1–2 Drinks Per Day Affect Lipoprotein Composition after 3 Weeks—Results from a Cross-Over Pilot Intervention Trial in Healthy Adults Using Nuclear Magnetic Resonance-Measured Lipoproteins and Apolipoproteins
Abstract
1. Introduction
2. Materials and Methods
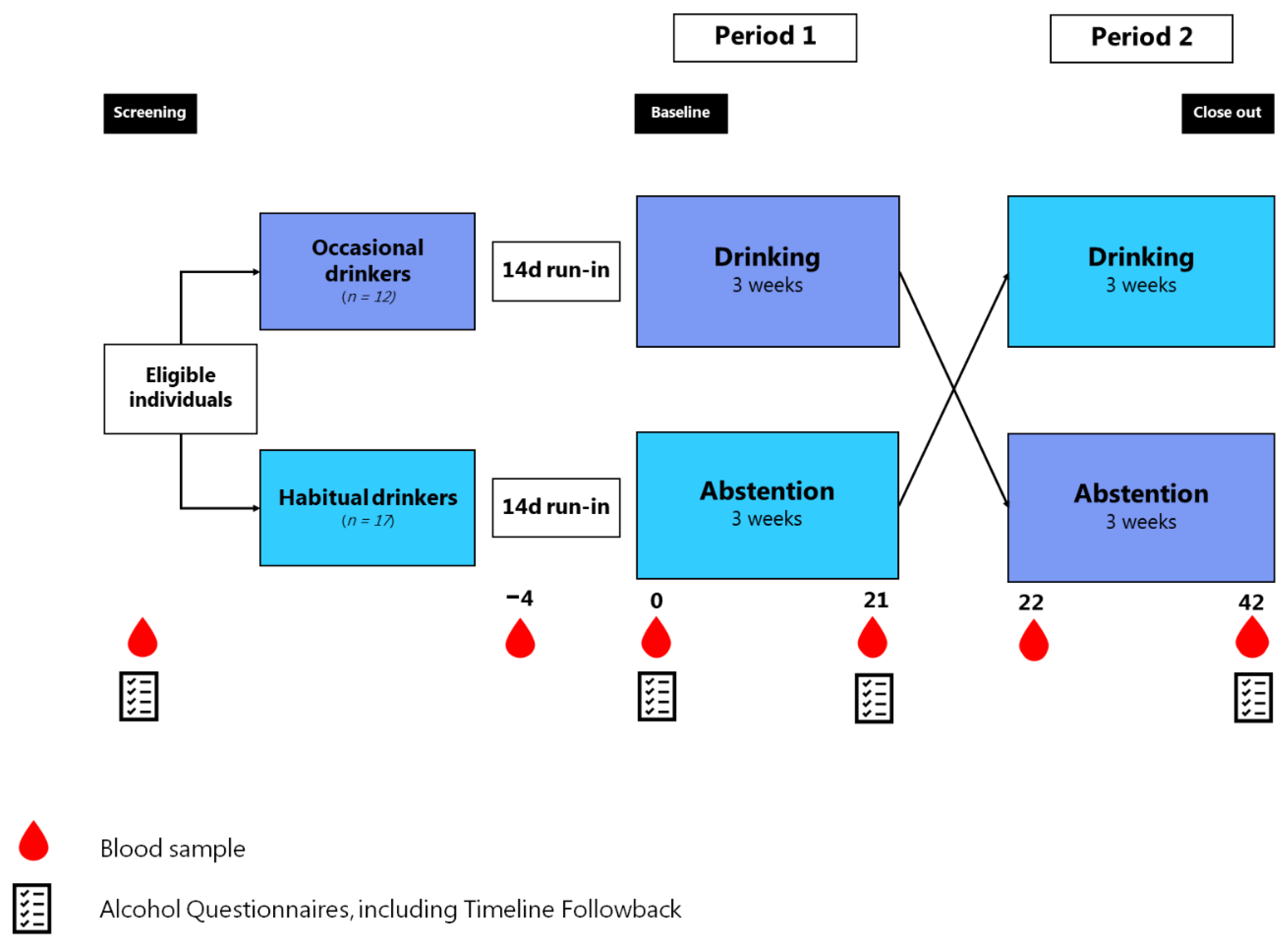
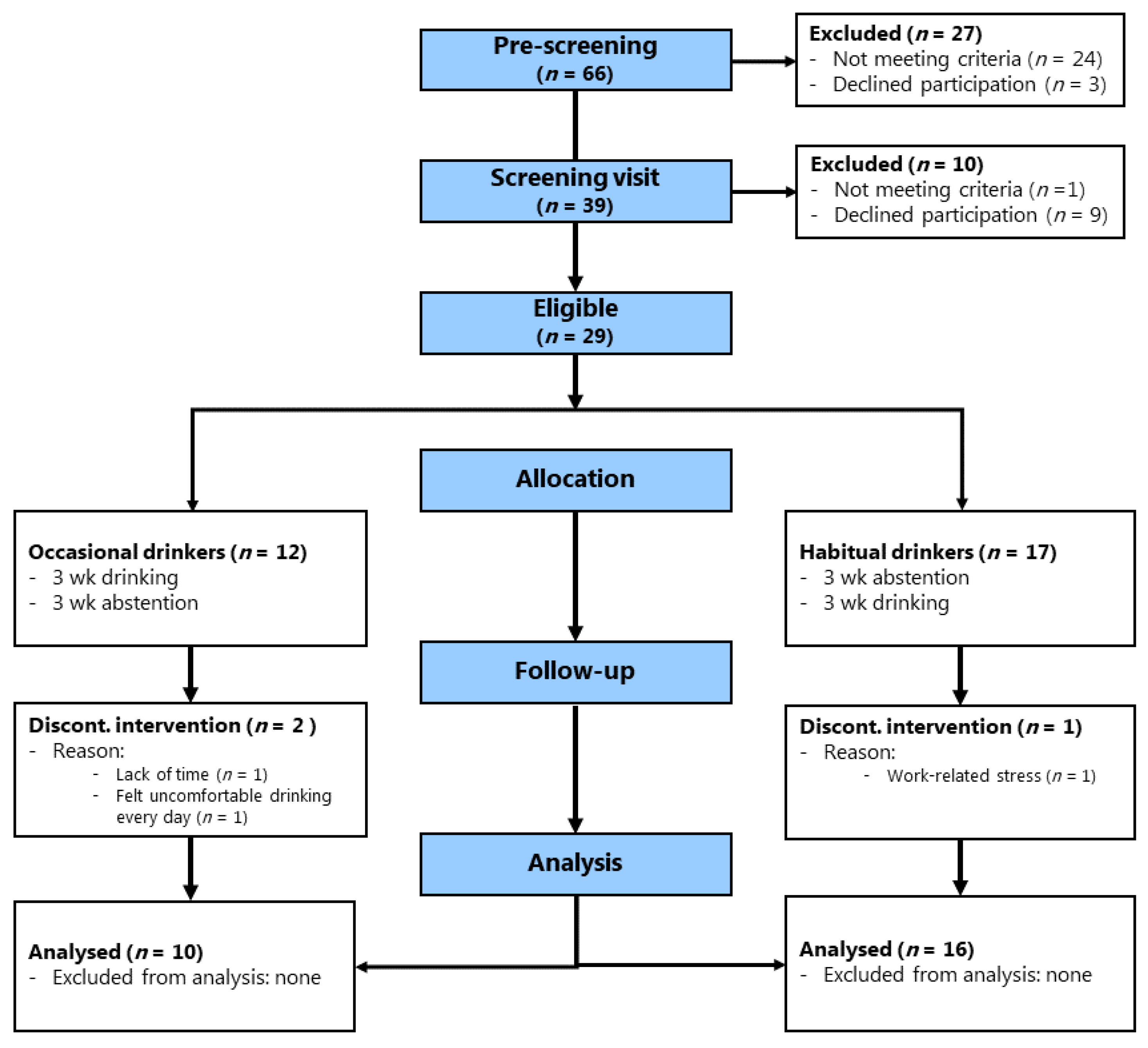
2.1. Study Design and Participants
2.2. Eligibility Criteria
2.3. Study Procedures
2.4. Intervention
2.5. Analysis of Lipids and Lipoproteins Using 1H NMR Spectroscopy
Quantification of Lipids and Lipoproteins Based on 1H NMR Spectra
2.6. Statistical Methods
Sample Size
3. Results
3.1. Baseline Characteristics
3.2. Compliance and Adverse Events
3.3. Whole Plasma Lipids
3.4. Lipoproteins and Lipoprotein Subfractions
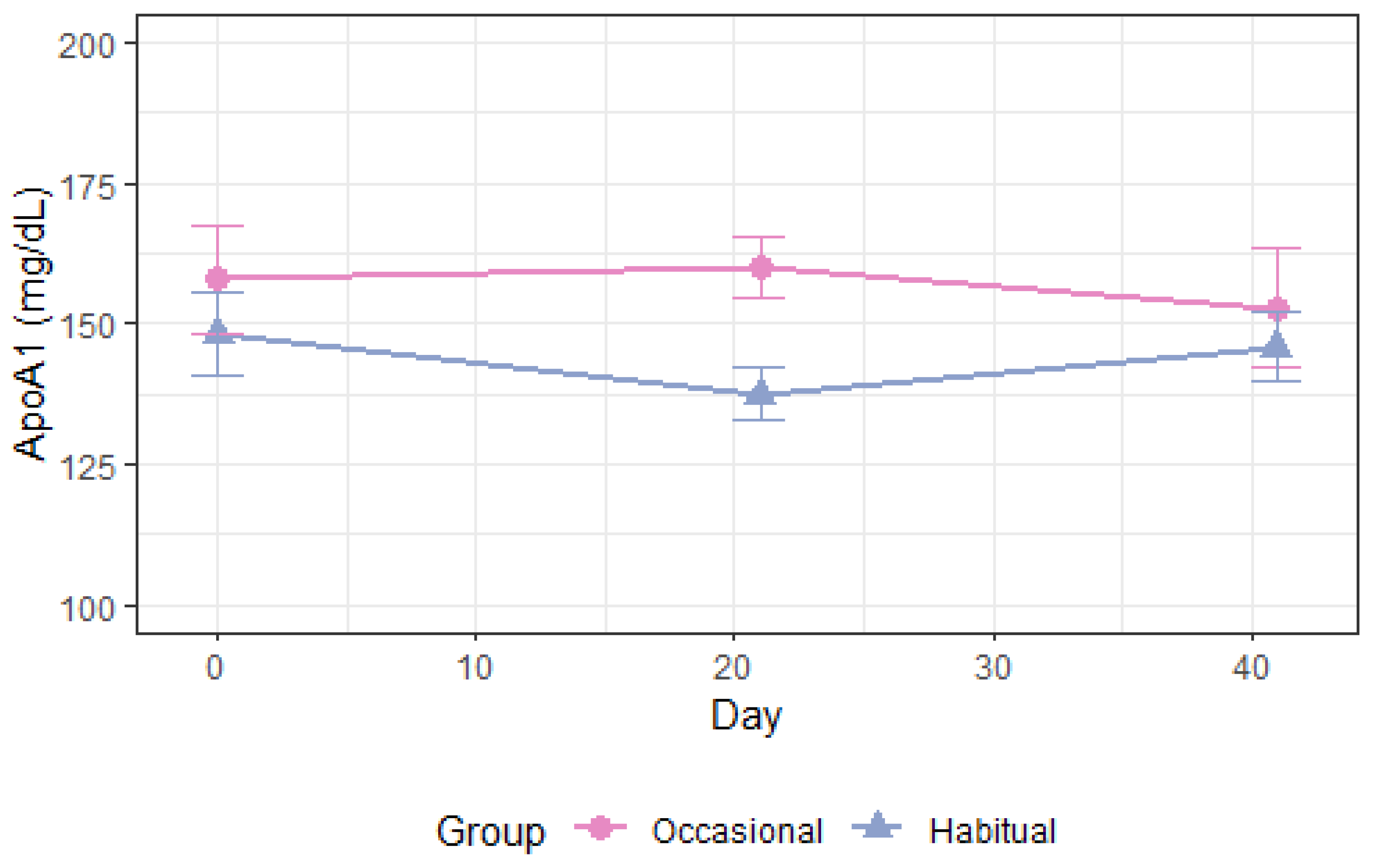
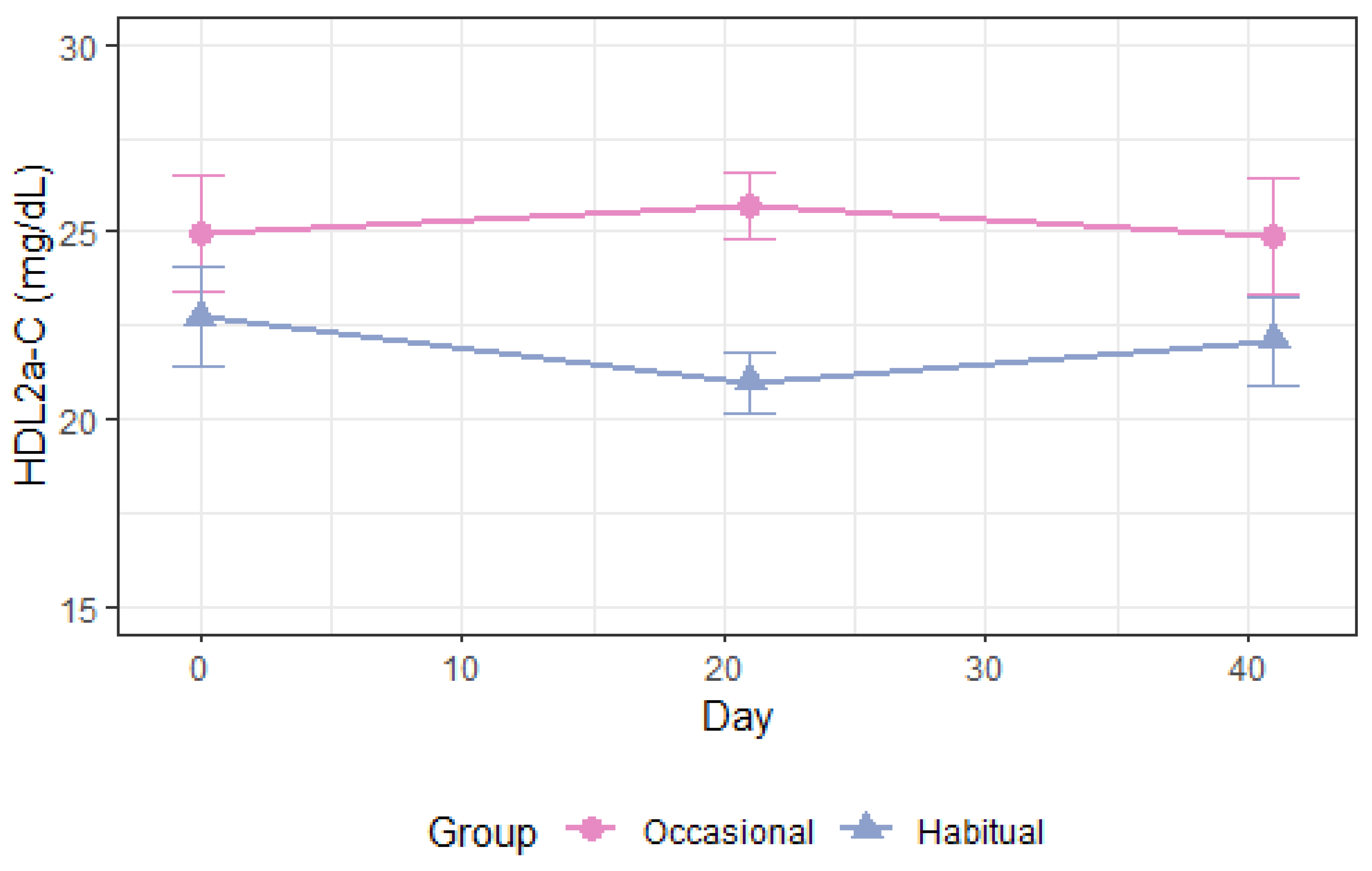
3.4.1. Apolipoprotein A1 and High-Density Lipoprotein
3.4.2. Apolipoprotein B and Apolipoprotein B-Containing Lipoproteins
3.4.3. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Maclure, M. Demonstration of Deductive Meta-Analysis: Ethanol Intake and Risk of Myocardial Infarction. Epidemiol. Rev. 1993, 15, 328–351. [Google Scholar] [CrossRef] [PubMed]
- Ronksley, P.E.; Brien, S.E.; Turner, B.J.; Mukamal, K.J.; Ghali, W. A Association of Alcohol Consumption with Selected Cardiovascular Disease Outcomes: A Systematic Review and Meta-Analysis. BMJ 2011, 342, d671. [Google Scholar] [CrossRef] [PubMed]
- Corrao, G.; Rubbiati, L.; Bagnardi, V.; Zambon, A.; Poikolainen, K. Alcohol and Coronary Heart Disease: A Meta-Analysis. Addiction 2000, 95, 1505–1523. [Google Scholar] [CrossRef]
- Reynolds, K.; Lewis, B.; Nolen, J.D.L.; Kinney, G.L.; Sathya, B.; He, J. Alcohol Consumption and Risk of Stroke. JAMA 2003, 289, 579. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, F.; Zhou, Y.; He, J. Association between Alcohol Consumption and the Risk of Incident Type 2 Diabetes: A Systematic Review and Dose-Response Meta-Analysis 1. Am. J. Clin. Nutr. 2016, 103, 818–829. [Google Scholar] [CrossRef]
- Sun, K.; Ren, M.; Liu, D.; Wang, C.; Yang, C.; Yan, L. Alcohol Consumption and Risk of Metabolic Syndrome: A Meta-Analysis of Prospective Studies. Clin. Nutr. 2014, 33, 596–602. [Google Scholar] [CrossRef]
- Di Castelnuovo, A.; Costanzo, S.; Bagnardi, V.; Donati, M.B.; Iacoviello, L.; De Gaetano, G. Alcohol Dosing and Total Mortality in Men and Women. Arch Int. Med. 2006, 166, 2437–2445. [Google Scholar] [CrossRef] [PubMed]
- International Alliance for Responsible Drinking (IARD). Drinking Guidelines: General Population. Available online: https://iard.org/science-resources/detail/Drinking-Guidelines-General-Population (accessed on 12 December 2020).
