Deciphering the Efficacy and Mechanism of Astragalus membranaceus on High Altitude Polycythemia by Integrating Network Pharmacology and In Vivo Experiments
Abstract
1. Introduction
2. Materials and Methods
2.1. Network Pharmacology Analysis
2.1.1. Collection and Analysis of Targets of AM and HAPC
2.1.2. Protein–Protein Interactions (PPI) Network and Core Target Analysis
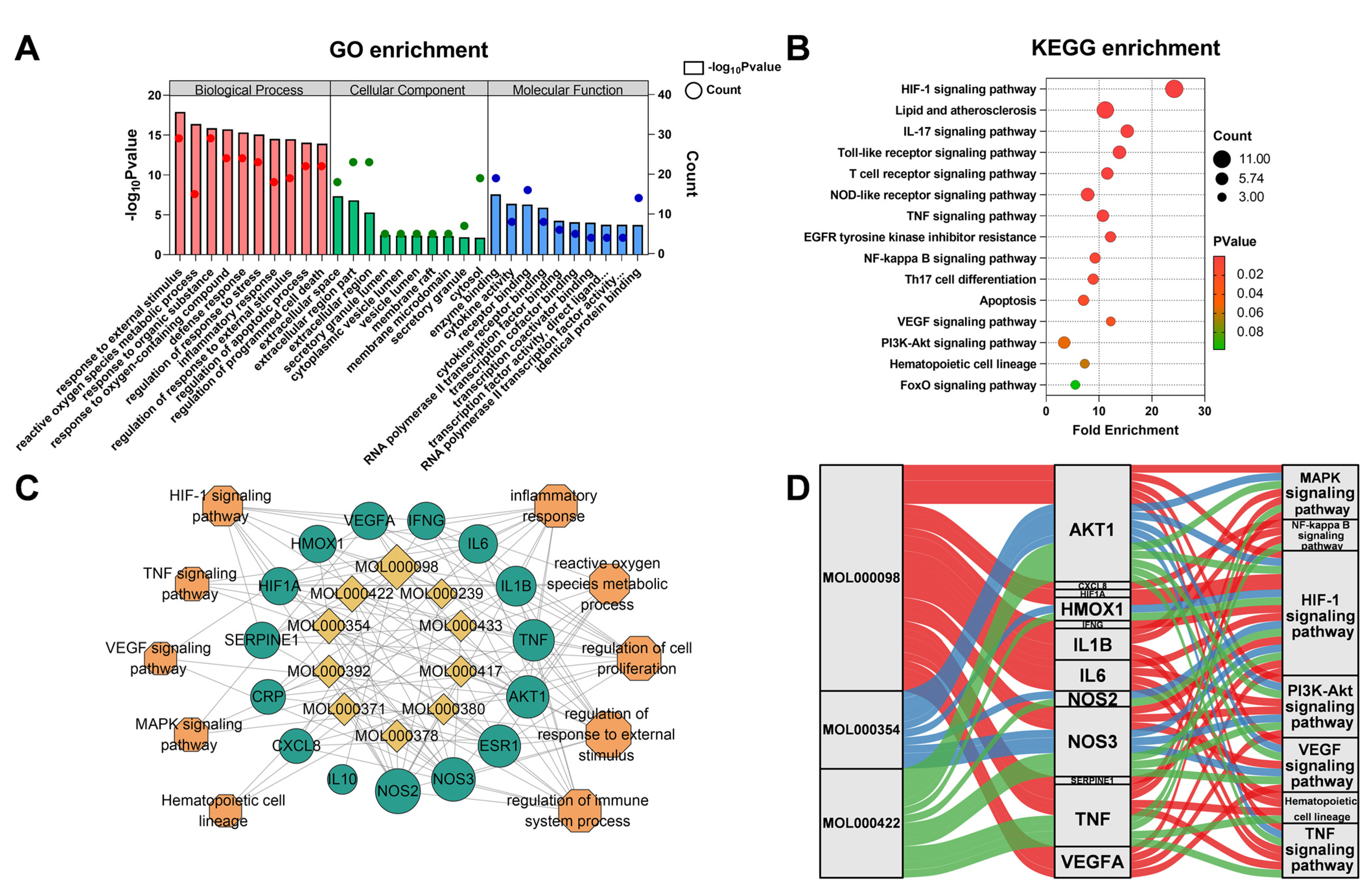
2.1.3. Analysis of Pathway Enrichment
