Fat-Soluble Vitamers: Parent-Child Concordance and Population Epidemiology in the Longitudinal Study of Australian Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Ethical Approval and Consent
2.2. Anthropometry
2.3. Sample Collection
2.4. Sample Randomization
2.5. Stock Preparation
2.6. FSV Extraction and Sample Preparation by Liquid Handling Robot
2.7. LC-MS/MS Analysis of FSV
2.8. Statistical Analyses
3. Results
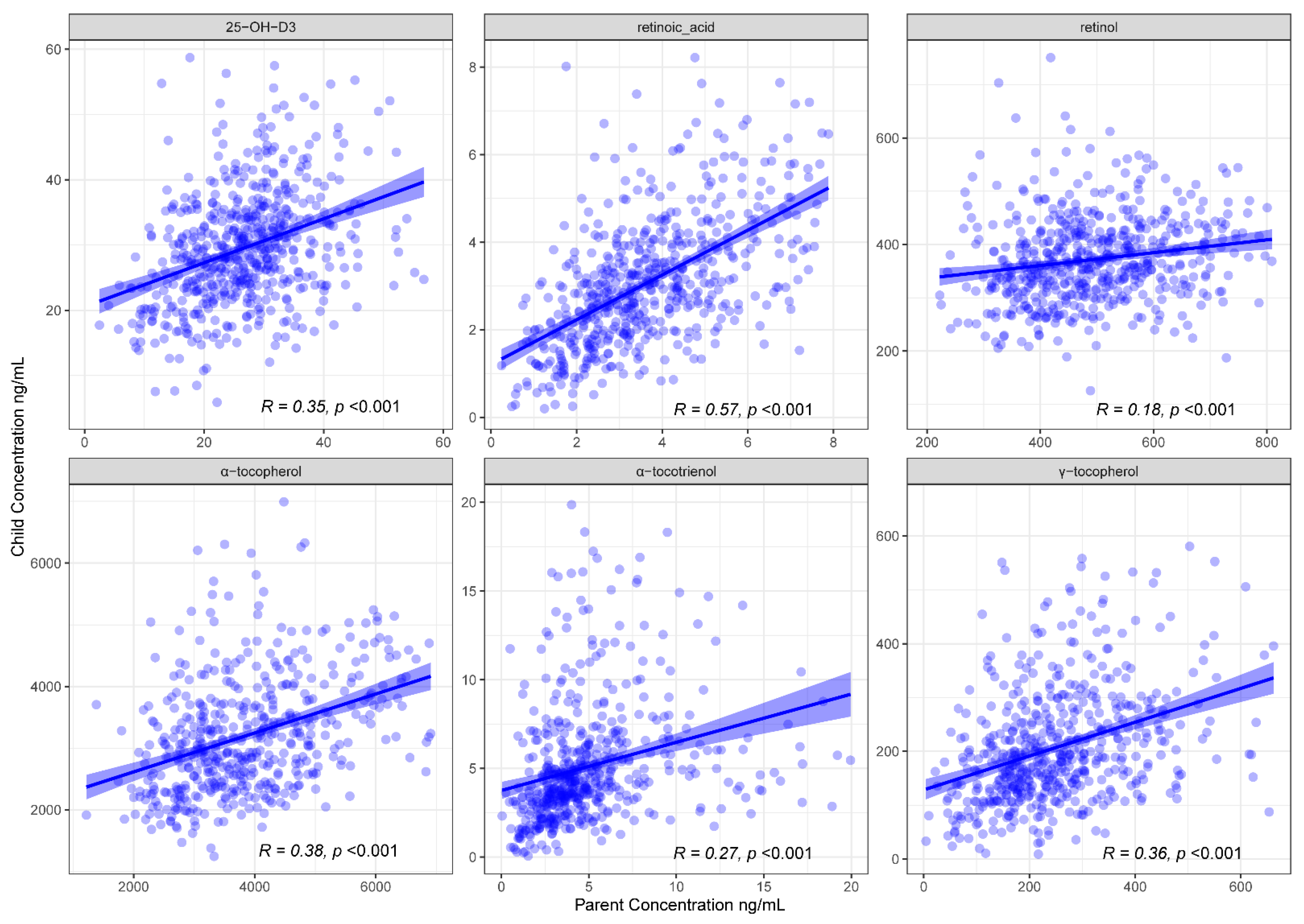
3.1. FSV Profiles Are Concordant between Children and Their Parents
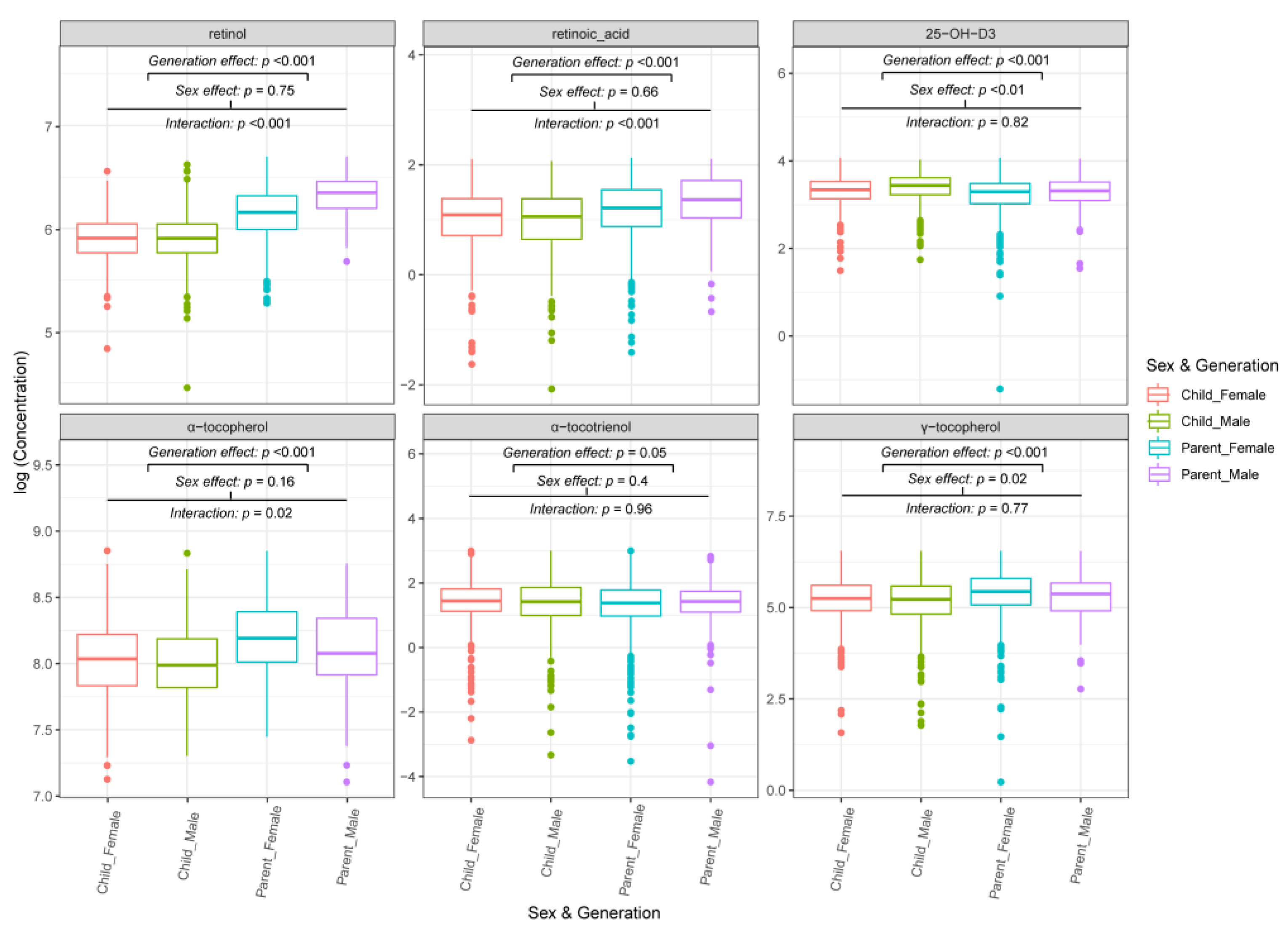
3.2. FSV Profiles from the Same Family Are Generation and Sex-Dependent
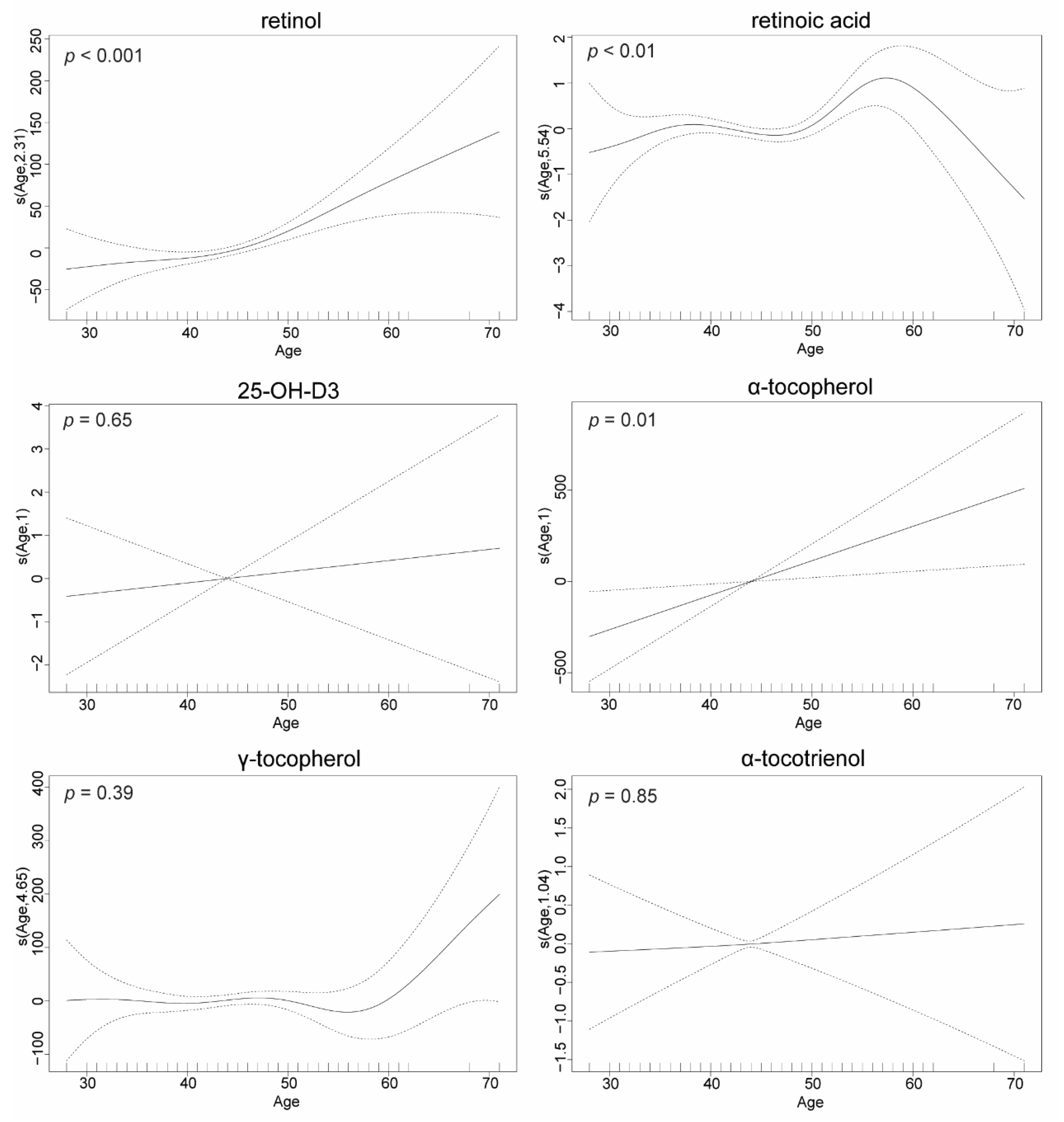
3.3. FSV Concentrations Are Age-Dependent
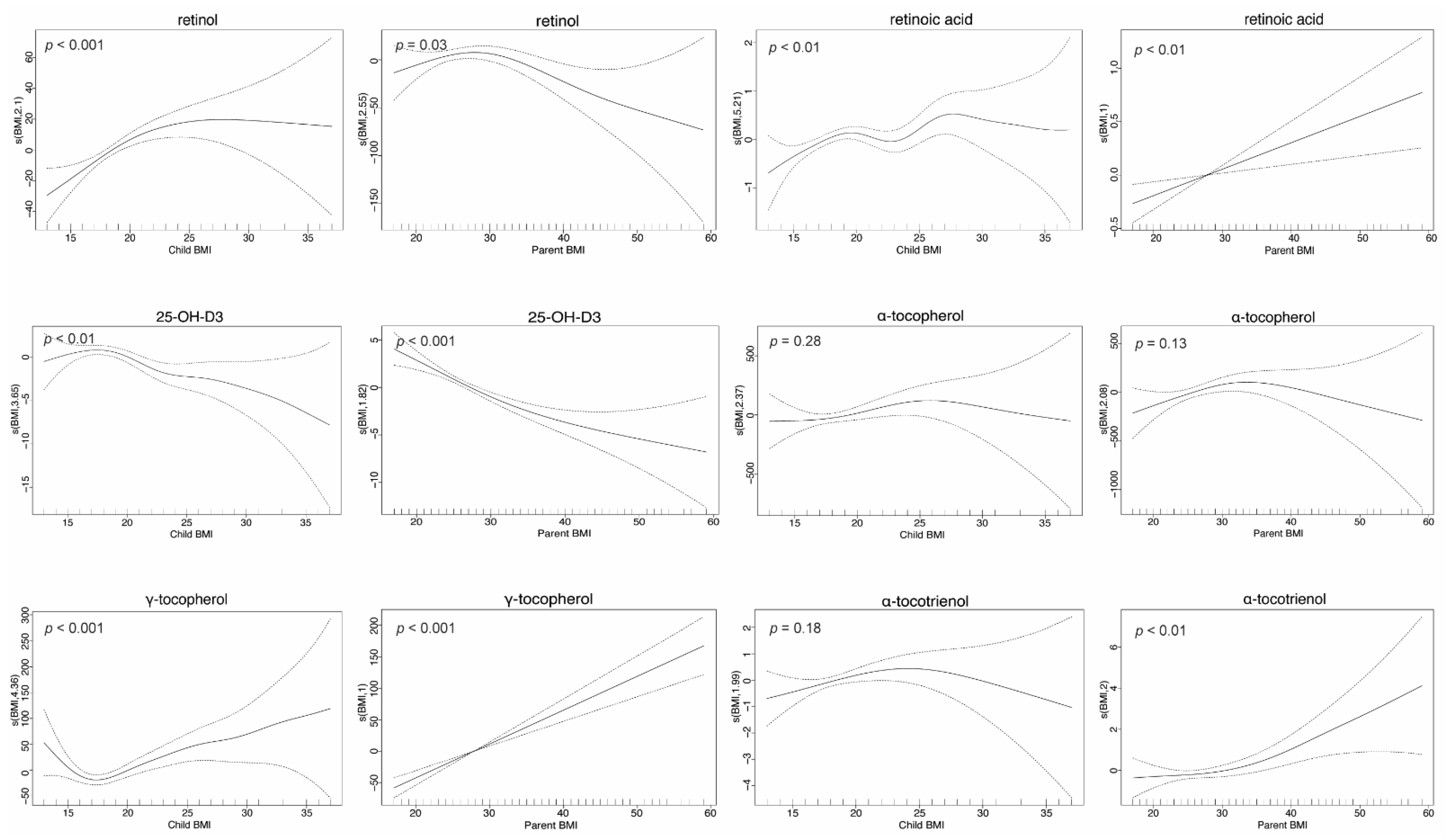
3.4. FSV Concentrations Are BMI Dependent
4. Discussion
4.1. Strengths and Limitations
4.2. Interpretation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Booth, S.L.; Tucker, K.L.; McKeown, N.M.; Davidson, K.W.; Dallal, G.E.; Sadowski, J.A. Relationships between Dietary Intakes and Fasting Plasma Concentrations of Fat-Soluble Vitamins in Humans. J. Nutr. 1997, 127, 587–592. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shea, M.K.; Booth, S.L.; Gundberg, C.M.; Peterson, J.W.; Waddell, C.; Dawson-Hughes, B.; Saltzman, E. Adulthood Obesity Is Positively Associated with Adipose Tissue Concentrations of Vitamin K and Inversely Associated with Circulating Indicators of Vitamin K Status in Men and Women. J. Nutr. 2010, 140, 1029–1034. [Google Scholar] [CrossRef]
- Kimmons, J.E.; Blanck, H.M.; Tohill, B.C.; Zhang, J.; Khan, L.K. Associations between Body Mass Index and the Prevalence of Low Micronutrient Levels among US Adults. Med. Gen. Med. 2006, 8, 59. [Google Scholar]
- Russell, R.M. Factors in Aging That Effect the Bioavailability of Nutrients. J. Nutr. 2001, 131, 1359S–1361S. [Google Scholar] [CrossRef]