- Brien, S.E.; Ronksley, P.E.; Turner, B.J.; Mukamal, K.J.; Ghali, W.A. Effect of Alcohol Consumption on Biological Markers Associated with Risk of Coronary Heart Disease: Systematic Review and Meta-Analysis of Interventional Studies. BMJ 2011, 342, d636. [Google Scholar] [CrossRef]
- Gepner, Y.; Golan, R.; Harman-Boehm, I.; Henkin, Y.; Schwarzfuchs, D.; Shelef, I.; Durst, R.; Kovsan, J.; Bolotin, A.; Leitersdorf, E.; et al. Effects of Initiating Moderate Alcohol Intake on Cardiometabolic Risk in Adults with Type 2 Diabetes: A 2-Year Randomized, Controlled Trial. Ann. Int. Med. 2015, 163, 569–579. [Google Scholar] [CrossRef]
- Pownall, H.J.; Rosales, C.; Gillard, B.K.; Gotto, A.M. Alcohol: A Nutrient with Multiple Salutary Effects. Nutrients 2015, 7, 1992–2000. [Google Scholar] [CrossRef]
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Bairey Merz, C.N.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.M.; et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2889–2934. [Google Scholar] [CrossRef] [PubMed]
- Rimm, E.B.; Williams, P.; Fosher, K.; Criqui, M.; Stampfer, M.J. Moderate Alcohol Intake and Lower Risk of Coronary Heart Disease: Meta-Analysis of Effects on Lipids and Haemostatic Factors. BMJ 1999, 319, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, Y.; Zheng, S.; Yang, X.; Wang, T.; Zeng, J. Moderate Alcohol Consumption and Atherosclerosis: Meta-Analysis of Effects on Lipids and Inflammation. Wien. Klin. Wochenschr. 2017, 129, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Spiegelman, D.; Lovato, L.C.; Khudyakov, P.; Wilkens, T.L.; Adebamowo, C.A.; Adebamowo, S.N.; Appel, L.J.; Beulens, J.W.J.; Coughlin, J.W.; Dragsted, L.O.; et al. The Moderate Alcohol and Cardiovascular Health Trial (MACH15): Design and Methods for a Randomized Trial of Moderate Alcohol Consumption and Cardiometabolic Risk. Eur. J. Prev. Cardiol. 2020, 27, 1967–1982. [Google Scholar] [CrossRef] [PubMed]
- Tall, A.R.; Rader, D.J. Trials and Tribulations of CETP Inhibitors. Circ. Res. 2018, 122, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Superko, H.R.; Pendyala, L.; Williams, P.T.; Momary, K.M.; King, S.B.; Garrett, B.C. High-Density Lipoprotein Subclasses and Their Relationship to Cardiovascular Disease. J. Clin. Lipidol. 2012, 6, 496–523. [Google Scholar] [CrossRef] [PubMed]
