Predictive Value of the Advanced Lipoprotein Profile and Glycated Proteins on Diabetic Retinopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Assessment
2.2. Assessment of Diabetes and Presence and Severity of Diabetic Retinopathy
2.3. Nuclear Magnetic Resonance (NMR) Molecular Profiling
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics in Diabetic Subjects with Diabetic Retinopathy
3.2. Advanced Lipoprotein and Glycoprotein Characteristics
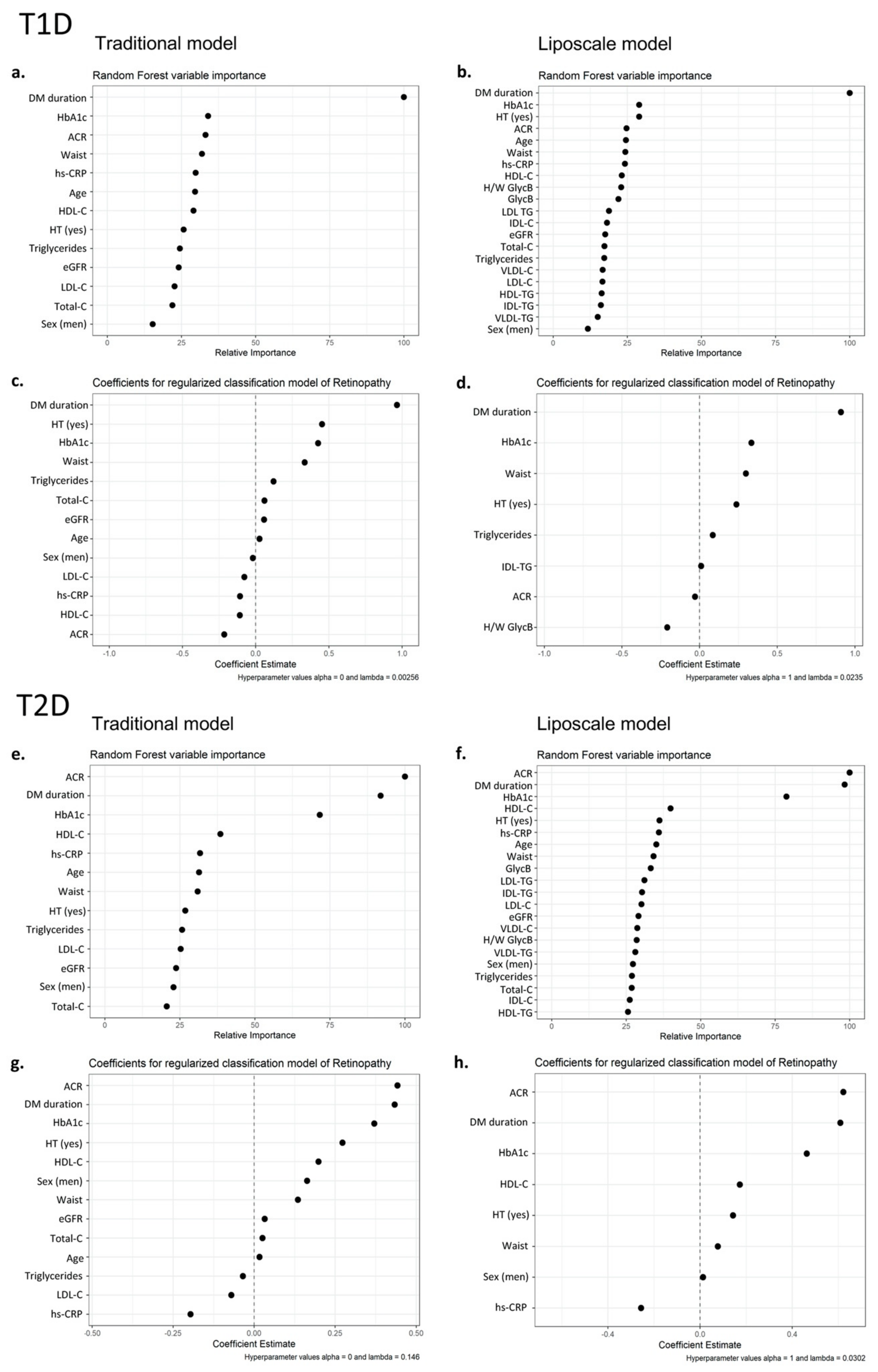
3.3. Contribution of Advanced Lipoprotein Characteristics and Glycoproteins to DR Prediction
3.4. Contribution of Advanced Lipoprotein Characteristics and Glycoproteins to DR Severity Prediction
3.4.1. Mild DR vs. Severe DR
3.4.2. No DR vs. Severe DR
3.4.3. DR Stages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yau, J.W.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; Chew, E.; Duh, E.J.; Sobrin, L.; Sun, J.K.; VanderBeek, B.L.; Wykoff, C.C.; Gardner, T.W. Diabetic Retinopathy: A Position Statement by the American Diabetes Association. Diabetes Care 2017, 40, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Sun, J.; Kawasaki, R.; Ruamviboonsuk, P.; Gupta, N.; Lansingh, V.C.; Maia, M.; Mathenge, W.; Moreker, S.; Muqit, M.M.K.; et al. Guidelines on Diabetic Eye Care: The International Council of Ophthalmology Recommendations for Screening, Follow-up, Referral, and Treatment Based on Resource Settings. Ophthalmology 2018, 125, 1608–1622. [Google Scholar] [CrossRef] [PubMed]
- Drinkwater, J.J.; Davis, T.M.E.; Davis, W.A. The relationship between carotid disease and retinopathy in diabetes: A systematic review. Cardiovasc. Diabetol. 2020, 19, 54. [Google Scholar] [CrossRef]
- Cheung, N.; Donaghue, K.C.; Liew, G.; Rogers, S.L.; Wang, J.J.; Lim, S.W.; Jenkins, A.J.; Hsu, W.; Li Lee, M.; Wong, T.Y. Quantitative assessment of early diabetic retinopathy using fractal analysis. Diabetes Care 2009, 32, 106–110. [Google Scholar] [CrossRef]
- Liew, G.; Wong, T.Y.; Mitchell, P.; Cheung, N.; Wang, J.J. Retinopathy predicts coronary heart disease mortality. Heart 2009, 95, 391–394. [Google Scholar] [CrossRef]
- Melo, L.G.N.; Morales, P.H.; Drummond, K.R.G.; Santos, D.C.; Pizarro, M.H.; Barros, B.S.V.; Mattos, T.C.L.; Pinheiro, A.A.; Mallmann, F.; Leal, F.S.L.; et al. Diabetic Retinopathy May Indicate an Increased Risk of Cardiovascular Disease in Patients With Type 1 Diabetes-A Nested Case-Control Study in Brazil. Front. Endocrinol. 2019, 10, 689. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Ambrosius, W.T.; Danis, R.; Ismail-Beigi, F.; Cushman, W.; Calles, J.; Banerji, M.; Schubart, U.; Chew, E.Y. Diabetic retinopathy, its progression, and incident cardiovascular events in the ACCORD trial. Diabetes Care 2013, 36, 1266–1271. [Google Scholar] [CrossRef]
