Abstract
Nausea and vomiting are the most common side effects of chemotherapy. They must be managed because they can increase the risk of malnutrition in patients, which can adversely affect treatment. The objective of this study was to evaluate the effect of ginger supplementation as an adjuvant treatment for alleviating chemo We checked. therapy-induced nausea and vomiting (CINV). This study searched for randomized controlled trials (RCTs) related to ginger supplement intake for CINV in three electronic databases (i.e., Medline (PubMed), Embase, and Web of Science). The search period ranged from each database’s first date of service to 5 November 2021. Two investigators independently performed abstract screenings, full-text screenings, data extraction, and risk of bias analyses (ROB). The Cochrane ROB tool was used for the assessment of ROB. This study systematically reviewed 23 RCTs. The effects of ginger supplementation were compared to those of placebo or antiemetic agents. This study conducted a meta-analysis after classifying the effects of ginger supplementation on acute and delayed CINV into subgroups due to the clinical heterogeneity between these RCTs. The results showed that the incidence of acute nausea (p = 0.53), the incidence of delayed nausea (p = 0.31), the incidence of acute vomiting (p = 0.09), and the incidence of delayed vomiting (p = 0.89) were not significantly different between the ginger supplement intake group and the control group. However, it was found that the ginger supplement intake group, which took not more than 1 g of ginger supplementation per day for above four days, had significantly less acute vomiting than the control group (OR 0.30; 95% CI 0.12 to 0.79; p = 0.02; I2 = 36%). Ginger supplementation may reduce the incidence of acute chemotherapy-induced vomiting. However, this study could not confirm the effects of ginger supplementation on the incidence of chemotherapy-induced nausea and delayed vomiting. Therefore, it will be necessary to conduct additional studies with sufficient sample sizes using high-quality RCTs to evaluate the effects of ginger supplementations based on the results of this study.
1. Introduction
Chemotherapy, along with surgery and radiation therapy, is one of the most common and effective cancer treatment methods [1,2]. It prohibits the growth of cancer cells, which spread throughout the body or kill them by using drugs. However, it may cause side effects because it can damage healthy cells as well as cancer cells [3]. Most of the patients who received chemotherapy (88%) experienced one or more side effects: fatigue (80%) was the most common side effect, followed by nausea and vomiting (48%) and pain (48%) [4]. Especially, it is necessary to pay more attention to nausea and vomiting because they can deteriorate the quality of life of the patient, negatively affect food intake [5], and increase the risk of malnutrition during treatment. This is because continuous persistent malnutrition can adversely affect treatment by lowering the patient’s immunity [6]. Prescribing an antiemetic drug is one way, but only approximately 26% of patients who were prescribed antiemetics experienced that they were effective in mitigating nausea and vomiting symptoms [7]. Additionally, taking them may cause side effects such as headache and sedation [8]. Consequently, many researchers have actively conducted studies on the effects of adjunct therapies using natural products that are effective for patients experiencing chemotherapy-induced nausea and vomiting (CINV) symptoms, with only a few side effects. Ginger (Zingiber Officinale Roscoe) is one of the most commonly used adjunct treatments for patients with CINV.
Ginger is originally from tropical Asia. It is a perennial crop, and it is widely used as a spice all over the world owing to the pungent and spicy taste of the rootstalk. Ginger is rich in a variety of components including phenolic compounds, polysaccharides, terpenes, lipids, and organic acids [9]. The main functional substances of ginger are phenolic compounds such as gingerols and shogaols [9]. They have various biological properties that are antioxidant, anti-inflammatory, antimicrobial, anticancer, neuroprotective, antidepressant, and antiemetic [9]. Due to these biological activities of ginger, it has been used as a traditional remedy for nausea, vomiting, indigestion, motion sickness, and morning sickness in various cultures for a long time [9,10]. It is known that ginger mitigates nausea and vomiting due to the inhibitory effects of gingerols or shogaols against 5-Hydroxytryptamine type 3 (5-HT3) receptors [11]. Gingerols and shogaols are presumed to have antiemetic effects by binding to the serotonin binding site through acting on the 5-HT3 receptor ion–channel complex [12].
A systematic review and meta-analysis study published in 2019 confirmed, after examining 18 parallel and crossover intervention trials, that ginger supplementation and CINV were not significantly associated [13]. However, the review study suffered from low reliability because studies included in the review were clinically heterogeneous due to different cancer types, chemotherapy, and antiemetics [13]. Therefore, there is a need to review recently updated publications. The objective of this study was to evaluate whether the ginger supplement was effective in reducing CINV compared to the use of a placebo or standard antiemetic medication in adult cancer patients receiving chemotherapy.
2. Materials and Methods
2.1. Data Sources and Searches
We conducted the literature search for randomized controlled trials (RCTs) related to ginger intake and chemotherapy-induced nausea and vomiting in 3 databases: Medline, Embase, and Web of Science (from inception to 5 November 2021; search on 5 November 2021). The complete search strategy is demonstrated in Table S1.
2.2. Study Eligibility Criteria
We included human intervention trials with ginger intake from dietary supplements or foods. To be eligible, studies must have compared the effect of ginger intake alone with that of a placebo or another active comparator. Additionally, studies must have information related to chemotherapy-induced nausea and vomiting.
