The Association of Serum L-Carnitine Concentrations with the Risk of Cancer in Chinese Adults with Hypertension
Abstract
1. Introduction
2. Methods
2.1. Study Population and Design
2.2. Nested Case-Control Study
2.3. Outcome Ascertainment
2.4. Exposure and Potential Confounders
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Participants
3.2. Association of LC with the Risk of Overall, Digestive, and Non-Digestive System Cancer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Stanaway, J.D.; Afshin, A.; Gakidou, E.; Lim, S.S.; Abate, D.; Abate, K.H.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Bleyer, A.; et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar]
- Liu, T.; Song, C.; Zhang, Y.; Siyin, S.T.; Zhang, Q.; Song, M.; Cao, L.; Shi, H. Hepatitis B virus infection and the risk of gastrointestinal cancers among Chinese population: A prospective cohort study. Int. J. Cancer 2022, 150, 1018–1028. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, Q.; Song, C.; Siyin, S.T.; Chen, S.; Zhang, Q.; Song, M.; Cao, L.; Shi, H. C-reactive protein trajectories and the risk of all cancer types: A prospective cohort study. Int. J. Cancer 2022, 151, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Karlic, H.; Lohninger, A. Supplementation of L-carnitine in athletes: Does it make sense? Nutrition 2004, 20, 709–715. [Google Scholar] [CrossRef]
- Sawicka, A.K.; Renzi, G.; Olek, R.A. The bright and the dark sides of L-carnitine supplementation: A systematic review. J. Int. Soc. Sports Nutr. 2020, 17, 49. [Google Scholar] [CrossRef]
- Ringseis, R.; Keller, J.; Eder, K. Mechanisms underlying the anti-wasting effect of L-carnitine supplementation under pathologic conditions: Evidence from experimental and clinical studies. Eur. J. Nutr. 2013, 52, 1421–1442. [Google Scholar] [CrossRef]