- Gao, L.; Zhao, W.; Yang, J.K.; Qin, M.Z. Proliferative diabetic retinopathy in patients with type 2 diabetes correlates with the presence of atherosclerosis cardiovascular disease. Diabetol. Metab. Syndr. 2021, 13, 48. [Google Scholar] [CrossRef]
- Tang, J.; Li, T.; Li, P.; Ma, Y.; Liu, M.; Shan, Q.; Guo, F.; Zhou, T.; Wei, Q. Early Assessment of the Risk Factors for Diabetic Retinopathy Can Reduce the Risk of Peripheral Arterial and Cardiovascular Diseases in Type 2 Diabetes. Ophthalmic Res. 2018, 59, 221–227. [Google Scholar] [CrossRef]
- Pease, A.; Earnest, A.; Ranasinha, S.; Nanayakkara, N.; Liew, D.; Wischer, N.; Andrikopoulos, S.; Zoungas, S. Burden of cardiovascular risk factors and disease among patients with type 1 diabetes: Results of the Australian National Diabetes Audit (ANDA). Cardiovasc. Diabetol. 2018, 17, 77. [Google Scholar] [CrossRef]
- Khazai, B.; Adabifirouzjaei, F.; Guo, M.; Ipp, E.; Klein, R.; Klein, B.; Cotch, M.F.; Wong, T.Y.; Swerdloff, R.; Wang, C.; et al. Relation between Retinopathy and Progression of Coronary Artery Calcium in Individuals with Versus Without Diabetes Mellitus (From the Multi-Ethnic Study of Atherosclerosis). Am. J. Cardiol. 2021, 149, 1–8. [Google Scholar] [CrossRef]
- Zhang, X.; Lim, S.C.; Tavintharan, S.; Yeoh, L.Y.; Sum, C.F.; Ang, K.; Yeo, D.; Low, S.; Kumari, N. Association of central arterial stiffness with the presence and severity of diabetic retinopathy in Asians with type 2 diabetes. Diab Vasc Dis Res 2019, 16, 498–505. [Google Scholar] [CrossRef]
- Carbonell, M.; Castelblanco, E.; Valldeperas, X.; Betriu, A.; Traveset, A.; Granado-Casas, M.; Hernandez, M.; Vazquez, F.; Martin, M.; Rubinat, E.; et al. Diabetic retinopathy is associated with the presence and burden of subclinical carotid atherosclerosis in type 1 diabetes. Cardiovasc. Diabetol. 2018, 17, 66. [Google Scholar] [CrossRef]
- Pena, A.S.; Liew, G.; Anderson, J.; Giles, L.C.; Gent, R.; Wong, T.Y.; Couper, J.J. Early atherosclerosis is associated with retinal microvascular changes in adolescents with type 1 diabetes. Pediatr. Diabetes 2018, 19, 1467–1470. [Google Scholar] [CrossRef]
- Alonso, N.; Traveset, A.; Rubinat, E.; Ortega, E.; Alcubierre, N.; Sanahuja, J.; Hernandez, M.; Betriu, A.; Jurjo, C.; Fernandez, E.; et al. Type 2 diabetes-associated carotid plaque burden is increased in patients with retinopathy compared to those without retinopathy. Cardiovasc. Diabetol. 2015, 14, 33. [Google Scholar] [CrossRef]
- Jung, C.H.; Baek, A.R.; Kim, K.J.; Kim, B.Y.; Kim, C.H.; Kang, S.K.; Mok, J.O. Association between Cardiac Autonomic Neuropathy, Diabetic Retinopathy and Carotid Atherosclerosis in Patients with Type 2 Diabetes. Endocrinol. Metab. 2013, 28, 309–319. [Google Scholar] [CrossRef]