2.3. Study Selection Process
Two investigators independently screened the abstract of all citations according to study eligibility criteria. To conduct the abstract screening, the researchers separately used the Rayyan program, the open source online software. Then, two investigators independently conducted the full-text screening according to the study eligibility criteria. We resolved the conflicts by our research group consensus. The study selection is presented in Figure 1.
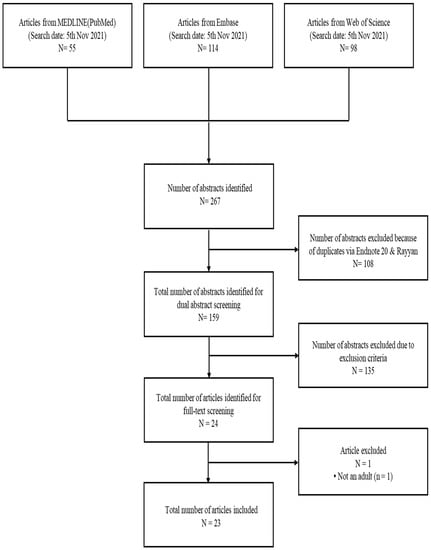
Figure 1.
Literature search and study selection process.
2.4. Data Extraction and Study Quality Assessment
The research conducted data extraction (e.g., study characteristics, odds ratio) of all the included studies by employing standardized data extraction forms. To examine the risk of bias (ROB) for each study, we employed the Cochrane ROB tool for randomized controlled trials. The Cochrane ROB tool consisted of five domains of bias including: (1) the randomization process; (2) deviations from the intended intervention; (3) missing outcome data; (4) the measurement of the outcome; and (5) the selection of the reported result. The researcher evaluated the ROB by selecting one of three options: low, high, or unclear risk. We conducted data reporting in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) reporting guidelines.
2.5. Quantitative Synthesis
Due to large methodological heterogeneity (e.g., different levels of ginger intake), we conducted a random-effects meta-analysis when there were at least two unique studies that stated sufficient quantitative information for the same outcome. To quantify the level of statistical heterogeneity, we conducted the Tau-square test, the Chi-square test, and the I-square test. We conducted all calculations and meta-analyses using the Cochrane’s Review Manager software. We considered values of less than 0.5 for two-tailed p values as statistically significant.
3. Results
We identified 267 citations by the initial search. After excluding dual abstracts, we produced 159 abstracts. After screening out 135 abstracts owing to the exclusion criteria, we identified 24 articles for full-text screening. Then, we finally included 23 abstracts for this systematic review. The flow chart of the study selection process is presented in Figure 1. The study characteristics of the included RCTs are stated in Table 1. The study risk of bias assessment for the included RCTs is in Table 2.

Table 1.
Characteristics of the included RCT.

Table 2.
Risk of bias assessment for the included RCTs.
3.1. Ginger Intake and Chemotherapy-Induced Nausea
3.1.1. Chemotherapy-Induced Acute Nausea
The meta-analysis result of five RCTs showed that the ginger intake group showed a tendency to slightly lower the incidence of acute nausea, but there was no significant difference (Odds Ratio [OR] = 0.82, 95% CI = 0.44 to 1.52, p = 0.53, I2 = 58%) (Figure 2).
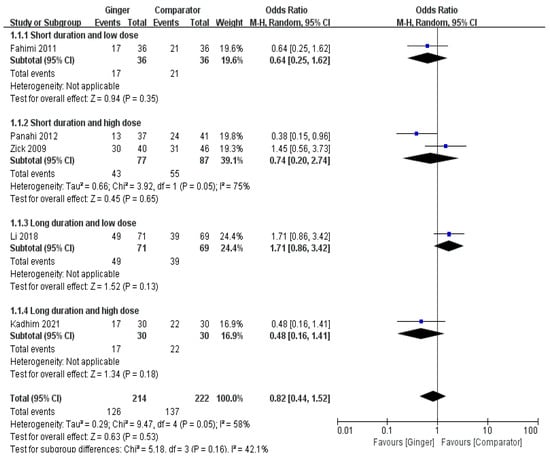
Figure 2.
Effects of ginger intake on chemotherapy-induced acute nausea. Short duration ≤ 4 days; Long duration > 4 days; Low dose ≤ 1 g/day; High dose > 1 g/day. Fahimi 2011 [19], Panahi 2012 [28], Li 2018 [23], Kadhim 2021 [21].
3.1.2. Chemotherapy-Induced Delayed Nausea
The meta-analysis result of six RCTs presented that the ginger group showed a tendency to marginally lower incidence of delayed nausea, there was no significant difference between the ginger and the control group (OR = 0.81, 95% CI = 0.53 to 1.22, p = 0.31, I2 = 10%) (Figure 3).
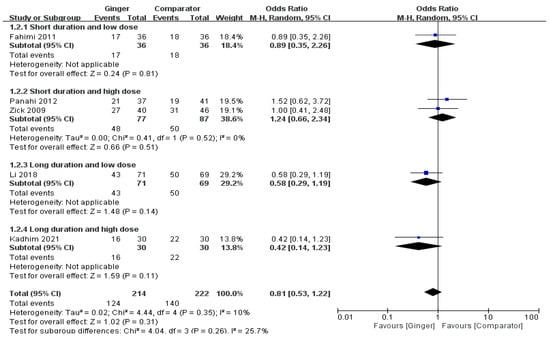
Figure 3.