- Hoogeveen, R.C.; Gaubatz, J.W.; Sun, W.; Dodge, R.C.; Crosby, J.R.; Jiang, J.; Couper, D.; Virani, S.S.; Kathiresan, S.; Boerwinkle, E.; et al. Small Dense Low-Density Lipoprotein-Cholesterol Concentrations Predict Risk for Coronary Heart Disease: The Atherosclerosis Risk In Communities (ARIC) Study. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Camont, L.; Chapman, M.J.; Kontush, A. Biological Activities of HDL Subpopulations and Their Relevance to Cardiovascular Disease. Trends Mol. Med. 2011, 17, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Aru, V.; Lam, C.; Khakimov, B.; Hoefsloot, H.C.J.; Zwanenburg, G.; Lind, M.V.; Schäfer, H.; van Duynhoven, J.; Jacobs, D.M.; Smilde, A.K.; et al. Quantification of Lipoprotein Profiles by Nuclear Magnetic Resonance Spectroscopy and Multivariate Data Analysis. TrAC—Trends Anal. Chem. 2017, 94, 210–219. [Google Scholar] [CrossRef]
- Flores-Guerrero, J.L.; Gruppen, E.G.; Connelly, M.A.; Shalaurova, I.; Otvos, J.D.; Garcia, E.; Bakker, S.J.L.; Dullaart, R.P.F. A Newly Developed Diabetes Risk Index, Based on Lipoprotein Subfractions and Branched Chain Amino Acids, Is Associated with Incident Type 2 Diabetes Mellitus in the PREVEND Cohort. J. Clin. Med. 2020, 9, 2781. [Google Scholar] [CrossRef]
- Rowland, C.M.; Shiffman, D.; Caulfield, M.; Garcia, V.; Melander, O.; Hastie, T. Association of Cardiovascular Events and Lipoprotein Particle Size: Development of a Risk Score Based on Functional Data Analysis. PLoS ONE 2019, 14, 1–16. [Google Scholar] [CrossRef]
- Muth, N.D.; Laughlin, G.A.; von Mühlen, D.; Smith, S.C.; Barrett-Connor, E. High-Density Lipoprotein Subclasses Are a Potential Intermediary between Alcohol Intake and Reduced Risk of Cardiovascular Disease: The Rancho Bernardo Study. Br. J. Nutr. 2010, 104, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Parlesak, A.; Eckoldt, J.; Winkler, K.; Bode, C.J.; Schäfer, C. Intercorrelations of Lipoprotein Subfractions and Their Covariation with Lifestyle Factors in Healthy Men. J. Clin. Biochem. Nutr. 2014, 54, 174–180. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haskell, W.L.; Camargo, C.; Williams, P.T.; Vranizan, K.M.; Krauss, R.M.; Lindgren, F.T.; Wood, P.D. The Effect of Cessation and Resumption of Moderate Alcohol Intake on Serum High-Density-Lipoprotein Subfractions. A Controlled Study. N. Engl. J. Med. 1984, 310, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Masarei, J.R.L.; Puddey, I.B.; Rouse, I.L.; Lynch, W.J.; Vandongen, R.; Benin, L.J. Effects of Alcohol Consumption on Serum Lipoprotein-Lipid and Apolipoprotein Concentrations. Results from an Intervention Study in Health Subjects. Atherosclerosis 1986, 60, 79–87. [Google Scholar] [CrossRef]
- Burr, M.L.; Fehily, A.M.; Butland, B.K.; Bolton, C.H.; Eastham, R.D. Alcohol and High-Density-Lipoprotein Cholesterol: A Randomized Controlled Trial. Br. J. Nutr. 1986, 56, 81–86. [Google Scholar] [CrossRef]