- Son, J.W.; Jang, E.H.; Kim, M.K.; Kim, I.T.; Roh, Y.J.; Baek, K.H.; Song, K.H.; Yoon, K.H.; Cha, B.Y.; Lee, K.W.; et al. Diabetic retinopathy is associated with subclinical atherosclerosis in newly diagnosed type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2011, 91, 253–259. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, S.; Li, M.; Wu, Y. Association Between Atherosclerosis and Diabetic Retinopathy in Chinese Patients with Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. Obes. 2020, 13, 1911–1920. [Google Scholar] [CrossRef]
- Krentz, A.J.; Clough, G.; Byrne, C.D. Interactions between microvascular and macrovascular disease in diabetes: Pathophysiology and therapeutic implications. Diabetes Obes. Metab. 2007, 9, 781–791. [Google Scholar] [CrossRef]
- Goldberg, I.J. Clinical review 124: Diabetic dyslipidemia: Causes and consequences. J. Clin. Endocrinol. Metab. 2001, 86, 965–971. [Google Scholar] [CrossRef]
- Kane, J.P.; Pullinger, C.R.; Goldfine, I.D.; Malloy, M.J. Dyslipidemia and diabetes mellitus: Role of lipoprotein species and interrelated pathways of lipid metabolism in diabetes mellitus. Curr. Opin. Pharmacol. 2021, 61, 21–27. [Google Scholar] [CrossRef]
- Amor, A.J.; Catalan, M.; Perez, A.; Herreras, Z.; Pinyol, M.; Sala-Vila, A.; Cofan, M.; Gilabert, R.; Ros, E.; Ortega, E. Nuclear magnetic resonance lipoprotein abnormalities in newly-diagnosed type 2 diabetes and their association with preclinical carotid atherosclerosis. Atherosclerosis 2016, 247, 161–169. [Google Scholar] [CrossRef]
- Nathan, D.M. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: Overview. Diabetes Care 2014, 37, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Savelieff, M.G.; Callaghan, B.C.; Feldman, E.L. The emerging role of dyslipidemia in diabetic microvascular complications. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Action to Control Cardiovascular Risk in Diabetes Follow-On (ACCORDION) Eye Study Group; The Action to Control Cardiovascular Risk in Diabetes Follow-On (ACCORDION) Study Group. Persistent Effects of Intensive Glycemic Control on Retinopathy in Type 2 Diabetes in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Follow-On Study. Diabetes Care 2016, 39, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, A.J.; Grant, M.B.; Busik, J.V. Lipids, hyperreflective crystalline deposits and diabetic retinopathy: Potential systemic and retinal-specific effect of lipid-lowering therapies. Diabetologia 2022, 665, 587–603. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R. Fenofibrate and statin therapy, compared with placebo and statin, slows the development of retinopathy in type 2 diabetes patients of 10 years duration: The ACCORD study. Evid. Based Med. 2011, 16, 45–46. [Google Scholar] [CrossRef]
- Chew, E.Y.; Davis, M.D.; Danis, R.P.; Lovato, J.F.; Perdue, L.H.; Greven, C.; Genuth, S.; Goff, D.C.; Leiter, L.A.; Ismail-Beigi, F.; et al. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: The Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology 2014, 121, 2443–2451. [Google Scholar] [CrossRef]
