Diet and Blood Concentrations of Essential and Non-Essential Elements among Rural Residents in Arctic Russia
Abstract
1. Introduction
2. Materials and Methods
2.1. Implementation
2.2. Food Frequency Questionnaire
2.3. Measurements of Elements in Whole Blood and Ferritin in Serum
2.4. Statistics
3. Results
4. Discussion
4.1. Traditional Food Consumption
4.2. Traditional Food Consumption and Elements in Whole Blood
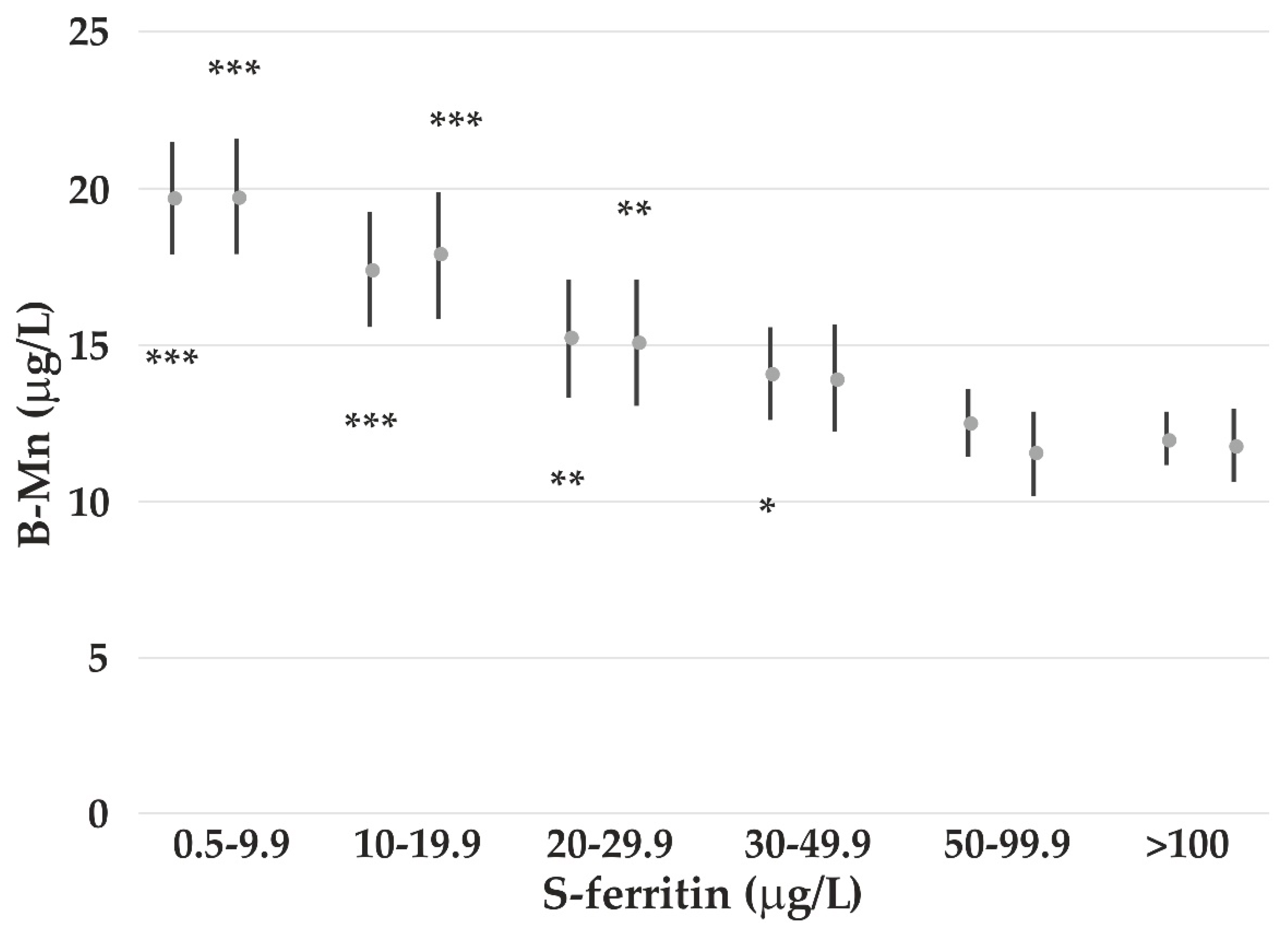
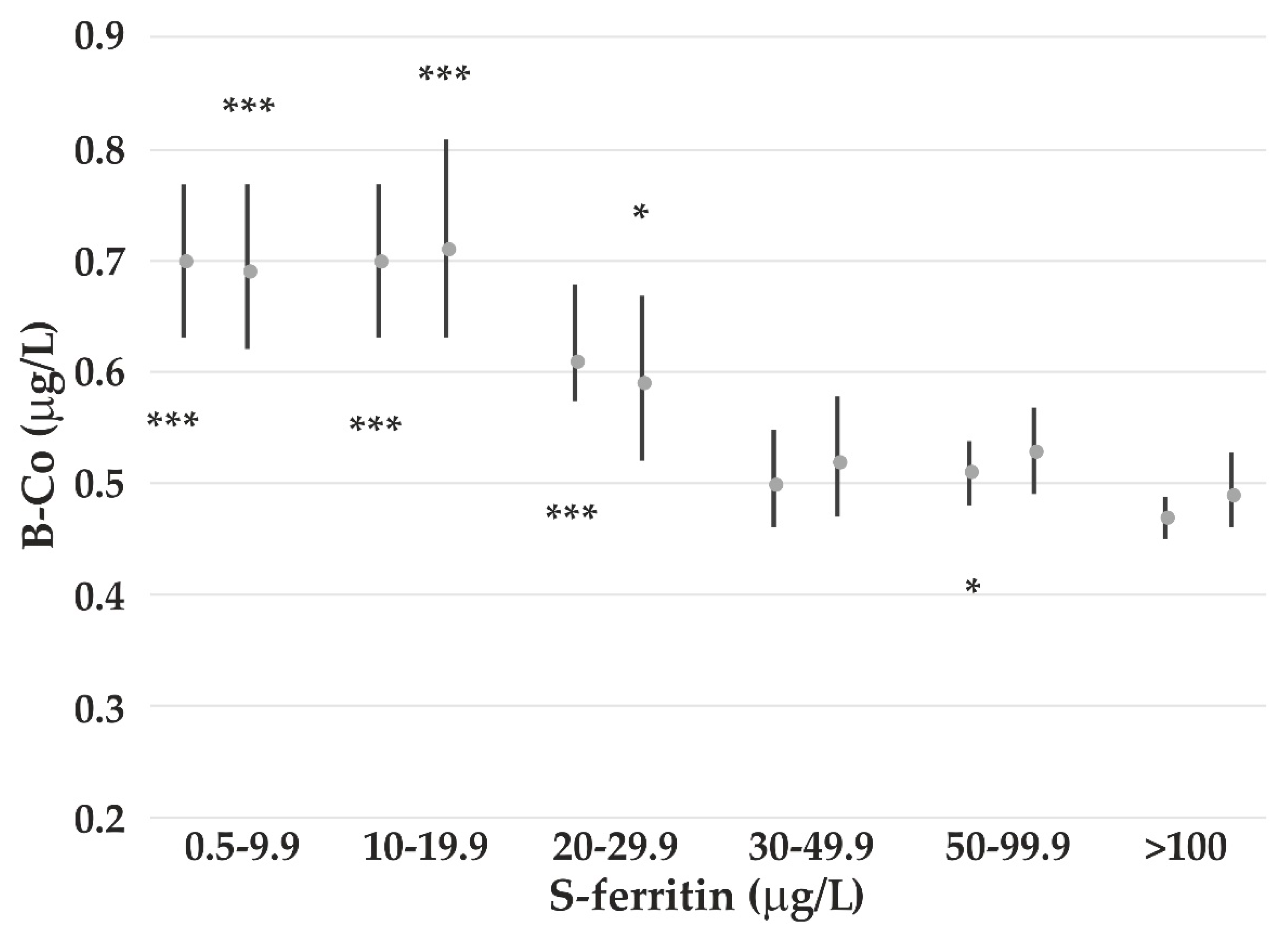
