Early-Adulthood Weight Change and Later Physical Activity in Relation to Cardiovascular and All-Cause Mortality: NHANES 1999–2014
Abstract
1. Introduction
2. Materials and Methods
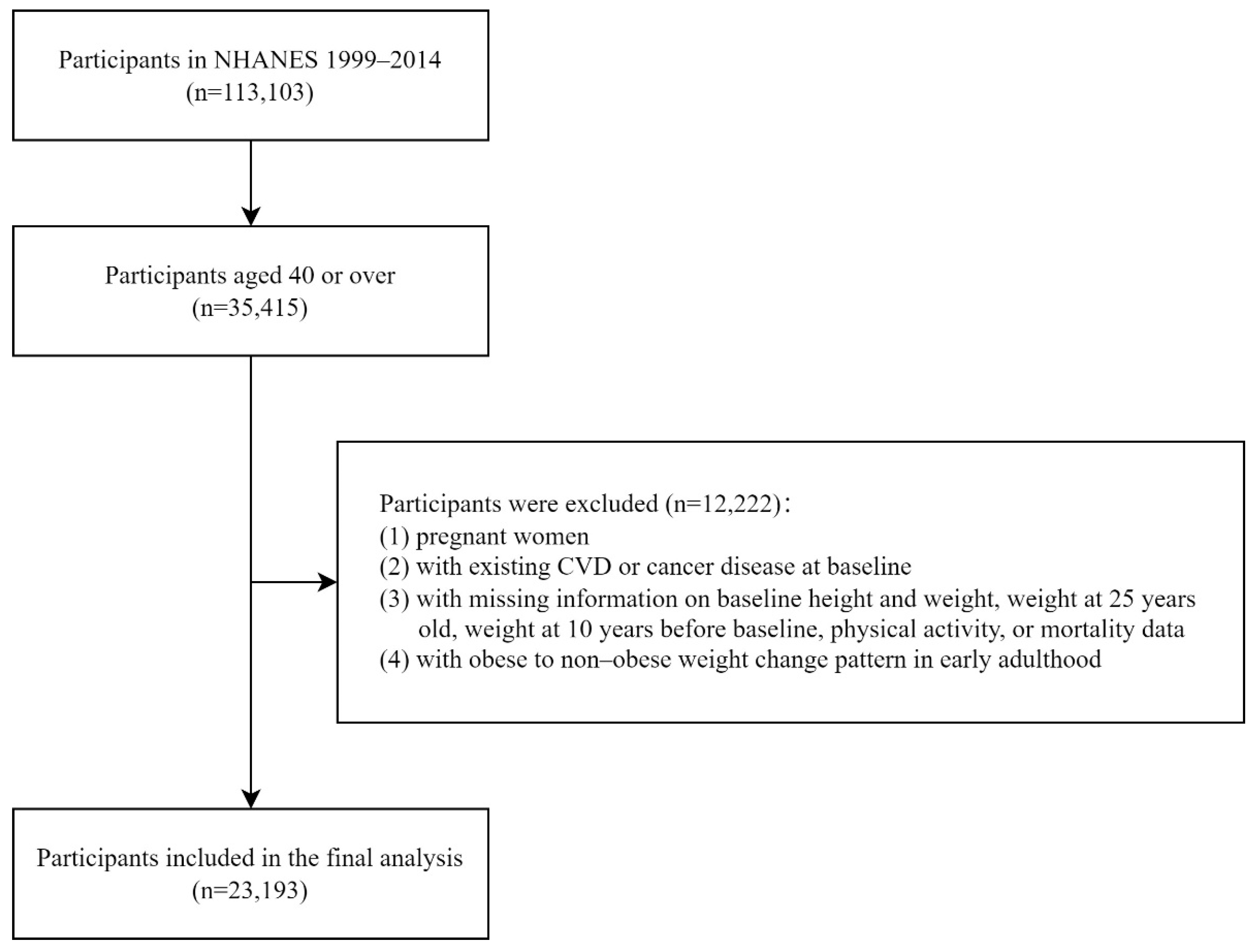
2.1. Study Population
2.2. Outcome Ascertainment
2.3. Assessments of Early-Adulthood Weight Change
2.4. Assessment of Later Physical Activity
2.5. Covariate Assessment
2.6. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Independent Association of Early-Adulthood Weight Change Pattern and Later Physical Activity with CVD and All-Cause Mortality
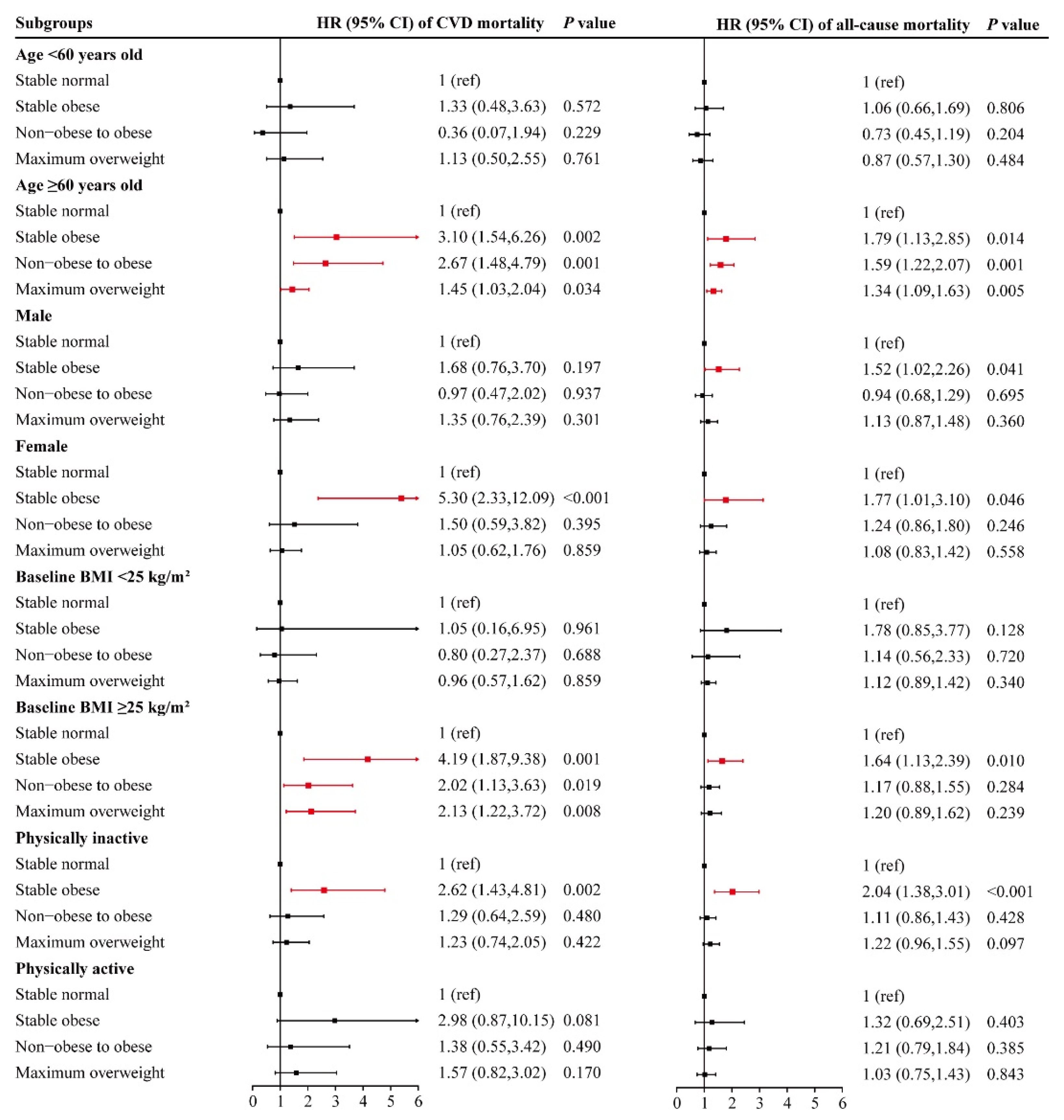
3.3. Associations of Early-Adulthood Weight Change Pattern with CVD and All-Cause Mortality among Different Subgroups
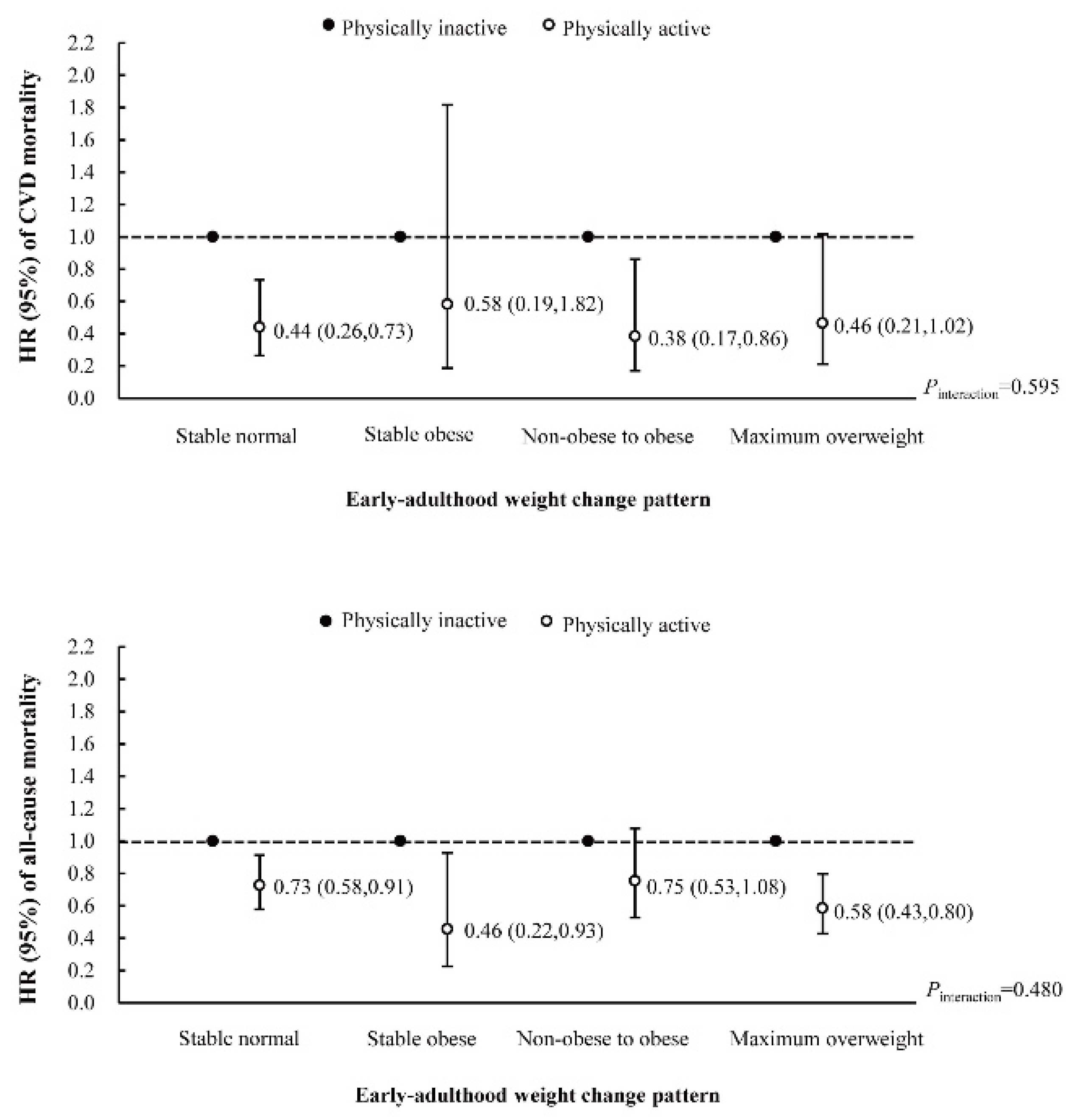
3.4. Interaction and Joint Analysis of Early-Adulthood Weight Change Pattern and Later Physical Activity with CVD and All-Cause Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jayedi, A.; Rashidy-Pour, A.; Khorshidi, M.; Shab-Bidar, S. Body mass index, abdominal adiposity, weight gain and risk of developing hypertension: A systematic review and dose-response meta-analysis of more than 2.3 million participants. Obes. Rev. 2018, 19, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yue, X.J.; Li, H.H.; Song, Z.X.; Yan, H.Q.; Zhang, P.; Gui, Y.K.; Chang, L.; Li, T. Overweight and Obesity in Young Adulthood and the Risk of Stroke: A Meta-analysis. J. Stroke Cerebrovasc. Dis. 2016, 25, 2995–3004. [Google Scholar] [CrossRef]
- Mongraw-Chaffin, M.L.; Peters, S.; Huxley, R.R.; Woodward, M. The sex-specific association between BMI and coronary heart disease: A systematic review and meta-analysis of 95 cohorts with 1.2 million participants. Lancet Diabetes Endocrinol. 2015, 3, 437–449. [Google Scholar] [CrossRef]
- French, S.A.; Folsom, A.R.; Jeffery, R.W.; Zheng, W.; Mink, P.J.; Baxter, J.E. Weight variability and incident disease in older women: The Iowa Women's Health Study. Int. J. Obes. Relat. Metab. Disord. 1997, 21, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Li, T.Y.; Rana, J.S.; Manson, J.E.; Willett, W.C.; Stampfer, M.J.; Colditz, G.A.; Rexrode, K.M.; Hu, F.B. Obesity as compared with physical activity in predicting risk of coronary heart disease in women. Circulation 2006, 113, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Okada, C.; Kubota, Y.; Eshak, E.S.; Cui, R.; Tamakoshi, A.; Iso, H. Weight Change and Mortality from Cardiovascular Diseases: The Japan Collaborative Cohort Study. J. Atheroscler. Thromb. 