Investigating the Effects of a Multinutrient Supplement on Cognition, Mood and Biochemical Markers in Middle-Aged Adults with ‘Optimal’ and ‘Sub-Optimal’ Diets: A Randomized Double Blind Placebo Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Dietary Assessment
2.3. Cognitive Assessments
2.3.1. Swinburne University Computerised Cognitive Aging Battery (SUCCAB)
2.3.2. Cognitive Demand Battery (CDB)
2.4. Mood Assessments
2.4.1. Perceived Stress Scale (PSS)
2.4.2. Profile of Mood States (POMS)
2.4.3. The Depression, Anxiety and Stress Scale (DASS)
2.4.4. Stress Reactivity
2.5. Biochemical Assessment
2.6. Investigational Product
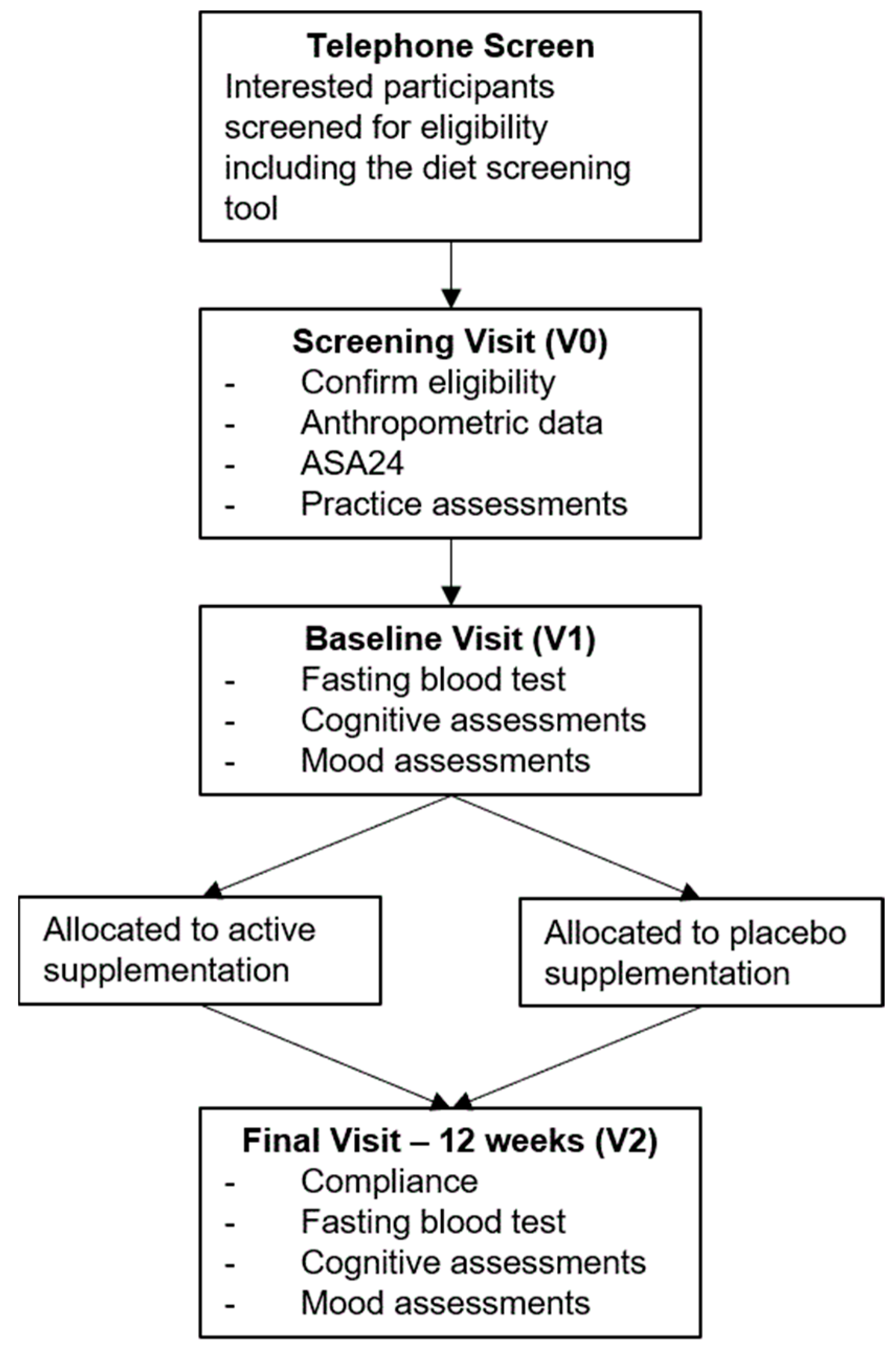
2.7. Procedure
2.8. Statistical Analysis
2.8.1. Sample Size Calculation
2.8.2. Analysis
2.8.3. Control Variables
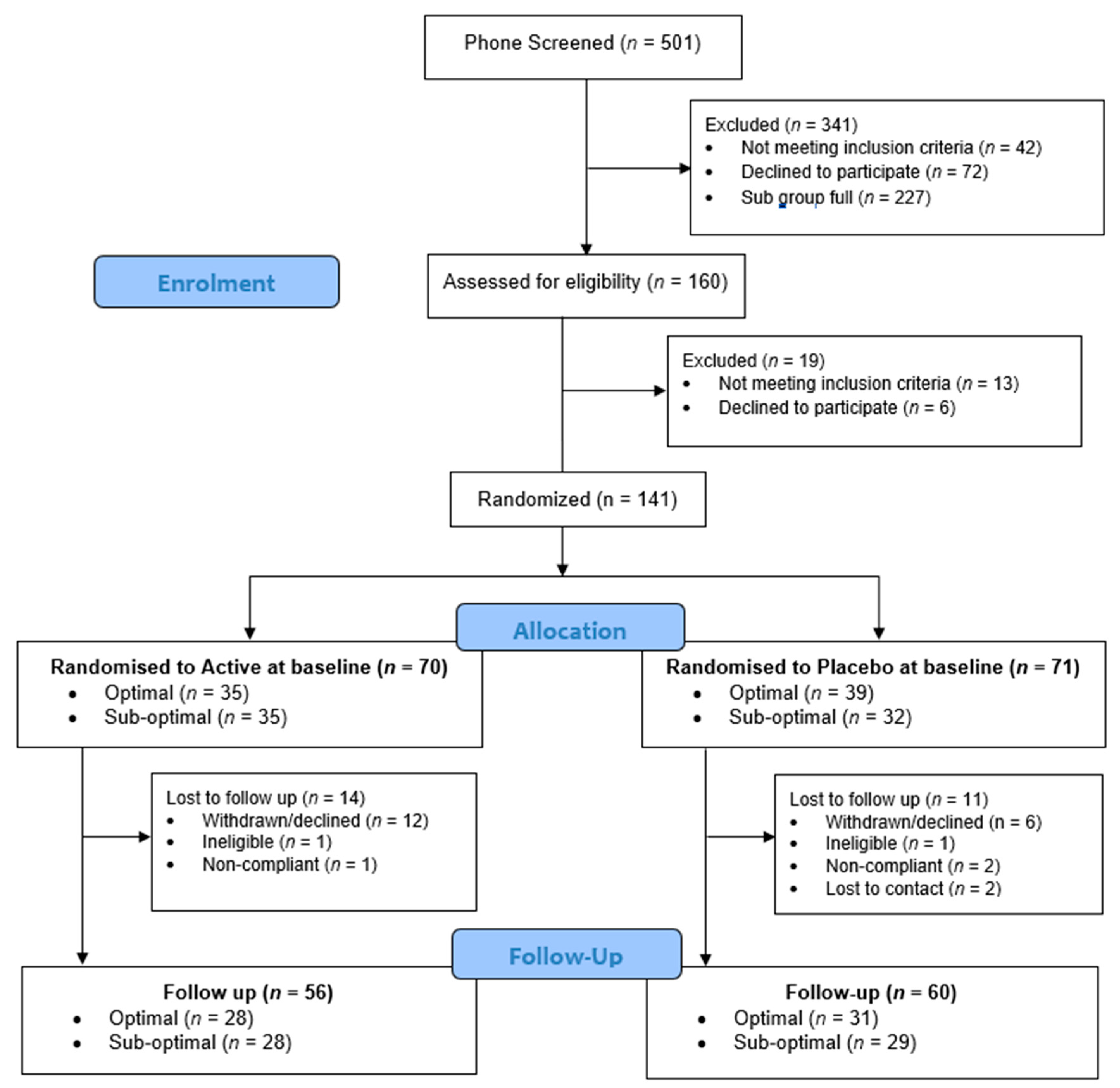
3. Results
3.1. Participant Characteristics
3.2. Primary Analysis
3.3. Secondary Analysis
3.3.1. Cognition
3.3.2. Mood and Stress Reactivity
3.3.3. Biochemical Markers of B Vitamins
3.4. Dietary Intake during Participation
3.5. Treatment Blinding
3.6. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gustaw-Rothenberg, K. Dietary patterns associated with Alzheimer’s disease: Population based study. Int. J. Environ. Res. Public Health 2009, 6, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef]
- Shakersain, B.; Santoni, G.; Larsson, S.C.; Faxén-Irving, G.; Fastbom, J.; Fratiglioni, L.; Xu, W. Prudent diet may attenuate the adverse effects of Western diet on cognitive decline. Alzheimer’s Dement. 2016, 12, 100–109. [Google Scholar] [CrossRef]
- Walsh, E.I.; Jacka, F.N.; Butterworth, P.; Anstey, K.J.; Cherbuin, N. The association between Western and Prudent dietary patterns and fasting blood glucose levels in type 2 diabetes and normal glucose metabolism in older Australian adults. Heliyon 2017, 3, e00315. [Google Scholar] [CrossRef]
- Francis, H.; Stevenson, R. The longer-term impacts of Western diet on human cognition and the brain. Appetite 2013, 63, 119–128. [Google Scholar] [CrossRef]
- Farooqui, A.A.; Farooqui, T. Neurochemical Effects of Western Diet Consumption on Human Brain. Diet and Exercise in Cognitive Function and Neurological Diseases; John Wiley & Sons, Inc.: Hoboken, NI, USA, 2015; pp. 15–28. [Google Scholar]
- Kanoski, S.E.; Davidson, T.L. Western diet consumption and cognitive impairment: Links to hippocampal dysfunction and obesity. Physiol. Behav. 2011, 103, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia prevention, intervention, and care. Lancet 2017, 390, 2673–2734. [Google Scholar]
- Kantor, E.D.; Rehm, C.D.; Du, M.; White, E.; Giovannucci, E.L. Trends in Dietary Supplement Use Among US Adults from 1999–2012. JAMA 2016, 316, 1464–1474. [Google Scholar] [CrossRef]
- Bailey, R.L.; Fulgoni, V.L., 3rd; Keast, D.R.; Dwyer, J.T. Examination of vitamin intakes among US adults by dietary supplement use. J. Acad. Nutr. Diet. 2012, 112, 657–663.e4. [Google Scholar] [CrossRef]
- Mishra, S.; Stierman, B.; Gahche, J.J.; Potischman, N. Dietary Supplement Use among Adults: United States, 2017–2018; Hyattsville, M.D., Ed.; US Department of Health and Human Services: Washington, DC, USA, 2021.
- Long, S.J.; Benton, D. Effects of vitamin and mineral supplementation on stress, mild psychiatric symptoms, and mood in nonclinical samples: A meta-analysis. Psychosom. Med. 2013, 75, 144–153. [Google Scholar] [CrossRef]
- Young, L.M.; Pipingas, A.; White, D.J.; Gauci, S.; Scholey, A. A Systematic Review and Meta-Analysis of B Vitamin Supplementation on Depressive Symptoms, Anxiety, and Stress: Effects on Healthy and ‘At-Risk’ Individuals. Nutrients 2019, 11, 2232. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.D.; Manson, J.E.; Rapp, S.R.; Sesso, H.D.; Gaussoin, S.A.; Shumaker, S.A.; Espeland, M.A. Effects of cocoa extract and a multivitamin on cognitive function: A randomized clinical trial. Alzheimer’s Dement. 2022, 1–12. [Google Scholar] [CrossRef]
- Macpherson, H.; Ellis, K.; Sali, A.; Pipingas, A. Memory improvements in elderly women following 16 weeks treatment with a combined multivitamin, mineral and herbal supplement: A randomized controlled trial. Psychopharmacology 2012, 220, 351–365. [Google Scholar] [CrossRef]
- Harris, E.; Kirk, J.; Rowsell, R.; Vitetta, L.; Sali, A.; Scholey, A.B.; Pipingas, A. The effect of multivitamin supplementation on mood and stress in healthy older men. Hum. Psychopharmacol. 2011, 26, 560–567. [Google Scholar] [CrossRef]