- Stephens, F.B.; Wall, B.T.; Marimuthu, K.; Shannon, C.E.; Constantin-Teodosiu, D.; Macdonald, I.A.; Greenhaff, P.L. Skeletal muscle carnitine loading increases energy expenditure, modulates fuel metabolism gene networks and prevents body fat accumulation in humans. J. Physiol. 2013, 591, 4655–4666. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Park, J.; Chang, H.; Lim, K. l-Carnitine supplement reduces skeletal muscle atrophy induced by prolonged hindlimb suspension in rats. Appl. Physiol. Nutr. Metab. 2016, 41, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Askarpour, M.; Hadi, A.; Miraghajani, M.; Symonds, M.E.; Sheikhi, A.; Ghaedi, E. Beneficial effects of l-carnitine supplementation for weight management in overweight and obese adults: An updated systematic review and dose-response meta-analysis of randomized controlled trials. Pharmacol. Res. 2020, 151, 104554. [Google Scholar] [CrossRef]
- Malaguarnera, M.; Cammalleri, L.; Gargante, M.P.; Vacante, M.; Colonna, V.; Motta, M. L-Carnitine treatment reduces severity of physical and mental fatigue and increases cognitive functions in centenarians: A randomized and controlled clinical trial. Am. J. Clin Nutr. 2007, 86, 1738–1744. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Heianza, Y.; Ma, W.; Manson, J.E.; Rexrode, K.M.; Qi, L. Gut Microbiota Metabolites and Risk of Major Adverse Cardiovascular Disease Events and Death: A Systematic Review and Meta-Analysis of Prospective Studies. J. Am. Heart Assoc. 2017, 6, e004947. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.W.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.S.; Levison, B.S.; Hazen, S.L. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res. 2015, 116, 448–455. [Google Scholar]