Effects of ginger intake on chemotherapy-induced delayed nausea. Short duration ≤ 4 days; Long duration > 4 days; Low dose ≤ 1 g/day; High dose > 1 g/day. Fahimi 2011 [19], Panahi 2012 [28], Li 2018 [23], Kadhim 2021 [21].
3.2. Ginger Intake and Chemotherapy-Induced Vomiting
3.2.1. Chemotherapy-Induced Acute Vomiting
The meta-analysis results of six RCTs presented that the ginger intake group showed a tendency to slightly lower the overall incidence of acute vomiting, but there was no significant difference (OR = 0.59, 95% CI = 0.33 to 1.09, p = 0.09, I2 = 52%). However, the odds ratio of acute vomiting was significantly decreased in subjects undertaking chemotherapy by 70% with ginger supplementation ≤ 1 g/day for > 4 days compared with the control groups (OR = 0.30, 95% CI = 0.12 to0.79, p = 0.02, I2 = 36%) (Figure 4).
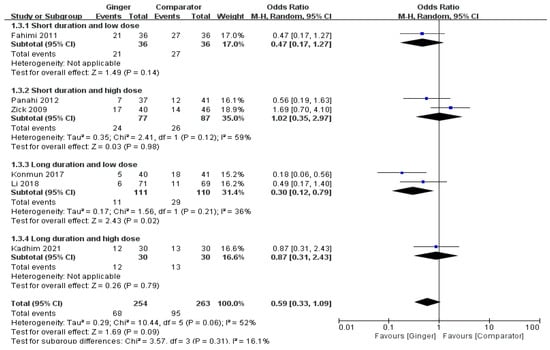
Figure 4.
Effects of ginger intake on chemotherapy-induced acute vomiting. Short duration ≤ 4 days; Long duration > 4 days; Low dose ≤ 1 g/day; High dose > 1 g/day. Fahimi 2011 [19], Panahi 2012 [28], Li 2018 [23], Kadhim 2021 [21].
3.2.2. Chemotherapy-Induced Delayed Vomiting
The meta-analysis results of six RCTs showed a non-significant difference of the effect of ginger on the onset of delayed vomiting (OR = 0.81, 95% CI = 0.39 to 1.69, p = 0.89, I2 = 71%) (Figure 5).
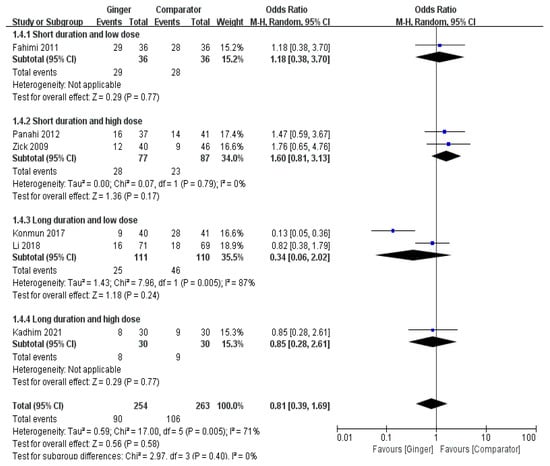
Figure 5.
Effects of ginger intake on chemotherapy-induced delayed vomiting. Short duration ≤ 4 days; Long duration > 4 days; Low dose ≤ 1 g/day; High dose > 1 g/day. Fahimi 2011 [19], Panahi 2012 [28], Li 2018 [23], Kadhim 2021 [21].
3.3. Reported Adverse Events after Intake of Ginger
Seven studies reported adverse events, including mild gastrointestinal symptoms, constipation, reflux, heartburn, bruising, flushing, rash, fever, fatigue, diarrhea, anemia, etc. [17,20,25,29,32,35,36] (Table 3). The incidence of these adverse events was similar between the ginger group and the placebo/control group, with no significant difference in adverse effects compared to the placebo. The distribution of serious adverse events between the ginger group and the placebo/control group was similar, with fewer events in the ginger group [17]. Nine studies reported no adverse events occurred during the study [15,16,18,22,23,24,30,33,34]. Seven studies reported no information regarding adverse events [14,19,21,26,27,28,31].

Table 3.
Reported adverse events after intake of ginger.
4. Discussion
This is the most recent study that systematically reviewed and conducted a meta-analysis on randomized controlled trial (RCT) studies, which evaluated the effectiveness of ginger supplements in reducing CINV compared to placebo and standard antiemetic medication in adult cancer patients receiving chemotherapy. The results of previous meta-analysis studies showed that taking at least 1 g of ginger supplements per day for three days or more reduced the chance of acute vomiting caused by chemotherapy by 60% [13]. However, when this review study carried out a meta-analysis of the six RCTs, ginger supplements did significantly decrease the possibility of acute chemotherapy-induced vomiting, as similar results were shown in the previous systematic review. The results of this study agreed with those of previous studies [1,13,37], which revealed that ginger supplements did not significantly decrease the occurrence and severity of chemotherapy-induced nausea, the occurrence and severity of delayed nausea, and the occurrence and severity of delayed vomiting.