2021, 28, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, E.; Berentzen, T.L.; Angquist, L.; Holst, C.; Sorensen, T.I. Long-term weight changes in obese young adult men and subsequent all-cause mortality. Int. J. Obes. 2013, 37, 1020–1025. [Google Scholar] [CrossRef][Green Version]
- Shimazu, T.; Kuriyama, S.; Ohmori-Matsuda, K.; Kikuchi, N.; Nakaya, N.; Tsuji, I. Increase in body mass index category since age 20 years and all-cause mortality: A prospective cohort study (the Ohsaki Study). Int. J. Obes. 2009, 33, 490–496. [Google Scholar] [CrossRef]
- Kimokoti, R.W.; Newby, P.K.; Gona, P.; Zhu, L.; McKeon-O’Malley, C.; Pablo, G.J.; D’Agostino, R.B.; Millen, B.E. Patterns of weight change and progression to overweight and obesity differ in men and women: Implications for research and interventions. Public Health Nutr. 2013, 16, 1463–1475. [Google Scholar] [CrossRef]
- Reas, D.L.; Nygard, J.F.; Svensson, E.; Sorensen, T.; Sandanger, I. Changes in body mass index by age, gender, and socio-economic status among a cohort of Norwegian men and women (1990–2001). BMC Public Health 2007, 7, 269. [Google Scholar] [CrossRef]
- Byberg, L.; Melhus, H.; Gedeborg, R.; Sundstrom, J.; Ahlbom, A.; Zethelius, B.; Berglund, L.G.; Wolk, A.; Michaelsson, K. Total mortality after changes in leisure time physical activity in 50 year old men: 35 year follow-up of population based cohort. BMJ 2009, 338, b688. [Google Scholar] [CrossRef] [PubMed]
- Schnohr, P.; O'Keefe, J.H.; Lange, P.; Jensen, G.B.; Marott, J.L. Impact of persistence and non-persistence in leisure time physical activity on coronary heart disease and all-cause mortality: The Copenhagen City Heart Study. Eur. J. Prev. Cardiol. 2017, 24, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Blond, K.; Brinklov, C.F.; Ried-Larsen, M.; Crippa, A.; Grontved, A. Association of high amounts of physical activity with mortality risk: A systematic review and meta-analysis. Br. J. Sports Med. 2020, 54, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Dattilo, A.M.; Kris-Etherton, P.M. Effects of weight reduction on blood lipids and lipoproteins: A meta-analysis. Am. J. Clin. Nutr. 1992, 56, 320–328. [Google Scholar] [CrossRef]