- Watson, J.; Lee, M.; Garcia-Casal, M.N. Consequences of Inadequate Intakes of Vitamin A, Vitamin B12, Vitamin D, Calcium, Iron, and Folate in Older Persons. Curr. Geriatr. Rep. 2018, 7, 103–113. [Google Scholar] [CrossRef]
- Talegawkar, S.A.; Johnson, E.J.; Carithers, T.; Taylor, H.A.; Bogle, M.L.; Tucker, K.L. Total α-Tocopherol Intakes Are Associated with Serum α-Tocopherol Concentrations in African American Adults. J. Nutr. 2007, 137, 2297–2303. [Google Scholar] [CrossRef]
- Andreozzi, P.; Verrusio, W.; Viscogliosi, G.; Summa, M.L.; Gueli, N.; Cacciafesta, M.; Albanese, C.V. Relationship between Vitamin D and Body Fat Distribution Evaluated by DXA in Postmenopausal Women. Nutrition 2016, 32, 687–692. [Google Scholar] [CrossRef]
- Paes-Silva, R.P.; Gadelha, P.C.F.P.; de Lemos, M.d.C.C.; de Castro, C.M.M.B.; de Arruda, I.K.G.; Diniz, A.d.S. Adiposity, Inflammation and Fat-Soluble Vitamins in Adolescents. J. Pediatr. (Rio. J.) 2019, 95, 575–583. [Google Scholar] [CrossRef]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased Bioavailability of Vitamin D in Obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Aasheim, E.T.; Hofsø, D.; Hjelmesæth, J.; Birkeland, K.I.; Bøhmer, T. Vitamin Status in Morbidly Obese Patients: A Cross-Sectional Study. Am. J. Clin. Nutr. 2008, 87, 362–369. [Google Scholar] [CrossRef]
- Nucci, A.M.; Russell, C.S.; Luo, R.; Ganji, V.; Olabopo, F.; Hopkins, B.; Holick, M.F.; Rajakumar, K. The Effectiveness of a Short Food Frequency Questionnaire in Determining Vitamin D Intake in Children. Dermato-Endocrinology 2013, 5, 205. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.R.; Rhodes, D.G.; Kramer, M.; Baer, D.J.; Rumpler, W.V. Validation of a Food Frequency Questionnaire by Direct Measurement of Habitual Ad Libitum Food Intake. Am. J. Epidemiol. 2005, 162, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Albahrani, A.A.; Greaves, R.F. Fat-Soluble Vitamins: Clinical Indications and Current Challenges for Chromatographic Measurement. Clin. Biochem. Rev. 2016, 37, 27–47. [Google Scholar]