- Bae, S.; Ulrich, C.M.; Neuhouser, M.L.; Malysheva, O.; Bailey, L.B.; Xiao, L.; Brown, E.C.; Cushing-Haugen, K.L.; Zheng, Y.; Caudill, M.A.; et al. Plasma choline metabolites and colorectal cancer risk in the Women’s Health Initiative Observational Study. Cancer Res. 2014, 74, 7442–7452. [Google Scholar] [CrossRef] [PubMed]
- Guertin, K.A.; Li, X.S.; Graubard, B.I.; Albanes, D.; Weinstein, S.J.; Goedert, J.J.; Wang, Z.; Hazen, S.L.; Sinha, R. Serum Trimethylamine N-oxide, Carnitine, Choline, and Betaine in Relation to Colorectal Cancer Risk in the Alpha Tocopherol, Beta Carotene Cancer Prevention Study. Cancer Epidemiol. Biomark. Prev. 2017, 26, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Baci, D.; Bruno, A.; Cascini, C.; Gallazzi, M.; Mortara, L.; Sessa, F.; Pelosi, G.; Albini, A.; Noonan, D.M. Acetyl-L-Carnitine downregulates invasion (CXCR4/CXCL12, MMP-9) and angiogenesis (VEGF, CXCL8) pathways in prostate cancer cells: Rationale for prevention and interception strategies. J. Exp. Clin. Cancer Res. 2019, 38, 464. [Google Scholar] [CrossRef]
- Baci, D.; Bruno, A.; Bassani, B.; Tramacere, M.; Mortara, L.; Albini, A.; Noonan, D.M. Acetyl-l-carnitine is an anti-angiogenic agent targeting the VEGFR2 and CXCR4 pathways. Cancer Lett. 2018, 429, 100–116. [Google Scholar] [CrossRef]
- Wenzel, U.; Nickel, A.; Daniel, H. Increased carnitine-dependent fatty acid uptake into mitochondria of human colon cancer cells induces apoptosis. J. Nutr. 2005, 135, 1510–1514. [Google Scholar] [CrossRef]
- A Cruciani, R.; Dvorkin, E.; Homel, P.; Culliney, B.; Malamud, S.; Shaiova, L.; Fleishman, S.; Lapin, J.; Klein, E.; Lesage, P.; et al. L-carnitine supplementation for the treatment of fatigue and depressed mood in cancer patients with carnitine deficiency: A preliminary analysis. Ann. N.Y. Acad. Sci. 2004, 1033, 168–176. [Google Scholar] [CrossRef]