- White, D.J.; Cox, K.H.; Peters, R.; Pipingas, A.; Scholey, A.B. Effects of four-week supplementation with a multi-vitamin/mineral preparation on mood and blood biomarkers in young adults: A randomised, double-blind, placebo-controlled trial. Nutrients 2015, 7, 9005–9017. [Google Scholar] [CrossRef]
- Macpherson, H.; Rowsell, R.; Cox, K.H.; Reddan, J.; Meyer, D.; Scholey, A.; Pipingas, A. The effects of four-week multivitamin supplementation on mood in healthy older women: A randomized controlled trial. Evid.-Based Complement. Altern. Med. 2016, 2016, 3092828. [Google Scholar] [CrossRef]
- Suh, S.W.; Kim, H.S.; Han, J.H.; Bin Bae, J.; Oh, D.J.; Kim, K.W. Efficacy of vitamins on cognitive function of non-demented people: A systematic review and meta-analysis. Nutrients 2020, 12, 1168. [Google Scholar] [CrossRef]
- Pipingas, A.; Camfield, D.A.; Stough, C.; Scholey, A.B.; Cox, K.H.; White, D. Effects of multivitamin, mineral and herbal supplement on cognition in younger adults and the contribution of B group vitamins. Hum. Psychopharmacol. 2014, 29, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Haskell, C.F.; Robertson, B.; Jones, E.; Forster, J.; Jones, R.; Wilde, A.; Maggini, S.; Kennedy, D.O. Effects of a multi-vitamin/mineral supplement on cognitive function and fatigue during extended multi-tasking. Hum. Psychopharmacol. 2010, 25, 448–461. [Google Scholar] [CrossRef]
- Wolters, M.; Hickstein, M.; Flintermann, A.; Tewes, U.; Hahn, A. Cognitive performance in relation to vitamin status in healthy elderly German women-the effect of 6-month multivitamin supplementation. Prev. Med. 2005, 41, 253–259. [Google Scholar] [CrossRef]
- McNeill, G.; Avenell, A.; Campbell, M.K.; A Cook, J.; Hannaford, P.C.; Kilonzo, M.M.; Milne, A.C.; Ramsay, C.R.; Seymour, D.G.; I Stephen, A.; et al. Effect of multivitamin and multimineral supplementation on cognitive function in men and women aged 65 years and over: A randomised controlled trial. Nutr. J. 2007, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Camfield, D.A.; Wetherell, M.A.; Scholey, A.B.; Cox, K.H.M.; Fogg, E.; White, D.J.; Sarris, J.; Kras, M.; Stough, C.; Sali, A.; et al. The effects of multivitamin supplementation on diurnal cortisol secretion and perceived stress. Nutrients 2013, 5, 4429–4450. [Google Scholar] [CrossRef] [PubMed]
- Cockle, S.M.; Haller, J.; Kimber, S.; Dawe, R.A.; Hindmarch, I. The influence of multivitamins on cognitive function and mood in the elderly. Aging Ment. Health 2000, 4, 339–353. [Google Scholar] [CrossRef]
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharmacogn Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Roodenrys, S.; Booth, D.; Bulzomi, S.; Phipps, A.; Micallef, C.; Smoker, J. Chronic effects of Brahmi (Bacopa monnieri) on human memory. Neuropsychopharmacology 2002, 27, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Sathyanarayanan, V.; Thomas, T.; Einöther, S.J.; Dobriyal, R.; Joshi, M.K.; Krishnamachari, S. Brahmi for the better? New findings challenging cognition and anti-anxiety effects of Brahmi (Bacopa monniera) in healthy adults. Psychopharmacology 2013, 227, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Stough, C.; Downey, L.A.; Lloyd, J.; Silber, B.; Redman, S.; Hutchison, C.; Wesnes, K.; Nathan, P.J. Examining the nootropic effects of a special extract of Bacopa monniera on human cognitive functioning: 90 day double-blind placebo-controlled randomized trial. Phytother. Res. 2008, 22, 1629–1634. [Google Scholar] [CrossRef]
- Stough, C.; Lloyd, J.; Clarke, J.; A Downey, L.; Hutchison, C.W.; Rodgers, T.; Nathan, P.J. The chronic effects of an extract of Bacopa monniera (Brahmi) on cognitive function in healthy human subjects. Psychopharmacology 2001, 156, 481–484. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Smith, S.J.; Ali, S.; Metse, A.P.; Kalns, J.; Drummond, P.D. Effects of a Bacopa monnieri extract (Bacognize®) on stress, fatigue, quality of life and sleep in adults with self-reported poor sleep: A randomised, double-blind, placebo-controlled study. J. Funct. Foods 2021, 85, 104671. [Google Scholar] [CrossRef]
- Lorca, C.; Mulet, M.; Arévalo-Caro, C.; Sanchez, M.; Perez, A.; Perrino, M.; Bach-Faig, A.; Aguilar-Martínez, A.; Vilella, E.; Gallart-Palau, X.; et al. Plant-derived nootropics and human cognition: A systematic review. Crit. Rev. Food Sci. Nutr. 2022, 1–25. [Google Scholar] [CrossRef]
- Liu, H.; Ye, M.; Guo, H. An Updated Review of Randomized Clinical Trials Testing the Improvement of Cognitive Function of Ginkgo biloba Extract in Healthy People and Alzheimer’s Patients. Front. Pharmacol. 2019, 10, 1688. [Google Scholar] [CrossRef]