- Arachchige, G.R.P.; Thorstensen, E.B.; Coe, M.; O’Sullivan, J.M.; Pook, C.J. Absolute Quantification of Eleven A, D, E and K Vitamers in Human Plasma Using Automated Extraction and UHPLC-Orbitrap MS. Anal. Chim. Acta 2021, 1181, 338877. [Google Scholar] [CrossRef]
- Edwards, B. Growing up in Australia: The Longitudinal Study of Australian Children: Entering Adolescence and Becoming a Young Adult. Fam. Matters 2014, 95, 5–14. [Google Scholar]
- Clifford, S.A.; Davies, S.; Wake, M. Child Health CheckPoint: Cohort Summary and Methodology of a Physical Health and Biospecimen Module for the Longitudinal Study of Australian Children. BMJ Open 2019, 9, 3–22. [Google Scholar] [CrossRef]
- Arachchige, G.R.P.; Thorstensen, E.B.; Coe, M.; McKenzie, E.J.; O’Sullivan, J.M.; Pook, C.J. LC-MS/MS Quantification of Fat Soluble Vitamers—A Systematic Review. Anal. Biochem. 2021, 613, 113980. [Google Scholar] [CrossRef]
- Edwards, B. Growing up in Australia: The Longitudinal Study of Australian Children: The first decade of life. Fam. Matters 2012, 91, 7–17. [Google Scholar]
- Ward, L.C. Bioelectrical Impedance Analysis for Body Composition Assessment: Reflections on Accuracy, Clinical Utility, and Standardisation. Eur. J. Clin. Nutr. 2019, 73, 194–199. [Google Scholar] [CrossRef]
- Andraos, S.; Lange, K.; Clifford, S.A.; Jones, B.; Thorstensen, E.B.; Kerr, J.A.; Wake, M.; Saffery, R.; Burgner, D.P.; O’Sullivan, J.M. Plasma Trimethylamine N-Oxide and Its Precursors: Population Epidemiology, Parent–Child Concordance, and Associations with Reported Dietary Intake in 11- to 12-Year-Old Children and Their Parents. Curr. Dev. Nutr. 2020, 4, nzaa103. [Google Scholar] [CrossRef] [PubMed]
- Committee for Medicinal Products for Human Use (CHMP). EMA Guideline on Bioanalytical Method Validation; Committee for Medicinal Products for Human Use (CHMP): London, UK, 2011. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 48. [Google Scholar] [CrossRef]
