5, 16–44. [Google Scholar] [CrossRef]
- Doi, R.; Oishi, K.; Ishida, N. CLOCK Regulates Circadian Rhythms of Hepatic Glycogen Synthesis through Transcriptional Activation ofGys2. J. Biol. Chem. 2010, 285, 22114–22121. [Google Scholar] [CrossRef]
- Ruddick-Collins, L.C.; Morgan, P.J.; Johnstone, A.M. Mealtime: A circadian disruptor and determinant of energy balance? J. Neuroendocrinol. 2020, 32, e12886. [Google Scholar] [CrossRef]
- Gill, S.; Panda, S. A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metab. 2015, 22, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Kant, A.K. Eating patterns of US adults: Meals, snacks, and time of eating. Physiol. Behav. 2018, 193, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.J.; Kumar, V.; Panda, S. A camera-phone based study reveals erratic eating pattern and disrupted daily eating-fasting cycle among adults in India. PLoS ONE 2017, 12, e0172852. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Rodríguez, V.A.; Rijo-Ferreira, F.; Green, C.B.; Takahashi, J.S. Importance of circadian timing for aging and longevity. Nat. Commun. 2021, 12, 2862. [Google Scholar] [CrossRef] [PubMed]
- Cienfuegos, S.; Gabel, K.; Kalam, F.; Ezpeleta, M.; Wiseman, E.; Pavlou, V.; Lin, S.; Oliveira, M.L.; Varady, K.A. Effects of 4- and 6-h Time-Restricted Feeding on Weight and Cardiometabolic Health: A Randomized Controlled Trial in Adults with Obesity. Cell Metab. 2020, 32, 366–378.e3. [Google Scholar] [CrossRef] [PubMed]
- Parr, E.B.; Devlin, B.L.; Radford, B.E.; Hawley, J.A. A Delayed Morning and Earlier Evening Time-Restricted Feeding Protocol for Improving Glycemic Control and Dietary Adherence in Men with Overweight/Obesity: A Randomized Controlled Trial. Nutrients 2020, 12, 505. [Google Scholar] [CrossRef]
- Parr, E.B.; Devlin, B.L.; Lim, K.H.C.; Moresi, L.N.Z.; Geils, C.; Brennan, L.; Palma, A.; Gentil, P.; Neri, M.; Paoli, A. Time-Restricted Eating as a Nutrition Strategy for Individuals with Type 2 Diabetes: A Feasibility Study. Nutrients 2020, 12, 3228. [Google Scholar] [CrossRef]
- Gabel, K.; Hoddy, K.K.; Haggerty, N.; Song, J.; Kroeger, C.M.; Trepanowski, J.F.; Panda, S.; Varady, K.A. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: A pilot study. Nutr. Healthy Aging 2018, 4, 345–353. [Google Scholar] [CrossRef]
- Moro, T.; Tinsley, G.; Bianco, A.; Marcolin, G.; Pacelli, Q.F.; Battaglia, G.; Palma, A.; Gentil, P.; Neri, M.; Paoli, A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med. 2016, 14, 290. [Google Scholar] [CrossRef]
- Anton, S.D.; Lee, S.A.; Donahoo, W.T.; McLaren, C.; Manini, T.; Leeuwenburgh, C.; Pahor, M. The Effects of Time Restricted Feeding on Overweight, Older Adults: A Pilot Study. Nutrients 2019, 11, 1500. [Google Scholar] [CrossRef]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018, 27, 1212–1221.e3. [Google Scholar] [CrossRef]
- Wilkinson, M.J.; Manoogian, E.N.C.; Zadourian, A.; Lo, H.; Fakhouri, S.; Shoghi, A.; Wang, X.; Fleischer, J.G.; Navlakha, S.; Panda, S.; et al. Ten-Hour Time-Restricted Eating Reduces Weight, Blood Pressure, and Atherogenic Lipids in Patients with Metabolic Syndrome. Cell Metab. 2020, 31, 92–104.e5. [Google Scholar] [CrossRef] [PubMed]
- Jamshed, H.; Beyl, R.A.; Della Manna, D.L.; Yang, E.S.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves 24-Hour Glucose Levels and Affects Markers of the Circadian Clock, Aging, and Autophagy in Humans. Nutrients 2019, 11, 1234. [Google Scholar] [CrossRef] [PubMed]
- Ravussin, E.; Beyl, R.A.; Poggiogalle, E.; Hsia, D.S.; Peterson, C.M. Early Time-Restricted Feeding Reduces Appetite and Increases Fat Oxidation but Does Not Affect Energy Expenditure in Humans. Obesity 2019, 27, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Kotarsky, C.J.; Johnson, N.R.; Mahoney, S.J.; Mitchell, S.L.; Schimek, R.L.; Stastny, S.N.; Hackney, K.J. Time-restricted eating and concurrent exercise training reduces fat mass and increases lean mass in overweight and obese adults. Physiol. Rep. 2021, 9, e14868. [Google Scholar] [CrossRef] [PubMed]
- LeCheminant, J.; Christenson, E.; Bailey, B.; Tucker, L.A. Restricting night-time eating reduces daily energy intake in healthy young men:a short-term cross-over study. Br. J. Nutr. 2013, 110, 2108–2113. [Google Scholar] [CrossRef]
- Tinsley, G.; Forsse, J.S.; Butler, N.K.; Paoli, A.; Bane, A.A.; La Bounty, P.M.; Morgan, G.B.; Grandjean, P.W. Time-restricted feeding in young men performing resistance training: A randomized controlled trial. Eur. J. Sport Sci. 2016, 17, 200–207. [Google Scholar] [CrossRef]
- Antoni, R.; Johnston, K.L.; Collins, A.L.; Robertson, M.D. Intermittent v. continuous energy restriction: Differential effects on postprandial glucose and lipid metabolism following matched weight loss in overweight/obese participants. Br. J. Nutr. 2018, 119, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, G.M.; Moore, M.L.; Graybeal, A.J.; Paoli, A.; Kim, Y.; Gonzales, J.U.; Harry, J.R.; VanDusseldorp, T.A.; Kennedy, D.N.; Cruz, M.R. Time-restricted feeding plus resistance training in active females: A randomized trial. Am. J. Clin. Nutr. 2019, 110, 628–640. [Google Scholar] [CrossRef]
- Kesztyüs, D.; Cermak, P.; Gulich, M.; Kesztyüs, T. Adherence to Time-Restricted Feeding and Impact on Abdominal Obesity in Primary Care Patients: Results of a Pilot Study in a Pre–Post Design. Nutrients 2019, 11, 2854. [Google Scholar] [CrossRef]
- McAllister, M.J.; Pigg, B.L.; Renteria, L.I.; Waldman, H.S. Time-restricted feeding improves markers of cardiometabolic health in physically active college-age men: A 4-week randomized pre-post pilot study. Nutr. Res. 2020, 75, 32–43. [Google Scholar] [CrossRef]