- Gschwind, Y.J.; Bridenbaugh, S.A.; Reinhard, S.; Granacher, U.; Monsch, A.U.; Kressig, R.W. Ginkgo biloba special extract LI 1370 improves dual-task walking in patients with MCI: A randomised, double-blind, placebo-controlled exploratory study. Aging Clin. Exp. Res. 2017, 29, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Laws, K.R.; Sweetnam, H.; Kondel, T.K. Is Ginkgo biloba a cognitive enhancer in healthy individuals? A meta-analysis. Hum. Psychopharmacol. 2012, 27, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Pase, M.P.; Kean, J.; Sarris, J.; Neale, C.; Scholey, A.B.; Stough, C. The cognitive-enhancing effects of Bacopa monnieri: A systematic review of randomized, controlled human clinical trials. J. Altern. Complement. Med. 2012, 18, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O. B Vitamins and the Brain: Mechanisms, Dose and Efficacy—A Review. Nutrients 2016, 8, 68. [Google Scholar] [CrossRef]
- Monti, J.M.; Moulton, C.J.; Cohen, N.J. The role of nutrition on cognition and brain health in ageing: A targeted approach. Nutr. Res. Rev. 2015, 28, 167–180. [Google Scholar] [CrossRef]
- Heaney, R.P. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr. Rev. 2014, 72, 48–54. [Google Scholar] [CrossRef]
- Morris, M.C.; Tangney, C.C. A potential design flaw of randomized trials of vitamin supplements. JAMA 2011, 305, 1348–1349. [Google Scholar] [CrossRef]
- Morris, M.C. Nutrition and risk of dementia: Overview and methodological issues. Ann. N. Y. Acad. Sci. 2016, 1367, 31–37. [Google Scholar] [CrossRef]
- Scholey, A. Nutrients for neurocognition in health and disease: Measures, methodologies and mechanisms. Proc. Nutr. Soc. 2018, 77, 73–83. [Google Scholar] [CrossRef]
- Young, L.M.; Gauci, S.; Scholey, A.; White, D.J.; Pipingas, A. Self-selection bias: An essential design consideration for nutrition trials in healthy populations. Front. Nutr. 2020, 7, 587983. [Google Scholar] [CrossRef]
- Jacka, F.N.; O’Neil, A.; Opie, R.; Itsiopoulos, C.; Cotton, S.; Mohebbi, M.; Castle, D.; Dash, S.; Mihalopoulos, C.; Chatterton, M.L.; et al. A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Med. 2017, 15, 23. [Google Scholar] [CrossRef]
- Bailey, R.L.; E Miller, P.; Mitchell, D.C.; Hartman, T.J.; Lawrence, F.R.; Sempos, C.T.; Smiciklas-Wright, H. Dietary screening tool identifies nutritional risk in older adults. Am. J. Clin. Nutr. 2009, 90, 177–183. [Google Scholar] [CrossRef]
- Ventura Marra, M.; Thuppal, S.V.; Johnson, E.J.; Bailey, R.L. Validation of a Dietary Screening Tool in a Middle-Aged Appalachian Population. Nutrients 2018, 10, 345. [Google Scholar] [CrossRef]
- Pipingas, A.; Harris, E.; Tournier, E.; King, R.; Kras, M.; Stough, C. Assessing the Efficacy of Nutraceutical Interventions on Cognitive Functioning in the Elderly. Curr. Top. Nutraceutical Res. 2010, 8, 79–88. [Google Scholar]
- Pase, M.; Grima, N.; Cockerell, R.; Stough, C.; Scholey, A.; Sali, A.; Pipingas, A. The effects of long-chain omega-3 fish oils and multivitamins on cognitive and cardiovascular function: A randomized, controlled clinical trial. J. Am. Coll. Nutr. 2015, 34, 21–31. [Google Scholar] [CrossRef]
- Kennedy, D.; Haskell, C.; Robertson, B.; Reay, J.; Brewster-Maund, C.; Luedemann, J.; Maggini, S.; Ruf, M.; Zangara, A.; Scholey, A. Improved cognitive performance and mental fatigue following a multi-vitamin and mineral supplement with added guaraná (Paullinia cupana). Appetite 2008, 50, 506–513. [Google Scholar] [CrossRef]
- Thorne, D.R. Throughput: A simple performance index with desirable characteristics. Behav. Res. Methods 2006, 38, 569–573. [Google Scholar] [CrossRef]
- Brébion, G. Language processing, slowing, and speed/accuracy trade-off in the elderly. Exp. Aging Res. 2001, 27, 137–150. [Google Scholar] [CrossRef]
- Cox, K.H.M.; White, D.J.; Pipingas, A.; Poorun, K.; Scholey, A. Further evidence of benefits to mood and working memory from lipidated curcumin in healthy older people: A 12-week, double-blind, placebo-controlled, partial replication study. Nutrients 2020, 12, 1678. [Google Scholar] [CrossRef]