- Wilkens, T.L.; Tranæs, K.; Eriksen, J.N.; Dragsted, L.O. Moderate Alcohol Consumption and Lipoprotein Subfractions: A Systematic Review of Intervention and Observational Studies. Nutr. Rev. 2021. [Google Scholar] [CrossRef]
- Goist, K.C.; Sutker, P.B. Acute Alcohol Intoxication and Body Composition in Women and Men. Pharmacol. Biochem. Behav. 1985, 22, 811–814. [Google Scholar] [CrossRef]
- Sobell, L.C.; Sobell, M.B. Self-Report Issues in Alcohol Abuse: State of the Art and Future Directions. Behav. Assess. 1990, 12, 77–90. [Google Scholar]
- Monsonis Centelles, S.; Hoefsloot, H.C.J.; Khakimov, B.; Ebrahimi, P.; Lind, M.V.; Kristensen, M.; De Roo, N.; Jacobs, D.M.; Van Duynhoven, J.; Cannet, C.; et al. Toward Reliable Lipoprotein Particle Predictions from NMR Spectra of Human Blood: An Interlaboratory Ring Test. Anal. Chem. 2017, 89, 8004–8012. [Google Scholar] [CrossRef]
- Dona, A.C.; Jiménez, B.; Schafer, H.; Humpfer, E.; Spraul, M.; Lewis, M.R.; Pearce, J.T.M.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Precision High-Throughput Proton NMR Spectroscopy of Human Urine, Serum, and Plasma for Large-Scale Metabolic Phenotyping. Anal. Chem. 2014, 86, 9887–9894. [Google Scholar] [CrossRef] [PubMed]
- Khakimov, B.; Mobaraki, N.; Trimigno, A.; Aru, V.; Engelsen, S.B. Signature Mapping (SigMa): An Efficient Approach for Processing Complex Human Urine 1H NMR Metabolomics Data. Anal. Chim. Acta 2020, 1108, 142–151. [Google Scholar] [CrossRef]
- Akoka, S.; Barantin, L.; Trierweiler, M. Concentration Measurement by Proton NMR Using the ERETIC Method. Anal. Chem. 1999, 71, 2554–2557. [Google Scholar] [CrossRef] [PubMed]
- Savorani, F.; Tomasi, G.; Engelsen, S.B. Icoshift: A Versatile Tool for the Rapid Alignment of 1D NMR Spectra. J. Magn. Reson. 2010, 202, 190–202. [Google Scholar] [CrossRef]
- Khakimov, B.; Hoefsloot, H.C.J.; Mobaraki, N.; Aru, V.; Kristensen, M.; Lind, M.V.; Holm, L.; Castro-Mejía, J.L.; Nielsen, D.S.; Jacobs, D.M.; et al. Human Blood Lipoprotein Predictions from 1 H NMR Spectra: Protocol, Model Performances, and Cage of Covariance. Anal. Chem. 2022, 94, 628–636. [Google Scholar] [CrossRef]
- Petersen, M.; Dyrby, M.; Toubro, S.; Engelsen, S.B.; Nørgaard, L.; Pedersen, H.T.; Dyerberg, J. Quantification of Lipoprotein Subclasses by Proton Nuclear Magnetic Resonance–Based Partial Least-Squares Regression Models. Clin. Chem. 2005, 51, 1457–1461. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; Version 3.5.1; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biometrical, J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Kassambara, A. Ggpubr: “ggplot2” Based Publication Ready Plots. 2018. Available online: https://rpkgs.datanovia.com/ggpubr/ (accessed on 20 November 2022).