- Chow, L.S.; Manoogian, E.N.C.; Alvear, A.; Fleischer, J.G.; Thor, H.; Dietsche, K.; Wang, Q.; Hodges, J.S.; Esch, N.; Malaeb, S.; et al. Time-Restricted Eating Effects on Body Composition and Metabolic Measures in Humans who are Overweight: A Feasibility Study. Obesity 2020, 28, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Lowe, D.A.; Wu, N.; Rohdin-Bibby, L.; Moore, A.H.; Kelly, N.; Liu, Y.E.; Philip, E.; Vittinghoff, E.; Heymsfield, S.B.; Olgin, J.E.; et al. Effects of Time-Restricted Eating on Weight Loss and Other Metabolic Parameters in Women and Men with Overweight and Obesity: The TREAT Randomized Clinical Trial. JAMA Intern. Med. 2020, 180, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Qin, Y.-L.; Shi, Z.-Y.; Chen, J.-H.; Zeng, M.-J.; Zhou, W.; Chen, R.-Q.; Chen, Z.-Y. Effects of alternate-day fasting on body weight and dyslipidaemia in patients with non-alcoholic fatty liver disease: A randomised controlled trial. BMC Gastroenterol. 2019, 19, 219. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xing, C.; Zhang, J.; Zhao, H.; Shi, W.; He, B. Eight-hour time-restricted feeding improves endocrine and metabolic profiles in women with anovulatory polycystic ovary syndrome. J. Transl. Med. 2021, 19, 148. [Google Scholar] [CrossRef]
- Martens, C.R.; Rossman, M.J.; Mazzo, M.R.; Jankowski, L.R.; Nagy, E.E.; Denman, B.A.; Richey, J.J.; Johnson, S.A.; Ziemba, B.P.; Wang, Y.; et al. Short-term time-restricted feeding is safe and feasible in non-obese healthy midlife and older adults. GeroScience 2020, 42, 667–686. [Google Scholar] [CrossRef]
- Peeke, P.M.; Greenway, F.L.; Billes, S.K.; Zhang, D.; Fujioka, K. Effect of time restricted eating on body weight and fasting glucose in participants with obesity: Results of a randomized, controlled, virtual clinical trial. Nutr. Diabetes 2021, 11, 6. [Google Scholar] [CrossRef]
- Phillips, N.; Mareschal, J.; Schwab, N.; Manoogian, E.; Borloz, S.; Ostinelli, G.; Gauthier-Jaques, A.; Umwali, S.; Rodriguez, E.G.; Aeberli, D.; et al. The Effects of Time-Restricted Eating versus Standard Dietary Advice on Weight, Metabolic Health and the Consumption of Processed Food: A Pragmatic Randomised Controlled Trial in Community-Based Adults. Nutrients 2021, 13, 1042. [Google Scholar] [CrossRef]
- Zeb, F.; Wu, X.; Chen, L.; Fatima, S.; Haq, I.-U.; Chen, A.; Majeed, F.; Feng, Q.; Li, M. Effect of time-restricted feeding on metabolic risk and circadian rhythm associated with gut microbiome in healthy males. Br. J. Nutr. 2020, 123, 1216–1226. [Google Scholar] [CrossRef]
- Jones, R.; Pabla, P.; Mallinson, J.; Nixon, A.; Taylor, T.; Bennett, A.; Tsintzas, K. Two weeks of early time-restricted feeding (eTRF) improves skeletal muscle insulin and anabolic sensitivity in healthy men. Am. J. Clin. Nutr. 2020, 112, 1015–1028. [Google Scholar] [CrossRef]
- Karras, S.N.; Koufakis, T.; Adamidou, L.; Antonopoulou, V.; Karalazou, P.; Thisiadou, K.; Mitrofanova, E.; Mulrooney, H.; Petróczi, A.; Zebekakis, P.; et al. Effects of orthodox religious fasting versus combined energy and time restricted eating on body weight, lipid concentrations and glycaemic profile. Int. J. Food Sci. Nutr. 2020, 72, 82–92. [Google Scholar] [CrossRef]
- Hutchison, A.T.; Regmi, P.; Manoogian, E.N.; Fleischer, J.G.; Wittert, G.A.; Panda, S.; Heilbronn, L.K. Time-Restricted Feeding Improves Glucose Tolerance in Men at Risk for Type 2 Diabetes: A Randomized Crossover Trial. Obesity 2019, 27, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Sun, Y.; Ye, Y.; Hu, D.; Zhang, H.; He, Z.; Zhao, H.; Yang, H.; Mao, Y. Randomized controlled trial for time-restricted eating in healthy volunteers without obesity. Nat. Commun. 2022, 13, 1003. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, D.; Barnea, M.; Wainstein, J.; Froy, O. High Caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity 2013, 21, 2504–2512. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Musso, G.; Beccuti, G.; Fadda, M.; Fedele, D.; Gambino, R.; Gentile, L.; Durazzo, M.; Ghigo, E.; Cassader, M. Consuming More of Daily Caloric Intake at Dinner Predisposes to Obesity. A 6-Year Population-Based Prospective Cohort Study. Claret M, editor. PLoS ONE 2014, 9, e108467. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento Queiroz, J.; Macedo, R.C.O.; Tinsley, G.M.; Reischak-Oliveira, A. Time-restricted eating and circadian rhythms: The biological clock is ticking. Crit. Rev. Food Sci. Nutr. 2020, 61, 2863–2875. [Google Scholar] [CrossRef]
- Froy, O. Metabolism and Circadian Rhythms—Implications for Obesity. Endocr. Rev. 2010, 31, 1–24. [Google Scholar] [CrossRef]
- Most, J.; Tosti, V.; Redman, L.M.; Fontana, L. Calorie restriction in humans: An update. Ageing Res. Rev. 2017, 39, 36–45. [Google Scholar] [CrossRef]
- Wing, R.R.; Blair, E.H.; Bononi, P.; Marcus, M.D.; Watanabe, R.; Bergman, R.N. Caloric Restriction Per Se Is a Significant Factor in Improvements in Glycemic Control and Insulin Sensitivity During Weight Loss in Obese NIDDM Patients. Diabetes Care 1994, 17, 30–36. [Google Scholar] [CrossRef]
- Fontana, L.; Meyer, T.E.; Klein, S.; Holloszy, J.O. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc. Natl. Acad. Sci. USA 2004, 101, 6659–6663. [Google Scholar] [CrossRef]
- Colman, R.J.; Beasley, T.M.; Kemnitz, J.W.; Johnson, S.C.; Weindruch, R.; Anderson, R.M. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat. Commun. 2014, 5, 3557. [Google Scholar] [CrossRef]
- Mattison, J.A.; Colman, R.J.; Beasley, T.M.; Allison, D.B.; Kemnitz, J.W.; Roth, G.S.; Ingram, D.K.; Weindruch, R.; de Cabo, R.; Anderson, R. Caloric restriction improves health and survival of rhesus monkeys. Nat. Commun. 2017, 8, 14063. [Google Scholar] [CrossRef] [PubMed]