- Pipingas, A.; Camfield, D.; Stough, C.; Cox, K.; Fogg, E.; Tiplady, B.; Sarris, J.; White, D.; Sali, A.; Wetherell, M.; et al. The effects of multivitamin supplementation on mood and general well-being in healthy young adults. A laboratory and at-home mobile phone assessment. Appetite 2013, 69, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Young, L.; Gauci, S.; Scholey, A.; White, D.; Lassemillante, A.-C.; Meyer, D.; Pipingas, A. Self-reported diet quality differentiates nutrient intake, blood nutrient status, mood, and cognition: Implications for identifying nutritional neurocognitive risk factors in middle age. Nutrients 2020, 12, 2964. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Smith, S.; De Jager, C.A.; Whitbread, P.; Johnston, C.; Agacinski, G.; Oulhaj, A.; Bradley, K.M.; Jacoby, R.; Refsum, H. Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: A randomized controlled trial. PLoS ONE 2010, 5, e12244. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Refsum, H.; Homocysteine, B. Vitamins, and Cognitive Impairment. Annu. Rev. Nutr. 2016, 36, 211–239. [Google Scholar] [CrossRef]
- Boisvert, W.A.; Mendoza, I.; Castaneda, C.; De Portocarrero, L.; Solomons, N.W.; Gershoff, S.N.; Russell, R.M. Riboflavin requirement of healthy elderly humans and its relationship to macronutrient composition of the diet. J. Nutr. 1993, 123, 915–925. [Google Scholar] [CrossRef]
- Powers, H.J. Riboflavin (vitamin B-2) and health. Am. J. Clin. Nutr. 2003, 77, 1352–1360. [Google Scholar] [CrossRef]
- Madigan, S.M.; Tracey, F.; McNulty, H.; Eaton-Evans, J.; Coulter, J.; McCartney, H.; Strain, J.J. Riboflavin and vitamin B-6 intakes and status and biochemical response to riboflavin supplementation in free-living elderly people. Am. J. Clin. Nutr. 1998, 68, 389–395. [Google Scholar] [CrossRef]
- Heaney, R.P. The nutrient problem. Nutr. Rev. 2012, 70, 165–169. [Google Scholar] [CrossRef]
- Tardy, A.-L.; Pouteau, E.; Marquez, D.; Yilmaz, C.; Scholey, A. Vitamins and Minerals for Energy, Fatigue and Cognition: A Narrative Review of the Biochemical and Clinical Evidence. Nutrients 2020, 12, 228. [Google Scholar] [CrossRef]
- Jacobs, D.R.; Steffen, L.M. Nutrients, foods, and dietary patterns as exposures in research: A framework for food synergy. Am. J. Clin. Nutr. 2003, 78, 508S–513S. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.R.; Tapsell, L.C. Food synergy: The key to a healthy diet. Proc. Nutr. Soc. 2013, 72, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Pipingas, A.; Cockerell, R.; Grima, N.; Sinclair, A.; Stough, C.; Scholey, A.; Myers, S.; Croft, K.; Sali, A.; Pase, M.P. Randomized controlled trial examining the effects of fish oil and multivitamin supplementation on the incorporation of n-3 and n-6 fatty acids into red blood cells. Nutrients 2014, 6, 1956–1970. [Google Scholar] [CrossRef] [PubMed]
- Oulhaj, A.; Jernerén, F.; Refsum, H.; Smith, A.D.; de Jager, C.A. Omega-3 Fatty Acid Status Enhances the Prevention of Cognitive Decline by B Vitamins in Mild Cognitive Impairment. J. Alzheimers Dis. 2016, 50, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Jernerén, F.; Elshorbagy, A.K.; Oulhaj, A.; Smith, S.M.; Refsum, H.; Smith, A.D. Brain atrophy in cognitively impaired elderly: The importance of long-chain ω-3 fatty acids and B vitamin status in a randomized controlled trial. Am. J. Clin. Nutr. 2015, 102, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Shi, Z.; Liu, G.; Chang, D.; Inglis, S.; Hall, J.; Schutte, A.; Byles, J.; Parker, D. The Joint Effects of Diet and Dietary Supplements in Relation to Obesity and Cardiovascular Disease over a 10-Year Follow-Up: A Longitudinal Study of 69,990 Participants in Australia. Nutrients 2021, 13, 944. [Google Scholar] [CrossRef] [PubMed]
- Meletis, C.D.; Barker, J.E. Synergy in nutrients. Altern. Complement. Ther. 2004, 10, 326–329. [Google Scholar] [CrossRef]
- Pistollato, F.; Iglesias, R.C.; Ruiz, R.; Aparicio, S.; Crespo, J.; Lopez, L.D.; Manna, P.P.; Giampieri, F.; Battino, M. Nutritional patterns associated with the maintenance of neurocognitive functions and the risk of dementia and Alzheimer’s disease: A focus on human studies. Pharmacol. Res. 2018, 131, 32–43. [Google Scholar] [CrossRef]
- Shao, A.; Drewnowski, A.; Willcox, D.C.; Krämer, L.; Lausted, C.; Eggersdorfer, M.; Mathers, J.; Bell, J.D.; Randolph, R.K.; Witkamp, R.; et al. Optimal nutrition and the ever-changing dietary landscape: A conference report. Eur. J. Nutr. 2017, 56 (Suppl. 1), 1–21. [Google Scholar] [CrossRef]
- Jacobs, D.R.; Tapsell, L.C. Food, not Nutrients, is the fundamental unit in nutrition. Nutr. Rev. 2007, 65, 439–450. [Google Scholar] [CrossRef]