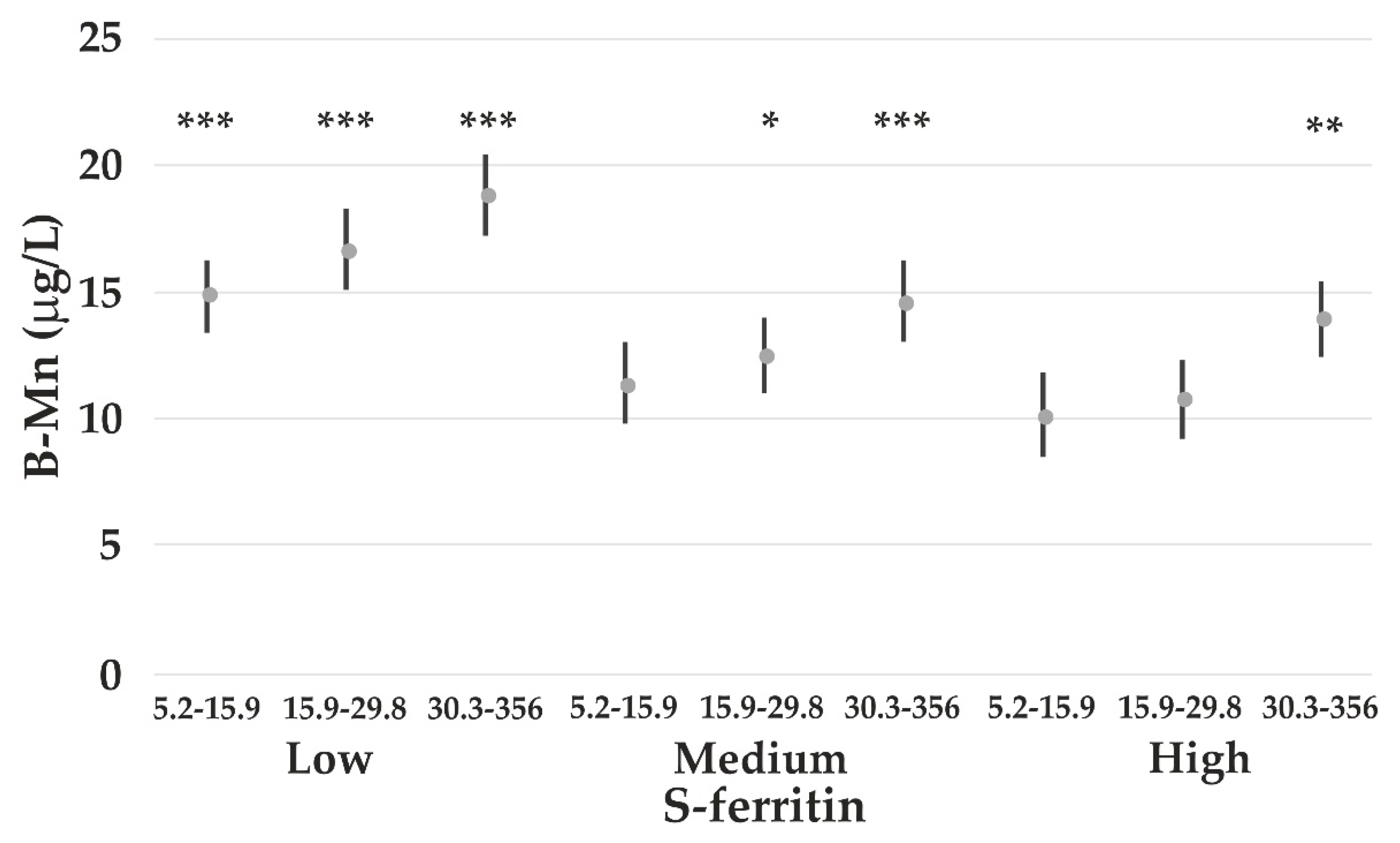
4.3. The Impact of Iron Status on Element Concentrations
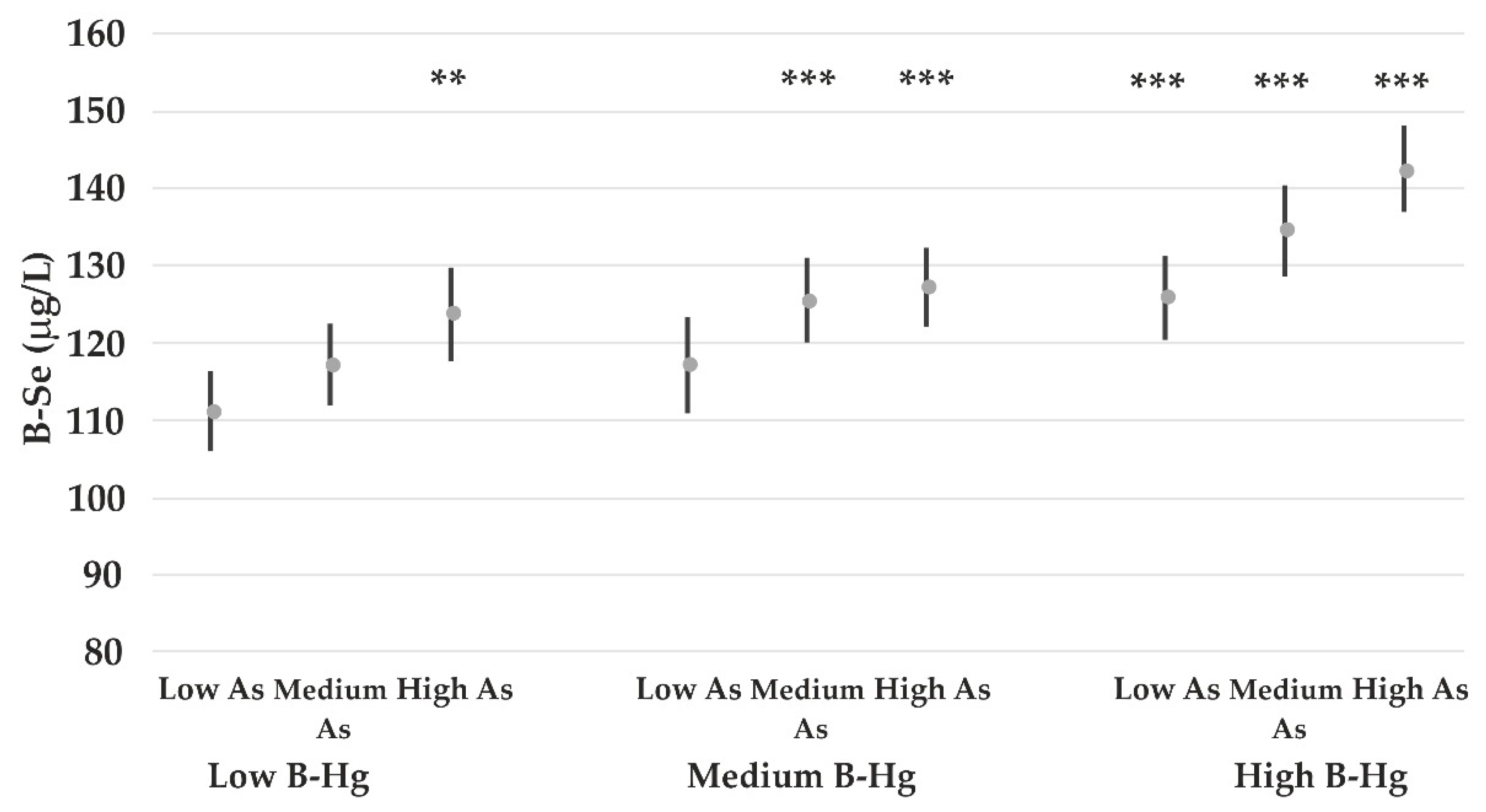
4.4. The Impact of Lead on Element Concentrations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dudarev, A.A.; Odland, J.O. Forty-Year Biomonitoring of Environmental Contaminants in Russian Arctic: Progress, Gaps and Perspectives. Int. J. Environ. Res. Public Health 2022, 19, 11951. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, W.; Stern, G.; Low, G.; Hendzel, M.; Boila, G.; Roach, P.; Evans, M.; Billeck, B.; DeLaronde, J.; Friesen, S.; et al. A history of total mercury in edible muscle of fish from lakes in northern Canada. Sci. Total Environ. 2005, 351–352, 427–463. [Google Scholar] [CrossRef] [PubMed]
- AMAP. AMAP Assessment 2015: Human Health in the Arctic, 2015. Arctic Monitoring and Assessment Programme (AMAP); AMAP: Oslo, Norway, 2015; ISBN 978-82-7971-093-6. Available online: www.amap.no (accessed on 13 May 2021).
