Anaemia and Nutritional Status during HIV and Helminth Coinfection among Adults in South Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Recruitment of Study Participants
2.2. Sample Collection
2.3. Parasite Detection
2.4. Detection of HIV Status, Viral Loads, and CD4+ Counts
2.5. Analysis of Anaemia and Nutritional Status
2.6. Data Analysis
3. Results
3.1. General and Clinical Profiles of Participants
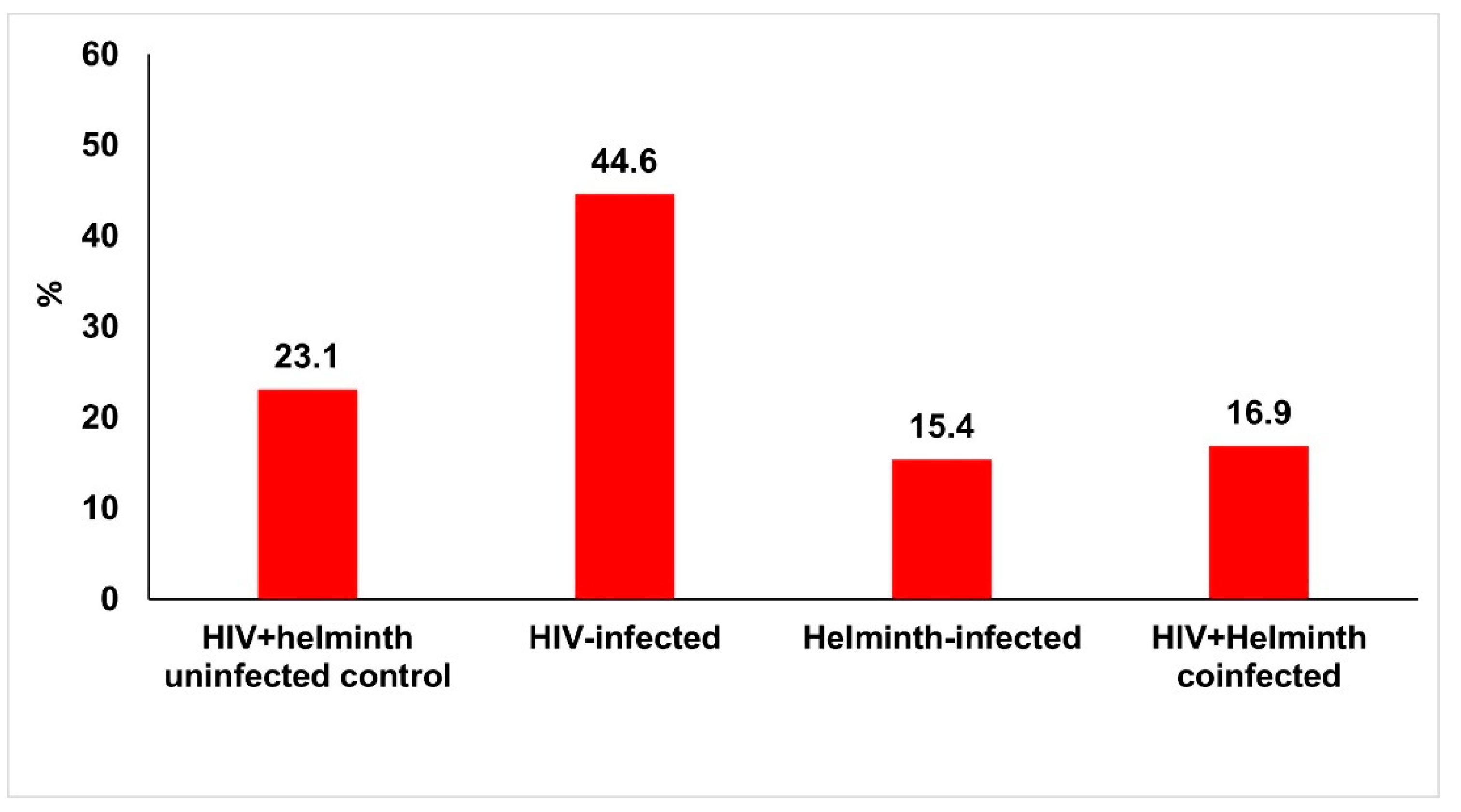
3.2. Anaemia Status