- Wickham, H.; François, R.; Henry, L.; Müller, K. Dplyr: A Grammar of Data Manipulation. 2018. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 20 November 2022).
- Schäfer, C.; Parlesak, A.; Eckoldt, J.; Bode, C.; Bode, J.C.; März, W.; Winkler, K. Beyond HDL-Cholesterol Increase: Phospholipid Enrichment and Shift from HDL3 to HDL2 in Alcohol Consumers. J. Lipid Res. 2007, 48, 1550–1558. [Google Scholar] [CrossRef]
- Nishiwaki, M.; Ishikawa, T.; Ito, T.; Shige, H.; Tomiyasu, K.; Nakajima, K.; Kondo, K.; Hashimoto, H.; Saitoh, K.; Manabe, M.; et al. Effects of Alcohol on Lipoprotein Lipase, Hepatic Lipase, Cholesteryl Ester Transfer Protein, and Lecithin:Cholesterol Acyltransferase in High-Density Lipoprotein Cholesterol Elevation. Atherosclerosis 1994, 111, 99–109. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira e Silva, E.R.; Foster, D.; Harper, M.M.; Seidman, C.E.; Smith, J.D.; Breslow, J.L.; Brinton, E.A. Alcohol Consumption Raises HDL Cholesterol Levels by Increasing the Transport Rate of Apolipoproteins A-I and A-II. Circulation 2000, 102, 2347–2352. [Google Scholar] [CrossRef]
- Hagiage, M.; Marti, C.; Rigaud, D.; Senault, C.; Fumeron, F.; Apfelbaum, M.; Girard-Globa, A. Effect of a Moderate Alcohol Intake on the Lipoproteins of Normotriglyceridemic Obese Subjects Compared with Normoponderal Controls. Metabolism. 1992, 41, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Beulens, J.W.J.J.; Sierksma, A.; van Tol, A.; Fournier, N.; van Gent, T.; Paul, J.-L.; Hendriks, H.F.J.J. Moderate Alcohol Consumption Increases Cholesterol Efflux Mediated by ABCA1. J. Lipid Res. 2004, 45, 1716–1723. [Google Scholar] [CrossRef]
- Khera, A.V.; Demler, O.V.; Adelman, S.J.; Collins, H.L.; Glynn, R.J.; Ridker, P.M.; Rader, D.J.; Mora, S. Cholesterol Efflux Capacity, High-Density Lipoprotein Particle Number, and Incident Cardiovascular Events. Circulation 2017, 135, 2494–2504. [Google Scholar] [CrossRef] [PubMed]
- Saleheen, D.; Scott, R.; Javad, S.; Zhao, W.; Rodrigues, A.; Picataggi, A.; Lukmanova, D.; Mucksavage, M.L.; Luben, R.; Billheimer, J.; et al. Association of HDL Cholesterol Efflux Capacity with Incident Coronary Heart Disease Events: A Prospective Case-Control Study. Lancet Diabetes Endocrinol. 2015, 3, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. ApoA1 and ApoA1-Specific Self-Antibodies in Cardiovascular Disease. Lab. Investig. 2016, 96, 708–718. [Google Scholar] [CrossRef]
- Sacks, F.M.; Jensen, M.K. From High-Density Lipoprotein Cholesterol to Measurements of Function: Prospects for the Development of Tests for High-Density Lipoprotein Functionality in Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 487–499. [Google Scholar] [CrossRef]
- Amarasuriya, R.N.; Gupta, A.K.; Civen, M.; Horng, Y.-C.; Maeda, T.; Kashyap, M.L. Ethanol Stimulates Apolipoprotein A-I Secretion by Human Hepatocytes: Implications for a Mechanism for Atherosclerosis Protection. Metabolism 1992, 41, 827–832. [Google Scholar] [CrossRef]
- Dashti, N.; Franklin, F.A.; Abrahamson, D.R. Effect of Ethanol on the Synthesis and Secretion of ApoA-I- and ApoB-Containing Lipoproteins in HepG2 Cells. J. Lipid Res. 1996, 37, 810–824. [Google Scholar] [CrossRef]
- Khodja, Y.; Samuels, M.E. Ethanol-Mediated Upregulation of APOA1 Gene Expression in HepG2 Cells Is Independent of de Novo Lipid Biosynthesis. Lipids Health Dis. 2020, 19, 1–13. [Google Scholar] [CrossRef]
- Senault, C.; Betoulle, D.; Luc, G.; Hauw, P.; Rigaud, D.; Fumeron, F. Beneficial Effects of a Moderate Consumption of Red Wine on Cellular Cholesterol Efflux in Young Men. Nutr. Metab. Cardiovasc. Dis. 2000, 10, 63–69. [Google Scholar]
- Zheng, J.-S.; Yang, J.; Huang, T.; Hu, X.-J.; Luo, M.; Li, D. Effects of Chinese Liquors on Cardiovascular Disease Risk Factors in Healthy Young Humans. Sci. World J. 2012, 2012, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Voight, B.F.; Peloso, G.M.; Orho-Melander, M.; Frikke-Schmidt, R.; Barbalic, M.; Jensen, M.K.; Hindy, G.; Hólm, H.; Ding, E.L.; Johnson, T.; et al. Plasma HDL Cholesterol and Risk of Myocardial Infarction: A Mendelian Randomisation Study. Lancet 2012, 380, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.H.; Toth, P.P.; Lirette, S.T.; Griswold, M.E.; Massaro, J.M.; Martin, S.S.; Blaha, M.J.; Kulkarni, K.R.; Khokhar, A.A.; Correa, A.; et al. Association of High-Density Lipoprotein Subclasses and Incident Coronary Heart Disease: The Jackson Heart and Framingham Offspring Cohort Studies. Eur. J. Prev. Cardiol. 2016, 23, 41–49. [Google Scholar] [CrossRef]