- Tapsell, L.C.; Neale, E.P.; Satija, A.; Hu, F.B. Foods, nutrients, and dietary patterns: Interconnections and implications for dietary guidelines. Adv. Nutr. 2016, 7, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Biesalski, H.K.; Aggett, P.J.; Anton, R.; Bernstein, P.S.; Blumberg, J.; Heaney, R.P.; Henry, J.; Nolan, J.M.; Richardson, D.P.; van Ommen, B.; et al. 26th Hohenheim Consensus Conference, 11 September 2010 Scientific substantiation of health claims: Evidence-based nutrition. Nutrition 2011, 27 (Suppl. 10), S1–S20. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, G.P.; Collins, F.S. Precision Nutrition—The Answer to “What to Eat to Stay Healthy”. JAMA 2020, 324, 735–736. [Google Scholar] [CrossRef] [PubMed]
| Whole Sample | Active | Placebo | ||||||
|---|---|---|---|---|---|---|---|---|
| n | M | SD | M | SD | M | SD | p | |
| Age | 141 | 52.84 | 6.87 | 53.02 | 6.78 | 52.67 | 7.01 | 0.763 |
| MMSE | 141 | 29.31 | 0.95 | 29.30 | 0.97 | 29.34 | 0.94 | 0.813 |
| Education years | 141 | 16.94 | 3.36 | 17.23 | 3.75 | 16.66 | 2.93 | 0.319 |
| BMI | 141 | 27.26 | 5.22 | 28.19 | 5.59 | 26.35 | 4.67 | 0.036 * |
| Waist Circumference | 140 | 95.91 | 14.77 | 97.97 | 15.74 | 93.85 | 13.54 | 0.099 |
| Hip Circumference | 140 | 107.21 | 9.15 | 105.35 | 10.03 | 106.06 | 8.08 | 0.139 |
| BDI | 141 | 4.00 | 4.37 | 3.97 | 4.40 | 4.03 | 4.36 | 0.939 |
| Diet quality | 141 | 62.10 | 13.00 | 60.40 | 12 | 63.77 | 13.31 | 0.124 |
| NART | 141 | 37.13 | 5.38 | 36.79 | 5.60 | 37.45 | 5.17 | 0.465 |
| STAI-trait | 141 | 31.48 | 7.42 | 31.43 | 7.65 | 31.54 | 7.25 | 0.932 |
| Brachial systolic | 141 | 119.28 | 12.36 | 120.30 | 12.67 | 118.28 | 12.05 | 0.334 |
| Brachial diastolic | 141 | 73.92 | 8.47 | 73.84 | 9.08 | 74.00 | 7.90 | 0.913 |
| Vitamin B1 | 94 | 126.60 | 33.80 | 126.22 | 36.85 | 127.00 | 30.56 | 0.912 |
| Vitamin B2 | 135 | 233.36 | 42.70 | 233.91 | 38.68 | 232.79 | 46.82 | 0.879 |
| RBC Folate | 62 | 1242.50 | 1285.15 | 152.06 | 1211.69 | 314.75 | 0.464 | |
| Vitamin B6 | 139 | 116.85 | 118.54 | 114.06 | 108.10 | 119.60 | 128.73 | 0.833 |
| Vitamin B12 | 139 | 321.09 | 114.11 | 329.33 | 99.74 | 312.96 | 126.89 | 0.271 |
| Homocysteine | 139 | 10.28 | 2.84 | 9.82 | 2.55 | 10.73 | 3.05 | 0.062 |
| High-sensitivity C-reactive protein | 135 | 1.77 | 2.98 | 2.12 | 3.56 | 1.43 | 2.28 | 0.180 |
| Active | Placebo | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Task | Subgroup | n | Baseline M (SD) | 12 Weeks M (SD) | Δ | n | Baseline M (SD) | 12 Weeks M (SD) | Δ | F | p |
| Spatial Working Memory Performance | All | 70 | 89.61 (25.64) | 92.28 (28.54) | 2.67 | 71 | 90.96 (28.60) | 95.50 (32.24) | 4.54 | 0.53 | 0.470 |
| Optimal | 35 | 87.63 (27.38) | 88.04 (29.51) | 0.41 | 39 | 87.18 (29.23) | 87.31 (29.53) | 0.13 | 0.08 | 0.772 | |
| Sub-Optimal | 35 | 91.59 (24.02) | 96.52 (27.41) | 4.93 | 32 | 95.57 (27.57) | 104.25 (33.20) | 8.68 | 0.54 | 0.467 | |
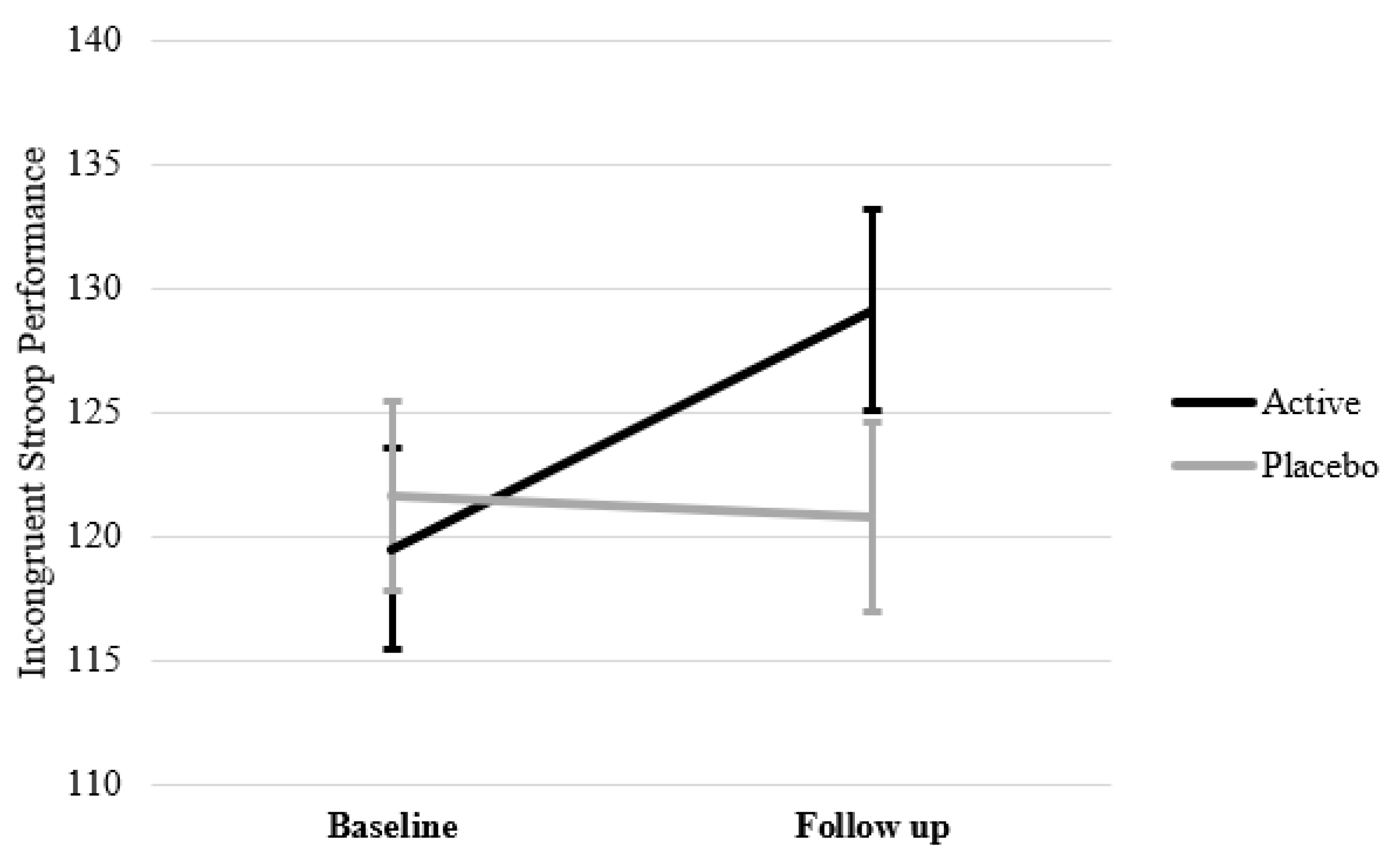
| Stroop Incongruent Performance | All | 69 | 121.40 (22.64) | 130.67 (21.09) | 9.27 | 70 | 119.01 (23.57) | 124.38 (25.90) | 5.37 | 1.89 | 0.171 |
| Optimal | 34 | 120.59 (24.32) | 130.38 (18.05) | 9.79 | 38 | 120.88 (23.22) | 120.66 (26.53) | −0.22 | 4.94 | 0.030 * | |
| Sub-Optimal | 35 | 122.19 (21.20) | 130.96 (24.08) | 8.77 | 32 | 116.79 (24.15) | 128.35 (25.06) | 11.56 | 0.37 | 0.544 | |
| Simple Reaction Time | All | 70 | 380.18 (35.34) | 380.23 (51.02) | 0.05 | 71 | 375.82 (57.09) | 375.37 (51.07) | −0.45 | 0.15 | 0.697 |
| Optimal | 35 | 384.60 (56.34) | 379.90 (56.47) | −4.70 | 39 | 379.71 (59.60) | 380.96 (54.68) | 1.25 | 0.99 | 0.323 | |
| Sub-Optimal | 35 | 375.75 (52.71) | 380.56 (45.97) | 4.81 | 32 | 371.08 (54.44) | 369.40 (47.12) | −1.68 | 0.26 | 0.609 | |
| Choice Reaction Time Performance | All | 70 | 220.35 (32.79) | 217.47 (32.60) | −2.88 | 71 | 211.97 (29.61) | 216.67 (31.69) | 4.70 | 2.02 | 0.158 |
| Optimal | 35 | 217.69 (36.03) | 215.71 (34.94) | −1.98 | 39 | 217.24 (28.28) | 214.80 (30.81) | −2.44 | 0.72 | 0.399 | |
| Sub-Optimal | 35 | 223.01 (29.47) | 219.23 (30.63) | −3.78 | 32 | 205.55 (30.36) | 218.67 (33.04) | 13.12 | 7.02 | 0.010 * | |
| Immediate Recognition Performance | All | 70 | 83.34 (16.46) | 87.06 (16.70) | 3.72 | 70 | 86.40 (19.95) | 90.02 (19.79) | 3.62 | 0.63 | 0.430 |
| Optimal | 35 | 84.53 (18.06) | 86.38 (15.61) | 1.85 | 39 | 84.24 (19.48) | 86.39 (20.51) | 2.15 | 0.00 | 0.998 | |
| Sub-Optimal | 35 | 82.16 (14.86) | 87.73 (17.99) | 5.57 | 31 | 89.12 (20.52) | 93.90 (18.54) | 4.78 | 1.21 | 0.276 | |
| Delayed Recognition Performance | All | 70 | 72.30 (16.06) | 68.07 (15.23) | −4.23 | 71 | 73.48 (17.16) | 71.77 (16.86) | −1.71 | 0.85 | 0.359 |