- Kraft, M.; Kraft, K.; Gärtner, S.; Mayerle, J.; Simon, P.; Weber, E.; Schütte, K.; Stieler, J.; Koula-Jenik, H.; Lerch, M.M.; et al. L-Carnitine-supplementation in advanced pancreatic cancer (CARPAN)--a randomized multicentre trial. Nutr. J. 2012, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Armenian, S.H.; Gelehrter, S.K.; Vase, T.; Venkatramani, R.; Landier, W.; Wilson, K.D.; Herrera, C.; Reichman, L.; Menteer, J.-D.; Mascarenhas, L.; et al. Carnitine and cardiac dysfunction in childhood cancer survivors treated with anthracyclines. Cancer Epidemiol Biomark. Prev. 2014, 23, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Iwase, S.; Kawaguchi, T.; Yotsumoto, D.; Miyara, K.; Odagiri, H.; Kitamura, K.; Ariyoshi, K.; Miyaji, T.; Ishiki, H.; Yamaguchi, T.; et al. Efficacy and safety of an amino acid jelly containing coenzyme Q10 and L-carnitine in controlling fatigue in breast cancer patients receiving chemotherapy: A multi-institutional, randomized, exploratory trial (JORTC-CAM01). Support Care Cancer 2016, 24, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Cruciani, R.A.; Zhang, J.J.; Manola, J.; Cella, D.; Ansari, B.; Fisch, M.J. L-carnitine supplementation for the management of fatigue in patients with cancer: An eastern cooperative oncology group phase III, randomized, double-blind, placebo-controlled trial. J. Clin. Oncol. 2012, 30, 3864–3869. [Google Scholar] [CrossRef]
- Gramignano, G.; Lusso, M.R.; Madeddu, C.; Massa, E.; Serpe, R.; Deiana, L.; Lamonica, G.; Dessì, M.; Spiga, C.; Astara, G.; et al. Efficacy of l-carnitine administration on fatigue, nutritional status, oxidative stress, and related quality of life in 12 advanced cancer patients undergoing anticancer therapy. Nutrition 2006, 22, 136–145. [Google Scholar] [CrossRef]
- Laviano, A.; Rianda, S.; Molfino, A.; Rossi Fanelli, F. Omega-3 fatty acids in cancer. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 156–161. [Google Scholar] [CrossRef]