- Wood, S.N.; Pya, N.; Säfken, B. Smoothing Parameter and Model Selection for General Smooth Models. J. Am. Stat. Assoc. 2016, 111, 1548–1563. [Google Scholar] [CrossRef]
- Wake, M.; Clifford, S.; York, E.; Mensah, F.; Gold, L.; Burgner, D.; Davies, S.; Azzopardi, P.; Baur, L.; Carlin, J.; et al. Introducing Growing up in Australia’s Child Health Checkpoint: A Physical Health and Biomarkers Module for the Longitudinal Study of Australian Children. Fam. Matters 2014, 95, 15–23. [Google Scholar]
- Root, M.M.; Hu, J.; Stephenson, L.S.; Parker, R.S.; Campbell, T.C. Determinants of Plasma Retinol Concentrations of Middle-Aged Women in Rural China. Nutrition 1999, 15, 101–107. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kuvibidila, S.R.; Gardner, R.; Velez, M.; Warrier, R. Clinical Observations, Plasma Retinol Concentrations, and In Vitro Lymphocyte Functions in Children With Sickle Cell Disease. Ochsner J. 2018, 18, 308–317. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Mu, D.; Fan, J.; Song, J.; Zhong, Y.; Li, D.; Xia, M. Circulating Retinoic Acid Levels and the Development of Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2016, 101, 1686–1692. [Google Scholar] [CrossRef]
- van Vliet, T.; Boelsma, E.; de Vries, A.J.; van den Berg, H. Retinoic Acid Metabolites in Plasma Are Higher after Intake of Liver Paste Compared with a Vitamin A Supplement in Women. J. Nutr. 2001, 131, 3197–3203. [Google Scholar] [CrossRef] [PubMed]
- Gilbert-Diamond, D.; Baylin, A.; Mora-Plazas, M.; Marin, C.; Arsenault, J.E.; Hughes, M.D.; Willett, W.C.; Villamor, E. Vitamin D Deficiency and Anthropometric Indicators of Adiposity in School-Age Children: A Prospective Study. Am. J. Clin. Nutr. 2010, 92, 1446–1451. [Google Scholar] [CrossRef]
- Littorin, B.; Blom, P.; Schölin, A.; Arnqvist, H.J.; Blohmé, G.; Bolinder, J.; Ekbom-Schnell, A.; Eriksson, J.W.; Gudbjörnsdottir, S.; Nyström, L.; et al. Lower Levels of Plasma 25-Hydroxyvitamin D among Young Adults at Diagnosis of Autoimmune Type 1 Diabetes Compared with Control Subjects: Results from the Nationwide Diabetes Incidence Study in Sweden (DISS). Diabetologia 2006, 49, 2847–2852. [Google Scholar] [CrossRef]