Although the certainty of the effect size estimated in this review was very low, it was believed that ginger supplements were effective in reducing the possibility of acute vomiting. However, the results are not conclusive because the clinician heterogeneity of the current evidence is high. Therefore, all results must be interpreted with caution until additional results are published from a study based on a solid experimental design and well-controlled samples.
Acute CINV, peaking at 5–6 h after chemotherapy, is related to 5-HT in the central and gastrointestinal tract, whereas delayed CINV, peaking at 72 h after chemotherapy, is mediated by SP in the central [38]. Ginger is rich in bioactive polyphenolic compounds, and 6-gingerol and 6-shogaol are the representative ingredients in gingerols that contribute to antiemetic actions against CINV [39]. The mechanism for the antiemetic action of ginger has not been fully understood yet. However, several mechanisms of the effect of ginger against CINV are proposed with the interactions of neurotransmitters in the central and peripheral, such as 5-hydroxytryptamine (5-HT), substance P (SP), and dopamine (DA), and the modulation of gastrointestinal motility [12,40,41,42].
Most of the monoamine neurotransmitter, 5-HT, is produced in the intestinal EC cells [43]. Chemotherapy stimulates EC cells to release 5-HT, and then activates 5-HT3R, resulting in nausea or vomiting [44]. Gingerols and its ingredients, especially 6-shogaol, significantly mitigate CINV by reducing 5-HT and blocking 5-HT3R expression [12]. Another mechanism is through mediating the substance P (SP) signaling pathway. The binding of SP to the neurokinin-1 receptor (NK-1R) results in vomiting [45]. SP is derived from substance P-precursor, preprotachykinin A (PPT-A) and Neprilysin (NEP) is an ectoenzyme that degrades tachykinins, such as SP [46]. The gingerols in ginger significantly ameliorated vomiting by reducing the SP level through decreasing of PPTA and increasing of NEP [41].
In addition to 5-HT and SP systems, the activation of the dopamine (DA) signaling pathway also leads to CINV. Tyrosine hydroxylase (TH) is the rate-limiting enzyme in DA synthesis, and DA activates D2-like dopamine receptors (D2R) through the dopamine transporter (DAT) resulting in an emetic response [47]. The effect of gingerols against CINV is partially due to the inhibition of DA synthesis and D2R activation caused by increasing TH and reducing DAT [47,48]. Chemotherapy may affect gastrointestinal motility, as well as that of neurotransmitters. It was reported that the delayed gastric emptying caused by chemotherapy may also be an important factor in explaining CINV [48,49,50]. The gingerols in ginger markedly improved delayed gastric emptying induced by cisplatin in a dose-dependent manner [42]. Despite several proposed mechanisms, further research is warranted to elucidate the underlying mechanism of the antiemetic action of ginger against CINV.
The studies reviewed in this study showed that the intake of ginger supplements varied from 160 mg/day to 15 g/day. It was also found that various intake methods were used: tea, capsulated ginger powder, taking the ginger powder with other foods such as yogurt, and capsulated ginger extract. Therefore, it is highly uncertain what is the most ideal dosage and method of taking ginger supplements for effectively reducing CINV because studies the clinical heterogeneity between studies is fairly high. It is believed that taking 1g or more of ginger supplements for 3 days or longer is potentially helpful in reducing the occurrence of acute vomiting.
Ginger is a spice that has been consumed by mankind for more than thousands of years [38]. Since people consume small amounts of it in daily life from cooked dishes, it does not require much caution. However, when consuming it in the form of a supplement, people may consume excessive amounts. Therefore, it is necessary to pay caution when consuming ginger supplements. Some randomized double-blind trials argued that ginger supplements were a non-pharmacological treatment that could reduce nausea and vomiting during pregnancy and its efficacy and safety were proven even for pregnant women [51,52,53,54]. However, these results should be interpreted with caution because the amount of intake varied between studies and the duration of intake was different between them. As a result, countries such as Finland prohibit pregnant women from taking ginger supplements due to the lack of solid scientific evidence for the benefits of ginger [55]. Moreover, the cytotoxicity [56,57,58] and mutagenic effects [59,60,61] of gingerols, the main functional substances of ginger, were tested in in vitro studies. Therefore, it is difficult to extrapolate these results to humans. However, these can be grounds that more research is needed to understand the caution against consuming ginger supplements that contain high levels of gingerol extract, which is sometimes pursued rather than consuming ginger in the diet and ensure the safety of ginger supplements and caution against an overdose of ginger supplements that contain high doses of gingerol extract, rather than consuming ginger in the diet.