- Arshad, A.; Isherwood, J.; Mann, C.; Cooke, J.; Pollard, C.; Runau, F.; Morgan, B.; Steward, W.; Metcalfe, M.; Dennison, A. Intravenous ω-3 Fatty Acids Plus Gemcitabine. JPEN J. Parenter Enteral. Nutr. 2017, 41, 398–403. [Google Scholar] [CrossRef]
- Georgescauld, F.; Mocan, I.; Lacombe, M.L.; Lascu, I. Rescue of the neuroblastoma mutant of the human nucleoside diphosphate kinase A/nm23-H1 by the natural osmolyte trimethylamine-N-oxide. FEBS Lett. 2009, 583, 820–824. [Google Scholar] [CrossRef]
- Kirby, T.W.; DeRose, E.F.; Beard, W.A.; Shock, D.D.; Wilson, S.H.; London, R.E. Substrate rescue of DNA polymerase β containing a catastrophic L22P mutation. Biochemistry 2014, 53, 2413–2422. [Google Scholar] [CrossRef]
- Kim, K.-B.; Yang, J.-Y.; Kwack, S.J.; Park, K.L.; Kim, H.S.; Ryu, D.H.; Kim, Y.-J.; Hwang, G.-S.; Lee, B.M. Toxicometabolomics of urinary biomarkers for human gastric cancer in a mouse model. J. Toxicol. Environ. Health A 2010, 73, 1420–1430. [Google Scholar] [CrossRef]
- Spence, J.D. Diet for stroke prevention. Stroke Vasc. Neurol. 2018, 3, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Talenezhad, N.; Mohammadi, M.; Ramezani-Jolfaie, N.; Mozaffari-Khosravi, H.; Salehi-Abargouei, A. Effects of l-carnitine supplementation on weight loss and body composition: A systematic review and meta-analysis of 37 randomized controlled clinical trials with dose-response analysis. Clin. Nutr. ESPEN 2020, 37, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Bodaghi-Namileh, V.; Sepand, M.R.; Omidi, A.; Aghsami, M.; Seyednejad, S.A.; Kasirzadeh, S.; Sabzevari, O. Acetyl-l-carnitine attenuates arsenic-induced liver injury by abrogation of mitochondrial dysfunction, inflammation, and apoptosis in rats. Environ. Toxicol. Pharmacol. 2018, 58, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, M.; Li, Q.; Zhao, H.; Wang, J.; Li, Y. Acetyl-l-carnitine inhibits TNF-alpha-induced insulin resistance via AMPK pathway in rat skeletal muscle cells. FEBS Lett. 2009, 583, 470–474. [Google Scholar] [CrossRef]