3.2.1. Frequency of Anaemia by Haemoglobin Analysis
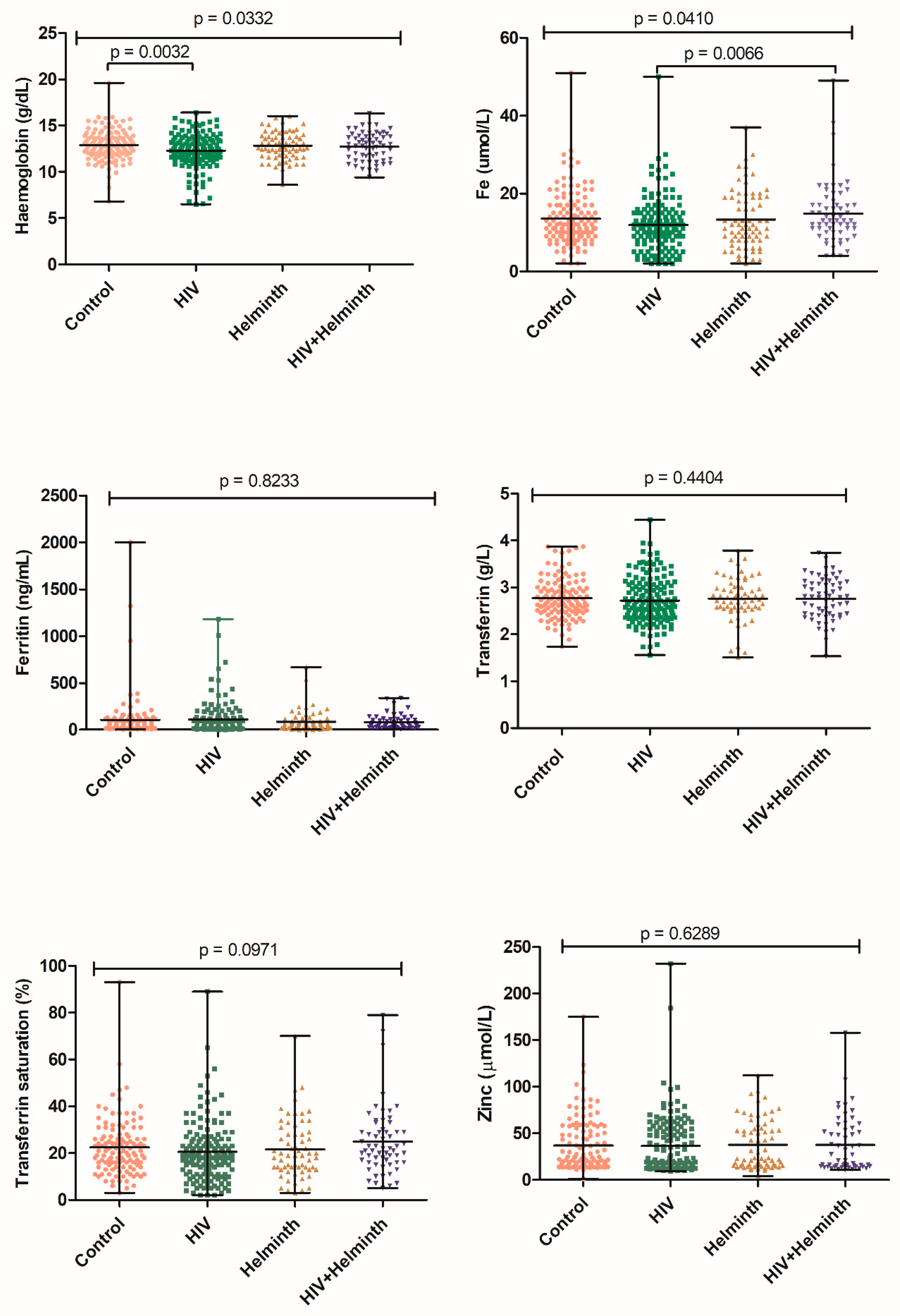
3.2.2. Haematological and Biochemical Analysis of Anaemia and Nutritional Status
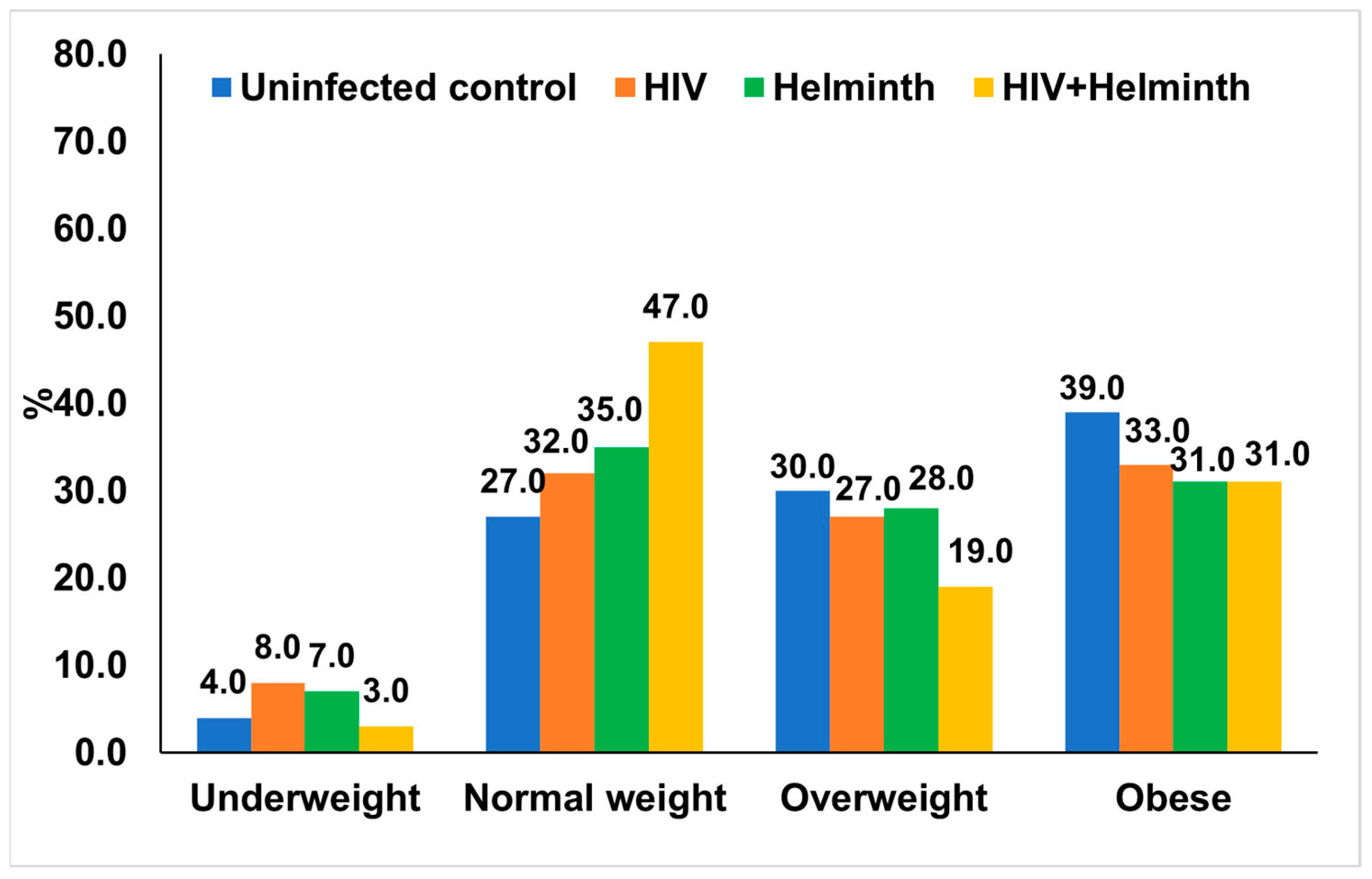
3.3. Nutritional Status
3.3.1. BMI Distribution and Nutritional Profiles in the Study Population
3.3.2. Biochemical Analysis of Nutritional Status
3.4. Multivariate Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Seitz, R. Human Immunodeficiency Virus (HIV). Transfus. Med. Hemotherapy 2016, 43, 203–222. [Google Scholar]
- Woodham, A.W.; Skeate, J.G.; Sanna, A.M.; Taylor, J.R.; Da Silva, D.M.; Cannon, P.M.; Kast, W.M. Human Immunodeficiency Virus Immune Cell Receptors, Coreceptors, and Cofactors: Implications for Prevention and Treatment. AIDS Patient Care STDS 2016, 30, 291–306. [Google Scholar] [CrossRef] [PubMed]
- UNAIDS. Global HIV Statistics—Fact Sheet. 2022. Available online: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf (accessed on 26 August 2022).