- Tchernof, A.; Nolan, A.; Sites, C.K.; Ades, P.A.; Poehlman, E.T. Weight loss reduces C-reactive protein levels in obese postmenopausal women. Circulation 2002, 105, 564–569. [Google Scholar] [CrossRef]
- Selvin, E.; Paynter, N.P.; Erlinger, T.P. The effect of weight loss on C-reactive protein: A systematic review. Arch. Intern. Med. 2007, 167, 31–39. [Google Scholar] [CrossRef]
- Gower, B.A.; Weinsier, R.L.; Jordan, J.M.; Hunter, G.R.; Desmond, R. Effects of weight loss on changes in insulin sensitivity and lipid concentrations in premenopausal African American and white women. Am. J. Clin. Nutr. 2002, 76, 923–927. [Google Scholar] [CrossRef]
- Mason, C.; Foster-Schubert, K.E.; Imayama, I.; Kong, A.; Xiao, L.; Bain, C.; Campbell, K.L.; Wang, C.Y.; Duggan, C.R.; Ulrich, C.M.; et al. Dietary weight loss and exercise effects on insulin resistance in postmenopausal women. Am. J. Prev. Med. 2011, 41, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.P.; Albert, S.G.; Reeds, D.N.; Kress, K.S.; McDaniel, J.L.; Klein, S.; Villareal, D.T. Effects of matched weight loss from calorie restriction, exercise, or both on cardiovascular disease risk factors: A randomized intervention trial. Am. J. Clin. Nutr. 2016, 104, 576–586. [Google Scholar] [CrossRef]
- Pojednic, R.; D'Arpino, E.; Halliday, I.; Bantham, A. The Benefits of Physical Activity for People with Obesity, Independent of Weight Loss: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 4981. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ye, Y.; Zhang, Y.; Pan, X.F.; Pan, A. Weight change across adulthood in relation to all cause and cause specific mortality: Prospective cohort study. BMJ 2019, 367, l5584. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.J.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.R.; Tucker, L.A.; Bailey, B.W.; LeCheminant, J.D. Physical Activity and Insulin Resistance in 6,500 NHANES Adults: The Role of Abdominal Obesity. J. Obes. 2020, 2020, 3848256. [Google Scholar] [CrossRef] [PubMed]