- Cooney, R.V.; Franke, A.A.; Wilkens, L.R.; Gill, J.; Kolonel, L.N. Elevated Plasma γ-Tocopherol and Decreased α-Tocopherol in Men Are Associated With Inflammatory Markers and Decreased Plasma 25-OH Vitamin D. Nutr. Cancer 2008, 60, 21–29. [Google Scholar] [CrossRef]
- Cuerq, C.; Restier, L.; Drai, J.; Blond, E.; Roux, A.; Charriere, S.; Michalski, M.-C.; Di Filippo, M.; Levy, E.; Lachaux, A.; et al. Establishment of Reference Values of α-Tocopherol in Plasma, Red Blood Cells and Adipose Tissue in Healthy Children to Improve the Management of Chylomicron Retention Disease, a Rare Genetic Hypocholesterolemia. Orphanet J. Rare Dis. 2016, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-N.; Cho, Y.-O. Vitamin E Status of 20- to 59-Year-Old Adults Living in the Seoul Metropolitan Area of South Korea. Nutr. Res. Pract. 2015, 9, 192. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, K.A.; Um, C.Y.; Gross, M.D.; Bostick, R.M. Circulating γ-Tocopherol Concentrations Are Inversely Associated with Antioxidant Exposures and Directly Associated with Systemic Oxidative Stress and Inflammation in Adults. J. Nutr. 2018, 148, 1453–1461. [Google Scholar] [CrossRef]
- Rasool, A.H.G.G.; YUEN, K.H.; YUSOFF, K.; Wong, A.R.; RAHMAN, A.R.A.A. Dose Dependent Elevation of Plasma Tocotrienol Levels and Its Effect on Arterial Compliance, Plasma Total Antioxidant Status, and Lipid Profile in Healthy Humans Supplemented with Tocotrienol Rich Vitamin E. J. Nutr. Sci. Vitaminol. 2006, 52, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Fairus, S.; Nor, R.M.; Cheng, H.M.; Sundram, K. Alpha-Tocotrienol Is the Most Abundant Tocotrienol Isomer Circulated in Plasma and Lipoproteins after Postprandial Tocotrienol-Rich Vitamin E Supplementation. Nutr. J. 2012, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Zahara, A.M.; Lee, C.C.; Fatimah, I.S.; Poh, B.K.; Khairul, O.; Das, S.; Mohamad, J. Plasma Vitamin C and Tocotrienols Changes in Response to Dietary Supplementation among Young Male Adults. Clin. Ter. 2010, 161, 121–124. [Google Scholar]