- World Health Organization. HIV. Fact Sheets. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/hiv-aids (accessed on 26 August 2022).
- The National Health Promotion Policy and Strategy 2015–2019; Department of Health Knowledge Hub: Pretoria, South Africa, 2015. Available online: https://www.knowledgehub.org.za/elibrary/national-health-promotion-policy-and-strategy-2015-2019 (accessed on 26 August 2022).
- Fong, D.; Chan, M.M. Soil-Transmitted Helminth Infections. In Human Parasites; WHO: Geneva, Switzerland, 2022; pp. 502–527. Available online: https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections (accessed on 26 August 2022).
- World Health Organization. Schistosomiasis and soil-transmitted helminthiases: Number of people treated in 2016. Wkly Epidemiol. Rec. 2017, 49, 749–760. [Google Scholar]
- Hotez, P.J.; Kamath, A. Neglected Tropical Diseases in Sub-Saharan Africa: Review of Their Prevalence, Distribution, and Disease Burden. PLoS Negl. Trop. Dis. 2009, 3, e412. [Google Scholar] [CrossRef]
- Takarinda, K.C.; Mutasa-Apollo, T.; Madzima, B.; Nkomo, B.; Chigumira, A.; Banda, M.; Muti, M.; Harries, A.D.; Mugurungi, O. Malnutrition status and associated factors among HIV-positive patients enrolled in ART clinics in Zimbabwe. BMC Nutr. 2017, 3, 15. [Google Scholar] [CrossRef]
- Adeleke, O.A.; Yogeswaran, P.; Wright, G. Intestinal helminth infections amongst HIV-infected adults in Mthatha General Hospital, South Africa. Afr. J. Prim. Health Care Fam. Med. 2015, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mkhize, B.T.; Mabaso, M.; Mamba, T.; Napier, C.E.; Mkhize-Kwitshana, Z.L. The Interaction between HIV and Intestinal Helminth Parasites Coinfection with Nutrition among Adults in KwaZulu-Natal, South Africa. Biomed. Res. Int. 2017, 2017, 9059523. [Google Scholar] [CrossRef]
- Gedle, D.; Gelaw, B.; Muluye, D.; Mesele, M. Prevalence of malnutrition and its associated factors among adult people living with HIV/AIDS receiving anti-retroviral therapy at Butajira Hospital, southern Ethiopia. BMC Nutr. 2015, 1, 5. [Google Scholar] [CrossRef]
- Lvers, L.C.; Cullen, K.A.; Freedberg, K.A.; Block, S.; Coates, J.; Webb, P. HIV/AIDS, Undernutrition and Food Insecurity. Clin. Infect. Dis. 2009, 49, 1096. [Google Scholar]
- Abdel-Rasoul, G.M.; Elgendy, F.M.; Elrazek, M.L.A. Iron deficiency anemia among preschool children (2–6 years) in a slum area (Alexandria, Egypt): An intervention study. Menoufia Med. J. 2017, 30, 213. [Google Scholar]
- Koethe, J.R.; Chi, B.H.; Megazzini, K.M.; Heimburger, D.C.; Stringer, J.S.A. Macronutrient Supplementation for Malnourished HIV-Infected Adults: A Review of the Evidence in Resource-Adequate and Resource-Constrained Settings. Clin. Infect. Dis. 2009, 49, 787–798. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stephenson, L.S.; Latham, M.C.; Ottesen, E.A. Malnutrition and parasitic helminth infections. Parasitology 2000, 121, S23–S38. [Google Scholar] [CrossRef] [PubMed]
- Mkhize-Kwitshana, Z.L.; Tadokera, R.; Mabaso, M.H.L. Helminthiasis: A Systematic Review of the Immune Interactions Present in Individuals Coinfected with HIV and/or Tuberculosis. In Human Helminthiasis; IntechOpen: London, UK, 2017; Available online: https://www.intechopen.com/state.item.id (accessed on 26 October 2022).