This is the most recent systematic review and meta-analysis study on RCTs to determine whether ginger supplements were effective in reducing CINV compared to placebos or standard antiemetic medication. Conclusively, it was found that ginger supplementation was effective in reducing the possibility of acute vomiting. However, since the subjects of the reviewed studies took ginger supplements, it may be difficult to observe the same effect by simply adding ginger to the daily diet. Although several studies were identified in this systematic review, substantial clinical heterogeneity was reported through a lack of reporting or large variations across studies in ginger interventions; the sources of clinical heterogeneity were varying CTx and antiemetic agents, as well as the type of cancer and groups of patients. It is challenging to draw reliable conclusions due to the large clinical heterogeneity between studies. Therefore, further studies are needed to gain enough confidence regarding the effects of ginger on CINV-related outcomes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14234982/s1, Table S1: Search strategy.
Author Contributions
J.C. took part in the study design, literature search, and selection, quality evaluation, data extraction, drafting of the manuscript, and revising the manuscript. J.L. designed a meta-analysis, drafting of the manuscript, and revised the manuscript. K.K. and H.-K.C. took part in drafting the manuscript and revising the manuscript. S.-A.L. contributed to literature search and selection, data extraction, and data analysis. H.-J.L. conceived the project, participated in the literature search and article selection, took charge of quality control, and revised the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Korea Food Research Institute (E0210400-02), Republic of Korea, and was partly supported by a grant (21162MFDS076) from Ministry of Food and Drug Safety in 2022.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Marx, W.M.; Teleni, L.; McCarthy, A.L.; Vitetta, L.; McKavanagh, D.; Thomson, D.; Isenring, E. Ginger (Zingiber officinale) and chemotherapy-induced nausea and vomiting: A systematic literature review. Nutr. Rev. 2013, 71, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Boterberg, T.; Lucas, J.; Panoff, J.; Valteau-Couanet, D.; Hero, B.; Bagatell, R.; Hill-Kayser, C.E. Neuroblastoma. Pediatr. Blood Cancer 2021, 68 (Suppl. S2), e28473. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A.; Haas, M.; Viney, R.; Pearson, S.A.; Haywood, P.; Brown, C.; Ward, R. Incidence and severity of self-reported chemotherapy side effects in routine care: A prospective cohort study. PLoS ONE 2017, 12, e0184360. [Google Scholar] [CrossRef]
- Henry, D.H.; Viswanathan, H.N.; Elkin, E.P.; Traina, S.; Wade, S.; Cella, D. Symptoms and treatment burden associated with cancer treatment: Results from a cross-sectional national survey in the U.S. Support Care Cancer 2008, 16, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Marx, W.; Kiss, N.; McCarthy, A.L.; McKavanagh, D.; Isenring, L. Chemotherapy-Induced Nausea and Vomiting: A Narrative Review to Inform Dietetics Practice. J. Acad. Nutr. Diet 2016, 116, 819–827. [Google Scholar] [CrossRef]
- Bourke, C.D.; Berkley, J.A.; Prendergast, A.J. Immune Dysfunction as a Cause and Consequence of Malnutrition. Trends Immunol. 2016, 37, 386–398. [Google Scholar] [CrossRef]
- Apfel, C.C.; Korttila, K.; Abdalla, M.; Kerger, H.; Turan, A.; Vedder, I.; Zernak, C.; Danner, K.; Jokela, R.; Pocock, S.J.; et al. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N. Engl. J. Med. 2004, 350, 2441–2451. [Google Scholar] [CrossRef]
- Navari, R.M. The safety of antiemetic medications for the prevention of chemotherapy-induced nausea and vomiting. Expert Opin. Drug. Saf. 2016, 15, 343–356. [Google Scholar] [CrossRef]
- Mao, Q.Q.; Xu, X.Y.; Cao, S.Y.; Gan, R.Y.; Corke, H.; Beta, T.; Li, H.B. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef]
- Giacosa, A.; Morazzoni, P.; Bombardelli, E.; Riva, A.; Bianchi Porro, G.; Rondanelli, M. Can nausea and vomiting be treated with ginger extract? Eur. Rev. Med. Pharm. Sci. 2015, 19, 1291–1296. [Google Scholar]