- Steeves, J.A.; Fitzhugh, E.C.; Bradwin, G.; McGlynn, K.A.; Platz, E.A.; Joshu, C.E. Cross-sectional association between physical activity and serum testosterone levels in US men: Results from NHANES 1999–2004. Andrology 2016, 4, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Y.; Jiang, H.; Liang, X.; Wang, Y.; Lu, W. Associations of BMI and Waist Circumference with All-Cause Mortality: A 22-Year Cohort Study. Obesity 2019, 27, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Kee, C.C.; Sumarni, M.G.; Lim, K.H.; Selvarajah, S.; Haniff, J.; Tee, G.; Gurpreet, K.; Faudzi, Y.A.; Amal, N.M. Association of BMI with risk of CVD mortality and all-cause mortality. Public Health Nutr. 2017, 20, 1226–1234. [Google Scholar] [CrossRef]
- Bhaskaran, K.; Dos-Santos-Silva, I.; Leon, D.A.; Douglas, I.J.; Smeeth, L. Association of BMI with overall and cause-specific mortality: A population-based cohort study of 3.6 million adults in the UK. Lancet Diabetes Endocrinol. 2018, 6, 944–953. [Google Scholar] [CrossRef]
- Strandberg, T.E.; Strandberg, A.; Salomaa, V.V.; Pitkala, K.; Miettinen, T.A. Impact of midlife weight change on mortality and quality of life in old age. Prospective cohort study. Int. J. Obes. Relat. Metab. Disord 2003, 27, 950–954. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Adams, K.F.; Leitzmann, M.F.; Ballard-Barbash, R.; Albanes, D.; Harris, T.B.; Hollenbeck, A.; Kipnis, V. Body mass and weight change in adults in relation to mortality risk. Am. J. Epidemiol. 2014, 179, 135–144. [Google Scholar] [CrossRef]
- Koster, A.; Harris, T.B.; Moore, S.C.; Schatzkin, A.; Hollenbeck, A.R.; van Eijk, J.T.; Leitzmann, M.F. Joint associations of adiposity and physical activity with mortality: The National Institutes of Health-AARP Diet and Health Study. Am. J. Epidemiol. 2009, 169, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cash, R.E.; Bower, J.K.; Focht, B.C.; Paskett, E.D. Physical activity and risk of cardiovascular disease by weight status among U.S adults. PLoS ONE 2020, 15, e232893. [Google Scholar] [CrossRef] [PubMed]
- Myrskyla, M.; Chang, V.W. Weight change, initial BMI, and mortality among middle- and older-aged adults. Epidemiology 2009, 20, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Kim, T.H.; Han, E. BMI, weight change, and incidence of disability among Korean adults: A nationwide retrospective cohort study. Disabil. Health J. 2021, 14, 101104. [Google Scholar] [CrossRef] [PubMed]
- Elagizi, A.; Kachur, S.; Carbone, S.; Lavie, C.J.; Blair, S.N. A Review of Obesity, Physical Activity, and Cardiovascular Disease. Curr Obes Rep 2020, 9, 571–581. [Google Scholar] [CrossRef]