- Nissapatorn, V.; Sawangjaroen, N. Parasitic infections in HIV infected individuals: Diagnostic & therapeutic challenges. Indian J. Med. Res. 2011, 134, 878–897. [Google Scholar] [PubMed]
- Kwitshana, Z.L.; Tsoka, J.M.; Mabaso, M.L.H. Intestinal parasitic infections in adult patients in KwaZulu-Natal. S. Afr. Med. J. 2008, 98, 709–711. [Google Scholar]
- Mpaka-Mbatha, M.; Naidoo, P.; Islam, M.; Singh, R.; Mkhize-Kwitshana, Z.L. Demographic profile of HIV and helminth coinfected adults in KwaZulu-Natal, South Africa. S. Afr. J. Infect. Dis. 2022. accepted. [Google Scholar]
- Mkhize-Kwitshana, Z.L.; Taylor, M.; Jooste, P.; Mabaso, M.L.H.; Walzl, G. The influence of different helminth infection phenotypes on immune responses against HIV in co-infected adults in South Africa. BMC Infect. Dis. 2011, 11, 273. [Google Scholar] [CrossRef]
- Mkhize-Kwitshana, Z.L.; Naidoo, P.; Nkwanyana, N.M.; Mabaso, M.L.H. Concurrent allergy and helminthiasis in underprivileged urban South African adults previously residing in rural areas. Parasite Immunol. 2022, 44, e12913. [Google Scholar] [CrossRef]
- HIV/AIDS. Available online: https://www.who.int/health-topics/hiv-aids#tab=tab_1 (accessed on 21 September 2022).
- Aregay, A.D.; Kidane, K.M.; Aregay, A.B.; Fenta, K.A.; Woldegebriel, A.G.; Godefay, H.; Woldearegay, T.W. Prediction of CD4 T-Lymphocyte Count Using WHO Clinical Staging among ART-Naïve HIV-Infected Adolescents and Adults in Northern Ethiopia: A Retrospective Study. AIDS Res. Treat. 2020, 2020, 2163486. [Google Scholar] [CrossRef]
- Akbarpour, E.; Paridar, Y.; Mohammadi, Z.; Mard, A.; Danehchin, L.; Abolnezhadian, F.; Azadpour, S.; Rahimi, Z.; Zamani, M.; Cheraghian, B.; et al. Anemia prevalence, severity, types, and correlates among adult women and men in a multiethnic Iranian population: The Khuzestan Comprehensive Health Study (KCHS). BMC Public Health 2022, 22, 168. [Google Scholar] [CrossRef]
- Yu, E.; Sharma, S. Physiology, Calcium; StatPearls Publishing: Treasure Island, FL, USA, 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482128/ (accessed on 29 September 2022).
- Favus, M.J.; Bushinsky, D.A.; Lemann, J., Jr. Regulation of calcium, magnesium, and phosphate metabolism. Disord Bone Miner. Metab. 2006, 6, 76–117. [Google Scholar]
- James, M.T.; Zhang, J.; Lyon, A.W.; Hemmelgarn, B.R. Derivation and internal validation of an equation for albumin-adjusted calcium. BMC Clin. Pathol. 2008, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Payne, R.B.; Little, A.J.; Williams, R.B.; Milner, J.R. Interpretation of serum calcium in patients with abnormal serum proteins. Br. Med. J. 1973, 4, 643–664. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.S.L.; Jefferson, L.S.; Taylor, J.M. Changes in plasma albumin concentration, synthesis rate, and mRNA level during acute inflammation. Am. J. Physiol.—Cell Physiol. 1986, 251, C928–C934. [Google Scholar] [CrossRef] [PubMed]
- Gyorkos, T.W.; Gilbert, N.L.; Larocque, R.; Casapía, M.; Montresor, A. Re-Visiting Trichuris trichiura Intensity Thresholds Based on Anemia during Pregnancy. PLoS Negl. Trop. Dis. 2012, 6, e1783. [Google Scholar] [CrossRef]
- Bhutta, Z.A. Effect of infections and environmental factors on growth and nutritional status in developing countries. J. Pediatr. Gastroenterol. Nutr. 2006, 43, S13–S21. [Google Scholar] [CrossRef]
- Osazuwa, F.; Ayo, O.M.; Imade, P. A significant association between intestinal helminth infection and anaemia burden in children in rural communities of Edo state, Nigeria. N. Am. J. Med. Sci. 2011, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Prevalence of Anaemia in Women of Reproductive Age (Aged 15–49) (%); WHO: Geneva, Switzerland, 2021. Available online: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/prevalence-of-anaemia-in-women-of-reproductive-age-(-) (accessed on 26 November 2021).