| Optimal | 35 | 76.67 (17.72) | 68.95 (13.57) | −7.72 | 38 | 72.59 (17.71) | 68.20 (16.12) | −4.39 | 0.84 | 0.364 | |
| Sub-Optimal | 35 | 67.93 (13.06) | 67.19 (16.93) | −0.74 | 32 | 74.53 (16.70) | 75.59 (17.06) | 1.06 | 0.18 | 0.670 | |
| Stroop Congruent Performance | All | 70 | 142.53 (20.82) | 148.42 (21.80) | 5.89 | 71 | 142.74 (22.28) | 144.65 (18.92) | 1.91 | 2.55 | 0.11 |
| Optimal | 35 | 138.29 (22.07) | 144.97 (21.17) | 6.68 | 39 | 142.87 (22.02) | 143.90 (18.79) | 1.03 | 3.27 | 0.076 ^ | |
| Sub-Optimal | 35 | 146.78 (18.85) | 151.88 (22.25) | 5.10 | 32 | 142.58 (22.94) | 145.46 (19.36) | 2.88 | 0.23 | 0.631 | |
| Contextual Memory Performance | All | 70 | 83.05 (22.53) | 90.02 (23.30) | 6.97 | 70 | 86.01 (23.00) | 93.32 (21.33) | 7.31 | 0.04 | 0.853 |
| Optimal | 35 | 83.71 (27.83) | 88.98 (26.97) | 5.27 | 38 | 83.41 (25.33) | 89.22 (23.51) | 5.81 | 1.79 | 0.186 | |
| Sub-Optimal | 35 | 82.40 (15.95) | 91.07 (19.39) | 8.67 | 32 | 89.10 (19.84) | 97.70 (18.11) | 8.61 | 1.16 | 0.285 | |
| Serial Threes correct (#) | All | 70 | 31.75 (13.83) | 33.10 (13.63) | 1.35 | 71 | 31.36 (13.85) | 33.71 (14.36) | 2.35 | 1.16 | 0.284 |
| Optimal | 35 | 30.75 (12.85) | 30.37 (12.65) | −0.38 | 39 | 30.61 (11.91) | 32.63 (11.85) | 2.02 | 1.49 | 0.227 | |
| Sub-Optimal | 35 | 32.75 (14.86) | 35.73 (14.22) | 2.98 | 32 | 32.27 (16.05) | 34.85 (16.79) | 2.58 | 0.04 | 0.835 | |
| Serial Threes incorrect (#) | All | 70 | 2.60 (2.16) | 2.84 (2.53) | 0.24 | 71 | 2.66 (2.10) | 3.18 (2.42) | 0.52 | 0.23 | 0.633 |
| Optimal | 35 | 2.63 (1.77) | 3.14 (3.00) | 0.51 | 39 | 2.80 (2.36) | 3.39 (2.41) | 0.59 | 0.01 | 0.957 | |
| Sub-Optimal | 35 | 2.58 (2.52) | 2.55 (1.98) | −0.03 | 32 | 2.48 (1.75) | 2.95 (2.45) | 0.47 | 0.43 | 0.515 | |
| Serial Sevens correct (#) | All | 70 | 20.86 (10.62) | 21.91 (11.34) | 1.05 | 70 | 20.80 (11.43) | 22.02 (12.46) | 1.22 | 0.02 | 0.893 |
| Optimal | 35 | 20.16 (9.92) | 20.10 (10.79) | −0.06 | 39 | 19.79 (10.04) | 21.63 (11.16) | 1.84 | 2.05 | 0.157 | |
| Sub-Optimal | 35 | 21.55 (11.38) | 23.65 (11.78) | 2.10 | 31 | 22.08 (13.03) | 22.43 (13.90) | 0.35 | 2.61 | 0.112 | |
| Serial Sevens incorrect (#) | All | 70 | 3.10 (1.76) | 3.24 (2.23) | 0.14 | 70 | 2.96 (2.11) | 3.87 (3.30) | 0.91 | 3.15 | 0.078 ^ |
| Optimal | 35 | 3.04 (1.84) | 3.59 (2.19) | 0.55 | 39 | 3.28 (2.23) | 4.17 (3.83) | 0.89 | 0.26 | 0.610 | |
| Sub-Optimal | 35 | 3.15 (1.70) | 2.90 (2.25) | −0.25 | 31 | 2.56 (1.91) | 3.55 (2.66) | 0.99 | 5.02 | 0.029 * | |
| RVIP Performance | All | 68 | 10.23 (4.96) | 11.20 (5.14) | 0.97 | 68 | 10.56 (4.47) | 11.79 (4.74) | 1.23 | 0.05 | 0.824 |
| Optimal | 33 | 10.76 (5.63) | 11.78 (5.77) | 1.02 | 37 | 10.62 (4.38) | 11.97 (4.55) | 1.35 | 0.04 | 0.840 | |
| Sub-Optimal | 35 | 9.74 (4.27) | 10.70 (4.57) | 0.96 | 31 | 10.50 (4.65) | 11.62 (4.99) | 1.12 | 0.35 | 0.554 | |
| RVIP False Alarms (#) | All | 68 | 8.57 (5.77) | 9.47 (10.14) | 0.90 | 68 | 8.52 (6.42) | 7.63 (5.70) | −0.89 | 0.02 | 0.895 |
| Optimal | 33 | 8.99 (7.55) | 8.19 (10.98) | −0.80 | 37 | 8.57 (6.34) | 7.68 (5.98) | −0.89 | 3.61 | 0.063 ^ | |
| Sub-Optimal | 35 | 8.17 (3.42) | 10.56 (9.43) | 2.39 | 31 | 8.46 (6.62) | 7.57 (5.53) | −0.89 | 2.28 | 0.137 | |
| RVIP Missed (#) | All | 68 | 21.87 (8.12) | 20.37 (8.69) | −1.50 | 68 | 20.91 (7.87) | 18.87 (8.24) | −2.04 | 0.28 | 0.600 |
| Optimal | 33 | 21.44 (9.18) | 19.40 (9.64) | −2.04 | 37 | 20.90 (7.55) | 18.76 (7.57) | −2.14 | 0.21 | 0.651 | |
| Sub-Optimal | 35 | 22.46 (7.08) | 21.20 (7.88) | −1.26 | 31 | 20.92 (8.35) | 18.98 (8.97) | −1.94 | 0.07 | 0.793 | |
| Active | Placebo | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Task | Subgroup | n | Baseline M (SD) | 12 Weeks M (SD) | Δ | n | Baseline M (SD) | 12 Weeks M (SD) | Δ | F | p |
| Perceived Stress Scale | All | 70 | 11.39 (5.82) | 11.98 (6.45) | 0.59 | 71 | 11.35 (5.93) | 11.23 (6.15) | −0.12 | 0.26 | 0.611 |
| Optimal | 35 | 10.77 (6.15) | 10.89 (5.70) | 0.12 | 39 | 10.85 (6.12) | 11.10 (6.25) | 0.25 | 0.33 | 0.569 | |
| Sub-Optimal | 35 | 12.00 (5.50) | 13.07 (7.05) | 1.07 | 32 | 11.97 (5.73) | 11.38 (6.15) | −0.59 | 1.22 | 0.273 | |
| DASS Total | All | 70 | 6.27 (6.79) | 6.93 (6.80) | 0.66 | 71 | 5.96 (5.47) | 5.28 (6.80) | −0.68 | 0.81 | 0.371 |
| Optimal | 35 | 5.97 (6.69) | 5.93 (6.93) | −0.04 | 39 | 5.90 (5.88) | 4.48 (4.25) | −1.42 | 0.01 | 0.927 | |
| Sub-Optimal | 35 | 6.57 (6.98) | 7.93 (6.65) | 1.36 | 32 | 6.03 (5.02) | 6.14 (8.75) | 0.11 | 1.36 | 0.247 | |
| DASS Stress | All | 70 | 3.26 (3.19) | 3.55 (3.42) | 0.29 | 71 | 3.00 (2.69) | 2.62 (3.06) | −0.38 | 0.96 | 0.329 |