- Tiozzo, E.; Gardener, H.; Hudson, B.I.; Dong, C.; Della-Morte, D.; Crisby, M.; Goldberg, R.B.; Elkind, M.S.V.; Cheung, Y.K.; Wright, C.B.; et al. High-Density Lipoprotein Subfractions and Carotid Plaque: The Northern Manhattan Study. Atherosclerosis 2014, 237, 163–168. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jensen, M.K.; Rimm, E.B.; Furtado, J.D.; Sacks, F.M. Apolipoprotein C-III as a Potential Modulator of the Association Between HDL-Cholesterol and Incident Coronary Heart Disease. J. Am. Heart Assoc. 2012, 1, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fournier, N.; Paul, J.L.; Atger, V.; Cogny, A.; Soni, T.; De La Llera-Moya, M.; Rothblat, G.; Moatti, N. HDL Phospholipid Content and Composition as a Major Factor Determining Cholesterol Efflux Capacity from Fu5AH Cells to Human Serum. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2685–2691. [Google Scholar] [CrossRef]
- Meikle, P.J.; Formosa, M.F.; Mellett, N.A.; Jayawardana, K.S.; Giles, C.; Bertovic, D.A.; Jennings, G.L.; Childs, W.; Reddy, M.; Carey, A.L.; et al. HDL Phospholipids, but Not Cholesterol Distinguish Acute Coronary Syndrome From Stable Coronary Artery Disease. J. Am. Heart Assoc. 2019, 8. [Google Scholar] [CrossRef]
- Karjalainen, M.K.; Holmes, M.V.; Wang, Q.; Anufrieva, O.; Kähönen, M.; Lehtimäki, T.; Havulinna, A.S.; Kristiansson, K.; Salomaa, V.; Perola, M.; et al. Apolipoprotein A-I Concentrations and Risk of Coronary Artery Disease: A Mendelian Randomization Study. Atherosclerosis 2020, 299, 56–63. [Google Scholar] [CrossRef]
- Mehta, A.; Shapiro, M.D. Apolipoproteins in Vascular Biology and Atherosclerotic Disease. Nat. Rev. Cardiol. 2021, 0123456789. [Google Scholar] [CrossRef]
- McQueen, M.J.; Hawken, S.; Wang, X.; Ounpuu, S.; Sniderman, A.; Probstfield, J.; Steyn, K.; Sanderson, J.E.; Hasani, M.; Volkova, E.; et al. Lipids, Lipoproteins, and Apolipoproteins as Risk Markers of Myocardial Infarction in 52 Countries (the INTERHEART Study): A Case-Control Study. Lancet 2008, 372, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Sniderman, A.D.; Thanassoulis, G.; Glavinovic, T.; Navar, A.M.; Pencina, M.; Catapano, A.; Ference, B.A. Apolipoprotein B Particles and Cardiovascular Disease: A Narrative Review. JAMA Cardiol. 2019, 1–9. [Google Scholar] [CrossRef]
- Jensen, M.K.; Aroner, S.A.; Mukamal, K.J.; Furtado, J.D.; Post, W.S.; Tsai, M.Y.; Tjønneland, A.; Polak, J.F.; Rimm, E.B.; Overvad, K.; et al. High-Density Lipoprotein Subspecies Defined by Presence of Apolipoprotein C-III and Incident Coronary Heart Disease in Four Cohorts. Circulation 2018, 137, 1364–1373. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Anderson, J.L.C.; Gruppen, E.G.; Lei, Y.; Bakker, S.J.L.; Dullaart, R.P.F.; Tietge, U.J.F. High-Density Lipoprotein Anti-Inflammatory Capacity and Incident Cardiovascular Events. Circulation 2021, 1935–1945. [Google Scholar] [CrossRef]
- Miller, M. Low-Density Lipoprotein Triglycerides. J. Am. Coll. Cardiol. 2018, 72, 170–172. [Google Scholar] [CrossRef] [PubMed]
- März, W.; Scharnagl, H.; Winkler, K.; Tiran, A.; Nauck, M.; Boehm, B.O.; Winkelmann, B.R. Low-Density Lipoprotein Triglycerides Associated with Low-Grade Systemic Inflammation, Adhesion Molecules, and Angiographic Coronary Artery Disease: The Ludwigshafen Risk and Cardiovascular Health Study. Circulation 2004, 110, 3068–3074. [Google Scholar] [CrossRef]
- Jin, J.-L.; Zhang, H.-W.; Cao, Y.-X.; Liu, H.-H.; Hua, Q.; Li, Y.-F.; Zhang, Y.; Guo, Y.-L.; Wu, N.-Q.; Zhu, C.-G.; et al. Long-Term Prognostic Utility of Low-Density Lipoprotein (LDL) Triglyceride in Real-World Patients with Coronary Artery Disease and Diabetes or Prediabetes. Cardiovasc. Diabetol. 2020, 19, 152. [Google Scholar] [CrossRef]