- Crompton, D.W.T.; Nesheim, M.C. Nutritional impact of intestinal helminthiasis during the human life cycle. Annu. Rev. Nutr. 2002, 22, 35–59. [Google Scholar] [CrossRef]
- Filteau, S.; NUSTART (Nutritional Support for Africans Starting Antiretroviral Therapy) Study Team; PrayGod, G.; Kasonka, L.; Woodd, S.; Rehman, A.M.; Chisenga, M.; Siame, J.; Koethe, J.R.; Changalucha, J.; et al. Effects on mortality of a nutritional intervention for malnourished HIV-infected adults referred for antiretroviral therapy: A randomised controlled trial. BMC Med. 2015, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Nutrient requirements for people living with HIV/AIDS. In WHO Library Cataloguing-in-Publication Data WHO Technical Consultation on Nutrient Requirements for People Living with HIV/AIDS; World Health Organization: Geneva, Switzerland, 2003; pp. 13–15. Available online: http://www.who.int/nutrition/publications/Content_nutrient_requirements.pdf (accessed on 5 September 2022).
- Forrester, J.E.; Sztam, K.A. Micronutrients in HIV/AIDS: Is there evidence to change the WHO 2003 recommendations? Am. Soc. Nutr. 2011, 94, 1683S–1689S. [Google Scholar] [CrossRef]
- Bais, R. Clinical Chemistry: Principles, Procedures, Correlations, 5th ed. Michael L. Bishop, Edward P. Fody, and Larry Schoeff. Baltimore, MD: Lippincott Williams & Wilkins, 2005, 756 pp., $76.95, hardcover. ISBN 0-7817-4611-6. Clin. Chem. 2005, 51, 1567. [Google Scholar]
- Harris, D.; Haboubi, N. Malnutrition screening in the elderly population. J. R. Soc. Med. 2005, 98, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Fiorino, A.S.; Atac, B. Paraproteinemia, plasmacytoma, myeloma and HIV infection. Leukemia 1997, 11, 2150–2156. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Adedeji, A.L.; Adenikinju, R.O.; Ajele, J.O.; Olawoye, T.L. Serum protein electrophoresis under effective control of HIV-1 disease progression. EXCLI J. 2014, 13, 761–771. [Google Scholar]
- Eisenhut, M. Malnutrition and variability in CD4+ cell counts in African populations. J. Infect. Diseases. Oxf. Acad. 2007, 195, 1550. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thimmapuram, R.; Lanka, S.; Esswein, A.; Dall, L. Correlation of Nutrition with Immune Status in Human Immunodeficiency Virus Outpatients. Mo. Med. 2019, 116, 336–339. [Google Scholar]
- Onoya, D.; Mokhele, I.; Sineke, T.; Mngoma, B.; Moolla, A.; Vujovic, M.; Bor, J.; Langa, J.; Fox, M.P. Health provider perspectives on the implementation of the same-day-ART initiation policy in the Gauteng province of South Africa. Heal. Res. Policy Syst. 2021, 19, 1–12. [Google Scholar] [CrossRef]
- Bahemuka, M.; Denham, M.J.; Hodkinson, H.M. Macrocytosis. MODGERIATR 1973, 3, 421–422. [Google Scholar]
- Koury, M.J.; Ponka, P. New insights into erythropoiesis: The roles of folate, vitamin B 12, and iron. Annu. Rev. Nutr. 2004, 24, 105–131. [Google Scholar] [CrossRef]