- Castelblanco, E.; Hernandez, M.; Ortega, E.; Amigo, N.; Real, J.; Granado-Casas, M.; Minambres, I.; Lopez, C.; Lecube, A.; Bermudez-Lopez, M.; et al. Outstanding improvement of the advanced lipoprotein profile in subjects with new-onset type 1 diabetes mellitus after achieving optimal glycemic control. Diabetes Res. Clin. Pract. 2021, 182, 109145. [Google Scholar] [CrossRef]
- Amor, A.J.; Vinagre, I.; Valverde, M.; Urquizu, X.; Meler, E.; Lopez, E.; Quiros, C.; Gimenez, M.; Codina, L.; Conget, I.; et al. Nuclear magnetic resonance lipoproteins are associated with carotid atherosclerosis in type 1 diabetes and pre-eclampsia. Diabetes Metab. Res. Rev. 2021, 37, e3362. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef]
- Chung, W.K.; Erion, K.; Florez, J.C.; Hattersley, A.T.; Hivert, M.F.; Lee, C.G.; McCarthy, M.I.; Nolan, J.J.; Norris, J.M.; Pearson, E.R.; et al. Precision medicine in diabetes: A Consensus Report from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2020, 63, 1671–1693. [Google Scholar] [CrossRef]
- Wilkinson, C.P.; Ferris, F.L., 3rd; Klein, R.E.; Lee, P.P.; Agardh, C.D.; Davis, M.; Dills, D.; Kampik, A.; Pararajasegaram, R.; Verdaguer, J.T. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003, 110, 1677–1682. [Google Scholar] [CrossRef]
- Carbonell, M.; Alonso, N.; Castelblanco, E.; Real, J.; Ramirez-Morros, A.; Simo, R.; Hernandez, C.; Jurjo, C.; Traveset, A.; Valldeperas, X.; et al. Assessment of Inner Retinal Layers and Choroidal Thickness in Type 1 Diabetes Mellitus: A Cross-Sectional Study. J. Clin. Med. 2019, 8, 1412. [Google Scholar] [CrossRef]
- Mallol, R.; Amigo, N.; Rodriguez, M.A.; Heras, M.; Vinaixa, M.; Plana, N.; Rock, E.; Ribalta, J.; Yanes, O.; Masana, L.; et al. Liposcale: A novel advanced lipoprotein test based on 2D diffusion-ordered 1H NMR spectroscopy. J. Lipid Res. 2015, 56, 737–746. [Google Scholar] [CrossRef]
- Fuertes-Martin, R.; Taverner, D.; Vallve, J.C.; Paredes, S.; Masana, L.; Correig Blanchar, X.; Amigo Grau, N. Characterization of (1)H NMR Plasma Glycoproteins as a New Strategy To Identify Inflammatory Patterns in Rheumatoid Arthritis. J. Proteome Res. 2018, 17, 3730–3739. [Google Scholar] [CrossRef]
- Amigo, N.; Fuertes-Martin, R.; Malo, A.I.; Plana, N.; Ibarretxe, D.; Girona, J.; Correig, X.; Masana, L. Glycoprotein Profile Measured by a (1)H-Nuclear Magnetic Resonance Based on Approach in Patients with Diabetes: A New Robust Method to Assess Inflammation. Life 2021, 11, 1407. [Google Scholar] [CrossRef]
- Couronne, R.; Probst, P.; Boulesteix, A.L. Random forest versus logistic regression: A large-scale benchmark experiment. BMC Bioinformatics 2018, 19, 270. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.r-project.org/ (accessed on 10 October 2021).