- Alpert, M.A. Obesity cardiomyopathy: Pathophysiology and evolution of the clinical syndrome. Am. J. Med. Sci. 2001, 321, 225–236. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Antoniades, C. The role of adipose tissue in cardiovascular health and disease. Nat. Rev. Cardiol. 2019, 16, 83–99. [Google Scholar] [CrossRef]
- Nakamura, K.; Fuster, J.J.; Walsh, K. Adipokines: A link between obesity and cardiovascular disease. J. Cardiol. 2014, 63, 250–259. [Google Scholar] [CrossRef]
- Gaesser, G.A.; Angadi, S.S. Obesity treatment: Weight loss versus increasing fitness and physical activity for reducing health risks. iScience 2021, 24, 102995. [Google Scholar] [CrossRef] [PubMed]
- Ramel, A.; Pumberger, C.; Martinez, A.J.; Kiely, M.; Bandarra, N.M.; Thorsdottir, I. Cardiovascular risk factors in young, overweight, and obese European adults and associations with physical activity and omega-3 index. Nutr. Res. 2009, 29, 305–312. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, L.; Wu, Y.; Wilson, R.F.; Weston, C.; Fawole, O.; Bleich, S.N.; Cheskin, L.J.; Showell, N.N.; Lau, B.D.; et al. What childhood obesity prevention programmes work? A systematic review and meta-analysis. Obes. Rev. 2015, 16, 547–565. [Google Scholar] [CrossRef] [PubMed]
- Top, F.U.; Kaya, B.; Tepe, B.; Avci, E. Physio-psychosocial and Metabolic Parameters of Obese Adolescents: Health-Promoting Lifestyle Education of Obesity Management. Community Ment. Health J. 2019, 55, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.J.; Perusse, L.; Sarzynski, M.A.; Fornage, M.; Sidney, S.; Sternfeld, B.; Rice, T.; Terry, J.G.; Jacobs, D.J.; Katzmarzyk, P.; et al. Genome-wide association studies suggest sex-specific loci associated with abdominal and visceral fat. Int. J. Obes. 2016, 40, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Doherty, A.; Smith-Byrne, K.; Ferreira, T.; Holmes, M.V.; Holmes, C.; Pulit, S.L.; Lindgren, C.M. GWAS identifies 14 loci for device-measured physical activity and sleep duration. Nat. Commun. 2018, 9, 5257. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Stable Normal | Stable Obese | Non-Obese to Obese | Maximum Overweight | ||||
|---|---|---|---|---|---|---|---|---|
| Inactive | Active | Inactive | Active | Inactive | Active | Inactive | Active | |