- Kim, A.H.; Jang, W.; Kim, Y.; Park, Y.J.; Han, K.; Oh, E.J. Mean corpuscular volume (MCV) values reflect therapeutic effectiveness in zidovudine-receiving HIV patients. J. Clin. Lab. Anal. 2013, 27, 373–378. [Google Scholar] [CrossRef]

| Parameters | Reference Ranges |
|---|---|
| Haemoglobin | Male: 13.0–15.3 g/dL |
| Female: 11.1–13.3 g/dL | |
| Mean Corpuscular Volume | 75.4–86.8 fL |
| Iron | 10.0–30.0 μmol/L |
| Ferritin | 30–400 ng/L |
| Transferrin | 2.0–3.60 g/L |
| Transferrin saturation | 20.0–55.0% |
| Calcium | 2.15–2.65 mmol/L |
| Phosphate | 0.80–1.40 mmol/L |
| Magnesium | 0.65–1.0 mmol/L |
| Zinc | 9.2–18.4 μmol/L |
| Vitamin A | 200–600 μg/L |
| Albumin | 35.0–52.0 g/L |
| Total protein | 60–85 g/L |
| Patient Characteristics | N, % (95% CI) | ||
|---|---|---|---|
| * HIV viral load (copies/mL) (n = 214) | Viral load lower than detection limit (≤20) | 141, 65.89 (59.31–71.19) | |
| Viral load higher than detection limit (≥20) | 73, 34.11 (28.09–40.69) | ||
| # CD4 counts (cells/uL) | Severe immunosuppression (<200) | 24, 5.8 (3.93–8.48) | |
| Advanced immunosuppression (200–349) | 30, 7.3 (5.12–10.16) | ||
| Mild immunosuppression (350–499) | 48, 11.6 (8.86–15.04) | ||
| Normal (>500) | 310, 74.9 (70.49–78.81) | ||
| ! Anaemia | Males (n = 138) | Severe (<7 g/dL) | 0, 0 (0.00–0.00) |
| Moderate (7–10.9 g/dL) | 3, 2.2 (0.74–6.20) | ||
| Mild (11.0–12.9) | 24, 17.4 (11.97–24.57) | ||
| Non anaemic (>13 g/dL) | 99, 71.7 (63.72–78.58) | ||
| Females (n = 276) | Severe (<7 g/dL) | 4, 1.45 (0.57–3.67) | |
| Moderate (7–9.9) | 12, 4.35 (2.50–7.44) | ||
| Mild (10–11.9) | 99, 35.9 (30.44–41.69) | ||
| Non anaemic (>12 g/dL) | 159, 57.6 (51.71–63.30) | ||
| Mean corpuscular volume (fL) | Low (<75.4) | 23, 5.56 (3.73–8.20) | |
| Normal (75.4–86.8) | 45, 10.87 (8.22–14.24) | ||
| High (>86.8) | 346, 83.36 (79.7–86.80) | ||
| $ Iron (μmol/L) | Low (<10.0) | 129, 31.2 (16.89–35.78) | |
| Normal (10.0–30.0) | 275, 66.4 (61.74–70.80) | ||
| High (>30) | 8, 1.9 (0.98–3.77) | ||
| $ Ferritin (ng/mL) | Low (<30) | 116, 28.0 (23.91–32.53) | |
| Normal (30–400) | 274, 66.2 (62.50–70.57) | ||
| High (>400) | 10, 2.42 (1.32–4.39) | ||
| $ Transferrin (g/L) | Low (<2.0) | 15, 3.6 (2.21–5.89) | |
| Normal (2.0–3.60) | 365, 88.2 (84.70–90.93) | ||
| High (>3.60) | 16, 3.9 (2.39–6.18) | ||
| $ Transferrin saturation (%) | Low (<20.0) | 192, 46.4 (41.62–51.19) | |
| Normal (20.0–55) | 194, 46.4 (41.63–51.19) | ||
| High (>55) | 9, 2.2 (1.14–4.07) | ||
| $ Haemoglobin (g/dL) | Low (<12.0) | 129, 31.2 (26.89–35.78) | |
| Normal (12.0–13.3) | 150, 36.2 (31.75–40.97) | ||
| High (>13.3) | 133, 32.1 (27.81–36.77) | ||
| $ Calcium (mmol/L) | Low (<2.15) | 27, 6.5 (4.52–9.32) | |
| Normal (2.15–2.62) | 385, 93.0 (90.12–95.08) | ||
| High (2.65) | 0, 0.0 (0.00–0.00) | ||
| $ Vitamin A (μg/L) | Low (<200) | 24, 5.8 (3.93–8.48) | |
| Normal (200–600) | 170, 41.1 (36.43–45.86) | ||
| High (>600) | 83, 20.0 (16.48–24.17) | ||
| $ Albumin (g/L) | Low (<35.0) | 33, 8.0 (5.73–10.98) | |