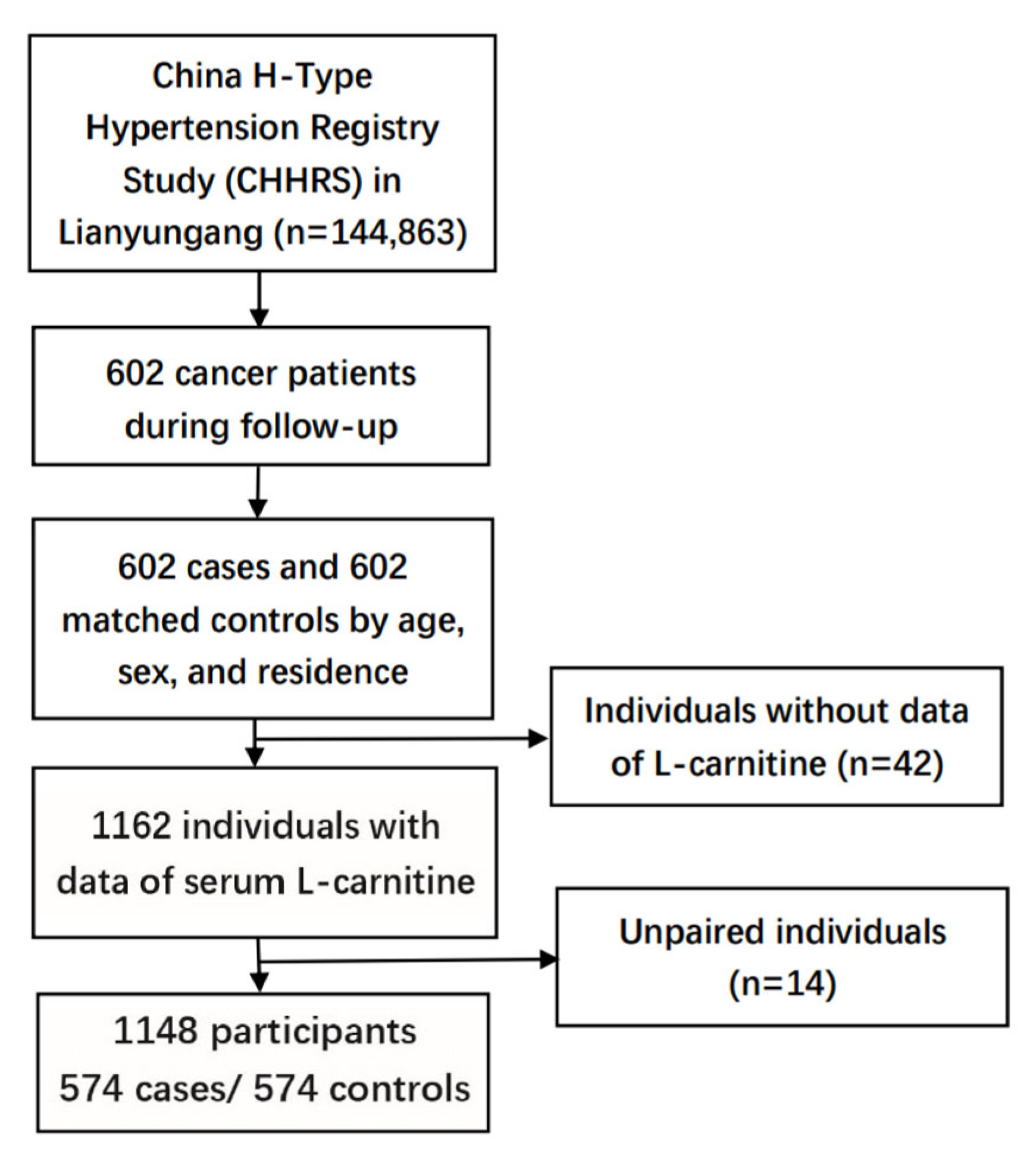
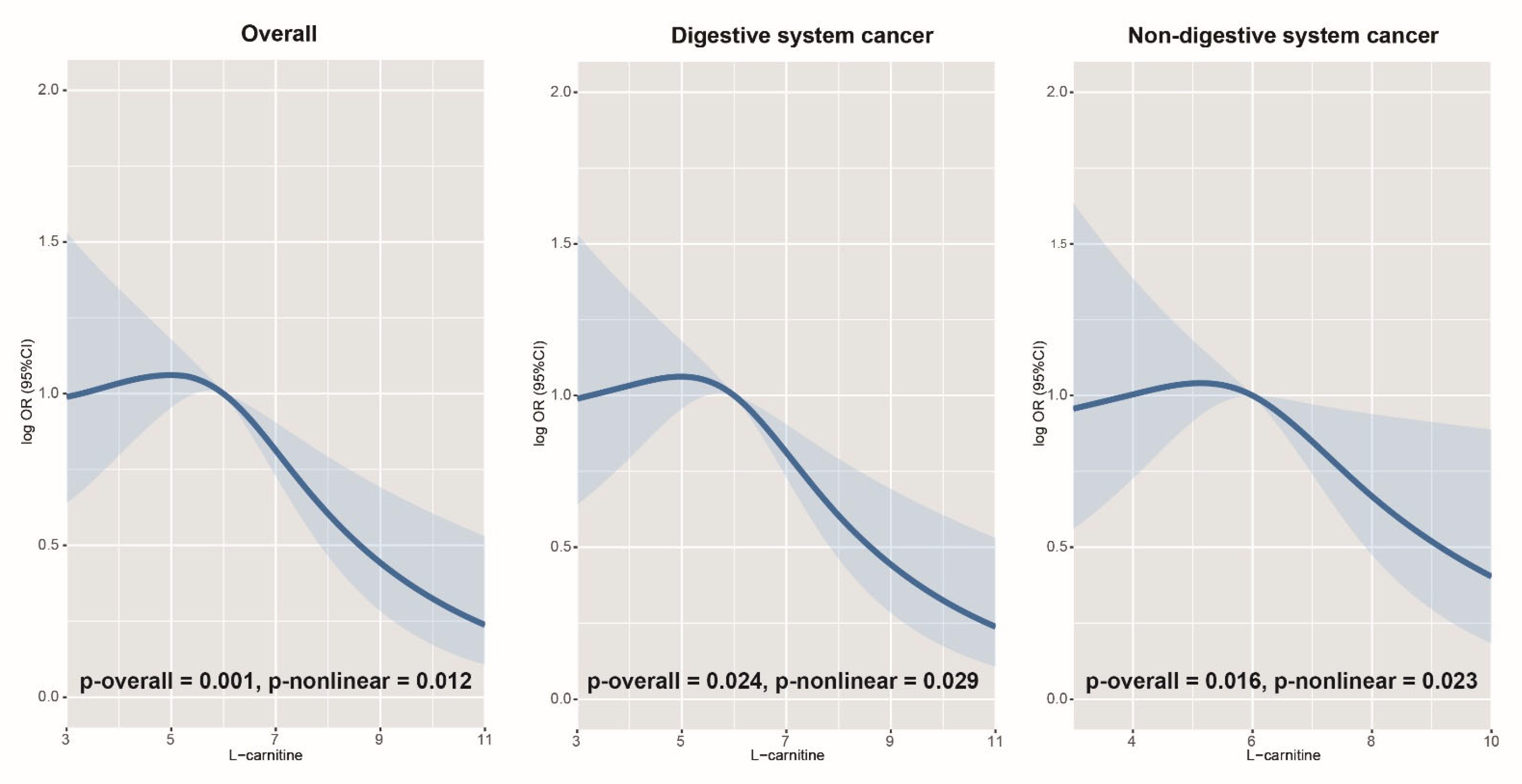
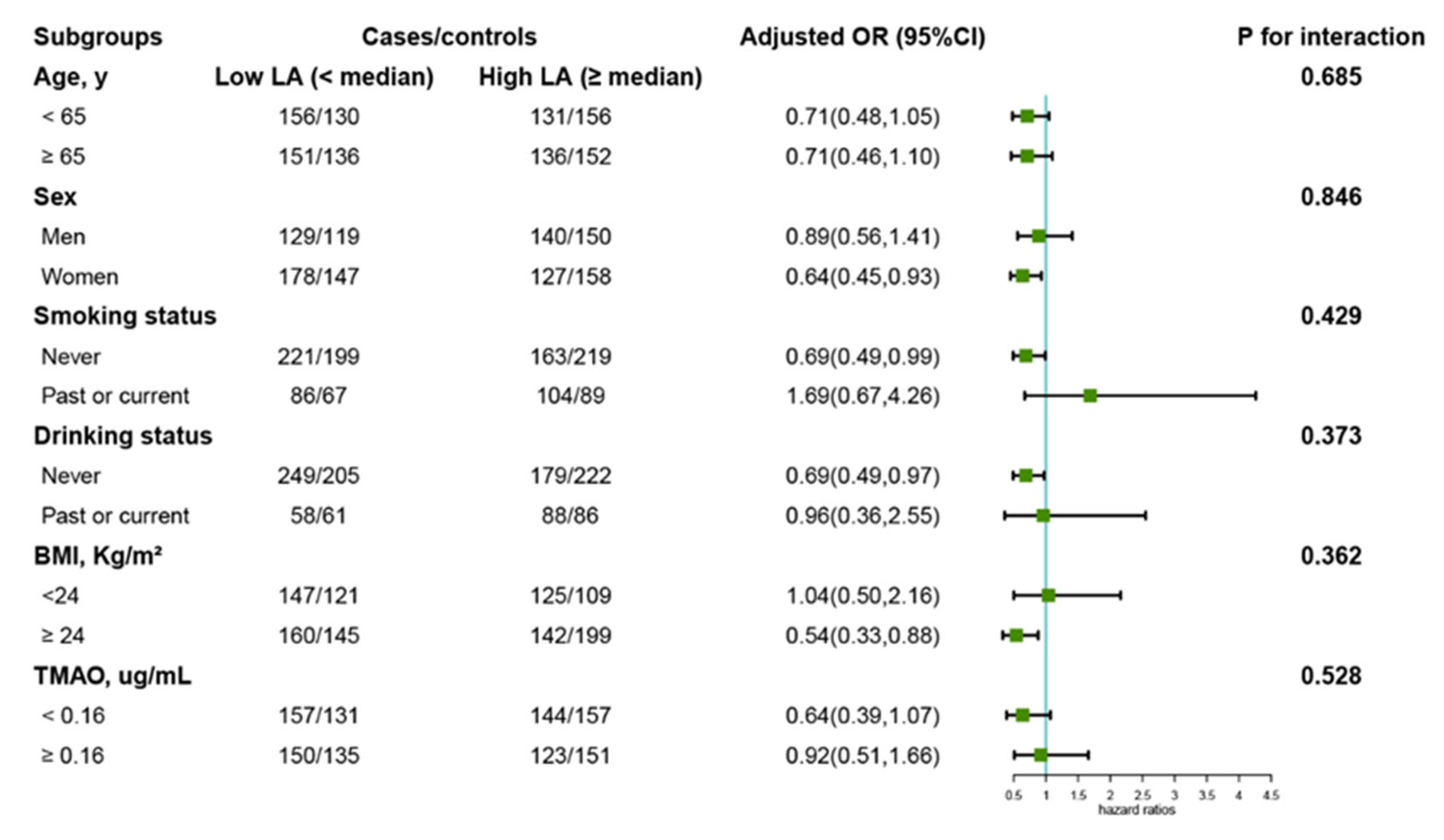
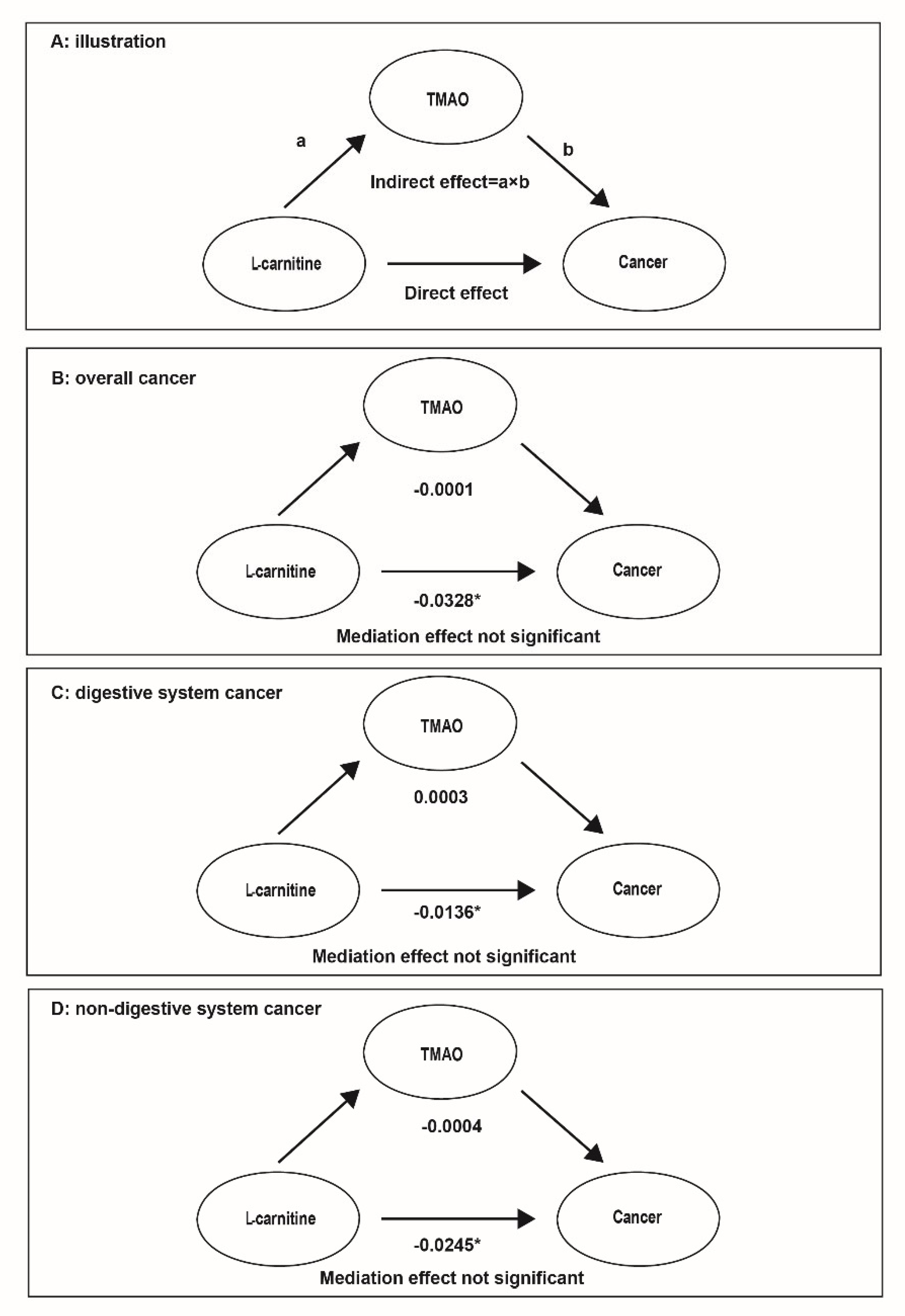
| Variables | Controls (n = 574) | Cases (n = 574) | p-Value |
|---|---|---|---|
| Age, year | 64.78 ± 9.53 | 64.78 ± 9.53 | 0.995 |
| Men, n (%) | 269 (46.86) | 269 (46.86) | 1.000 |
| BMI, kg/m2 | 24.89 ± 3.33 | 24.55 ± 3.95 | 0.114 |
| Baseline SBP, mmHg | 139.46 ± 18.59 | 138.60 ± 18.77 | 0.438 |
| Baseline DBP, mmHg | 86.00 ± 13.64 | 85.84 ± 14.53 | 0.845 |
| ALT, U/L | 15.0 (12.0, 21.0) | 15.0 (11.0, 21.0) | 0.564 |
| ALB, g/L | 44.61 ± 2.42 | 44.10 ± 2.94 | 0.002 |
| TG, mmol/L | 1.41 (0.92, 2.29) | 1.30 (0.88, 2.14) | 0.258 |
| TC, mmol/L | 5.60 ± 1.08 | 5.54 ± 1.04 | 0.322 |
| UA, umol/L | 305.0 (259.0, 356.0) | 305.0 (255.0, 358.0) | 0.863 |
| HDL-C, mmol/L | 1.15 ± 0.25 | 1.15 ± 0.26 | 0.795 |
| FBG, mmol/L | 6.36 ± 1.75 | 6.29 ± 1.47 | 0.493 |
| Creatinine, umol/L | 53.0 (46.0, 62.0) | 53.0 (47.0, 62.0) | 0.756 |
| TMAO, μg/mL | 0.16 (0.10,0.25) | 0.16 (0.09,0.26) | 0.612 |
| L-carnitine, μg/mL | 6.07 ± 1.81 | 5.65 ± 1.49 | <0.001 |
| Marital status, (married, n (%)) | 495 (86.24) | 487 (84.84) | 0.502 |
| High school or above, n (%) | 32 (5.57) | 23 (4.01) | 0.214 |
| Current smoker, n (%) | 109 (18.99) | 148 (25.78) | 0.022 |
| Current drinker, n (%) | 120 (20.91) | 110 (19.16) | 0.423 |
| History of CKD, n (%) | 4 (0.70) | 6 (1.05) | 0.525 |
| History of CHD, n (%) | 0 (0) | 39 (6.79) | <0.001 * |
| History of stroke, n (%) | 0 (0) | 70 (12.20) | <0.001 * |
| Family history of cancer, n (%) | 18 (3.14) | 26 (4.53) | 0.170 |
| Poor sleep quality, n (%) | 66 (11.50) | 97 (16.90) | 0.015 |
| Antihypertensive drug usage, n (%) | 193 (33.62) | 186 (32.40) | 0.660 |