- Seres-Noriega, T.; Gimenez, M.; Perea, V.; Blanco, J.; Vinagre, I.; Pane, A.; Ruiz, S.; Cofan, M.; Mesa, A.; Esmatjes, E.; et al. Quantification of glycoproteins by nuclear magnetic resonance associated with preclinical carotid atherosclerosis in patients with type 1 diabetes. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2099–2108. [Google Scholar] [CrossRef]
- Henricsson, M.; Nilsson, A.; Groop, L.; Heijl, A.; Janzon, L. Prevalence of diabetic retinopathy in relation to age at onset of the diabetes, treatment, duration and glycemic control. Acta Ophthalmol. Scand 1996, 74, 523–527. [Google Scholar] [CrossRef]
- Song, K.H.; Jeong, J.S.; Kim, M.K.; Kwon, H.S.; Baek, K.H.; Ko, S.H.; Ahn, Y.B. Discordance in risk factors for the progression of diabetic retinopathy and diabetic nephropathy in patients with type 2 diabetes mellitus. J. Diabetes Investig. 2019, 10, 745–752. [Google Scholar] [CrossRef]
- Jeng, C.J.; Hsieh, Y.T.; Yang, C.M.; Yang, C.H.; Lin, C.L.; Wang, I.J. Diabetic Retinopathy in Patients with Diabetic Nephropathy: Development and Progression. PLoS ONE 2016, 11, e0161897. [Google Scholar] [CrossRef]
- Edwards, M.S.; Wilson, D.B.; Craven, T.E.; Stafford, J.; Fried, L.F.; Wong, T.Y.; Klein, R.; Burke, G.L.; Hansen, K.J. Associations between retinal microvascular abnormalities and declining renal function in the elderly population: The Cardiovascular Health Study. Am. J. Kidney Dis. 2005, 46, 214–224. [Google Scholar] [CrossRef]
- Pedro, R.A.; Ramon, S.A.; Marc, B.B.; Juan, F.B.; Isabel, M.M. Prevalence and relationship between diabetic retinopathy and nephropathy, and its risk factors in the North-East of Spain, a population-based study. Ophthalmic Epidemiol. 2010, 17, 251–265. [Google Scholar] [CrossRef]
- Park, Y.H.; Shin, J.A.; Han, J.H.; Park, Y.M.; Yim, H.W. The association between chronic kidney disease and diabetic retinopathy: The Korea National Health and Nutrition Examination Survey 2008-2010. PLoS ONE 2015, 10, e0125338. [Google Scholar] [CrossRef]
- Hanssen, K.F.; Bangstad, H.J.; Brinchman-Hansen, O.; Dahl-Jorgensen, K. Glycemic control, incipient diabetic nephropathy, and diabetic retinopathy. J. Diabetes Complicat. 1990, 4, 66–67. [Google Scholar] [CrossRef]
- Guo, F.; Zhou, T.; Tang, J.; Dong, M.; Wei, Q. Related Risk Factors between Subclinical Carotid Atherosclerosis and Diabetic Retinopathy in Newly Diagnosed Type 2 Diabetes Mellitus in China. Exp. Clin. Endocrinol. Diabetes 2021, 129, 283–288. [Google Scholar] [CrossRef]
- Tada, H.; Kawashiri, M.A.; Nomura, A.; Yoshimura, K.; Itoh, H.; Komuro, I.; Yamagishi, M. Serum triglycerides predict first cardiovascular events in diabetic patients with hypercholesterolemia and retinopathy. Eur. J. Prev. Cardiol. 2018, 25, 1852–1860. [Google Scholar] [CrossRef]