- Saeed, A.; Feofanova, E.V.; Yu, B.; Sun, W.; Virani, S.S.; Nambi, V.; Coresh, J.; Guild, C.S.; Boerwinkle, E.; Ballantyne, C.M.; et al. Remnant-Like Particle Cholesterol, Low-Density Lipoprotein Triglycerides, and Incident Cardiovascular Disease. J. Am. Coll. Cardiol. 2018, 72, 156–169. [Google Scholar] [CrossRef]
- Ding, X.-H.; Ye, P.; Wang, X.-N.; Cao, R.-H.; Yang, X.; Xiao, W.-K.; Zhang, Y.; Bai, Y.-Y.; Wu, H.-M. The Predictive Value of Baseline LDL-TG Level on Major Adverse Cardiovascular Events in a Followed up Cohort Population. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1060–1064. [Google Scholar]
- Albers, J.J.; Slee, A.; Fleg, J.L.; O’Brien, K.D.; Marcovina, S.M. Relationship of Baseline HDL Subclasses, Small Dense LDL and LDL Triglyceride to Cardiovascular Events in the AIM-HIGH Clinical Trial. Atherosclerosis 2016, 251, 454–459. [Google Scholar] [CrossRef][Green Version]
- Baliunas, D.O.; Taylor, B.J.; Irving, H.; Roerecke, M.; Patra, J.; Mohapatra, S.; Rehm, J. Alcohol as a Risk Factor for Type 2 Diabetes: A Systematic Review and Meta-Analysis. Diabetes Care 2009, 32, 2123–2132. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, A.-S.; Qamar, A.; Hutchins, K.; Crowley, M.J.; Batch, B.C.; Guyton, J.R. Triglycerides: Emerging Targets in Diabetes Care? Review of Moderate Hypertriglyceridemia in Diabetes. Curr. Diab. Rep. 2019, 19, 13. [Google Scholar] [CrossRef] [PubMed]
- Schrieks, I.C.; Heil, A.L.J.; Hendriks, H.F.J.; Mukamal, K.J.; Beulens, J.W.J. The Effect of Alcohol Consumption on Insulin Sensitivity and Glycemic Status: A Systematic Review and Meta-Analysis of Intervention Studies. Diabetes Care 2015, 38, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Dragsted, L.O.; Krath, B.; Haren GRVogel, U.B.; Vinggaard, A.M.; Loft, S.; Rasmussen, S.E.; Pedersen, A.; Sandstrom, B. Biological Effects of Fruit and Vegetables. Proc. Nutr. Soc. 2006, 65, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ravn-Haren, G.; Dragsted, L.O.; Buch-Andersen, T.; Jensen, E.N.; Németh-Balogh, M.; Paulovicsová, B.; Bergström, A.; Wilcks, A.; Licht, T.R.; Markowski, J.; et al. Intake of whole apples or clear apple juice has contrasting effects on plasma lipids in healthy volunteers. Eur. J. Nutr. 2013, 52, 1875–1889. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.; Petocz, P.; Duve, E.; Abbott, K.; Cassettari, T.; Blumfield, M.; Fayet-Moore, F. The Effect of Replacing Refined Grains with Whole Grains on Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis of Randomized Controlled Trials with GRADE Clinical Recommendation. J. Acad. Nutr. Diet. 2020, 120, 1859–1883.e31. [Google Scholar] [CrossRef]
- Conrad, M.; McNamara, P.; King, A. Alternative Substance Paradigm: Effectiveness of Beverage Blinding and Effects on Acute Alcohol Responses. Exp. Clin. Psychopharmacol. 2012, 20, 382–389. [Google Scholar] [CrossRef]
- Monošík, R.; Dragsted, L.O. Dried Urine Swabs as a Tool for Monitoring Metabolite Excretion. Bioanalysis 2018, 10, 1371–1381. [Google Scholar] [CrossRef]
| Type | Alcohol Volume % | Volume of One Drink (mL) | Alcohol Content (g) Δ | |
|---|---|---|---|---|
| Pilsner beer | 4.6 | 330 | 12 | |
| Strong beer | 7.2–8 | 250 | 14.2–15.8 | |
| Cider | 4.6 | 330 | 12 | |
| Red wine | 12–15 | 130 | 12.3–15.4 | |
| White wine | 12–15 | 130 | 12.3–15.4 | |
| Dessert wine | 17–22 | 80 | 10.7–13.9 | |
| Gin | 37–40 | 40 | 11.6–12.6 | |
| Whiskey | 40–46 | 40 | 12.6–14.5 | |
| Snaps | 40–46 | 40 | 12.6–14.5 |
| Demographic Characteristics | A Occasional Drinkers (n = 10) | B Habitual Drinkers (n = 16) | p B vs. A | C Habitual Drinkers—Women (n = 9) | p C vs. A |
|---|---|---|---|---|---|
| Age, median years (IQR) | 30 (22–59) | 32 (23–64) | 0.38 | 29 (23–62) | 0.90 |
| Female, n (%) | 10 (100) | 9 (56) | 0.02 | 9 (100) | NA |
| Alcohol intake 14 days run-in, mean drinks * (SD) | 5.4 ± 7.3 | 12.7 ± 8.6 | 0.03 | 10.8 ± 7.2 | 0.12 |
| Smoking status, n (%) | |||||
| 6 (60) | 9 (56) | 0.86 | 5 (56) | 0.84 |
| 4 (40) | 6 (38) | 0.90 | 3 (33) | 0.76 |
| 0 (0) | 1 (6) | 0.42 | 1 (11) | 0.28 |
| Weight, mean kg (SD) | 71 (±17) | 69 (±15) | 0.76 | 58 (±7) | 0.05 |
| Height, mean cm (SD) | 166 (±6) | 174 (±9) | 0.01 | 168 (±5) | 0.36 |
| BMI, mean kg/m2 (SD) | 26 (±5) | 22 (±3) | 0.10 | 21 (±2) | 0.01 |