| Number of participants | 4415 | 4673 | 717 | 580 | 2119 | 1761 | 4513 | 4415 |
| Age (years), n (%) | ||||||||
| <60 | 2880(77.07) | 3294(79.62) | 494(81.29) | 467(87.15) | 1010(62.49) | 994(68.86) | 2450(68.88) | 2704(73.17) |
| ≥60 | 1535(22.93) | 1379(20.38) | 223(18.71) | 113(12.85) | 1109(37.51) | 767(31.14) | 2063(31.12) | 1711(26.83) |
| Sex, n (%) | ||||||||
| Male | 1658(35.38) | 1930(36.96) | 316(45.64) | 347(66.41) | 905(43.68) | 966(56.41) | 2493(56.91) | 2949(69.00) |
| Female | 2757(64.62) | 2743(63.04) | 401(54.36) | 233(33.59) | 1214(56.32) | 795(43.59) | 2020(43.09) | 1466(31.00) |
| Race/ethnicity, n (%) | ||||||||
| Mexican-American | 638(4.22) | 518(3.48) | 117(5.18) | 83(5.60) | 419(6.33) | 352(6.56) | 983(6.93) | 711(5.14) |
| Non-Hispanic white | 2177(73.67) | 2620(78.82) | 285(68.89) | 277(72.55) | 959(74.36) | 848(76.12) | 2038(72.78) | 2320(78.62) |
| Non-Hispanic black | 957(10.29) | 787(7.24) | 245(17.64) | 164(13.35) | 543(12.59) | 377(9.80) | 977(11.14) | 817(8.33) |
| Other | 643(11.81) | 748(10.46) | 70(8.29) | 56(8.49) | 198(6.73) | 184(7.52) | 515(9.16) | 567(7.91) |
| Education, n (%) | ||||||||
| <High school | 1099(14.57) | 854(10.79) | 205(16.30) | 125(12.58) | 678(19.06) | 402(12.77) | 1392(17.99) | 949(12.54) |
| High school | 998(22.28) | 989(20.35) | 183(28.01) | 158(29.88) | 508(27.64) | 428(26.85) | 1026(24.67) | 966(21.89) |
| >High school | 2313(63.15) | 2827(68.86) | 327(55.68) | 297(57.54) | 930(53.30) | 931(60.38) | 2092(57.34) | 2494(65.57) |
| Ratio of family income to poverty, n (%) | ||||||||
| ≤1.30 | 965(14.43) | 961(13.07) | 195(20.66) | 158(19.13) | 528(16.87) | 369(12.61) | 989(14.39) | 848(11.81) |
| 1.31–3.50 | 1432(31.61) | 1371(27.38) | 247(34.86) | 195(36.06) | 727(32.36) | 628(36.26) | 1505(30.72) | 1351(27.80) |
| >3.50 | 1659(53.96) | 2008(59.55) | 205(44.49) | 197(44.81) | 691(50.77) | 660(51.13) | 1688(54.89) | 1901(60.39) |
| Baseline BMI (kg/m2), mean ± SD | 24.95 ± 4.09 | 24.49 ± 3.65 | 39.54 ± 8.86 | 36.75 ± 7.43 | 34.66 ± 5.96 | 33.63 ± 5.30 | 29.28 ± 4.23 | 29.06 ± 4.02 |
| Total cholesterol (mg/dL), mean ± SD | 207.97 ± 40.34 | 207.72 ± 40.07 | 195.06 ± 43.04 | 197.69 ± 41.21 | 202.93 ± 44.77 | 203.55 ± 40.90 | 207.03 ± 41.2 | 208.39 ± 41.7 |
| HDL cholesterol (mg/dL), mean ± SD | 58.31 ± 17.95 | 59.52 ± 17.55 | 49.05 ± 13.51 | 47.58 ± 12.81 | 50.35 ± 15.11 | 50.11 ± 13.54 | 51.42 ± 14.57 | 52.21 ± 15.76 |