- Rynders, C.A.; Thomas, E.A.; Zaman, A.; Pan, Z.; Catenacci, V.A.; Melanson, E.L. Effectiveness of Intermittent Fasting and Time-Restricted Feeding Compared to Continuous Energy Restriction for Weight Loss. Nutrients 2019, 11, 2442. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.J.; VanWormer, J.J.; Crain, A.L.; Boucher, J.L.; Histon, T.; Caplan, W.; Bowman, J.D.; Pronk, N.P. Weight-Loss Outcomes: A Systematic Review and Meta-Analysis of Weight-Loss Clinical Trials with a Minimum 1-Year Follow-Up. J. Am. Diet. Assoc. 2007, 107, 1755–1767. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xie, C.; Lu, S.; Nichols, R.G.; Tian, Y.; Li, L.; Patel, D.; Ma, Y.; Brocker, C.N.; Yan, T.; et al. Intermittent Fasting Promotes White Adipose Browning and Decreases Obesity by Shaping the Gut Microbiota. Cell Metab. 2017, 26, 672–685.e4. [Google Scholar] [CrossRef]
- Patterson, R.E.; Sears, D.D. Metabolic Effects of Intermittent Fasting. Annu. Rev. Nutr. 2017, 37, 371–393. [Google Scholar] [CrossRef]
- Flanagan, A.; Bechtold, D.A.; Pot, G.K.; Johnston, J.D. Chrono-nutrition: From molecular and neuronal mechanisms to human epidemiology and timed feeding patterns. J. Neurochem. 2020, 157, 53–72. [Google Scholar] [CrossRef]
- Lessan, N.; Ali, T. Energy Metabolism and Intermittent Fasting: The Ramadan Perspective. Nutrients 2019, 11, 1192. [Google Scholar] [CrossRef]
- Anton, S.D.; Moehl, K.; Donahoo, W.T.; Marosi, K.; Lee, S.A.; Mainous, A.G., 3rd; Leeuwenburgh, C.; Mattson, M.P. Flipping the Metabolic Switch: Understanding and Applying the Health Benefits of Fasting. Obesity 2018, 26, 254–268. [Google Scholar] [CrossRef]
- Vasim, I.; Majeed, C.N.; DeBoer, M.D. Intermittent Fasting and Metabolic Health. Nutrients 2022, 14, 631. [Google Scholar] [CrossRef]
- Carter, S.; Clifton, P.M.; Keogh, J.B. Effect of Intermittent Compared with Continuous Energy Restricted Diet on Glycemic Control in Patients with Type 2 Diabetes. JAMA Netw. Open 2018, 1, e180756. [Google Scholar] [CrossRef]
- Catenacci, V.A.; Pan, Z.; Ostendorf, D.; Brannon, S.; Gozansky, W.S.; Mattson, M.P.; Martin, B.; MacLean, P.S.; Melanson, E.L.; Donahoo, W.T. A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity 2016, 24, 1874–1883. [Google Scholar] [CrossRef] [PubMed]
- Harvie, M.N.; Pegington, M.; Mattson, M.P.; Frystyk, J.; Dillon, B.; Evans, G.; Cuzick, J.; Jebb, S.A.; Martin, B.; Cutler, R.G.; et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: A randomized trial in young overweight women. Int. J. Obes. 2010, 35, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Headland, M.L.; Clifton, P.M.; Keogh, J.B. Effect of intermittent compared to continuous energy restriction on weight loss and weight maintenance after 12 months in healthy overweight or obese adults. Int. J. Obes. 2018, 43, 2028–2036. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, A.T.; Liu, B.; Wood, R.E.; Vincent, A.D.; Thompson, C.H.; O’Callaghan, N.J.; Wittert, G.A.; Heilbronn, L.K. Effects of Intermittent Versus Continuous Energy Intakes on Insulin Sensitivity and Metabolic Risk in Women with Overweight. Obesity 2018, 27, 50–58. [Google Scholar] [CrossRef]
- Trepanowski, J.F.; Kroeger, C.M.; Barnosky, A.; Klempel, M.C.; Bhutani, S.; Hoddy, K.K.; Gabel, K.; Freels, S.; Rigdon, J.; Rood, J.; et al. Effect of Alternate-Day Fasting on Weight Loss, Weight Maintenance, and Cardioprotection Among Metabolically Healthy Obese Adults. JAMA Intern. Med. 2017, 177, 930. [Google Scholar] [CrossRef]
- Schübel, R.; Nattenmüller, J.; Sookthai, D.; Nonnenmacher, T.; Graf, M.E.; Riedl, L.; Schlett, C.L.; von Stackelberg, O.; Johnson, T.; Nabers, D.; et al. Effects of intermittent and continuous calorie restriction on body weight and metabolism over 50 wk: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 933–945. [Google Scholar] [CrossRef]
- Di Francesco, A.; Di Germanio, C.; Bernier, M.; de Cabo, R. A time to fast. Science 2018, 362, 770–775. [Google Scholar] [CrossRef]
- Mitchell, S.J.; Bernier, M.; Mattison, J.A.; Aon, M.A.; Kaiser, T.A.; Anson, R.M.; Ikeno, Y.; Anderson, R.M.; Ingram, D.K.; de Cabo, R. Daily Fasting Improves Health and Survival in Male Mice Independent of Diet Composition and Calories. Cell Metab. 2019, 29, 221–228.e3. [Google Scholar] [CrossRef]
- Chaix, A.; Manoogian, E.N.C.; Melkani, G.C.; Panda, S. Time-Restricted Eating to Prevent and Manage Chronic Metabolic Diseases. Annu. Rev. Nutr. 2019, 39, 291–315. [Google Scholar] [CrossRef]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.; et al. Time-Restricted Feeding without Reducing Caloric Intake Prevents Metabolic Diseases in Mice Fed a High-Fat Diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef]
- Regmi, P.; Heilbronn, L.K. Time-Restricted Eating: Benefits, Mechanisms, and Challenges in Translation. iScience 2020, 23, 101161. [Google Scholar] [CrossRef] [PubMed]
- Boyd, P.; O’Connor, S.G.; Heckman-Stoddard, B.M.; Sauter, E.R. Time-Restricted Feeding Studies and Possible Human Benefit. JNCI Cancer Spectr. 2022, 6, pkac032. [Google Scholar] [CrossRef] [PubMed]
- Chaix, A.; Zarrinpar, A.; Miu, P.; Panda, S. Time-Restricted Feeding Is a Preventative and Therapeutic Intervention against Diverse Nutritional Challenges. Cell Metab. 2014, 20, 991–1005. [Google Scholar] [CrossRef] [PubMed]
- Olsen, M.K.; Choi, M.H.; Kulseng, B.; Zhao, C.-M.; Chen, D. Time-restricted feeding on weekdays restricts weight gain: A study using rat models of high-fat diet-induced obesity. Physiol. Behav. 2017, 173, 298–304. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, S.G.; Boyd, P.; Bailey, C.P.; Shams-White, M.M.; Agurs-Collins, T.; Hall, K.; Reedy, J.; Sauter, E.R.; Czajkowski, S.M. Perspective: Time-Restricted Eating Compared with Caloric Restriction: Potential Facilitators and Barriers of Long-Term Weight Loss Maintenance. Adv. Nutr. 2021, 12, 325–333. [Google Scholar] [CrossRef]
- Parr, E.B.; Devlin, B.L.; Hawley, J.A. Time-Restricted Eating: Integrating the What with the When. Adv. Nutr. 2022, 13, 699–711. [Google Scholar] [PubMed]