| Waist circumference, mean cm (SD) | 97 (±31) | 83 (±10) | 0.21 | 77 (±5) | 0.08 |
| HDL-C, mean mg/dL (SD) | 62 (±15) | 57 (±20) | 0.53 | 63 (±20) | 0.86 |
| LDL-C, mean mg/dL (SD) | 91 (±18) | 90 (±22) | 0.92 | 76 (±16) | 0.08 |
| TG, mean mg/dL (SD) | 100 (±33) | 105 (±46) | 0.76 | 86 (±26) | 0.33 |
| TC, mean mg/dL (SD) | 183 (±28) | 179 (±33) | 0.73 | 165 (±33) | 0.24 |
| Systolic BP, mean mmHg (SD) | 108 (±9) | 117 (±12) | 0.07 | 112 (±10) | 0.44 |
| Diastolic BP, mean mmHg (SD) | 70 (±6) | 72 (±8) | 0.68 | 71 (±8) | 0.88 |
| Pulse, mean bpm (SD) | 75 (±10) | 67 (±9) | 0.07 | 70 (±9) | 0.29 |
| Period 1 | Period 2 | Period 1 + 2 | ||||
|---|---|---|---|---|---|---|
| ΔMean1 £ | p | ΔMean2 ¤ | p | ΔMean Drinking vs. Abstention * | p | |
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | ||||
| HDL-C, mg/dL Occ. vs. Hab. All | −4.8 (−13.1–3.5) | 0.26 | −3.1 (−11.3–5.2) | 0.46 | −3.9 (−9.8–1.9) | 0.19 |
| HDL2b-C, mg/dL Occ. vs. Hab. All | −1.6 (−7.2–4.1) | 0.59 | 2.3 (−3.4–7.9) | 0.43 | 0.4 (−3.6–4.4) | 0.86 |
| HDL2a-C, mg/dL Occ. vs. Hab. All | −2.7 (−5.0–[−0.5]) | 0.02 | −1.9 (−4.1–0.3) | 0.09 | −2.3 (−3.9–[−0.7]) | 0.004 |
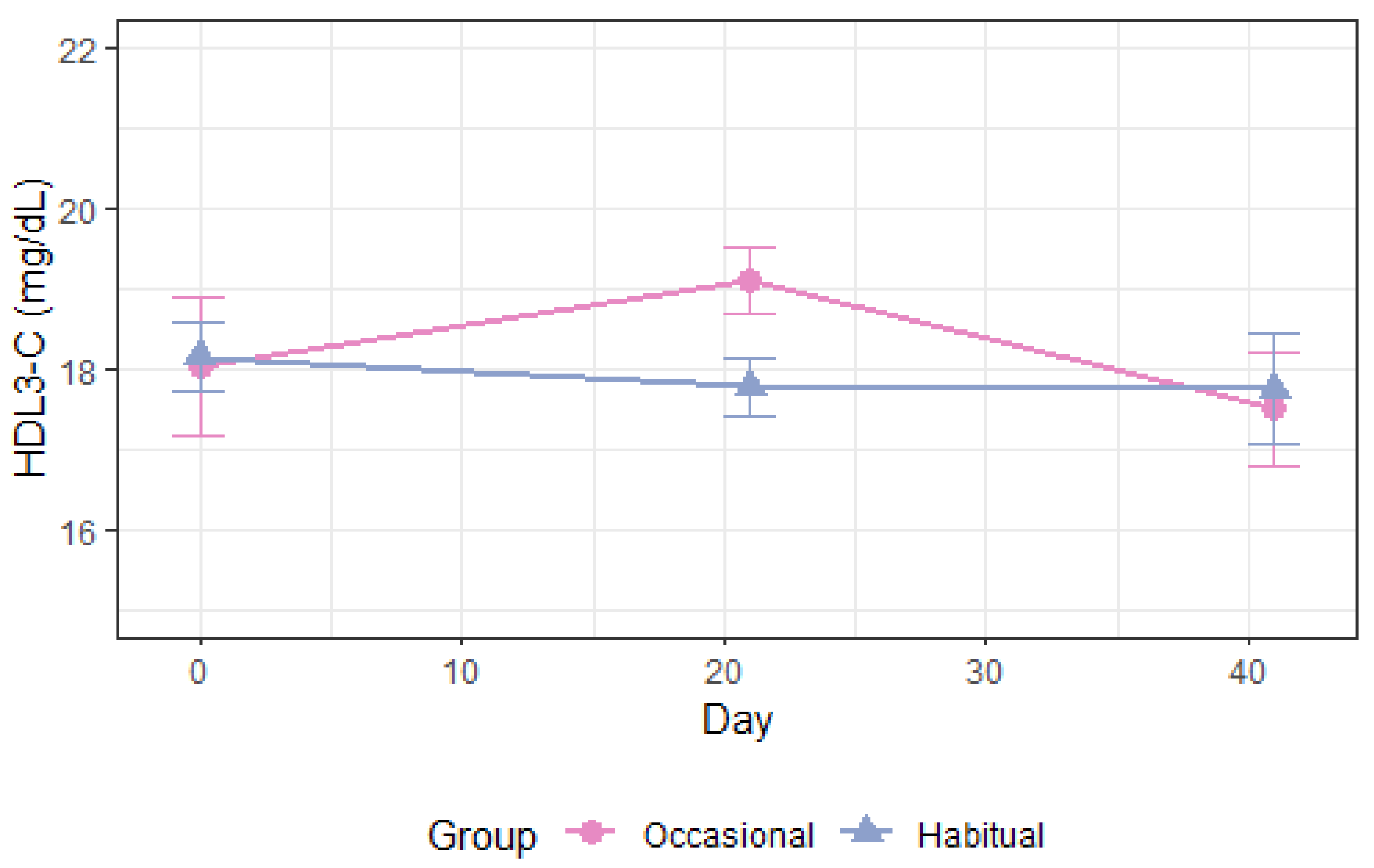
| HDL3-C, mg/dL Occ. vs. Hab. All | −1.6 (−3.2–0.09) | 0.06 | −1.6 (−3.2–0.04) | 0.056 | −1.6 (−2.7–[−0.4]) | 0.008 |
| HDL2b-TG, mg/dL Occ. vs. Hab. All | 0.2 (−0.6–1.1) | 0.62 | −0.1 (−1.0–0.7) | 0.77 | 0.04 (−0.6–0.6) | 0.89 |
| HDL2a-TG, mg/dL Occ. vs. Hab. All | −0.001 (−0.7–0.7) | 0.99 | −0.8 (−1.5–[−0.05]) | 0.04 | −0.4 (−0.9–0.1) | 0.14 |
| HDL-PL, mg/dL Occ. vs. Hab. All | −7.8 (−21.9–6.3) | 0.28 | −5.4 (−19.4–8.6) | 0.45 | −6.6 (−16.5–3.3) | 0.19 |
| HDL2b-PL, mg/dL Occ. vs. Hab. All | −3.5 (−13.8–6.7) | 0.50 | 1.1 (−9.1–11.2) | 0.83 | −1.2 (−8.4–6.0) | 0.74 |
| HDL2a-PL, mg/dL Occ. vs. Hab. All | −4.5 (−9.7–0.78) | 0.10 | −3.3 (−8.5–1.9) | 0.22 | −3.9 (−7.6–[−0.2]) | 0.04 |
| ApoA1, mg/dL Occ. vs. Hab. All | −13.8 (−27.9–0.3) | 0.055 | −15.5 (−29.5–[−1.5]) | 0.03 | −14.7 (−24.6–[−4.7]) | 0.004 |
| HDL-apoA1, mg/dL Occ. vs. Hab. All | −12.1 (−24.7–0.4) | 0.058 | −12.1 (−24.5–0.4) | 0.057 | −12.1 (−21.0–[−3.3]) | 0.007 |
| HDL2b-apoA1, mg/dL Occ. vs. Hab. All | −3.4 (−12.0–5.1) | 0.43 | 1.2 (−7.2–9.7) | 0.78 | −1.1 (−7.1–4.9) | 0.72 |
| HDL2a-apoA1, mg/dL Occ. vs. Hab. All | −5.6 (−10.2–[−1.0]) | 0.02 # | −5.3 (−9.8–[−0.7]) | 0.02 | −5.4 (−8.7–[−2.2]) | 0.001 # |
| HDL3-apoA1, mg/dL Occ. vs. Hab. All | −3.2 (−7.9–1.5) | 0.18 | −7.8 (−12.4–[−3.1]) | 0.001 | −5.5 (−8.8–[−2.2]) | 0.001 |
| ApoB/apoA1, mg/dL Occ. vs. Hab. All | 0.11 (0.03–0.19) | 0.01 | 0.08 (−0.005–0.16) | 0.07 | 0.09 (0.03–0.15) | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilkens, T.L.; Ziegler, Z.; Aru, V.; Khakimov, B.; Overgaard, S.L.; Engelsen, S.B.; Dragsted, L.O. 1–2 Drinks Per Day Affect Lipoprotein Composition after 3 Weeks—Results from a Cross-Over Pilot Intervention Trial in Healthy Adults Using Nuclear Magnetic Resonance-Measured Lipoproteins and Apolipoproteins. Nutrients 2022, 14, 5043. https://doi.org/10.3390/nu14235043
Wilkens TL, Ziegler Z, Aru V, Khakimov B, Overgaard SL, Engelsen SB, Dragsted LO. 1–2 Drinks Per Day Affect Lipoprotein Composition after 3 Weeks—Results from a Cross-Over Pilot Intervention Trial in Healthy Adults Using Nuclear Magnetic Resonance-Measured Lipoproteins and Apolipoproteins. Nutrients. 2022; 14(23):5043. https://doi.org/10.3390/nu14235043
Chicago/Turabian StyleWilkens, Trine Levring, Zabrina Ziegler, Violetta Aru, Bekzod Khakimov, Snædís Lilja Overgaard, Søren Balling Engelsen, and Lars Ove Dragsted. 2022. "1–2 Drinks Per Day Affect Lipoprotein Composition after 3 Weeks—Results from a Cross-Over Pilot Intervention Trial in Healthy Adults Using Nuclear Magnetic Resonance-Measured Lipoproteins and Apolipoproteins" Nutrients 14, no. 23: 5043. https://doi.org/10.3390/nu14235043
APA StyleWilkens, T. L., Ziegler, Z., Aru, V., Khakimov, B., Overgaard, S. L., Engelsen, S. B., & Dragsted, L. O. (2022). 1–2 Drinks Per Day Affect Lipoprotein Composition after 3 Weeks—Results from a Cross-Over Pilot Intervention Trial in Healthy Adults Using Nuclear Magnetic Resonance-Measured Lipoproteins and Apolipoproteins. Nutrients, 14(23), 5043. https://doi.org/10.3390/nu14235043