| Carbohydrate intake (gm), median (IQR) | 225.7(134.1) | 235.6(145.3) | 224.6(144.0) | 253.3(167.9) | 213.7(137.7) | 231.8(138.9) | 228.8(136.7) | 242.6(147.3) |
| Dietary fiber (gm), median (IQR) | 13.8(10.8) | 15.5(13.2) | 13.6(10.8) | 14.7(11.8) | 13.7(10.7) | 15.3(12.2) | 14.5(11.7) | 16.0(12.0) |
| Protein intake (gm), median (IQR) | 68.6(44.0) | 71.8(40.4) | 73.3(36.5) | 67.6(54.0) | 72.9(50.8) | 73.9(54.6) | 76.8(45.3) | 77.8(48.2) |
| Fat intake (gm), median (IQR) | 65.3(49.2) | 69(53.5) | 73.4(59.3) | 81.9(66.9) | 65.8(52.3) | 78.9(56.7) | 70.2(53.2) | 75.5(54.0) |
| Energy intake (kcal), median (IQR) | 1828.5(1050.8) | 1911(1079.0) | 1938(1186.6) | 2187.5(1248.0) | 1732.0(1097.0) | 2014.0(1155.0) | 1918.0(1155.0) | 2046.5(1195.5) |
| Alcohol drinking status, n (%) | ||||||||
| Nondrinker | 3005(68.49) | 2923(61.70) | 575(80.78) | 422(73.09) | 1633(76.63) | 1266(71.42) | 3118(67.30) | 2867(63.40) |
| Moderate drinking | 446(11.40) | 569(12.80) | 34(7.11) | 42(7.89) | 176(10.91) | 179(10.68) | 480(12.19) | 552(13.75) |
| Heavy drinking | 737(20.10) | 913(25.50) | 78(12.11) | 90(19.02) | 202(12.46) | 257(17.90) | 699(20.52) | 809(22.85) |
| Smoking status, n (%) | ||||||||
| Nonsmoker | 2290(52.71) | 2350(51.01) | 408(55.94) | 299(54.49) | 1184(56.10) | 928(52.43) | 2305(50.67) | 2307(53.55) |
| Former smoker | 1149(26.79) | 1295(28.28) | 188(25.59) | 143(26.32) | 647(29.21) | 564(32.16) | 1385(30.95) | 1412(32.40) |
| Current smoker | 971(20.50) | 1026(20.71) | 120(18.48) | 138(19.20) | 287(14.69) | 269(15.41) | 820(18.37) | 695(14.05) |
| Hypertension, n (%) | ||||||||
| Yes | 1228(22.83) | 1087(21.00) | 426(61.08) | 294(46.23) | 1206(51.36) | 874(45.57) | 1812(34.81) | 1641(33.17) |
| No | 3178(77.17) | 3575(79.00) | 283(38.92) | 285(53.77) | 906(48.64) | 882(54.43) | 2684(65.19) | 2768(66.83) |
| Diabetes, n (%) | ||||||||
| Yes | 319(4.95) | 253(3.58) | 262(31.74) | 196(29.11) | 693(24.81) | 449(19.28) | 802(12.37) | 599(9.54) |
| No | 4096(95.05) | 4420(96.42) | 455(68.26) | 384(70.89) | 1426(75.19) | 1312(80.72) | 3711(87.63) | 3816(90.46) |
| Characteristic | Early-Adulthood Weight Change Pattern | Later Physical Activity | ||||
|---|---|---|---|---|---|---|
| Stable Normal | Stable Obese | Non-Obese to Obese | Maximum Overweight | Inactive | Active | |