- Sobolev, N.; Aksenov, A.; Sorokina, T.; Chashchin, V.; Ellingsen, D.G.; Nieboer, E.; Varakina, Y.; Veselkina, E.; Kotsur, D.; Thomassen, Y. Essential and non-essential trace elements in fish consumed by indigenous peoples of the European Russian Arctic. Environ. Pollut. 2019, 253, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Olmedo, P.; Hernández, A.; Pla, A.; Femia, P.; Navas-Acien, A.; Gil, F. Determination of essential elements (copper, manganese, selenium and zinc) in fish and shellfish samples. Risk and nutritional assessment and mercury–selenium balance. Food Chem. Toxicol. 2013, 62, 299–307. [Google Scholar] [CrossRef]
- Hassan, A.A.; Sandanger, T.M.; Brustad, M. Selected Vitamins and Essential Elements in Meat from Semi-Domesticated Reindeer (Rangifer tarandus tarandus L.) in Mid- and Northern Norway: Geographical Variations and Effect of Animal Population Density. Nutrients 2012, 4, 724–739. [Google Scholar] [CrossRef]
- Rosstat. Federal State Statistics Service. 2021. Available online: https://showdata.gks.ru/finder/ (accessed on 13 May 2021).
- Kozlov, A.; Khabarova, Y.; Vershubsky, G.; Ateeva, Y.; Ryzhaenkov, V. Vitamin D status of northern indigenous people of Russia leading traditional and “modernized” way of life. Int. J. Circumpolar Health 2014, 73, 26038. [Google Scholar] [CrossRef]
- Andronov, S.; Lobanov, A.; Popov, A.; Luo, Y.; Shaduyko, O.; Fesyun, A.; Lobanova, L.; Bogdanova, E.; Kobel’Kova, I. Changing diets and traditional lifestyle of Siberian Arctic Indigenous Peoples and effects on health and well-being. Ambio 2020, 50, 2060–2071. [Google Scholar] [CrossRef]
- Bellona. Industrial Pollution in Russia’s Barents Sea Areas, Belona Foundation Report. 2014. Available online: https://network.bellona.org/content/uploads/sites/3/IndustrialPollutioninRussiasBarentsSeaAreas2014_ENG.pdf (accessed on 7 September 2022).
- Ionova, I.E.; Agbalyan, E.V. Sovremennye tendenciji haraktera pitaniya i lipidnogo spectra krovi zhitelej korennoj natsionalnosti Kraijnego Severa. Profil. Zabol. I Ukrepleniya Zdoroviya 2005, 3, 16–19. [Google Scholar]
- Sobolev, N.; Ellingsen, D.G.; Belova, N.; Aksenov, A.; Sorokina, T.; Trofimova, A.; Varakina, Y.; Kotsur, D.; Grjibovski, A.M.; Chashchin, V.; et al. Essential and non-essential elements in biological samples of inhabitants residing in Nenets Autonomous Okrug of the Russian Arctic. Environ. Int. 2021, 152, 106510. [Google Scholar] [CrossRef]
- Finley, J.W. Manganese absorption and retention by young women is associated with serum ferritin concentration. Am. J. Clin. Nutr. 1999, 70, 37–43. [Google Scholar] [CrossRef]
- Bárány, E.; Bergdahl, I.; Bratteby, L.-E.; Lundh, T.; Samuelson, G.; Skerfving, S.; Oskarsson, A. Iron status influences trace element levels in human blood and serum. Environ. Res. 2005, 98, 215–223. [Google Scholar] [CrossRef]
- Meltzer, H.M.; Brantsæter, A.L.; Borch-Iohnsen, B.; Ellingsen, D.G.; Alexander, J.; Thomassen, Y.; Stigum, H.; Ydersbond, T.A. Low iron stores are related to higher blood concentrations of manganese, cobalt and cadmium in non-smoking, Norwegian women in the HUNT 2 study. Environ. Res. 2010, 110, 497–504. [Google Scholar] [CrossRef]
- Lee, B.-K.; Kim, Y. Sex-specific Profiles of Blood Metal Levels Associated with Metal–Iron Interactions. Saf. Health Work 2014, 5, 113–117. [Google Scholar] [CrossRef]
- Kim, Y.; Lobdell, D.T.; Wright, C.W. Blood metal concentrations of manganese, lead and cadmium in relation to serum ferritin levels in Ohio residents. Biol. Trace Elem. Res. 2015, 165, 1–9. [Google Scholar] [CrossRef]
- AMAP. Human Health in the Arctic; Arctic Monitoring and Assessment Programme (AMAP): Oslo, Norway, 2009; Available online: https://www.amap.no/documents/doc/amap-assessment-2009-human-health-in-the-arctic/98 (accessed on 7 December 2020).
- Sobolev, N.; Nieboer, E.; Aksenov, A.; Sorokina, T.; Chashchin, V.; Ellingsen, D.G.; Varakina, Y.; Plakhina, E.; Kotsur, D.; Kosheleva, A.; et al. Concentration dataset for 4 essential and 5 non-essential elements in fish collected in Arctic and sub-Arctic territories of the Nenets Autonomous and Arkhangelsk regions of Russia. Data Brief 2019, 27, 104631. [Google Scholar] [CrossRef]
- Åkesson, A.; Berglund, M.; Schütz, A.; Bjellerup, P.; Bremme, K.; Vahter, M. Cadmium Exposure in Pregnancy and Lactation in Relation to Iron Status. Am. J. Public Health 2002, 92, 284–287. [Google Scholar] [CrossRef]
- Cade, J.; Thompson, R.; Burley, V.; Warm, D. Development, validation and utilisation of food-frequency questionnaires—A review. Public Health Nutr. 2002, 5, 567–587. [Google Scholar] [CrossRef]
- Berglund, M.; Lind, B.; Björnberg, K.A.; Palm, B.; Einarsson, O.; Vahter, M. Inter-individual variations of human mercury exposure biomarkers: A cross-sectional assessment. Environ. Health 2005, 4, 20. [Google Scholar] [CrossRef]
- Wiseman, C.; Parnia, A.; Chakravartty, D.; Archbold, J.; Copes, R.; Cole, D. Total, methyl and inorganic mercury concentrations in blood and environmental exposure sources in newcomer women in Toronto, Canada. Environ. Res. 2019, 169, 261–271. [Google Scholar] [CrossRef]
- Mahaffey, K.R.; Clickner, R.P.; Bodurow, C.C. Blood organic mercury and dietary mercury intake: National Health and Nutrition Examination Survey, 1999 and 2000. Environ. Health Perspect. 2004, 112, 562–570. [Google Scholar] [CrossRef]
- Dallmann, W.K.; Peskov, V.V.; Murashko, O.A. Monitoring of Development of Traditional Indigenous Land Use Areas in the Nenets Autonomous Okrug, NW Russia; Project Report 138; Norwegian Polar Institute: Tromsø, Norway, 2010; ISBN 13:978-82-7666-293-1. Available online: https://hdl.handle.net/11250/173430 (accessed on 2 September 2022).