| Optimal | 35 | 3.00 (3.08) | 2.68 (3.14) | −0.32 | 39 | 3.03 (2.84) | 2.29 (2.19) | −0.74 | 0.01 | 0.961 | |
| Sub-Optimal | 35 | 3.51 (3.33) | 4.43 (3.51) | 0.92 | 32 | 2.97 (2.53) | 2.97 (3.79) | 0.00 | 2.04 | 0.159 | |
| DASS Anxiety | All | 70 | 1.06 (1.65) | 1.45 (1.72) | 0.39 | 71 | 0.85 (1.67) | 0.88 (1.67) | 0.03 | 0.17 | 0.685 |
| Optimal | 35 | 1.14 (1.65) | 1.50 (1.93) | 0.36 | 39 | 1.05 (1.99) | 0.84 (1.13) | −0.21 | 0.06 | 0.816 | |
| Sub-Optimal | 35 | 0.97 (1.67) | 1.39 (1.50) | 0.42 | 32 | 0.59 (1.16) | 0.93 (2.12) | 0.34 | 0.07 | 0.796 | |
| DASS Depression | All | 70 | 1.96 (2.88) | 1.93 (2.88) | −0.03 | 71 | 2.11 (2.46) | 1.78 (2.92) | −0.33 | 0.31 | 0.582 |
| Optimal | 35 | 1.83 (2.77) | 1.75 (3.09) | −0.08 | 39 | 1.82 (2.14) | 1.35 (2.04) | −0.47 | 0.01 | 0.973 | |
| Sub-Optimal | 35 | 2.09 (3.02) | 1.39 (1.50) | −0.70 | 32 | 2.47 (2.81) | 2.24 (3.62) | −0.23 | 0.84 | 0.362 | |
| POMS Total Mood Disturbance | All | 70 | 7.46 (25.09) | 5.38 (24.26) | −2.08 | 71 | 3.58 (21.30) | −0.87 (18.07) | −4.45 | 0.00 | 0.999 |
| Optimal | 35 | 3.57 (23.63) | −1.18 (22.28) | −4.75 | 39 | 1.69 (23.96) | −3.03 (17.60) | −4.72 | 1.18 | 0.282 | |
| Sub-Optimal | 35 | 11.34 (26.23) | 11.93 (24.77) | 0.59 | 32 | 5.88 (17.64) | 1.45 (18.58) | −4.43 | 1.08 | 0.303 | |
| STAI-S (State Anxiety) | All | 70 | 27.87 (7.11) | 27.84 (8.09) | −0.03 | 71 | 28.46 (6.40) | 29.62 (8.55) | 1.16 | 0.91 | 0.341 |
| Optimal | 35 | 28.74 (8.20) | 26.14 (6.91) | −2.60 | 39 | 28.69 (7.35) | 29.68 (8.41) | 0.99 | 6.12 | 0.016 * | |
| Sub-Optimal | 35 | 27.00 (5.80) | 29.54 (8.93) | 2.54 | 32 | 28.19 (5.13) | 29.55 (8.84) | 1.36 | 0.68 | 0.412 | |
| Bond Lader Alertness | All | 70 | 70.87 (16.09) | 71.17 (15.05) | 0.30 | 71 | 71.23 (14.72) | 73.06 (14.65) | 1.83 | 0.00 | 0.989 |
| Optimal | 35 | 70.66 (16.92) | 73.08 (14.26) | 2.42 | 39 | 69.09 (16.36) | 71.43 (14.28) | 2.34 | 0.19 | 0.668 | |
| Sub-Optimal | 35 | 71.07 (15.46) | 69.26 (15.83) | −1.81 | 32 | 73.86 (12.18) | 74.80 (15.08) | 0.94 | 0.06 | 0.806 | |
| Bond Lader Calmness | All | 70 | 75.42 (14.09) | 71.86 (16.60) | −3.56 | 71 | 72.18 (13.57) | 73.10 (15.16) | 0.92 | 2.04 | 0.155 |
| Optimal | 35 | 75.24 (15.27) | 75.98 (15.45) | 0.74 | 39 | 69.62 (13.45) | 72.50 (13.74) | 2.88 | 0.37 | 0.548 | |
| Sub-Optimal | 35 | 75.60 (13.02) | 67.73 (16.94) | −7.87 | 32 | 75.30 (13.27) | 73.74 (16.76) | −1.56 | 1.76 | 0.325 | |
| Bond Lader Contentedness | All | 70 | 78.03 (15.24) | 76.31 (13.81) | −1.72 | 71 | 77.41 (12.83) | 74.95 (14.79) | −2.46 | 0.57 | 0.452 |
| Optimal | 35 | 77.54 (16.25) | 77.33 (14.09) | −0.21 | 39 | 75.64 (13.60) | 74.29 (14.30) | −1.35 | 0.46 | 0.498 | |
| Sub-Optimal | 35 | 78.53 (14.38) | 75.29 (13.71) | −3.24 | 32 | 79.58 (11.68) | 75.66 (15.52) | −3.92 | 0.19 | 0.665 | |
| Bond Lader Stress | All | 70 | 20.94 (21.39) | 21.41 (18.77) | 0.47 | 71 | 19.25 (16.52) | 25.25 (19.87) | 6.00 | 1.94 | 0.166 |
| Optimal | 35 | 21.17 (21.61) | 18.93 (17.66) | −2.24 | 39 | 22.95 (18.55) | 27.19 (17.82) | 4.24 | 1.57 | 0.215 | |
| Sub-Optimal | 35 | 20.71 (21.47) | 23.89 (19.84) | 3.18 | 32 | 14.75 (12.50) | 23.17 (21.98) | 8.42 | 0.51 | 0.477 | |
| Bond Lader Anxiety | All | 70 | 16.94 (15.37) | 21.36 (19.37) | 4.42 | 71 | 20.35 (18.48) | 24.07 (20.10) | 3.72 | 0.04 | 0.847 |
| Optimal | 35 | 18.37 (17.03) | 16.86 (17.78) | −1.51 | 39 | 23.95 (19.93) | 26.55 (18.35) | 2.60 | 2.85 | 0.097 ^ | |
| Sub-Optimal | 35 | 15.51 (13.60) | 25.86 (20.15) | 10.35 | 32 | 15.97 (15.75) | 21.41 (21.82) | 5.44 | 1.25 | 0.269 | |
| Bond Lader Mental Fatigue | All | 70 | 31.71 (24.10) | 27.84 (19.72) | −3.87 | 71 | 28.83 (21.79) | 28.70 (19.57) | −0.13 | 1.04 | 0.311 |
| Optimal | 35 | 33.91 (25.70) | 24.29 (17.39) | −9.62 | 39 | 31.46 (23.61) | 31.74 (18.96) | 0.28 | 2.89 | 0.094 ^ | |
| Sub-Optimal | 35 | 29.51 (22.55) | 31.39 (21.52) | 1.88 | 32 | 25.63 (19.23) | 25.45 (20.02) | −0.18 | 0.04 | 0.848 | |
| Bond Lader Concentration | All | 70 | 67.80 (19.62) | 65.95 (18.13) | −1.85 | 71 | 69.96 (18.48) | 69.73 (15.94) | −0.23 | 0.04 | 0.847 |
| Optimal | 35 | 65.57 (20.33) | 66.25 (17.94) | 0.68 | 39 | 67.41 (20.39) | 67.71 (15.55) | 0.30 | 0.32 | 0.574 | |
| Sub-Optimal | 35 | 70.03 (18.92) | 65.64 (18.64) | −4.39 | 32 | 73.06 (15.61) | 71.90 (16.35) | −1.16 | 0.03 | 0.862 | |
| Bond Lader Mental Stamina | All | 70 | 68.30 (19.04) | 65.23 (18.09) | −3.07 | 71 | 68.59 (18.16) | 69.28 (15.69) | 0.69 | 0.45 | 0.506 |