| CVD mortality | ||||||
| No. of participants | 9088 | 1297 | 3880 | 8928 | 11,764 | 11,429 |
| Deaths/person-years | 149/83,876 | 40/10,157 | 112/32,028 | 232/80,340 | 370/108,840 | 163/97,561 |
| Unadjusted | 1.00 | 3.58 (1.79,7.16) | 2.20 (1.37,3.54) | 1.89 (1.30,2.76) | 1.00 | 0.47 (0.32,0.71) |
| Model 1 | 1.00 | 3.75 (1.81,7.76) | 1.62 (1.00,2.64) | 1.40 (0.94,2.08) | 1.00 | 0.47 (0.31,0.71) |
| Model 2 | 1.00 | 2.51 (1.41,4.47) | 1.20 (0.66,2.16) | 1.30 (0.88,1.93) | 1.00 | 0.51 (0.33,0.81) |
| All-cause mortality | ||||||
| No. of participants | 9088 | 1297 | 3880 | 8928 | 11,764 | 11,429 |
| Deaths/person-years | 938/83,876 | 142/10,157 | 482/32,028 | 1172/80,340 | 1771/108,840 | 963/97,561 |
| Unadjusted | 1.00 | 1.68 (1.26,2.26) | 1.53 (1.25,1.87) | 1.40 (1.17,1.69) | 1.00 | 0.62 (0.52,0.75) |
| Model 1 | 1.00 | 1.89 (1.41,2.54) | 1.15 (0.95,1.39) | 1.10 (0.90,1.33) | 1.00 | 0.62 (0.52,0.75) |
| Model 2 | 1.00 | 1.69 (1.22,2.35) | 1.11 (0.90,1.38) | 1.14 (0.92,1.40) | 1.00 | 0.67 (0.55,0.81) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, X.; Tang, C.; Zhai, X.; Li, S.; Ma, W.; Liu, K.; Kokoro, S.; Sheerah, H.A.; Zhu, H.; Cao, J. Early-Adulthood Weight Change and Later Physical Activity in Relation to Cardiovascular and All-Cause Mortality: NHANES 1999–2014. Nutrients 2022, 14, 4974. https://doi.org/10.3390/nu14234974
Xiao X, Tang C, Zhai X, Li S, Ma W, Liu K, Kokoro S, Sheerah HA, Zhu H, Cao J. Early-Adulthood Weight Change and Later Physical Activity in Relation to Cardiovascular and All-Cause Mortality: NHANES 1999–2014. Nutrients. 2022; 14(23):4974. https://doi.org/10.3390/nu14234974
Chicago/Turabian StyleXiao, Xinyu, Chengyao Tang, Xiaobing Zhai, Shiyang Li, Wenzhi Ma, Keyang Liu, Shirai Kokoro, Haytham A. Sheerah, Huiping Zhu, and Jinhong Cao. 2022. "Early-Adulthood Weight Change and Later Physical Activity in Relation to Cardiovascular and All-Cause Mortality: NHANES 1999–2014" Nutrients 14, no. 23: 4974. https://doi.org/10.3390/nu14234974
APA StyleXiao, X., Tang, C., Zhai, X., Li, S., Ma, W., Liu, K., Kokoro, S., Sheerah, H. A., Zhu, H., & Cao, J. (2022). Early-Adulthood Weight Change and Later Physical Activity in Relation to Cardiovascular and All-Cause Mortality: NHANES 1999–2014. Nutrients, 14(23), 4974. https://doi.org/10.3390/nu14234974