- Bradley, M.A.; Barst, B.D.; Basu, N. A Review of Mercury Bioavailability in Humans and Fish. Int. J. Environ. Res. Public Health 2017, 14, 169. [Google Scholar] [CrossRef] [PubMed]
- Gochfeld, M.; Burger, J. Mercury interactions with selenium and sulfur and the relevance of the Se:Hg molar ratio to fish consumption advice. Environ. Sci. Pollut. Res. 2021, 28, 18407–18420. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in Human Health and Disease. Antioxidants Redox Signal. 2011, 14, 1337–1383. [Google Scholar] [CrossRef] [PubMed]
- Thomson, C.D. Assessment of requirements for selenium and adequacy of selenium status: A review. Eur. J. Clin. Nutr. 2004, 58, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Falandysz, J. Selenium in Edible Mushrooms. J. Environ. Sci. Health Part C 2008, 26, 256–299. [Google Scholar] [CrossRef]
- Brantsæter, A.L.; Haugen, M.; Thomassen, Y.; Ellingsen, D.G.; A Ydersbond, T.; Hagve, T.-A.; Alexander, J.; Meltzer, H.M. Exploration of biomarkers for total fish intake in pregnant Norwegian women. Public Health Nutr. 2009, 13, 54–62. [Google Scholar] [CrossRef]
- Yamashita, M.; Yamashita, Y.; Ando, T.; Wakamiya, J.; Akiba, S. Identification and Determination of Selenoneine, 2-Selenyl-N α, N α, N α -Trimethyl-l-Histidine, as the Major Organic Selenium in Blood Cells in a Fish-Eating Population on Remote Japanese Islands. Biol. Trace Element Res. 2013, 156, 36–44. [Google Scholar] [CrossRef]
- Klein, M.; Ouerdane, L.; Bueno, M.; Pannier, F. Identification in human urine and blood of a novel selenium metabolite, Se-methylselenoneine, a potential biomarker of metabolization in mammals of the naturally occurring selenoneine, by HPLC coupled to electrospray hybrid linear ion trap-orbital ion trap MS. Metallomics 2011, 3, 513–520. [Google Scholar] [CrossRef]
- Little, M.; Achouba, A.; Dumas, P.; Ouellet, N.; Ayotte, P.; Lemire, M. Determinants of selenoneine concentration in red blood cells of Inuit from Nunavik (Northern Québec, Canada). Environ. Int. 2019, 127, 243–252. [Google Scholar] [CrossRef]
- Achouba, A.; Dumas, P.; Ouellet, N.; Little, M.; Lemire, M.; Ayotte, P. Selenoneine is a major selenium species in beluga skin and red blood cells of Inuit from Nunavik. Chemosphere 2019, 229, 549–558. [Google Scholar] [CrossRef]
- Ye, Q.; Park, J.E.; Gugnani, K.; Betharia, S.; Pino-Figueroa, A.; Kim, J. Influence of iron metabolism on manganese transport and toxicity. Metallomics 2017, 9, 1028–1046. [Google Scholar] [CrossRef]
- Aschner, J.L.; Aschner, M. Nutritional aspects of manganese homeostasis. Mol. Asp. Med. 2005, 26, 353–362. [Google Scholar] [CrossRef]
- Aschner, M.; Erikson, K. Manganese. Adv. Nutr. Int. Rev. J. 2017, 8, 520–521. [Google Scholar] [CrossRef]