| Meat intake per week, n (%) | 0.104 | ||
| Seldom | 265 (46.17) | 302 (52.61) | |
| 1–2 times | 201 (35.02) | 181 (31.53) | |
| ≥3 times | 108 (18.81) | 91 (15.95) | |
| Type of meat consumed, n (%) | 0.028 | ||
| None | 136 (23.69) | 166 (28.92) | |
| Lean meat | 309 (53.83) | 281 (48.95) | |
| Mixed lean and fatty | 92 (16.03) | 74 (12.89) | |
| Fatty meat | 37 (6.45) | 53 (9.23) |
| L-Carnitine (μg/mL) | Cases/Controls (Ratio 1:1) | Crude Model | Adjusted Model | ||
|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | ||
| Per 1-unit increment | 574/574 | 0.87 (0.81, 0.93) | <0.001 | 0.86 (0.79, 0.94) | 0.001 |
| Quartiles | |||||
| Q1 (<4.72) | 160/130 | Ref. | Ref. | ||
| Q2 (4.72–5.78) | 147/136 | 0.90 (0.63, 1.27) | 0.543 | 0.83 (0.56, 1.24) | 0.338 |
| Q3 (5.79–6.84) | 137/151 | 0.75 (0.53, 1.06) | 0.106 | 0.72 (0.49, 1.07) | 0.105 |
| Q4 (≥6.85) | 130/157 | 0.70 (0.50, 0.98) | 0.037 | 0.67 (0.46, 0.99) | 0.046 |
| Categories | |||||
| Low (<5.78, median) | 307/266 | Ref. | Ref. | ||
| High (≥5.78, median) | 267/308 | 0.76 (0.60, 0.97) | 0.026 | 0.75 (0.57, 0.99) | 0.039 |
| L-Carnitine (μg/mL) | Digestive System | Non-Digestive System | ||||
|---|---|---|---|---|---|---|
| CASES/CONTROLS | OR (95% CI) | p-Value | Cases/Controls | OR (95% CI) | p-Value | |
| Per 1-unit increment | 214/214 | 0.82 (0.69, 0.97) | 0.024 | 361/361 | 0.87 (0.78, 0.98) | 0.016 |
| Quartiles | ||||||
| Q1 | 60/46 | Ref. | 98/83 | Ref. | ||
| Q2 | 59/48 | 0.88 (0.44, 1.75) | 0.633 | 92/89 | 0.83 (0.51, 1.34) | 0.440 |
| Q3 | 50/56 | 0.58 (0.28, 1.22) | 0.152 | 88/92 | 0.78 (0.48, 1.28) | 0.329 |
| Q4 | 44/63 | 0.48 (0.23, 0.99) | 0.049 | 83/97 | 0.61 (0.38, 0.99) | 0.043 |
| Categories | ||||||
| Low (<median *) | 119/94 | Ref. | 190/172 | Ref. | ||
| High (≥median *) | 94/119 | 0.57 (0.34, 0.97) | 0.038 | 171/189 | 0.81 (0.58, 1.13) | 0.211 |