- Isenmann, E.; Dissemond, J.; Geisler, S. The Effects of a Macronutrient-Based Diet and Time-Restricted Feeding (16:8) on Body Composition in Physically Active Individuals—A 14-Week Randomised Controlled Trial. Nutrients 2021, 13, 3122. [Google Scholar] [CrossRef]
- Adafer, R.; Messaadi, W.; Meddahi, M.; Patey, A.; Haderbache, A.; Bayen, S.; Messaadi, N. Food Timing, Circadian Rhythm and Chrononutrition: A Systematic Review of Time-Restricted Eating’s Effects on Human Health. Nutrients 2020, 12, 3770. [Google Scholar] [CrossRef]
- Lanfray, D.; Richard, D. Emerging Signaling Pathway in Arcuate Feeding-Related Neurons: Role of the Acbd7. Front. Neurosci. 2017, 11, 328. [Google Scholar] [CrossRef]
- Varela, L.; Horvath, T.L. Leptin and insulin pathways in POMC and AgRP neurons that modulate energy balance and glucose homeostasis. EMBO Rep. 2012, 13, 1079–1086. [Google Scholar] [CrossRef]
- Silva, A.D.; Bloom, S.R. Gut Hormones and Appetite Control: A Focus on PYY and GLP-1 as Therapeutic Targets in Obesity. Gut Liver 2012, 6, 10–20. [Google Scholar] [CrossRef]
- Yanagi, S.; Sato, T.; Kangawa, K.; Nakazato, M. The Homeostatic Force of Ghrelin. Cell Metab. 2018, 27, 786–804. [Google Scholar] [CrossRef] [PubMed]
- LeSauter, J.; Hoque, N.; Weintraub, M.; Pfaff, D.W.; Silver, R. Stomach ghrelin-secreting cells as food-entrainable circadian clocks. Proc. Natl. Acad. Sci. USA 2009, 106, 13582–13587. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, H.; Tong, J.; Tschöp, M.H.; Pfluger, P.T. Ghrelin and PYY in the regulation of energy balance and metabolism: Lessons from mouse mutants. Am. J. Physiol.-Endocrinol. Metab. 2010, 298, E909–E919. [Google Scholar] [CrossRef] [PubMed]
- Bodosi, B.; Gardi, J.; Hajdu, I.; Szentirmai, E.; Obal, F.; Krueger, J.M. Rhythms of ghrelin, leptin, and sleep in rats: Effects of the normal diurnal cycle, restricted feeding, and sleep deprivation. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2004, 287, R1071–R1079. [Google Scholar] [CrossRef]
- Sinha, M.K.; Ohannesian, J.P.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Magosin, S.; Marco, C.; Caro, J.F. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J. Clin. Investig. 1996, 97, 1344–1347. [Google Scholar] [CrossRef]
- Suriagandhi, V.; Nachiappan, V. Protective Effects of Melatonin against Obesity-Induced by Leptin Resistance. Behav. Brain Res. 2022, 417, 113598. [Google Scholar] [CrossRef]
- Kettner Nicole, M.; Mayo Sara, A.; Hua, J.; Lee, C.; Moore David, D.; Fu, L. Circadian Dysfunction Induces Leptin Resistance in Mice. Cell Metab. 2015, 22, 448–459. [Google Scholar] [CrossRef]
- Shapiro, A.; Mu, W.; Roncal, C.; Cheng, K.-Y.; Johnson, R.J.; Scarpace, P.J. Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2008, 295, R1370–R1375. [Google Scholar] [CrossRef]
- Lin, S.; Thomas, T.; Storlien, L.; Huang, X. Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice. Int. J. Obes. 2000, 24, 639–646. [Google Scholar] [CrossRef]
- Chan, J.L.; Heist, K.; DePaoli, A.M.; Veldhuis, J.D.; Mantzoros, C.S. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J. Clin. Investig. 2003, 111, 1409–1421. [Google Scholar] [CrossRef] [PubMed]
- Spezani, R.; da Silva, R.R.; Martins, F.F.; de Souza Marinho, T.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Intermittent fasting, adipokines, insulin sensitivity, and hypothalamic neuropeptides in a dietary overload with high-fat or high-fructose diet in mice. J. Nutr. Biochem. 2020, 83, 108419. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Hong, N.; Kim, K.-W.; Cho, S.J.; Lee, M.; Lee, Y.-H.; Lee, Y.H.; Kang, E.S.; Cha, B.S.; Lee, B.W. The Effectiveness of Intermittent Fasting to Reduce Body Mass Index and Glucose Metabolism: A Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 1645. [Google Scholar] [CrossRef] [PubMed]
- Albosta, M.; Bakke, J. Intermittent fasting: Is there a role in the treatment of diabetes? A review of the literature and guide for primary care physicians. Clin. Diabetes Endocrinol. 2021, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Kahn, B.B.; Alquier, T.; Carling, D.; Hardie, D.G. AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005, 1, 15–25. [Google Scholar] [CrossRef]
- Minokoshi, Y.; Alquier, T.; Furukawa, N.; Kim, Y.-B.; Lee, A.; Xue, B.; Mu, J.; Foufelle, F.; Ferré, P.; Birnbaum, M.J.; et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 2004, 428, 569–574. [Google Scholar] [CrossRef]
- Scarpace, P.J.; Matheny, M.; Pollock, B.H.; Tümer, N. Leptin increases uncoupling protein expression and energy expenditure. Am. J. Physiol. 1997, 273 Pt 1, E226–E230. [Google Scholar] [CrossRef]
- Biancolin, A.D.; Martchenko, A.; Mitova, E.; Gurges, P.; Michalchyshyn, E.; Chalmers, J.A.; Doria, A.; Mychaleckyj, J.C.; Adriaenssens, A.E.; Reimann, F.; et al. The core clock gene, Bmal1, and its downstream target, the SNARE regulatory protein secretagogin, are necessary for circadian secretion of glucagon-like peptide-1. Mol. Metab. 2020, 31, 124–137. [Google Scholar] [CrossRef]
- Hill, B.R.; De Souza, M.J.; Williams, N.I. Characterization of the diurnal rhythm of peptide YY and its association with energy balance parameters in normal-weight premenopausal women. Am. J. Physiol.-Endocrinol. Metab. 2011, 301, E409–E415. [Google Scholar] [CrossRef]
- Wever, M.C.; van Meer, F.; Charbonnier, L.; Crabtree, D.R.; Buosi, W.; Giannopoulou, A.; Androutsos, O.; Johnstone, A.M.; Manios, Y.; Meek, C.L.; et al. Associations between ghrelin and leptin and neural food cue reactivity in a fasted and sated state. NeuroImage 2021, 240, 118374. [Google Scholar] [CrossRef]