- Pertz, H.H.; Lehmann, J.; Roth-Ehrang, R.; Elz, S. Effects of ginger constituents on the gastrointestinal tract: Role of cholinergic M3 and serotonergic 5-HT3 and 5-HT4 receptors. Planta Med. 2011, 77, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, H.; Windeck, T.; Ploch, M.; Verspohl, E.J. Mode of action of gingerols and shogaols on 5-HT3 receptors: Binding studies, cation uptake by the receptor channel and contraction of isolated guinea-pig ileum. Eur. J. Pharm. 2006, 530, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Crichton, M.; Marshall, S.; Marx, W.; McCarthy, A.L.; Isenring, E. Efficacy of Ginger (Zingiber officinale) in Ameliorating Chemotherapy-Induced Nausea and Vomiting and Chemotherapy-Related Outcomes: A Systematic Review Update and Meta-Analysis. J. Acad. Nutr. Diet 2019, 119, 2055–2068. [Google Scholar] [CrossRef] [PubMed]
- Alparslan, C.B.; Ozkarman, A.; Eskin, N.; Yilmaz, S.; Akay, M.; Acikgoz, A.; Orsal, O. Effect of ginger on chemotherapy-induced nausea and/or vomiting in cancer patients. J. Aust. Tradit.-Med. Soc. 2012, 18, 15–18. [Google Scholar]
- Ansari, M.; Porouhan, P.; Mohammadianpanah, M.; Omidvari, S.; Mosalaei, A.; Ahmadloo, N.; Nasrollahi, H.; Hamedi, S.H. Efficacy of Ginger in Control of Chemotherapy Induced Nausea and Vomiting in Breast Cancer Patients Receiving Doxorubicin-Based Chemotherapy. Asian Pac. J. Cancer Prev. 2016, 17, 3877–3880. [Google Scholar] [PubMed]
- Arslan, M.; Ozdemir, L. Oral intake of ginger for chemotherapy-induced nausea and vomiting among women with breast cancer. Clin. J. Oncol. Nurs. 2015, 19, E92–E97. [Google Scholar] [CrossRef]
- Bossi, P.; Cortinovis, D.; Fatigoni, S.; Cossu Rocca, M.; Fabi, A.; Seminara, P.; Ripamonti, C.; Alfieri, S.; Granata, R.; Bergamini, C.; et al. A randomized, double-blind, placebo-controlled, multicenter study of a ginger extract in the management of chemotherapy-induced nausea and vomiting (CINV) in patients receiving high-dose cisplatin. Ann. Oncol. 2017, 28, 2547–2551. [Google Scholar] [CrossRef]
- Das, S.; Banra, M.P.; Joseph, N.M. Effect of ginger tea on chemotherapy-induced nausea and vomiting among cancer patients in selected hospitals, Bhubaneswar, Odisha. Int. J. Res. Pharm. Sci. 2020, 11, 1165–1171. [Google Scholar]
- Fahimi, F.; Khodadad, K.; Amini, S.; Naghibi, F.; Salamzadeh, J.; Baniasadi, S. Evaluating the effect of zingiber officinalis on nausea and vomiting in patients receiving Cisplatin based regimens. Iran. J. Pharm. Res. 2011, 10, 379–384. [Google Scholar]
- Shokri, F.; Mostafa Gharebaghi, P.; Esfhanai, A.; Sayyah-Melli, M.; Jafari Shobeiri, M.; Ouladsahebmadarek, E.; Ghojazadeh, M. Comparison of the Complications of Platinum-Based Adjuvant Chemotherapy with and Without Ginger in a Pilot Study on Ovarian Cancer Patients. Int. J. Women's Health Reprod. Sci. 2016, 5, 324–331. [Google Scholar] [CrossRef][Green Version]
- Kadhim, R.A.; Ali, B.M.; Kadhim, M.A.; Mohammed, S.J. Effect of Ginger Tea on Chemotherapy-Induced Nausea and Vomiting among Patients Attending the Oncology Teaching Hospital, Baghdad 2020. Indian J. Forensic Med. Toxicol. 2021, 15, 1463–1470. [Google Scholar] [CrossRef]
- Konmun, J.; Danwilai, K.; Ngamphaiboon, N.; Sripanidkulchai, B.; Sookprasert, A.; Subongkot, S. A phase II randomized double-blind placebo-controlled study of 6-gingerol as an anti-emetic in solid tumor patients receiving moderately to highly emetogenic chemotherapy. Med. Oncol. 2017, 34, 69. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qin, Y.; Liu, W.; Zhou, X.Y.; Li, Y.N.; Wang, L.Y. Efficacy of Ginger in Ameliorating Acute and Delayed Chemotherapy-Induced Nausea and Vomiting Among Patients with Lung Cancer Receiving Cisplatin-Based Regimens: A Randomized Controlled Trial. Integr. Cancer 2018, 17, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Manusirivithaya, S.; Sripramote, M.; Tangjitgamol, S.; Sheanakul, C.; Leelahakorn, S.; Thavaramara, T.; Tangcharoenpanich, K. Antiemetic effect of ginger in gynecologic oncology patients receiving cisplatin. Int. J. Gynecol. Cancer 2004, 14, 1063–1069. [Google Scholar] [CrossRef]
- Marx, W.; McCarthy, A.L.; Ried, K.; McKavanagh, D.; Vitetta, L.; Sali, A.; Lohning, A.; Isenring, E. The Effect of a Standardized Ginger Extract on Chemotherapy-Induced Nausea-Related Quality of Life in Patients Undergoing Moderately or Highly Emetogenic Chemotherapy: A Double Blind, Randomized, Placebo Controlled Trial. Nutrients 2017, 9, 867. [Google Scholar] [CrossRef]