| Normal (35.0–52.0) | 273, 65.9 (61.25–70.34) | ||
| High (52.0) | 0, 0.0 (0.00–0.91) | ||
| $ Zinc (μmol/L) | Low (9.2) | 1, 0.2 (0.04–1.35) | |
| Normal (9.2–18.4) | 160, 38.6 (34.08–43.42) | ||
| High (>18.4) | 226, 54.6 (49.77–59.32) | ||
| $ Total Protein (g/L) | Low (<60) | 1, 0.2 (0.04–1.35) | |
| Normal (60–85) | 349, 84.3 (80.48–87.49) | ||
| High (>85) | 50, 12.1 (9.28–15.57) | ||
| $ Magnesium (mmol/L) | Low (0.65) | 8, 1.9 (0.98–3.77) | |
| Normal (0.65–1.10) | 402, 97.1 (95.00–98.33) | ||
| High (>1.0) | 3, 0.7 (0.25–2.11) | ||
| $ Phosphate (mmol/L) | Low (<0.80) | 32, 7.7 (5.53–10.71) | |
| Normal (0.80–1.40) | 368, 88.9 (85.50–91.57) | ||
| High (>1.40) | 13, 3.1 (1.84–5.30) | ||
| Unstandardized β—Coefficient Values | |||||||
|---|---|---|---|---|---|---|---|
| Parameters | Uninfected Controls | HIV-Infected | Helminth-Infected | HIV/Helminth Coinfected | |||
| β | p-Value | β | p-Value | β | p-Value | ||
| Haemoglobin (g/dL) | Reference group | 0.28 | 0.745 | −0.24 | 0.242 | −0.07 | 0.737 |
| Iron (μmol/L) | −1.06 | 0.237 | −1.04 | 0.314 | 1.40 | 0.244 | |
| Transferrin (g/L) | 2.16 | 0.434 | 0.03 | 0.669 | −0.03 | 0.699 | |
| Transferrin Saturation (%) | −1.25 | 0.436 | −2.62 | 0.163 | 2.74 | 0.203 | |
| Ferritin (ng/mL) | −9.21 | 0.567 | 21.82 | 0.317 | −23.69 | 0.113 | |
| Calcium (mmol/L) | −0.04 | 0.005 | −0.02 | 0.254 | −0.05 | 0.003 | |
| Total Protein (g/L) | 2.45 | 0.003 | −0.64 | 0.432 | 2.01 | 0.040 | |
| Phosphate (mmol/L) | 0.02 | 0.388 | −0.00 | 0.997 | −0.00 | 0.862 | |
| Magnesium (mmol/L) | 0.44 | 0.301 | 0.00 | 0.757 | −0.00 | 0.850 | |
| Vitamin A (μg/L) | −18.21 | 0.636 | −18.66 | 0.680 | −91.44 | 0.040 | |
| Albumin (g/L) | −2.83 | 0.000 | −0.74 | 0.197 | −2.13 | 0.001 | |
| C-Reactive Protein (mg/L) | 3.73 | 0.320 | 6.74 | 0.177 | −0.45 | 0.874 | |
| Zinc (μmol/L) | 0.49 | 0.907 | −0.52 | 0.906 | −0.11 | 0.951 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mpaka-Mbatha, M.N.; Naidoo, P.; Islam, M.M.; Singh, R.; Mkhize-Kwitshana, Z.L. Anaemia and Nutritional Status during HIV and Helminth Coinfection among Adults in South Africa. Nutrients 2022, 14, 4970. https://doi.org/10.3390/nu14234970
Mpaka-Mbatha MN, Naidoo P, Islam MM, Singh R, Mkhize-Kwitshana ZL. Anaemia and Nutritional Status during HIV and Helminth Coinfection among Adults in South Africa. Nutrients. 2022; 14(23):4970. https://doi.org/10.3390/nu14234970
Chicago/Turabian StyleMpaka-Mbatha, Miranda N., Pragalathan Naidoo, Md Mazharul Islam, Ravesh Singh, and Zilungile L. Mkhize-Kwitshana. 2022. "Anaemia and Nutritional Status during HIV and Helminth Coinfection among Adults in South Africa" Nutrients 14, no. 23: 4970. https://doi.org/10.3390/nu14234970
APA StyleMpaka-Mbatha, M. N., Naidoo, P., Islam, M. M., Singh, R., & Mkhize-Kwitshana, Z. L. (2022). Anaemia and Nutritional Status during HIV and Helminth Coinfection among Adults in South Africa. Nutrients, 14(23), 4970. https://doi.org/10.3390/nu14234970