- Hassan, A.A.; Rylander, C.; Brustad, M.; Sandanger, T.M. Persistent organic pollutants in meat, liver, tallow and bone marrow from semi-domesticated reindeer (Rangifer tarandus tarandus L.) in Northern Norway. Acta Veter-Scand. 2013, 55, 57. [Google Scholar] [CrossRef]
- Tsuji, L.J.; Wainman, B.C.; Martin, I.D.; Sutherland, C.; Weber, J.-P.; Dumas, P.; Nieboer, E. Lead shot contribution to blood lead of First Nations people: The use of lead isotopes to identify the source of exposure. Sci. Total Environ. 2008, 405, 180–185. [Google Scholar] [CrossRef]
- Johansen, P.; Pedersen, H.S.; Asmund, G.; Riget, F. Lead shot from hunting as a source of lead in human blood. Environ. Pollut. 2006, 142, 93–97. [Google Scholar] [CrossRef]
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and its importance for human health: An integrative review. J. Res. Med. Sci. 2013, 18, 144–157. [Google Scholar]
- Bost, M.; Houdart, S.; Oberli, M.; Kalonji, E.; Huneau, J.-F.; Margaritis, I. Dietary copper and human health: Current evidence and unresolved issues. J. Trace Elements Med. Biol. 2016, 35, 107–115. [Google Scholar] [CrossRef]
- Zoller, H.; Weiss, G.; Theurl, I.; Koch, R.O.; Vogel, W.; Obrist, P.; Pietrangelo, A.; Montosi, G.; Haile, D.J. Expression of the duodenal iron transporters divalent-metal transporter 1 and ferroportin 1 in iron deficiency and iron overload. Gastroenterology 2001, 120, 1412–1419. [Google Scholar] [CrossRef]
- Tholin, K.; Sandström, B.; Palm, R.; Hallmans, G. Changes in Blood Manganese Levels During Pregnancy in Iron Supplemented and non Supplemented Women. J. Trace Elements Med. Biol. 1995, 9, 13–17. [Google Scholar] [CrossRef]
- Finley, J.W.; E Johnson, P.; Johnson, L.K. Sex affects manganese absorption and retention by humans from a diet adequate in manganese. Am. J. Clin. Nutr. 1994, 60, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.; Landrigan, P.J.; Baker, E.L.; Orenstein, W.A.; von Lindern, I.H. Lead-induced anemia: Dose-response relationships and evidence for a threshold. Amer. J. Pub. Health 1990, 80, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Skerfving, S.; Bergdahl, I.A. Lead. In Handbook on the Toxicology of Metals, 4th ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 655–666. [Google Scholar]
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicol. 2012, 5, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Labbé, R.F.; Vreman, H.J.; Stevenson, D.K. Zinc Protoporphyrin: A Metabolite with a Mission. Clin. Chem. 2000, 45, 2060–2072. [Google Scholar] [CrossRef]