- Montazeri, A.S.; Raei, M.; Ghanbari, A.; Dadgari, A.; Montazeri, A.S.; Hamidzadeh, A. Effect of herbal therapy to intensity chemotherapy-induced nausea and vomiting in cancer patients. Iran. Red. Crescent Med. J. 2013, 15, 101–106. [Google Scholar] [CrossRef][Green Version]
- Muthia, R.; Wahyu, W.; Dachriyanus. Effect of Ginger Infusion on Chemotherapy Induced Nausea and Vomiting in Breast Cancer Patients. J. Biol. Agric. Healthc. 2013, 3, 42–46. [Google Scholar]
- Panahi, Y.; Saadat, A.; Sahebkar, A.; Hashemian, F.; Taghikhani, M.; Abolhasani, E. Effect of ginger on acute and delayed chemotherapy-induced nausea and vomiting: A pilot, randomized, open-label clinical trial. Integr. Cancer 2012, 11, 204–211. [Google Scholar] [CrossRef]
- Ryan, J.L.; Heckler, C.E.; Roscoe, J.A.; Dakhil, S.R.; Kirshner, J.; Flynn, P.J.; Hickok, J.T.; Morrow, G.R. Ginger (Zingiber officinale) reduces acute chemotherapy-induced nausea: A URCC CCOP study of 576 patients. Support Care Cancer 2012, 20, 1479–1489. [Google Scholar] [CrossRef]
- Sontakke, S.; Thawani, V.; Naik, M.S. Ginger as an antiemetic in nausea and vomiting induced by chemotherapy: A randomized, cross-over, double blind study. Indian J. Pharmacol. 2003, 35, 32–36. [Google Scholar]
- Sanaati, F.; Najafi, S.; Kashaninia, Z.; Sadeghi, M. Effect of Ginger and Chamomile on Nausea and Vomiting Caused by Chemotherapy in Iranian Women with Breast Cancer. Asian Pac. J. Cancer Prev. 2016, 17, 4125–4129. [Google Scholar]
- Thamlikitkul, L.; Srimuninnimit, V.; Akewanlop, C.; Ithimakin, S.; Techawathanawanna, S.; Korphaisarn, K.; Chantharasamee, J.; Danchaivijitr, P.; Soparattanapaisarn, N. Efficacy of ginger for prophylaxis of chemotherapy-induced nausea and vomiting in breast cancer patients receiving adriamycin-cyclophosphamide regimen: A randomized, double-blind, placebo-controlled, crossover study. Support Care Cancer 2017, 25, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Uthaipaisanwong, A.; Oranratanaphan, S.; Musigavong, N. Effects of ginger adjunct to the standard prophylaxis on reducing carboplatin and paclitaxel-induced nausea vomiting: A randomized controlled study. Support Care Cancer 2020, 28, 3831–3838. [Google Scholar] [CrossRef]
- Wazqar, D.Y.; Thabet, H.A.; Safwat, A.M. A Quasi-Experimental Study of the Effect of Ginger Tea on Preventing Nausea and Vomiting in Patients with Gynecological Cancers Receiving Cisplatin-Based Regimens. Cancer Nurs. 2021, 44, E513–E519. [Google Scholar] [CrossRef] [PubMed]
- Yekta, Z.P.; Ebrahimi, S.M.; Hosseini, M.; Nasrabadi, A.N.; Sedighi, S.; Surmaghi, M.H.; Madani, H. Ginger as a miracle against chemotherapy-induced vomiting. Iran. J. Nurs. Midwifery Res. 2012, 17, 325–329. [Google Scholar] [PubMed]
- Zick, S.M.; Ruffin, M.T.; Lee, J.; Normolle, D.P.; Siden, R.; Alrawi, S.; Brenner, D.E. Phase II trial of encapsulated ginger as a treatment for chemotherapy-induced nausea and vomiting. Support Care Cancer 2009, 17, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Oh, H. Ginger as an antiemetic modality for chemotherapy-induced nausea and vomiting: A systematic review and meta-analysis. Oncol. Nurs. Forum. 2013, 40, 163–170. [Google Scholar] [CrossRef]
- Natale, J.J. Overview of the prevention and management of CINV. Am. J. Manag. Care 2018, 24, S391–S397. [Google Scholar]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A.M. Gingerols and shogaols: Important nutraceutical principles from ginger. Phytochemistry 2015, 117, 554–568. [Google Scholar] [CrossRef]
- Navari, R.M.; Aapro, M. Antiemetic Prophylaxis for Chemotherapy-Induced Nausea and Vomiting. N. Engl. J. Med. 2016, 374, 1356–1367. [Google Scholar] [CrossRef]
- Tian, L.; Qian, W.; Qian, Q.; Zhang, W.; Cai, X. Gingerol inhibits cisplatin-induced acute and delayed emesis in rats and minks by regulating the central and peripheral 5-HT, SP, and DA systems. J. Nat. Med. 2020, 74, 353–370. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Cai, X.; Wang, Y.; Zhang, X.; Zhao, H.; Qian, Q.; Yang, Z.; Liu, Z.; Hasegawa, J. Effect of Gingerol on Cisplatin-Induced Pica Analogous to Emesis Via Modulating Expressions of Dopamine 2 Receptor, Dopamine Transporter and Tyrosine Hydroxylase in the Vomiting Model of Rats. Yonago Acta Med. 2016, 59, 100–110. [Google Scholar] [PubMed]