- Itoh, H.; Komuro, I.; Takeuchi, M.; Akasaka, T.; Daida, H.; Egashira, Y.; Fujita, H.; Higaki, J.; Hirata, K.I.; Ishibashi, S.; et al. Intensive Treat-to-Target Statin Therapy in High-Risk Japanese Patients With Hypercholesterolemia and Diabetic Retinopathy: Report of a Randomized Study. Diabetes Care 2018, 41, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Hermans, M.P.; Fioretto, P.; Valensi, P.; Davis, T.; Horton, E.; Wanner, C.; Al-Rubeaan, K.; Aronson, R.; Barzon, I.; et al. Association between plasma triglycerides and high-density lipoprotein cholesterol and microvascular kidney disease and retinopathy in type 2 diabetes mellitus: A global case-control study in 13 countries. Circulation 2014, 129, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Sasso, F.C.; Pafundi, P.C.; Gelso, A.; Bono, V.; Costagliola, C.; Marfella, R.; Sardu, C.; Rinaldi, L.; Galiero, R.; Acierno, C.; et al. High HDL cholesterol: A risk factor for diabetic retinopathy? Findings from NO BLIND study. Diabetes Res. Clin. Pract. 2019, 150, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, A.; Morita, H.; Sato, T.; Kawahara, T.; Takeda, N.; Kato, S.; Itoh, H.; Komuro, I. Small Dense Low-Density Lipoprotein Cholesterol is a Potential Marker for Predicting Laser Treatment for Retinopathy in Diabetic Patients. J. Atheroscler. Thromb. 2021, 29, 678–691. [Google Scholar] [CrossRef]
- Jansson Sigfrids, F.; Dahlstrom, E.H.; Forsblom, C.; Sandholm, N.; Harjutsalo, V.; Taskinen, M.R.; Groop, P.H. Remnant cholesterol predicts progression of diabetic nephropathy and retinopathy in type 1 diabetes. J. Intern. Med. 2021, 290, 632–645. [Google Scholar] [CrossRef]
- Curcio, C.A.; Johnson, M.; Huang, J.D.; Rudolf, M. Apolipoprotein B-containing lipoproteins in retinal aging and age-related macular degeneration. J. Lipid Res. 2010, 51, 451–467. [Google Scholar] [CrossRef]
- Shan, Y.; Wang, Q.; Zhang, Y.; Tong, X.; Pu, S.; Xu, Y.; Gao, X. High remnant cholesterol level is relevant to diabetic retinopathy in type 2 diabetes mellitus. Lipids Health Dis. 2022, 21, 12. [Google Scholar] [CrossRef]
- Verges, B. Dyslipidemia in Type 1 Diabetes: AMaskedDanger. Trends Endocrinol. Metab. 2020, 31, 422–434. [Google Scholar] [CrossRef]
- Poznyak, A.; Grechko, A.V.; Poggio, P.; Myasoedova, V.A.; Alfieri, V.; Orekhov, A.N. The Diabetes Mellitus-Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int. J. Mol. Sci. 2020, 21, 1835. [Google Scholar] [CrossRef]
- Storti, F.; Pulley, J.; Kuner, P.; Abt, M.; Luhmann, U.F.O. Circulating Biomarkers of Inflammation and Endothelial Activation in Diabetic Retinopathy. Transl. Vis. Sci. Technol. 2021, 10, 8. [Google Scholar] [CrossRef]
| Characteristics | T1D | T2D | ||||
|---|---|---|---|---|---|---|
| Non-DR | DR | p-Value | Non-DR | DR | p-Value | |
| n = 181 | n = 128 | n = 139 | n = 125 | |||
| Age (years) | 44.0 (10.9) | 48.9 (12.1) | <0.001 | 57.4 (10.0) | 59.5 (8.58) | 0.073 |
| Sex (women) | 95 (52.5%) | 68 (53.1%) | 1 | 66 (47.5%) | 60 (48.0%) | 1 |
| BMI (kg/m2) | 25.4 (3.86) | 26.8 (4.26) | 0.003 | 31.3 (5.25) | 31.8 (5.66) | 0.505 |
| Waist circumference (cm) | 87.8 (11.7) | 91.9 (12.9) | 0.005 | 104 (12.1) | 107 (10.6) | 0.085 |
| sBP (mmHg) | 125 (17.5) | 132 (18.7) | 0.001 | 134 (15.7) | 144 (20.7) | <0.001 |
| dBP (mmHg) | 74.6 (10.1) | 73.8 (10.1) | 0.485 | 76.4 (10.2) | 77.0 (10.8) | 0.601 |
| Hypertension (yes) | 35 (19.3%) | 53 (41.4%) | <0.001 | 68 (48.9%) | 81 (64.8%) | 0.013 |