- Wibowo, A.A.E.; Sallé, H.J.A.; Del Castilho, P.; Zielhuis, R.L. An effect of erythrocyte protoporphyrin on blood manganese in lead-exposed children and adults. Int. Arch. Occup. Environ. Health 1979, 43, 177–182. [Google Scholar] [CrossRef]
- Borg, D.C.; Cotzias, G.C. Incorporation of Manganese into Erythrocytes as Evidence for a Manganese Porphyrin in Man. Nature 1958, 182, 1677–1678. [Google Scholar] [CrossRef]
- Hancock, R.; Fritze, K. Chromatographic evidence for the existence of a manganese porphyrin in erythrocytes. Bioinorg. Chem. 1973, 3, 77–87. [Google Scholar] [CrossRef]
- Plante, C.; Blanchet, C.; Rochette, L.; O’Brien, H.T. Prevalence of anemia among Inuit women in Nunavik, Canada. Int. J. Circumpolar Health 2011, 70, 154–165. [Google Scholar] [CrossRef][Green Version]
| Non-Nenets n = 136 | Nenets n = 161 | Women n = 204 | Men n = 93 | Coastal n = 148 | Inland n = 149 | |
|---|---|---|---|---|---|---|
| Prevalence (%) | Prevalence (%) | Prevalence (%) | ||||
| Fish | ||||||
| Anadromous | 88.9 * | 78.9 | 84.2 | 81.7 | 88.4 * | 78.5 |
| Semi-anadromous | 61.5 ** | 44.1 | 47.8 * | 61.3 | 44.9 * | 59.1 |
| Marine | 67.4 | 60.9 | 65.0 | 61.3 | 74.8 *** | 53.0 |
| Freshwater | 59.3 * | 46.6 | 52.2 | 52.7 | 29.7 *** | 70.3 |
| Meat Reindeer Other local meat (hare, elk, wild boar) | 88.1 * 16.3 | 75.2 11.8 | 81.3 12.3 | 80.6 17.2 | 78.2 10.2 | 83.9 17.4 |
| Birds Goose Other birds (duck and partridge) | 81.5 52.6 | 82.5 43.5 | 82.2 44.3 | 81.7 54.8 | 88.4 ** 45.6 | 75.8 49.7 |
| Berries and mushroom Mushroom Blueberries Cloudberries Cowberries Other berries | 31.9 47.8 78.4 73.1 34.3 * | 26.7 49.1 82.6 66.5 22.4 | 31.0 50.5 84.2 * 73.8 * 30.7 | 24.7 44.1 73.1 60.2 21.5 | 36.7 * 36.3 *** 77.4 58.9 *** 30.1 | 21.5 60.4 83.9 79.9 25.5 |
| Whole blood concentrations c | AM (min–max) μg/L | AM (min–max) μg/L | AM (min–max) μg/L | AM (min–max) μg/L | AM (min–max) μg/L | AM (min–max) μg/L |
| Co b Cu a Mn Se Zn a As b Cd b Hg Pb b S-ferritin | 0.5 (0.3–1.7) 1.05 (0.58–1.9) ** 12.1 (4.4–27) *** 128 (89–227) ** 9.1 (4.2–13.7) ** 5.9 (0.5–143) ** 0.3 (<DL-4.2) ** 4.6 (0.5–18) ** 20 (5.4–356) *** 105 (3–506) | 0.5 (0.3–2.5) 1.13 (0.73–2.4) 15.1 (4.3–35) 122 (85–192) 8.6 (4.1–14.5) 3.9 (0.5–163) 0.4 (<DL-3.0) 5.6 (0.3–24) 29 (5.2–281) 95 (0.5–506) | 0.6 (0.3–2.5) *** 1.14 (0.69–2.4) *** 14.0 (4.3–35) 125 (85–227) *** 8.6 (4.2–14.5) 5.2 (0.5–163) 0.3 (<DL-4.2) 5.1 (0.3–24) 21 (5.2–356) *** 80 (0.5–463) *** | 0.5 (0.3–0.9) 1.01 (0.58–1.5) 13.2 (4.5–24) 124 (89–192) 9.4 (4.1–12.9) 3.9 (0.5–143) 0.4 (<DL-2.6) 5.4 (0.6–24) 35 (5.4–281) 143 (13–506) | 0.5 (0.3–2.5) 1.07 (0.58–2.4) ** 15.4 (5.3–35) *** 127 (85–192) 9.3 (4.9–13.7) *** 7.6 (0.5–163) *** 0.3 (<DL-4.2) 3.8 (0.3–21) *** 34 (7.2–356) *** 88 (0.5–463) * | 0.5 (0.3–1.2) 1.13 (0.69–1.9) 12.0 (4.3–27) 123 (89–227) 8.4 (4.1–14.5) 3.0 (0.5–50) 0.3 (0.1–3.0) 6.5 (0.9–24) 17 (5.2–164) 111 (3–506) |