| TMAO (μg/mL) | Cases/Controls (Ratio 1:1) | Crude Model | Adjusted Model | ||
|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | ||
| Continuous (1-unit increment) | 574/574 | 1.07 (0.65, 1.76) | 0.801 | 1.02 (0.57, 1.83) | 0.950 |
| Quartiles | |||||
| Q1 (<0.10) | Ref. | Ref. | |||
| Q2 (0.10–0.15) | 0.84 (0.61, 1.17) | 0.300 | 0.80 (0.55, 1.17) | 0.249 | |
| Q3 (0.16–0.25) | 0.71 (0.50, 1.03) | 0.070 | 0.57 (0.37, 0.90) | 0.016 | |
| Q4 (≥0.26) | 0.91 (0.64, 1.29) | 0.598 | 0.75 (0.49, 1.14) | 0.176 | |
| p for trend | 0.285 | 0.173 | |||
| Categories | |||||
| Low (<median *) | Ref. | Ref. | |||
| High (≥median *) | 0.89 (0.69, 1.16) | 0.391 | 0.77 (0.55, 1.04) | 0.085 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Liu, C.; Wang, X.; Wei, Y.; Li, S.; Song, Y.; Chen, P.; Liu, L.; Wang, B.; Shi, H. The Association of Serum L-Carnitine Concentrations with the Risk of Cancer in Chinese Adults with Hypertension. Nutrients 2022, 14, 4999. https://doi.org/10.3390/nu14234999
Liu T, Liu C, Wang X, Wei Y, Li S, Song Y, Chen P, Liu L, Wang B, Shi H. The Association of Serum L-Carnitine Concentrations with the Risk of Cancer in Chinese Adults with Hypertension. Nutrients. 2022; 14(23):4999. https://doi.org/10.3390/nu14234999
Chicago/Turabian StyleLiu, Tong, Chenan Liu, Xiaomeng Wang, Yaping Wei, Shuqun Li, Yun Song, Ping Chen, Lishun Liu, Binyan Wang, and Hanping Shi. 2022. "The Association of Serum L-Carnitine Concentrations with the Risk of Cancer in Chinese Adults with Hypertension" Nutrients 14, no. 23: 4999. https://doi.org/10.3390/nu14234999
APA StyleLiu, T., Liu, C., Wang, X., Wei, Y., Li, S., Song, Y., Chen, P., Liu, L., Wang, B., & Shi, H. (2022). The Association of Serum L-Carnitine Concentrations with the Risk of Cancer in Chinese Adults with Hypertension. Nutrients, 14(23), 4999. https://doi.org/10.3390/nu14234999