- Minami, M.; Endo, T.; Hirafuji, M.; Hamaue, N.; Liu, Y.; Hiroshige, T.; Nemoto, M.; Saito, H.; Yoshioka, M. Pharmacological aspects of anticancer drug-induced emesis with emphasis on serotonin release and vagal nerve activity. Pharmacol. Ther. 2003, 99, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Browning, K.N. Role of central vagal 5-HT3 receptors in gastrointestinal physiology and pathophysiology. Front. Neurosci. 2015, 9, 413. [Google Scholar] [CrossRef]
- Muñoz, M.; Coveñas, R. Involvement of substance P and the NK-1 receptor in human pathology. Amino Acids 2014, 46, 1727–1750. [Google Scholar] [CrossRef]
- Patak, E.; Candenas, M.L.; Pennefather, J.N.; Ziccone, S.; Lilley, A.; Martín, J.D.; Flores, C.; Mantecón, A.G.; Story, M.E.; Pinto, F.M. Tachykinins and tachykinin receptors in human uterus. Br. J. Pharm. 2003, 139, 523–532. [Google Scholar] [CrossRef]
- Daubner, S.C.; Le, T.; Wang, S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch. Biochem. Biophys. 2011, 508, 1–12. [Google Scholar] [CrossRef]
- Wu, C.T.; Liao, J.M.; Ko, J.L.; Lee, Y.L.; Chang, H.Y.; Wu, C.H.; Ou, C.C. D-Methionine Ameliorates Cisplatin-Induced Muscle Atrophy via Inhibition of Muscle Degradation Pathway. Integr. Cancer 2019, 18, 1534735419828832. [Google Scholar] [CrossRef]
- Ahmad, A.; Khushtar, M.; Kumar, R.; Badruddeen; Riyaz, A.; Khan, M.I.; Rahman, A. Augmented Reversal of Cisplatin-Induced Delayed Gastric Emptying by Amla (Emblica Officinalis) Fruit Extract in Sprague-Dawley Rats. J. Diet Suppl. 2018, 15, 684–691. [Google Scholar] [CrossRef]
- Wong, Y.S.; Lin, M.Y.; Liu, P.F.; Ko, J.L.; Huang, G.T.; Tu, D.G.; Ou, C.C. D-methionine improves cisplatin-induced anorexia and dyspepsia syndrome by attenuating intestinal tryptophan hydroxylase 1 activity and increasing plasma leptin concentration. Neurogastroenterol. Motil. 2020, 32, e13803. [Google Scholar] [CrossRef]
- Ensiyeh, J.; Sakineh, M.A. Comparing ginger and vitamin B6 for the treatment of nausea and vomiting in pregnancy: A randomised controlled trial. Midwifery 2009, 25, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Chittumma, P.; Kaewkiattikun, K.; Wiriyasiriwach, B. Comparison of the effectiveness of ginger and vitamin B6 for treatment of nausea and vomiting in early pregnancy: A randomized double-blind controlled trial. J. Med. Assoc. Thai 2007, 90, 15–20. [Google Scholar] [PubMed]
- Vutyavanich, T.; Kraisarin, T.; Ruangsri, R. Ginger for nausea and vomiting in pregnancy: Randomized, double-masked, placebo-controlled trial. Obs. Gynecol. 2001, 97, 577–582. [Google Scholar] [CrossRef]
- Ozgoli, G.; Goli, M.; Simbar, M. Effects of ginger capsules on pregnancy, nausea, and vomiting. J. Altern. Complement. Med. 2009, 15, 243–246. [Google Scholar] [CrossRef]
- Stanisiere, J.; Mousset, P.Y.; Lafay, S. How Safe Is Ginger Rhizome for Decreasing Nausea and Vomiting in Women during Early Pregnancy? Foods 2018, 7, 50. [Google Scholar] [CrossRef]
- Peng, F.; Tao, Q.; Wu, X.; Dou, H.; Spencer, S.; Mang, C.; Xu, L.; Sun, L.; Zhao, Y.; Li, H.; et al. Cytotoxic, cytoprotective and antioxidant effects of isolated phenolic compounds from fresh ginger. Fitoterapia 2012, 83, 568–585. [Google Scholar] [CrossRef]
- Wei, Q.Y.; Ma, J.P.; Cai, Y.J.; Yang, L.; Liu, Z.L. Cytotoxic and apoptotic activities of diarylheptanoids and gingerol-related compounds from the rhizome of Chinese ginger. J. Ethnopharmacol. 2005, 102, 177–184. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, S.I.; Park, H.W.; Yang, J.H.; Shin, T.Y.; Kim, Y.C.; Baek, N.I.; Kim, S.H.; Choi, S.U.; Kwon, B.M.; et al. Cytotoxic components from the dried rhizomes of Zingiber officinale Roscoe. Arch. Pharm. Res. 2008, 31, 415–418. [Google Scholar] [CrossRef]
- Abudayyak, M.; Özdemir Nath, E.; Özhan, G. Toxic potentials of ten herbs commonly used for aphrodisiac effect in Turkey. Turk. J. Med. Sci. 2015, 45, 496–506. [Google Scholar] [CrossRef]
- Yang, G.; Zhong, L.; Jiang, L.; Geng, C.; Cao, J.; Sun, X.; Ma, Y. Genotoxic effect of 6-gingerol on human hepatoma G2 cells. Chem. Biol. Interact 2010, 185, 12–17. [Google Scholar] [CrossRef]
- Soudamini, K.K.; Unnikrishnan, M.C.; Sukumaran, K.; Kuttan, R. Mutagenicity and anti-mutagenicity of selected spices. Indian J. Physiol. Pharm. 1995, 39, 347–353. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).