| Co | Cu | Zn | Cd | Hg | Pb | As | Se | |
|---|---|---|---|---|---|---|---|---|
| Mn | 0.19 ** | −0.20 ** | 0.22 ** | 0.11 | −0.08 | 0.31 ** | 0.22 ** | 0.06 |
| Co | - | 0.26 ** | −0.13 * | 0.26 ** | −0.17 ** | −0.02 | −0.09 | −0.16 ** |
| Cu | - | −0.33 ** | 0.05 | 0.06 | −0.11* | −0.14 * | −0.11 | |
| Zn | - | 0.14 * | 0.02 | 0.30 ** | 0.10 | 0.23 ** | ||
| Cd | - | 0.02 | 0.25 ** | −0.11 | −0.07 | |||
| Hg | - | 0.15 ** | 0.06 | 0.33 ** | ||||
| Pb | - | 0.15 ** | 0.16 ** | |||||
| As | - | 0.32 ** |
| α | β | |||||||
|---|---|---|---|---|---|---|---|---|
| S-ferritin | Sex | B-Pb (lg) | Smoke | Age | BMI | Food item | ||
| B-Mn | 6.8 *** | −0.02 *** | - | 5.5 *** | - | - | - | 1.8 Cloudberry ** |
| B-Co (lg) | −0.20 *** | −0.0004 *** | −0.05 ** | - | - | −0.001 * | - | 0.04 Reindeer * |
| B-Se | 97 *** | 0.02 * | - | 7.3 * | - | - | 0.45 * | 6.0 Mushroom * 7.3 Local meat * |
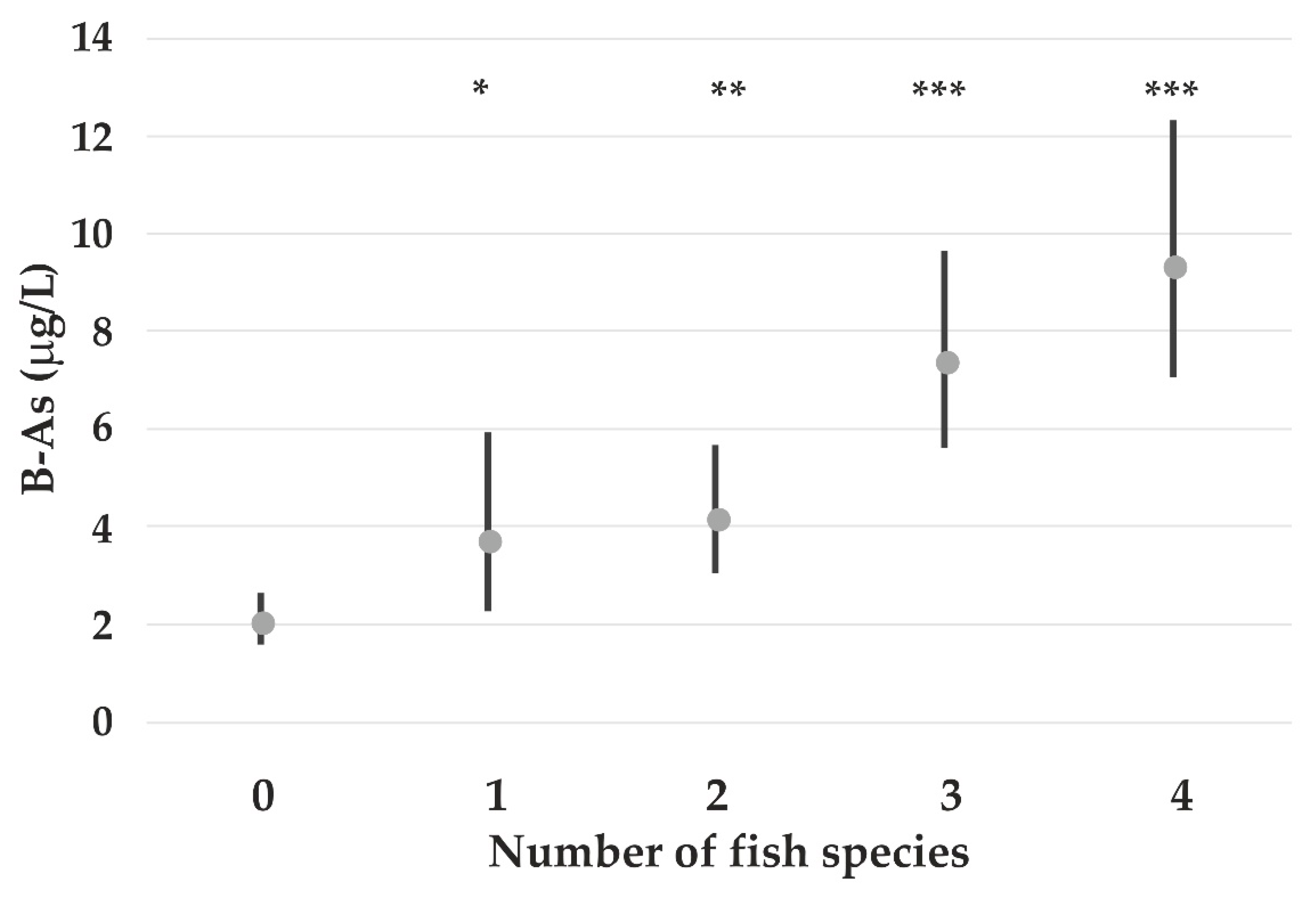
| B-As (lg) | −0.31 ns | - | - | 0.23 ** | - | 0.008 *** | - | 0.44 Marine fish *** |
| B-Hg | 0.18 ns | 0.009 *** | - | 2.05 ** | - | - | - | 2.4 Freshw. Fish *** |
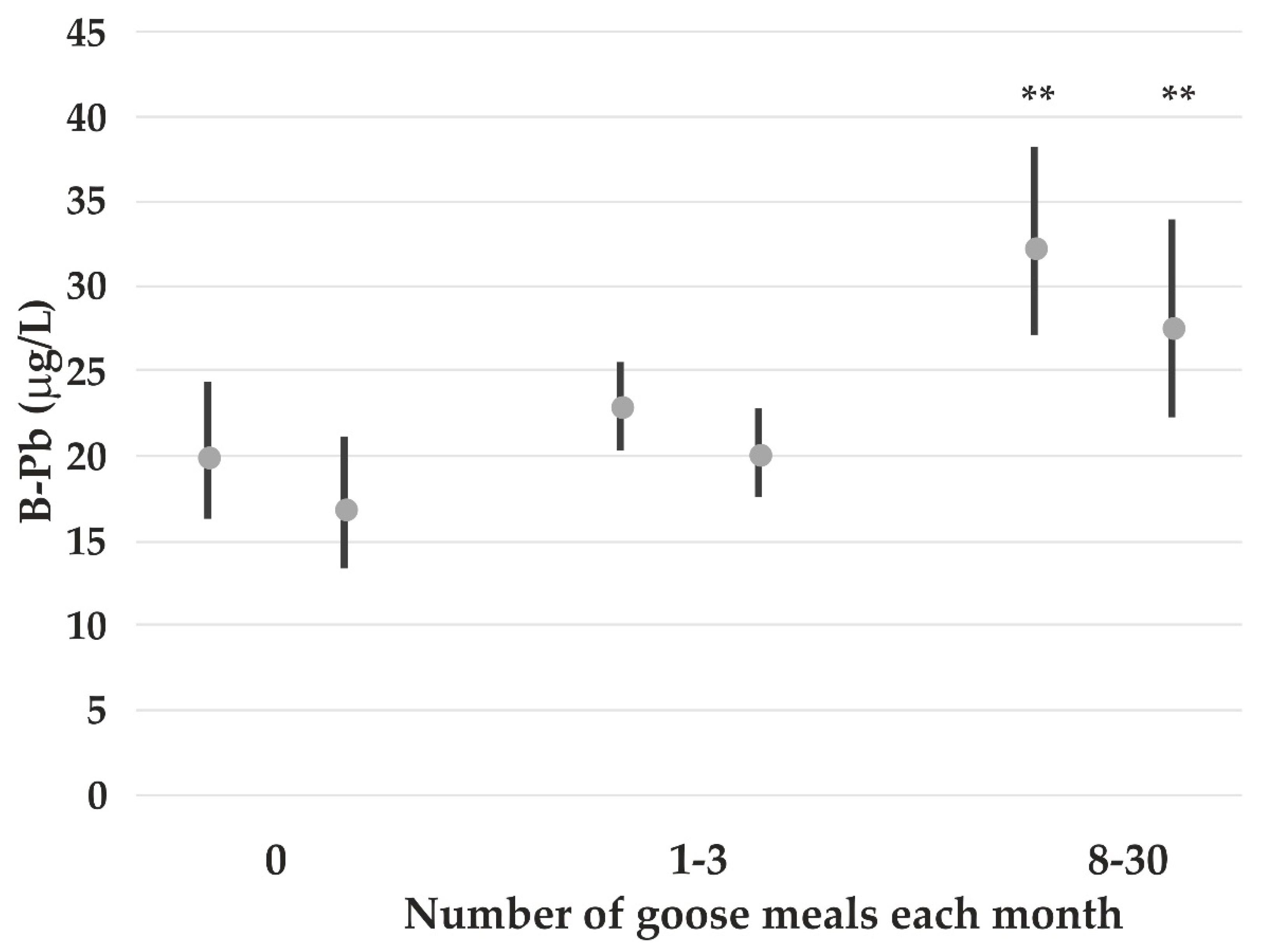
| B-Pb | 1.20 *** | - | 0.21 *** | ♦ | 0.11 * | - | - | 0.10 Goose * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorokina, T.; Sobolev, N.; Belova, N.; Aksenov, A.; Kotsur, D.; Trofimova, A.; Varakina, Y.; Grjibovski, A.M.; Chashchin, V.; Korobitsyna, R.; et al. Diet and Blood Concentrations of Essential and Non-Essential Elements among Rural Residents in Arctic Russia. Nutrients 2022, 14, 5005. https://doi.org/10.3390/nu14235005
Sorokina T, Sobolev N, Belova N, Aksenov A, Kotsur D, Trofimova A, Varakina Y, Grjibovski AM, Chashchin V, Korobitsyna R, et al. Diet and Blood Concentrations of Essential and Non-Essential Elements among Rural Residents in Arctic Russia. Nutrients. 2022; 14(23):5005. https://doi.org/10.3390/nu14235005
Chicago/Turabian StyleSorokina, Tatiana, Nikita Sobolev, Natalia Belova, Andrey Aksenov, Dmitriy Kotsur, Anna Trofimova, Yulia Varakina, Andrej M. Grjibovski, Valerii Chashchin, Rimma Korobitsyna, and et al. 2022. "Diet and Blood Concentrations of Essential and Non-Essential Elements among Rural Residents in Arctic Russia" Nutrients 14, no. 23: 5005. https://doi.org/10.3390/nu14235005
APA StyleSorokina, T., Sobolev, N., Belova, N., Aksenov, A., Kotsur, D., Trofimova, A., Varakina, Y., Grjibovski, A. M., Chashchin, V., Korobitsyna, R., & Thomassen, Y. (2022). Diet and Blood Concentrations of Essential and Non-Essential Elements among Rural Residents in Arctic Russia. Nutrients, 14(23), 5005. https://doi.org/10.3390/nu14235005