- Zelenková, M.; Zima, T.; Kalousová, M. Determination of Retinoic Acid in Human Serum and Plasma by High-Performance Liquid Chromatography. Mon. Für Chem.-Chem. Mon. 2019, 150, 1731–1735. [Google Scholar] [CrossRef]
- Cavalier, E. Long-Term Stability of 25-Hydroxyvitamin D: Importance of the Analytical Method and of the Patient Matrix. Clin. Chem. Lab. Med. 2021, 59, e389–e391. [Google Scholar] [CrossRef]
- Jezequel-Cuer, M.; Le Moël, G.; Covi, G.; Lepage, S.; Peynet, J.; Gousson-Evstigneeff, T.; Laureaux, C.; Troupel, S. [Stability of Alpha-Tocopherol: Pre-Analytical Conditions in Its Determination in Blood Samples]. Ann. Biol. Clin. 1994, 52, 271–276. [Google Scholar]
- Craft, N.E.; Brown, E.D.; Smith, J.C. Effects of Storage and Handling Conditions on Concentrations of Individual Carotenoids, Retinol, and Tocopherol in Plasma. Clin. Chem. 1988, 34, 44–48. [Google Scholar] [CrossRef]
- Edmonds, B.K.; Nierenberg, D.W. Serum Concentrations of Retinol, d-α-Tocopherol and β-Carotene: Effects of Storage at —70 °C for Five Years. J. Chromatogr. B Biomed. Sci. Appl. 1993, 614, 169–174. [Google Scholar] [CrossRef]
- Bates, C.J.; Mishra, G.D.; Prentice, A. γ-Tocopherol as a Possible Marker for Nutrition-Related Risk: Results from Four National Diet and Nutrition Surveys in Britain. Br. J. Nutr. 2004, 92, 137–150. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Garry, P.J.; Hunt, W.C.; Bandrofchak, J.L.; VanderJagt, D.; Goodwin, J.S. Vitamin A Intake and Plasma Retinol Levels in Healthy Elderly Men and Women. Am. J. Clin. Nutr. 1987, 46, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Hallfrisch, J.; Muller, D.C.; Singh, V.N. Vitamin A and E Intakes and Plasma Concentrations of Retinol, β- Carotene, and α-Tocopherol in Men and Women of the Baltimore Longitudinal Study of Aging. Am. J. Clin. Nutr. 1994, 60, 176–182. [Google Scholar] [CrossRef]