- Cummings, D.E.; Weigle, D.S.; Frayo, R.S.; Breen, P.A.; Ma, M.K.; Dellinger, E.P.; Purnell, J.Q. Plasma Ghrelin Levels after Diet-Induced Weight Loss or Gastric Bypass Surgery. N. Engl. J. Med. 2002, 346, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Sumithran, P.; Prendergast, L.A.; Delbridge, E.; Purcell, K.; Shulkes, A.; Kriketos, A.; Proietto, J. Long-Term Persistence of Hormonal Adaptations to Weight Loss. N. Engl. J. Med. 2011, 365, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Lean, M.E.J.; Malkova, D. Altered gut and adipose tissue hormones in overweight and obese individuals: Cause or consequence? Int. J. Obes. 2015, 40, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Al-Rawi, N.; Madkour, M.; Jahrami, H.; Salahat, D.; Alhasan, F.; BaHammam, A.; Faris, M.A.-I. Effect of diurnal intermittent fasting during Ramadan on ghrelin, leptin, melatonin, and cortisol levels among overweight and obese subjects: A prospective observational study. PLoS ONE 2020, 15, e0237922. [Google Scholar] [CrossRef] [PubMed]
- Alzoghaibi, M.A.; Pandi-Perumal, S.R.; Sharif, M.M.; BaHammam, A.S. Diurnal Intermittent Fasting during Ramadan: The Effects on Leptin and Ghrelin Levels. PLoS ONE 2014, 9, e92214. [Google Scholar] [CrossRef]
- Omisade, A.; Buxton, O.M.; Rusak, B. Impact of acute sleep restriction on cortisol and leptin levels in young women. Physiol. Behav. 2010, 99, 651–656. [Google Scholar] [CrossRef]
- Nedeltcheva, A.V.; Kilkus, J.M.; Imperial, J.; Schoeller, D.A.; Penev, P.D. Insufficient Sleep Undermines Dietary Efforts to Reduce Adiposity. Ann. Intern. Med. 2010, 153, 435. [Google Scholar] [CrossRef]
- Hayes, A.L.; Xu, F.; Babineau, D.; Patel, S.R. Sleep Duration and Circulating Adipokine Levels. Sleep 2011, 34, 147–152. [Google Scholar] [CrossRef]
- Ali, R.; Tariq, S.; Kareem, O.; Fayaz, F.; Aziz, T.; Pottoo, F.H.; Siddiqui, N. Nutraceuticals for Sleep Disorders. Comb. Chem. High Throughput Screen. 2021, 24, 1583–1592. [Google Scholar] [CrossRef]
- Godos, J.; Ferri, R.; Castellano, S.; Angelino, D.; Mena, P.; Del Rio, D.; Caraci, F.; Galvano, F.; Grosso, G. Specific Dietary (Poly)phenols Are Associated with Sleep Quality in a Cohort of Italian Adults. Nutrients 2020, 12, 1226. [Google Scholar] [CrossRef]
- Cantó, C.; Auwerx, J. Calorie Restriction: Is AMPK a Key Sensor and Effector? Physiology 2011, 26, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.-J.; Logunova, L.; Maywood, E.S.; Gallego, M.; Lebiecki, J.; Brown, T.M.; Sládek, M.; Semikhodskii, A.S.; Glossop, N.R.; Piggins, H.D.; et al. Setting Clock Speed in Mammals: The CK1ε tau Mutation in Mice Accelerates Circadian Pacemakers by Selectively Destabilizing PERIOD Proteins. Neuron 2008, 58, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Lamia, K.A.; Sachdeva, U.M.; DiTacchio, L.; Williams, E.C.; Alvarez, J.G.; Egan, D.F.; Vasquez, D.S.; Juguilon, H.; Panda, S.; Shaw, R.J.; et al. AMPK Regulates the Circadian Clock by Cryptochrome Phosphorylation and Degradation. Science 2009, 326, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Sherman, H.; Genzer, Y.; Cohen, R.; Chapnik, N.; Madar, Z.; Froy, O. Timed high-fat diet resets circadian metabolism and prevents obesity. FASEB J. 2012, 26, 3493–3502. [Google Scholar] [CrossRef] [PubMed]
- Poljsak, B. NAMPT-Mediated NAD Biosynthesis as the Internal Timing Mechanism: In NAD+ World, Time Is Running in Its Own Way. Rejuvenation Res. 2018, 21, 210–224. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Yoshino, J. Adipose tissue NAD+biology in obesity and insulin resistance: From mechanism to therapy. BioEssays 2017, 39, 1600227. [Google Scholar] [CrossRef]
- Levine, D.C.; Ramsey, K.M.; Bass, J. Circadian NAD(P)(H) cycles in cell metabolism. Semin. Cell Dev. Biol. 2022, 126, 15–26. [Google Scholar] [CrossRef]
- Ramsey, K.M.; Yoshino, J.; Brace, C.S.; Abrassart, D.; Kobayashi, Y.; Marcheva, B.; Hong, H.-K.; Chong, J.L.; Buhr, E.D.; Lee, C.; et al. Circadian Clock Feedback Cycle Through NAMPT-Mediated NAD+ Biosynthesis. Science 2009, 324, 651–654. [Google Scholar] [CrossRef]
- Fulco, M.; Cen, Y.; Zhao, P.; Hoffman, E.P.; McBurney, M.W.; Sauve, A.A.; Sartorelli, A. Glucose Restriction Inhibits Skeletal Myoblast Differentiation by Activating SIRT1 through AMPK-Mediated Regulation of Nampt. Dev. Cell 2008, 14, 661–673. [Google Scholar] [CrossRef]
- Yoshino, J.; Mills Kathryn, F.; Yoon, M.; Imai, S. Nicotinamide Mononucleotide, a Key NAD+ Intermediate, Treats the Pathophysiology of Diet- and Age-Induced Diabetes in Mice. Cell Metab. 2011, 14, 528–536. [Google Scholar] [CrossRef]
- Canto, C.; Jiang, L.Q.; Deshmukh, A.S.; Mataki, C.; Coste, A.; Lagouge, M.; Zierath, J.R.; Auwerx, J. Interdependence of AMPK and SIRT1 for Metabolic Adaptation to Fasting and Exercise in Skeletal Muscle. Cell Metab. 2010, 11, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Nakahata, Y.; Sahar, S.; Astarita, G.; Kaluzova, M.; Sassone-Corsi, P. Circadian Control of the NAD+ Salvage Pathway by CLOCK-SIRT1. Science 2009, 324, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Poljsak, B.; Kovač, V.; Milisav, I. Healthy Lifestyle Recommendations: Do the Beneficial Effects Originate from NAD+ Amount at the Cellular Level? Myers JN, editor. Oxidative Med. Cell. Longev. 2020, 2020, 8819627. [Google Scholar] [CrossRef]
- Houtkooper, R.H.; Pirinen, E.; Auwerx, J. Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol. 2012, 13, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Bordone, L.; Guarente, L. Calorie restriction, SIRT1 and metabolism: Understanding longevity. Nat. Rev. Mol. Cell Biol. 2005, 6, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Guarente, L. Sirtuins and calorie restriction. Nat. Rev. Mol. Cell Biol. 2012, 13, 207. [Google Scholar] [CrossRef]
- Guarente, L. Calorie restriction and sirtuins revisited. Genes Dev. 2013, 27, 2072–2085. [Google Scholar] [CrossRef]
- Yu, W.; Qin, J.; Chen, C.; Fu, Y.; Wang, W. Moderate calorie restriction attenuates age-associated alterations and improves cardiac function by increasing SIRT1 and SIRT3 expression. Mol. Med. Rep. 2018, 18, 4087–4094. [Google Scholar] [CrossRef]