| Optimal | 35 | 66.66 (20.43) | 67.04 (18.28) | 0.38 | 39 | 65.69 (19.63) | 67.77 (14.85) | 2.08 | 0.01 | 0.935 | |
| Sub-Optimal | 35 | 69.94 (17.68) | 63.43 (18.05) | −6.51 | 32 | 72.13 (15.78) | 70.90 (16.65) | −1.23 | 0.53 | 0.469 | |
| Active | Placebo | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Measure | Subgroup | n | Baseline M (SD) | 12 Weeks M (SD) | Δ | n | Baseline M (SD) | 12 Weeks M (SD) | Δ | F | p |
| Vitamin B1 (nmol/L) | All | 49 | 126.22 (36.85) | 175.72 (52.03) | 49.5 | 45 | 127.00 (30.56) | 130.08 (50.86) | 3.08 | 17.59 | 0.000 * |
| Optimal | 25 | 126.68 (32.16) | 183.63 (56.08) | 56.95 | 27 | 129.70 (33.26) | 140.28 (64.21) | 10.58 | 4.90 | 0.031 * | |
| Sub-Optimal | 24 | 125.75 (41.87) | 169.46 (48.88) | 43.71 | 18 | 122.94 (26.39) | 120.27 (31.79) | −2.67 | 20.59 | 0.002 * | |
| Vitamin B2 (nmol/L) | All | 69 | 233.91 (38.68) | 282.30 (46.00) | 48.39 | 66 | 232.79 (46.82) | 261.05 (47.56) | 28.26 | 3.13 | 0.079 ^ |
| Optimal | 35 | 231.29 (36.73) | 286.96 (42.94) | 55.67 | 35 | 230.00 (33.38) | 263.79 (55.76) | 33.79 | 3.34 | 0.072 ^ | |
| Sub-Optimal | 34 | 236.62 (40.97) | 277.96 (49.05) | 41.34 | 31 | 235.94 (58.88) | 258.11 (37.67) | 22.17 | 0.48 | 0.490 | |
| Vitamin B6 (nmol/L) | All | 69 | 114.06 (108.10) | 608.71 (250.14) | 494.65 | 70 | 119.60 (128.73) | 121.24 (139.91) | 1.64 | 181.00 | 0.000 * |
| Optimal | 35 | 137.29 (145.09) | 612.21 (278.16) | 474.92 | 38 | 139.79 (164.18) | 153.96 (190.24) | 14.17 | 42.60 | 0.000 * | |
| Sub-Optimal | 34 | 90.15 (35.09) | 605.96 (231.70) | 515.81 | 32 | 95.63 (60.15) | 89.77 (47.99) | −5.86 | 263.95 | 0.000 * | |
| Vitamin B12 (pmol/L) | All | 69 | 329.33 (99.74) | 510.57 (195.90) | 181.24 | 70 | 312.96 (126.89) | 307.73 (140.82) | −5.23 | 73.54 | 0.000 * |
| Optimal | 35 | 316.94 (90.03) | 510.50 (185.34) | 193.56 | 39 | 299.79 (124.34) | 299.33 (86.87) | −0.46 | 39.86 | 0.000 * | |
| Sub-Optimal | 34 | 342.09 (108.71) | 510.64 (208.62) | 168.55 | 31 | 329.52 (130.15) | 316.13 (180.70) | −13.39 | 34.09 | 0.000 * | |
| Homocysteine (μmol/L) | All | 69 | 9.82 (2.55) | 9.68 (2.93) | −0.14 | 70 | 10.73 (3.05) | 10.55 (2.97) | −0.18 | 0.00 | 0.964 |
| Optimal | 35 | 9.41 (2.37) | 9.70 (2.77) | 0.29 | 39 | 10.58 (2.81) | 10.73 (2.72) | 0.15 | 0.00 | 0.938 | |
| Sub-Optimal | 34 | 10.25 (2.69) | 9.67 (3.14) | −0.58 | 31 | 10.92 (3.36) | 10.37 (3.26) | −0.55 | 0.00 | 0.982 | |
| All | 66 | 1.02 (0.09) | 0.98 (0.10) | −0.04 | 69 | 1.01 (0.9) | 1.00 (0.10) | −0.01 | 0.10 | 0.754 | |
| High-sensitivity C-reactive protein (mg/L) | Optimal | 34 | 0.95 (0.13) | 0.97 (0.14) | 0.02 | 39 | 0.98 (0.12) | 0.93 (0.13) | −0.05 | 0.25 | 0.620 |
| Sub-Optimal | 32 | 1.06(0.13) | 0.95 (0.14) | −0.11 | 30 | 1.09(0.14) | 1.12(0.14) | 0.03 | 0.54 | 0.467 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Young, L.M.; Gauci, S.; Arnoldy, L.; Martin, L.; Perry, N.; White, D.J.; Meyer, D.; Lassemillante, A.-C.; Ogden, E.; Silber, B.; et al. Investigating the Effects of a Multinutrient Supplement on Cognition, Mood and Biochemical Markers in Middle-Aged Adults with ‘Optimal’ and ‘Sub-Optimal’ Diets: A Randomized Double Blind Placebo Controlled Trial. Nutrients 2022, 14, 5079. https://doi.org/10.3390/nu14235079
Young LM, Gauci S, Arnoldy L, Martin L, Perry N, White DJ, Meyer D, Lassemillante A-C, Ogden E, Silber B, et al. Investigating the Effects of a Multinutrient Supplement on Cognition, Mood and Biochemical Markers in Middle-Aged Adults with ‘Optimal’ and ‘Sub-Optimal’ Diets: A Randomized Double Blind Placebo Controlled Trial. Nutrients. 2022; 14(23):5079. https://doi.org/10.3390/nu14235079
Chicago/Turabian StyleYoung, Lauren M., Sarah Gauci, Lizanne Arnoldy, Laura Martin, Naomi Perry, David J. White, Denny Meyer, Annie-Claude Lassemillante, Edward Ogden, Beata Silber, and et al. 2022. "Investigating the Effects of a Multinutrient Supplement on Cognition, Mood and Biochemical Markers in Middle-Aged Adults with ‘Optimal’ and ‘Sub-Optimal’ Diets: A Randomized Double Blind Placebo Controlled Trial" Nutrients 14, no. 23: 5079. https://doi.org/10.3390/nu14235079
APA StyleYoung, L. M., Gauci, S., Arnoldy, L., Martin, L., Perry, N., White, D. J., Meyer, D., Lassemillante, A.-C., Ogden, E., Silber, B., Scholey, A., & Pipingas, A. (2022). Investigating the Effects of a Multinutrient Supplement on Cognition, Mood and Biochemical Markers in Middle-Aged Adults with ‘Optimal’ and ‘Sub-Optimal’ Diets: A Randomized Double Blind Placebo Controlled Trial. Nutrients, 14(23), 5079. https://doi.org/10.3390/nu14235079