- Didriksen, A.; Burild, A.; Jakobsen, J.; Fuskevåg, O.M.; Jorde, R. Vitamin D3 Increases in Abdominal Subcutaneous Fat Tissue after Supplementation with Vitamin D3. Eur. J. Endocrinol. 2015, 172, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P.; Horst, R.L.; Cullen, D.M.; Armas, L.A.G. Vitamin D3 Distribution and Status in the Body. J. Am. Coll. Nutr. 2009, 28, 252–256. [Google Scholar] [CrossRef]
| Vitamin | Vitamer | Sex | n | Mean Concentration (ng/mL) | SD (ng/mL) | Gen | n | Mean Concentration (ng/mL) | SD (ng/mL) | Sex & Gen | n | Mean Concentration (ng/mL) | SD (ng/mL) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | retinol | Female | 1733 | 452 | 128 | Child | 1154 | 375 | 84.4 | female child | 596 | 375 | 85.3 |
| female parent | 1137 | 492 | 129 | ||||||||||
| Male | 731 | 425 | 133 | Parent | 1310 | 505 | 133 | male child | 558 | 374 | 83.5 | ||
| male parent | 173 | 589 | 131 | ||||||||||
| D | retinoic acid | Female | 1722 | 3.53 | 1.72 | Child | 1145 | 3.14 | 1.57 | female child | 589 | 3.18 | 1.57 |
| female parent | 1133 | 3.71 | 1.78 | ||||||||||
| Male | 729 | 3.38 | 1.77 | Parent | 1306 | 3.78 | 1.83 | male child | 556 | 3.10 | 1.57 | ||
| male parent | 173 | 4.28 | 2.07 | ||||||||||
| 25-OH-D3 | Female | 1735 | 28.0 | 10.3 | Child | 1155 | 30.3 | 9.49 | female child | 595 | 29.1 | 9.30 | |
| female parent | 1140 | 27.4 | 10.8 | ||||||||||
| Male | 733 | 30.8 | 9.55 | Parent | 1313 | 27.5 | 10.6 | male child | 560 | 31.6 | 9.52 | ||
| male parent | 173 | 28.1 | 9.16 | ||||||||||
| E | α-tocopherol | Female | 1548 | 3702 | 1275 | Child | 1017 | 3173 | 937 | female child | 527 | 3233 | 952 |
| female parent | 1021 | 3945 | 1351 | ||||||||||
| Male | 641 | 3222 | 1032 | Parent | 1172 | 3899 | 1346 | male child | 490 | 3109 | 916 | ||
| male parent | 151 | 3590 | 1277 | ||||||||||
| α-tocotrienol | Female | 1709 | 5.39 | 5.57 | Child | 1150 | 5.70 | 5.17 | female child | 590 | 5.64 | 5.17 | |