- Cantó, C. Dietary restriction and Sirtuin 1 in metabolic health: Connections and divergences. Proc. Nutr. Soc. 2015, 75, 30–37. [Google Scholar] [CrossRef]
- Watroba, M.; Szukiewicz, D. Sirtuins at the Service of Healthy Longevity. Front. Physiol. 2021, 12, 724506. [Google Scholar] [CrossRef]
- Asher, G.; Gatfield, D.; Stratmann, M.; Reinke, H.; Dibner, C.; Kreppel, F.; Mostoslavsky, R.; Alt, F.W.; Schibler, U. SIRT1 Regulates Circadian Clock Gene Expression through PER2 Deacetylation. Cell 2008, 134, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Nakahata, Y.; Kaluzova, M.; Grimaldi, B.; Sahar, S.; Hirayama, J.; Chen, D.; Guarente, L.P.; Sassone-Corsi, P. The NAD+-Dependent Deacetylase SIRT1 Modulates CLOCK-Mediated Chromatin Remodeling and Circadian Control. Cell 2008, 134, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-C.; Guarente, L. SIRT1 Mediates Central Circadian Control in the SCN by a Mechanism that Decays with Aging. Cell 2013, 153, 1448–1460. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-H.; Zhao, T.; Cui, K.; Hu, G.; Chen, Q.; Chen, W.; Wang, X.-W.; Soto-Gutierrez, A.; Zhao, K.; Deng, C.-X. Negative reciprocal regulation between Sirt1 and Per2 modulates the circadian clock and aging. Sci. Rep. 2016, 6, 28633. [Google Scholar] [CrossRef]
- Terada, S.; Goto, M.; Kato, M.; Kawanaka, K.; Shimokawa, T.; Tabata, I. Effects of low-intensity prolonged exercise on PGC-1 mRNA expression in rat epitrochlearis muscle. Biochem. Biophys. Res. Commun. 2002, 296, 350–354. [Google Scholar] [CrossRef]
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef]
- Jäger, S.; Handschin, C.; St-Pierre, J.; Spiegelman, B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc. Natl. Acad. Sci. USA 2007, 104, 12017–12022. [Google Scholar] [CrossRef]
- Borra, M.T.; Smith, B.C.; Denu, J.M. Mechanism of human SIRT1 activation by resveratrol. J. Biol. Chem. 2005, 280, 17187–17195. [Google Scholar] [CrossRef]
- Gertz, M.; Nguyen, G.T.T.; Fischer, F.; Suenkel, B.; Schlicker, C.; Fränzel, B.; Tomaschewski, J.; Aladini, F.; Becker, C.; Wolters, D.; et al. A Molecular Mechanism for Direct Sirtuin Activation by Resveratrol. PLoS ONE 2012, 7, e49761. [Google Scholar] [CrossRef]
- Iside, C.; Scafuro, M.; Nebbioso, A.; Altucci, L. SIRT1 Activation by Natural Phytochemicals: An Overview. Front. Pharmacol. 2020, 11, 1225. [Google Scholar] [CrossRef]
- Akan, O.D.; Qin, D.; Guo, T.; Lin, Q.; Luo, F. Sirtfoods: New Concept Foods, Functions, and Mechanisms. Foods 2022, 11, 2955. [Google Scholar] [CrossRef] [PubMed]
- Pallauf, K.; Giller, K.; Huebbe, P.; Rimbach, G. Nutrition and Healthy Ageing: Calorie Restriction or Polyphenol-Rich “MediterrAsian” Diet? Oxidative Med. Cell. Longev. 2013, 2013, 707421. [Google Scholar] [CrossRef]
- Chung, S.; Yao, H.; Caito, S.; Hwang, J.; Arunachalam, G.; Rahman, I. Regulation of SIRT1 in cellular functions: Role of polyphenols. Arch. Biochem. Biophys. 2010, 501, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Sarubbo, F.; Esteban, S.; Miralles, A.; Moranta, D. Effects of Resveratrol and other Polyphenols on Sirt1: Relevance to Brain Function During Aging. Curr. Neuropharmacol. 2018, 16, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Xu, H.; Xie, Z.; Wang, L.; Sun, Y.; Yang, H.; Hu, D.; Mao, Y. Time-Restricted Feeding Reduces the Detrimental Effects of a High-Fat Diet, Possibly by Modulating the Circadian Rhythm of Hepatic Lipid Metabolism and Gut Microbiota. Front. Nutr. 2020, 7, 596285. [Google Scholar] [CrossRef]
- Cantó, C.; Menzies Keir, J.; Auwerx, J. NAD+ Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab. 2015, 22, 31–53. [Google Scholar] [CrossRef]
- Yang, Y.; Sauve, A.A. NAD+ metabolism: Bioenergetics, signaling and manipulation for therapy. Biochim. Biophys. Acta 2016, 1864, 1787–1800. [Google Scholar] [CrossRef]
- Bai, P.; Cantó, C.; Oudart, H.; Brunyánszki, A.; Cen, Y.; Thomas, C.; Yamamoto, H.; Huber, A.; Kiss, B.; Houtkooper, R.H.; et al. PARP-1 Inhibition Increases Mitochondrial Metabolism through SIRT1 Activation. Cell Metab. 2011, 13, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Szántó, M.; Bai, P. The role of ADP-ribose metabolism in metabolic regulation, adipose tissue differentiation, and metabolism. Genes Dev. 2020, 34, 321–340. [Google Scholar] [CrossRef]
- Vernochet, C.; Peres, S.B.; Davis, K.E.; McDonald, M.E.; Qiang, L.; Wang, H.; Scherer, P.E.; Farmer, S.R. C/EBPα and the Corepressors CtBP1 and CtBP2 Regulate Repression of Select Visceral White Adipose Genes during Induction of the Brown Phenotype in White Adipocytes by Peroxisome Proliferator-Activated Receptor γ Agonists. Mol. Cell. Biol. 2009, 29, 4714–4728. [Google Scholar] [CrossRef]
- Kajimura, S. Promoting brown and beige adipocyte biogenesis through the PRDM16 pathway. Int. J. Obes. Suppl. 2015, 5 (Suppl. 1), S11–S14. [Google Scholar] [CrossRef] [PubMed]
- Bournat, J.C.; Brown, C.W. Mitochondrial dysfunction in obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Breininger, S.P.; Malcomson, F.C.; Afshar, S.; Turnbull, D.M.; Greaves, L.; Mathers, J.C. Effects of obesity and weight loss on mitochondrial structure and function and implications for colorectal cancer risk. Proc. Nutr. Soc. 2019, 78, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.T.; Puigserver, P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc. Natl. Acad. Sci. USA 2007, 104, 12861–12866. [Google Scholar] [CrossRef] [PubMed]
- Gerhart-Hines, Z.; Rodgers, J.T.; Bare, O.; Lerin, C.; Kim, S.-H.; Mostoslavsky, R.; Alt, F.W.; Wu, Z.; Puigserver, P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1α. EMBO J. 2007, 26, 1913–1923. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.T.; Lerin, C.; Haas, W.; Gygi, S.P.; Spiegelman, B.M.; Puigserver, P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 2005, 434, 113–118. [Google Scholar] [CrossRef]