| Dyslipidemia (yes) | 68 (37.6%) | 66 (51.6%) | 0.020 | 61 (43.9%) | 63 (50.4%) | 0.350 |
| Smoking: | 0.596 | 0.496 | ||||
| Active smoker | 47 (26.0%) | 31 (24.2%) | 29 (20.9%) | 26 (20.8%) | ||
| Former smoker | 43 (23.8%) | 37 (28.9%) | 50 (36.0%) | 37 (29.6%) | ||
| DM duration (years) | 17.9 (9.72) | 27.2 (9.96) | <0.001 | 6.88 (5.48) | 13.6 (9.51) | <0.001 |
| Glucose (mg/dL) | 163 (71.6) | 170 (78.5) | 0.398 | 148 (49.2) | 166 (58.1) | 0.010 |
| Creatinine (mg/dL) | 0.77 (0.16) | 0.77 (0.14) | 0.900 | 0.81 (0.17) | 0.81 (0.17) | 0.901 |
| eGFR (mL/min/1.73 m2) | 103 (13.9) | 99.9 (14.0) | 0.026 | 92.0 (14.5) | 90.4 (14.5) | 0.381 |
| Triglycerides (mg/dL) | 72.6 (29.5) | 83.3 (48.4) | 0.028 | 136 (68.9) | 133 (70.7) | 0.762 |
| Total C (md/dL) | 179 (28.1) | 181 (34.4) | 0.708 | 186 (36.4) | 185 (36.6) | 0.913 |
| HDL-C (mg/dL) | 64.3 (14.4) | 62.7 (17.2) | 0.378 | 48.1 (10.6) | 52.2 (15.5) | 0.013 |
| LDL-C (mg/dL) | 101 (23.1) | 102 (28.1) | 0.684 | 112 (30.6) | 107 (30.2) | 0.200 |
| HbA1c (%) | 7.48 (0.94) | 7.88 (1.09) | 0.001 | 7.29 (1.16) | 8.36 (1.46) | <0.001 |
| HbA1c (mmol/mol) | 58.3 (10.3) | 62.6 (11.9) | 0.001 | 56.1 (12.7) | 67.9 (16.0) | <0.001 |
| hs-CRP (mg/L) | 3.11 (6.88) | 2.66 (3.85) | 0.465 | 4.44 (4.47) | 3.86 (5.22) | 0.339 |
| Plaque: | 0.001 | <0.001 | ||||
| Multiple plaques | 21 (11.6%) | 33 (25.8%) | 28 (20.1%) | 54 (43.2%) | ||
| No plaque | 134 (74.0%) | 69 (53.9%) | 69 (49.6%) | 42 (33.6%) | ||
| One plaque | 26 (14.4%) | 26 (20.3%) | 42 (30.2%) | 29 (23.2%) | ||
| FLI | 24.3 (22.1) | 32.9 (26.1) | 0.003 | 66.8 (22.7) | 69.4 (23.0) | 0.349 |
| Microalbuminuria (mg/L) | 9.26 (24.4) | 13.9 (25.2) | 0.108 | 11.1 (14.7) | 30.1 (37.2) | <0.001 |
| ACR (mg/g) | 4.48 (11.0) | 6.27 (19.4) | 0.349 | 9.93 (18.6) | 29.7 (39.6) | <0.001 |
| Advanced Lipoprotein Profile | T1D | T2D | ||||
|---|---|---|---|---|---|---|
| Non-DR | DR | p-Value | Non-DR | DR | p-Value | |
| n = 181 | n = 128 | n = 139 | n = 125 | |||
| VLDL-P number (nmol/L) | ||||||
| Total | 30.2 (14.6) | 34.8 (24.1) | 0.059 | 69.1 (45.2) | 69.6 (56.4) | 0.935 |
| Large | 0.81 (0.32) | 0.89 (0.47) | 0.081 | 1.63 (0.89) | 1.62 (1.21) | 0.949 |
| Medium | 3.11 (1.79) | 3.51 (3.29) | 0.213 | 6.26 (6.68) | 6.49 (8.04) | 0.804 |
| Small | 26.3 (12.7) | 30.4 (20.5) | 0.050 | 61.2 (38.6) | 61.5 (48.0) | 0.956 |
| VLDL-P composition | ||||||
| VLDL-C (mg/dL) | 7.62 (5.65) | 9.13 (8.68) | 0.087 | 17.1 (13.4) | 17.4 (15.0) | 0.875 |
| VLDL-TG (mg/dL) | 43.0 (20.1) | 49.1 (34.2) | 0.071 | 98.8 (68.0) | 99.6 (86.9) | 0.928 |
| VLDL-P size (nm) | 42.2 (0.23) | 42.1 (0.23) | 0.044 | 42.0 (0.21) | 42.0 (0.22) | 0.315 |
| LDL-P number (nmol/L) | ||||||
| Total | 1265 (193) | 1285 (230) | 0.415 | 1355 (252) | 1276 (262) | 0.013 |
| Large | 182 (29.6) | 182 (31.9) | 0.871 | 174 (31.5) | 173 (35.4) | 0.743 |
| Medium | 413 (106) | 409 (123) | 0.768 | 392 (129) | 379 (137) | 0.428 |
| Small | 669 (95.1) | 694 (115) | 0.046 | 789 (124) | 724 (134) | <0.001 |
| LDL-P composition | ||||||
| LDL-C (mg/dL) | 125 (19.7) | 125 (23.4) | 0.895 | 127 (25.2) | 120 (26.2) | 0.026 |
| LDL-TG (mg/dL) | 15.8 (4.31) | 16.5 (4.76) | 0.167 | 17.4 (4.74) | 17.9 (5.45) | 0.476 |