| female parent | 1119 | 5.26 | 5.77 | ||||||||||
| Male | 731 | 5.57 | 4.80 | Parent | 1290 | 5.21 | 5.50 | male child | 560 | 5.77 | 5.17 | ||
| male parent | 171 | 4.92 | 3.21 | ||||||||||
| γ-tocopherol | Female | 1742 | 255 | 168 | Child | 1160 | 222 | 157 | female child | 599 | 229 | 174 | |
| female parent | 1143 | 269 | 164 | ||||||||||
| Male | 733 | 221 | 146 | Parent | 1315 | 265 | 165 | male child | 561 | 215 | 136 | ||
| male parent | 172 | 244 | 172 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arachchige, G.R.P.; Pook, C.J.; Jones, B.; Coe, M.; Saffery, R.; Wake, M.; Thorstensen, E.B.; O’Sullivan, J.M.; on behalf of the Child CheckPoint Team. Fat-Soluble Vitamers: Parent-Child Concordance and Population Epidemiology in the Longitudinal Study of Australian Children. Nutrients 2022, 14, 4990. https://doi.org/10.3390/nu14234990
Arachchige GRP, Pook CJ, Jones B, Coe M, Saffery R, Wake M, Thorstensen EB, O’Sullivan JM, on behalf of the Child CheckPoint Team. Fat-Soluble Vitamers: Parent-Child Concordance and Population Epidemiology in the Longitudinal Study of Australian Children. Nutrients. 2022; 14(23):4990. https://doi.org/10.3390/nu14234990
Chicago/Turabian StyleArachchige, Githal Randunu Porawakara, Chris James Pook, Beatrix Jones, Margaret Coe, Richard Saffery, Melissa Wake, Eric Bruce Thorstensen, Justin Martin O’Sullivan, and on behalf of the Child CheckPoint Team. 2022. "Fat-Soluble Vitamers: Parent-Child Concordance and Population Epidemiology in the Longitudinal Study of Australian Children" Nutrients 14, no. 23: 4990. https://doi.org/10.3390/nu14234990
APA StyleArachchige, G. R. P., Pook, C. J., Jones, B., Coe, M., Saffery, R., Wake, M., Thorstensen, E. B., O’Sullivan, J. M., & on behalf of the Child CheckPoint Team. (2022). Fat-Soluble Vitamers: Parent-Child Concordance and Population Epidemiology in the Longitudinal Study of Australian Children. Nutrients, 14(23), 4990. https://doi.org/10.3390/nu14234990