- Popov, L. Mitochondrial biogenesis: An update. J. Cell. Mol. Med. 2020, 24, 4892–4899. [Google Scholar] [CrossRef]
- Higashida, K.; Kim, S.H.; Jung, S.R.; Asaka, M.; Holloszy, J.O.; Han, D.-H. Effects of Resveratrol and SIRT1 on PGC-1α Activity and Mitochondrial Biogenesis: A Reevaluation. PLoS Biol. 2013, 11, e1001603. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Auwerx, J. PGC-1α, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009, 20, 98–105. [Google Scholar] [CrossRef]
- Gurd, B.J. Deacetylation of PGC-1α by SIRT1: Importance for skeletal muscle function and exercise-induced mitochondrial biogenesis. Appl. Physiol. Nutr. Metab. 2011, 36, 589–597. [Google Scholar] [CrossRef]
- Tang, B. Sirt1 and the Mitochondria. Mol. Cells 2016, 39, 87–95. [Google Scholar] [PubMed]
- Sanchis-Gomar, F.; Garcia-Gimenez, J.; Gomez-Cabrera, M.; Pallardo, F. Mitochondrial Biogenesis in Health and Disease. Molecular and Therapeutic Approaches. Curr. Pharm. Des. 2014, 20, 5619–5633. [Google Scholar] [CrossRef] [PubMed]
- Onyango, I.G.; Lu, J.; Rodova, M.; Lezi, E.; Crafter, A.B.; Swerdlow, R.H. Regulation of neuron mitochondrial biogenesis and relevance to brain health. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2010, 1802, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, R.M.; Corum, D.; Beeson, C.C.; Schnellmann, R.G. Mitochondrial Biogenesis as a Pharmacological Target: A New Approach to Acute and Chronic Diseases. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 229–249. [Google Scholar] [CrossRef] [PubMed]
- Goede, P.; Wüst, R.C.I.; Schomakers, B.V.; Denis, S.; Vaz, F.M.; Pras-Raves, M.L.; Weeghel, M.; Yi, C.; Kalsbeek, A.; Houtkooper, R.H. Time-restricted feeding during the inactive phase abolishes the daily rhythm in mitochondrial respiration in rat skeletal muscle. FASEB J. 2022, 36, e22133. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; De Stefani, D.; De Vivo, I.; Scapagnini, G. Polyphenols as Caloric Restriction Mimetics Regulating Mitochondrial Biogenesis and Mitophagy. Trends Endocrinol. Metab. 2020, 31, 536–550. [Google Scholar] [CrossRef]
- Chodari, L.; Aytemir, M.D.; Vahedi, P.; Alipour, M.; Vahed, S.Z.; Khatibi, S.M.H.; Ahmadian, E.; Ardalan, M.; Eftekhari, A. Targeting Mitochondrial Biogenesis with Polyphenol Compounds. de Oliveira FL, editor. Oxidative Med. Cell. Longev. 2021, 2021, 4946711. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Z.; Liu, J.; Hao, Y.; Sun, B.; Wang, J. Potential Health Benefits of Whole Grains: Modulation of Mitochondrial Biogenesis and Energy Metabolism. J. Agric. Food Chem. 2021, 69, 14065–14074. [Google Scholar] [CrossRef]
- Cypess, A.M.; Kahn, C.R. The role and importance of brown adipose tissue in energy homeostasis. Curr. Opin. Pediatr. 2010, 22, 478–484. [Google Scholar] [CrossRef]
- Lo, K.; Sun, L. Turning WAT into BAT: A review on regulators controlling the browning of white adipocytes. Biosci. Rep. 2013, 33, 711–719. [Google Scholar] [CrossRef]
- Van der Veen, D.R.; Laing, E.E.; Bae, S.-E.; Johnston, J.D.; Dijk, D.-J.; Archer, S.N. A Topological Cluster of Differentially Regulated Genes in Mice Lacking PER3. Front. Mol. Neurosci. 2020, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M.; Rosen, C.J. PPARγ: A circadian transcription factor in adipogenesis and osteogenesis. Nat. Rev. Endocrinol. 2010, 6, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yu, Q.; Wang, G.-X.; Lin, J.D. The Biological Clock is Regulated by Adrenergic Signaling in Brown Fat but is Dispensable for Cold-Induced Thermogenesis. PLoS ONE 2013, 8, e70109. [Google Scholar] [CrossRef]
- Heyde, I.; Begemann, K.; Oster, H. Contributions of white and brown adipose tissues to the circadian regulation of energy metabolism. Endocrinology 2021, 162, bqab009. [Google Scholar] [CrossRef] [PubMed]
- Seale, P.; Kajimura, S.; Yang, W.; Chin, S.; Rohas, L.M.; Uldry, M.; Tavernier, G.; Langin, D.; Spiegelman, B.M. Transcriptional Control of Brown Fat Determination by PRDM16. Cell Metab. 2007, 6, 38–54. [Google Scholar] [CrossRef]
- Ohno, H.; Shinoda, K.; Spiegelman Bruce, M.; Kajimura, S. PPARγ agonists Induce a White-to-Brown Fat Conversion through Stabilization of PRDM16 Protein. Cell Metab. 2012, 15, 395–404. [Google Scholar] [CrossRef]
- Seale, P.; Bjork, B.; Yang, W.; Kajimura, S.; Chin, S.; Kuang, S.; Scimè, A.; Devarakonda, S.; Conroe, H.M.; Erdjument-Bromage, H.; et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008, 454, 961–967. [Google Scholar] [CrossRef]
- Monnier, C.; Auclair, M.; Le Cam, G.; Garcia, M.; Antoine, B. The nuclear retinoid-related orphan receptor ROR α controls circadian thermogenic programming in white fat depots. Physiol. Rep. 2018, 6, e13678. [Google Scholar] [CrossRef]
- Weger, M.; Diotel, N.; Dorsemans, A.-C.; Dickmeis, T.; Weger, B.D. Stem cells and the circadian clock. Dev. Biol. 2017, 431, 111–123. [Google Scholar] [CrossRef]
- Nam, D.; Chatterjee, S.; Yin, H.; Liu, R.; Lee, J.; Yechoor, V.K.; Ma, K. Novel Function of Rev-erbα in Promoting Brown Adipogenesis. Sci. Rep. 2015, 5, 11239. [Google Scholar] [CrossRef]
- Lettieri Barbato, D.; Baldelli, S.; Pagliei, B.; Aquilano, K.; Ciriolo, M.R. Caloric Restriction and the Nutrient-Sensing PGC-1αin Mitochondrial Homeostasis: New Perspectives in Neurodegeneration. Int. J. Cell Biol. 2012, 2012, 759583. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.N.; Clark, J.P.; Martin, S.A.; Howell, P.R.; Burhans, M.S.; Haws, S.A.; Johnson, N.B.; Rhoads, T.W.; Pavelec, D.M.; Eliceiri, K.W.; et al. PGC-1a integrates a metabolism and growth network linked to caloric restriction. Aging Cell 2019, 18, e12999. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Deguchi, Y.; Nozaki, Y.; Higami, Y. Contribution of PGC-1α to Obesity- and Caloric Restriction-Related Physiological Changes in White Adipose Tissue. Int. J. Mol. Sci. 2021, 22, 6025. [Google Scholar] [CrossRef] [PubMed]