| LDL-P size (nm) | 21.1 (0.24) | 21.0 (0.25) | 0.043 | 20.8 (0.23) | 20.9 (0.32) | 0.010 |
| HDL-P number (nmol/L) | ||||||
| Total | 32.7 (5.95) | 33.0 (7.02) | 0.771 | 27.1 (5.01) | 27.7 (6.02) | 0.348 |
| Large | 0.28 (0.05) | 0.29 (0.05) | 0.117 | 0.26 (0.04) | 0.27 (0.05) | 0.354 |
| Medium | 10.8 (2.42) | 11.0 (2.67) | 0.614 | 8.04 (1.34) | 8.49 (2.15) | 0.045 |
| Small | 21.6 (4.21) | 21.7 (5.03) | 0.908 | 18.8 (4.24) | 18.9 (4.71) | 0.734 |
| HDL-P composition | ||||||
| HDL-C (mg/dL) | 65.6 (13.4) | 65.5 (16.6) | 0.938 | 49.4 (9.47) | 51.1 (12.7) | 0.214 |
| HDL-TG (mg/dL) | 13.8 (3.98) | 14.8 (3.83) | 0.032 | 14.0 (4.58) | 14.4 (4.79) | 0.471 |
| HDL-P size (nm) | 8.23 (0.06) | 8.24 (0.06) | 0.447 | 8.20 (0.07) | 8.21 (0.07) | 0.302 |
| IDL-P composition | ||||||
| IDL-C (mg/dL) | 9.38 (4.43) | 10.8 (5.03) | 0.011 | 13.1 (4.83) | 13.5 (5.37) | 0.572 |
| IDL-TG (mg/dL) | 10.7 (3.37) | 11.8 (3.87) | 0.011 | 14.5 (4.13) | 15.0 (4.34) | 0.377 |
| Other atherogenic variables | ||||||
| Non-HDL-P (nmol/L) | 1262 (198) | 1287 (233) | 0.332 | 1397 (254) | 1318 (262) | 0.013 |
| Total-P/HDL-P | 41.0 (10.9) | 41.7 (11.3) | 0.597 | 54.5 (15.1) | 50.9 (14.9) | 0.046 |
| LDL-P/HDL-P | 40.0 (10.5) | 40.6 (10.8) | 0.680 | 51.8 (14.3) | 48.1 (14.1) | 0.035 |
| Total C (mg/dL) | 208 (26.2) | 211 (32.1) | 0.366 | 207 (32.0) | 202 (33.5) | 0.245 |
| Total TG (mg/dL) | 83.3 (27.0) | 92.2 (41.5) | 0.034 | 142 (62.2) | 142 (65.2) | 0.983 |
| GlycA | 5.12 (1.03) | 5.18 (1.13) | 0.620 | 6.71 (1.51) | 6.71 (1.51) | 0.969 |
| GlycB | 2.02 (0.40) | 1.99 (0.39) | 0.492 | 2.25 (0.32) | 2.30 (0.37) | 0.297 |
| H/W GlycA | 17.1 (3.45) | 16.9 (2.91) | 0.607 | 21.8 (3.78) | 21.9 (4.44) | 0.940 |
| H/W GlycB | 4.88 (0.93) | 4.76 (0.78) | 0.222 | 5.93 (0.92) | 5.94 (0.93) | 0.892 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Julve, J.; Rossell, J.; Correig, E.; Rojo-Lopez, M.I.; Amigó, N.; Hernández, M.; Traveset, A.; Carbonell, M.; Alonso, N.; Mauricio, D.; et al. Predictive Value of the Advanced Lipoprotein Profile and Glycated Proteins on Diabetic Retinopathy. Nutrients 2022, 14, 3932. https://doi.org/10.3390/nu14193932
Julve J, Rossell J, Correig E, Rojo-Lopez MI, Amigó N, Hernández M, Traveset A, Carbonell M, Alonso N, Mauricio D, et al. Predictive Value of the Advanced Lipoprotein Profile and Glycated Proteins on Diabetic Retinopathy. Nutrients. 2022; 14(19):3932. https://doi.org/10.3390/nu14193932
Chicago/Turabian StyleJulve, Josep, Joana Rossell, Eudald Correig, Marina Idalia Rojo-Lopez, Nuria Amigó, Marta Hernández, Alicia Traveset, Marc Carbonell, Nuria Alonso, Didac Mauricio, and et al. 2022. "Predictive Value of the Advanced Lipoprotein Profile and Glycated Proteins on Diabetic Retinopathy" Nutrients 14, no. 19: 3932. https://doi.org/10.3390/nu14193932
APA StyleJulve, J., Rossell, J., Correig, E., Rojo-Lopez, M. I., Amigó, N., Hernández, M., Traveset, A., Carbonell, M., Alonso, N., Mauricio, D., & Castelblanco, E. (2022). Predictive Value of the Advanced Lipoprotein Profile and Glycated Proteins on Diabetic Retinopathy. Nutrients, 14(19), 3932. https://doi.org/10.3390/nu14193932