- Lira, V.A.; Benton, C.R.; Yan, Z.; Bonen, A. PGC-1α regulation by exercise training and its influences on muscle function and insulin sensitivity. Am. J. Physiol.-Endocrinol. Metab. 2010, 299, E145–E161. [Google Scholar] [CrossRef]
- Summermatter, S.; Handschin, C. PGC-1α and exercise in the control of body weight. Int. J. Obes. 2012, 36, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Vidal, P.; Stanford, K.I. Exercise-Induced Adaptations to Adipose Tissue Thermogenesis. Front. Endocrinol. 2020, 11, 270. [Google Scholar] [CrossRef]
- Aouichat, S.; Chayah, M.; Bouguerra-Aouichat, S.; Agil, A. Time-Restricted Feeding Improves Body Weight Gain, Lipid Profiles, and Atherogenic Indices in Cafeteria-Diet-Fed Rats: Role of Browning of Inguinal White Adipose Tissue. Nutrients 2020, 12, 2185. [Google Scholar] [CrossRef]
- Goede, P.; Hellings, T.P.; Coopmans, T.V.; Ritsema, W.I.G.R.; Kalsbeek, A. After-Effects of Time-Restricted Feeding on Whole-Body Metabolism and Gene Expression in Four Different Peripheral Tissues. Obesity 2020, 28, S68–S80. [Google Scholar] [CrossRef]
- Persinger, A.; Butawan, M.; Faietti, M.; Pryke, A.; Rose, K.; van der Merwe, M.; Bloomer, R.; Puppa, M. Effects of Feeding Time on Markers of Muscle Metabolic Flexibility Following Acute Aerobic Exercise in Trained Mice Undergoing Time Restricted Feeding. Nutrients 2021, 13, 1717. [Google Scholar] [CrossRef]
- Vieira, R.F.L.; Muñoz, V.R.; Junqueira, R.L.; Oliveira, F.; Gaspar, R.C.; Nakandakari, S.C.B.R.; Costa, S.D.O.; Torsoni, M.A.; da Silva, A.S.; Cintra, D.E.; et al. Time-restricted feeding combined with aerobic exercise training can prevent weight gain and improve metabolic disorders in mice fed a high-fat diet. J. Physiol. 2021, 600, 797–813. [Google Scholar] [CrossRef]
- Rodrigues, L.G.F.; de Araujo, L.D.; Roa, S.L.R.; Bueno, A.C.; Uchoa, E.T.; Antunes-Rodrigues, J.; Moreira, A.C.; Elias, L.L.K.; de Castro, M.; Martins, C.S. Restricted feeding modulates peripheral clocks and nutrient sensing pathways in rats. Arch. Endocrinol. Metab. 2021, 65, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Wood dos Santos, T.; Cristina Pereira, Q.; Teixeira, L.; Gambero, A.; Villena, J.A.; Lima Ribeiro, M. Effects of Polyphenols on Thermogenesis and Mitochondrial Biogenesis. Int. J. Mol. Sci. 2018, 19, 2757. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Sapere, N.; Visentin, M.; Zella, D.; Scapagnini, G. Enhancement of mitochondrial biogenesis with polyphenols: Combined effects of resveratrol and equol in human endothelial cells. Immun. Ageing 2013, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Voigt, R.M.; Forsyth, C.B.; Green, S.J.; Mutlu, E.; Engen, P.; Vitaterna, M.H.; Turek, F.W.; Keshavarzian, A. Circadian Disorganization Alters Intestinal Microbiota. PLoS ONE 2014, 9, e97500. [Google Scholar] [CrossRef]
- Voigt, R.M.; Summa, K.C.; Forsyth, C.B.; Green, S.J.; Engen, P.; Naqib, A.; Vitaterna, M.H.; Turek, F.W.; Keshavarzian, A. The CircadianClockMutation Promotes Intestinal Dysbiosis. Alcohol. Clin. Exp. Res. 2016, 40, 335–347. [Google Scholar] [CrossRef]
- Thaiss Christoph, A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler Anouk, C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom Control of Microbiota Diurnal Oscillations Promotes Metabolic Homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef]
- Zarrinpar, A.; Chaix, A.; Yooseph, S.; Panda, S. Diet and Feeding Pattern Affect the Diurnal Dynamics of the Gut Microbiome. Cell Metab. 2014, 20, 1006–1017. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, W.; Wang, J.; Yu, J.; Yang, L. Intermittent Fasting Improves Lipid Metabolism Through Changes in Gut Microbiota in Diet-Induced Obese Mice. Med. Sci. Monit. 2020, 26, e926789-1–e926789-11. [Google Scholar] [CrossRef]
- Patterson, R.E.; Laughlin, G.A.; LaCroix, A.Z.; Hartman, S.J.; Natarajan, L.; Senger, C.M.; Martínez, M.E.; Villaseñor, A.; Sears, D.D.; Marinac, C.R.; et al. Intermittent Fasting and Human Metabolic Health. J. Acad. Nutr. Diet. 2015, 115, 1203–1212. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2008, 457, 480–484. [Google Scholar] [CrossRef]
- Cui, Y.; Li, S.; Yin, Y.; Li, X.; Li, X. Daytime restricted feeding promotes circadian desynchrony and metabolic disruption with changes in bile acids profiles and gut microbiota in C57BL/6 Male Mice. J. Nutr. Biochem. 2022, 109, 109121. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef]
- Molinari, R.; Merendino, N.; Costantini, L. Polyphenols as modulators of pre-established gut microbiota dysbiosis: State-of-the-art. BioFactors 2021, 48, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Hills, R.D.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-Generated Metabolites Promote Metabolic Benefits via Gut-Brain Neural Circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Kumar Singh, A.; Cabral, C.; Kumar, R.; Ganguly, R.; Kumar Rana, H.; Gupta, A.; Lauro, M.R.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial Effects of Dietary Polyphenols on Gut Microbiota and Strategies to Improve Delivery Efficiency. Nutrients 2019, 11, 2216. [Google Scholar] [CrossRef]
- Leone, V.; Gibbons, S.M.; Martinez, K.; Hutchison, A.L.; Huang, E.Y.; Cham, C.M.; Pierre, J.F.; Heneghan, A.F.; Nadimpalli, A.; Hubert, N.; et al. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe 2015, 17, 681–689. [Google Scholar] [CrossRef]
- Effects of alternate-day fasting, time-restricted fasting and intermittent energy restriction DSS-induced on colitis and behavioral disorders. Redox Biol. 2020, 32, 101535. [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).