2.2. Molecular Docking of Active Components-Core Targets
2.3. Animals
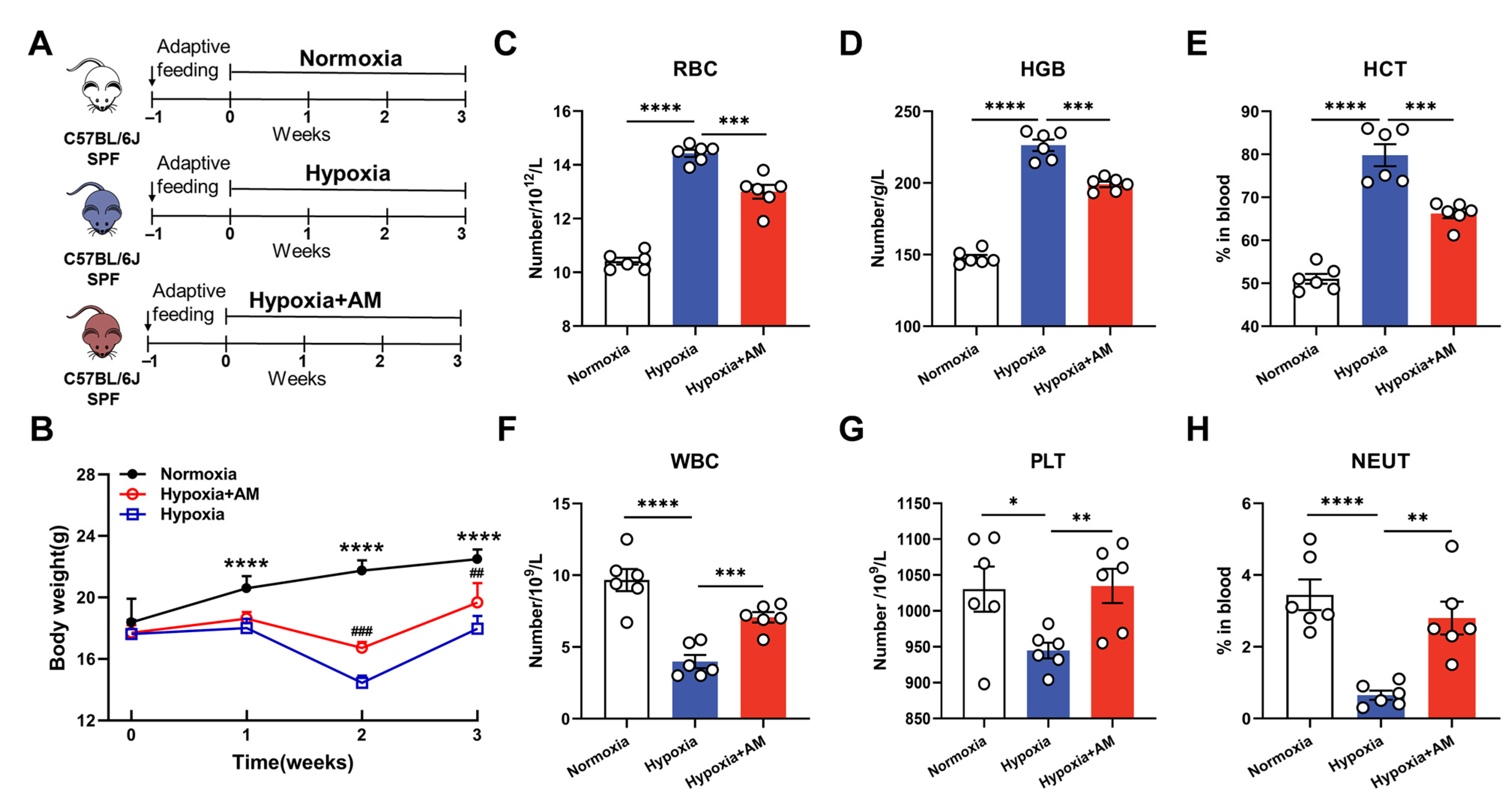
2.4. Establishment of HAPC Model
2.5. Blood Counts
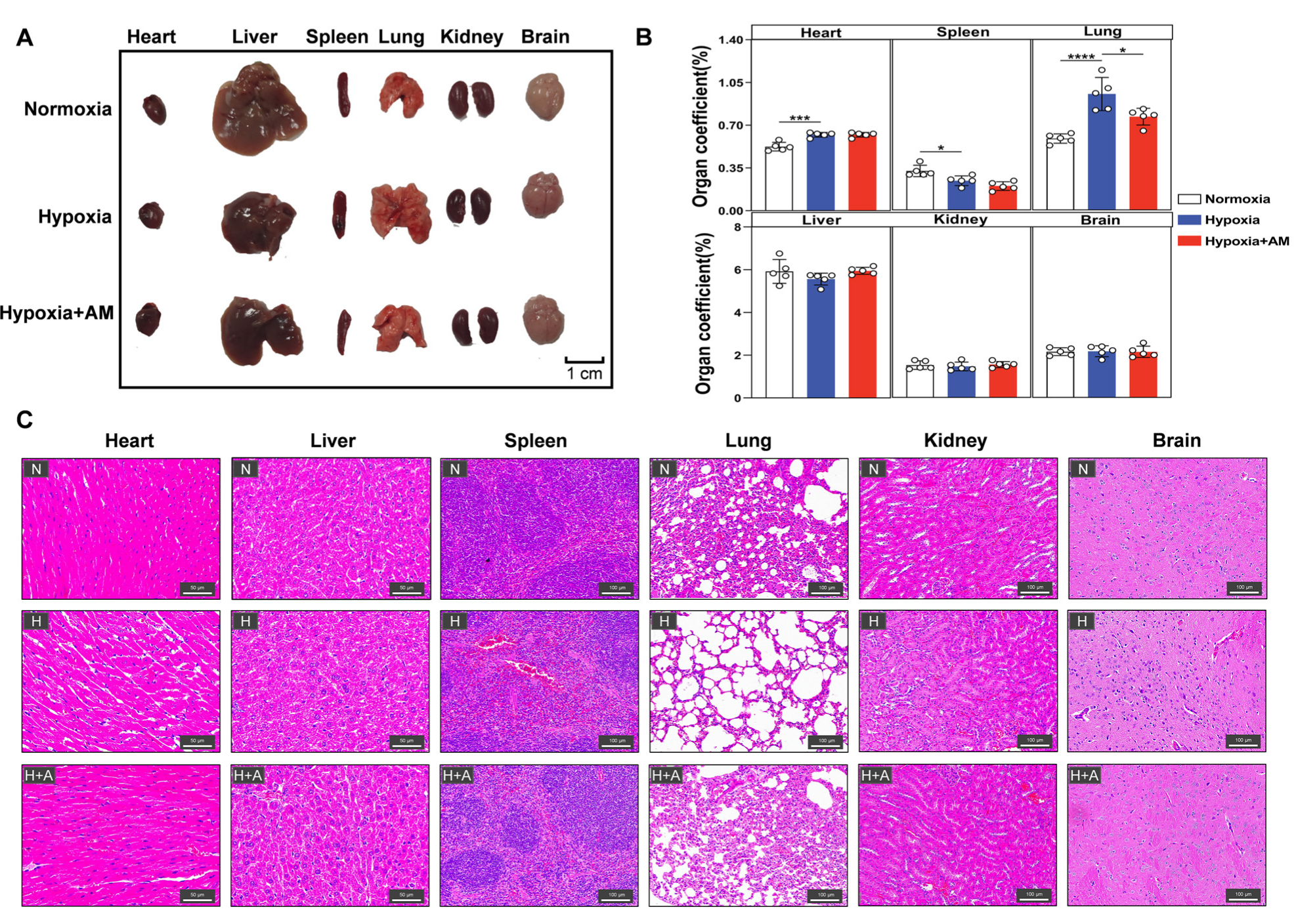
2.6. Organ Morphology
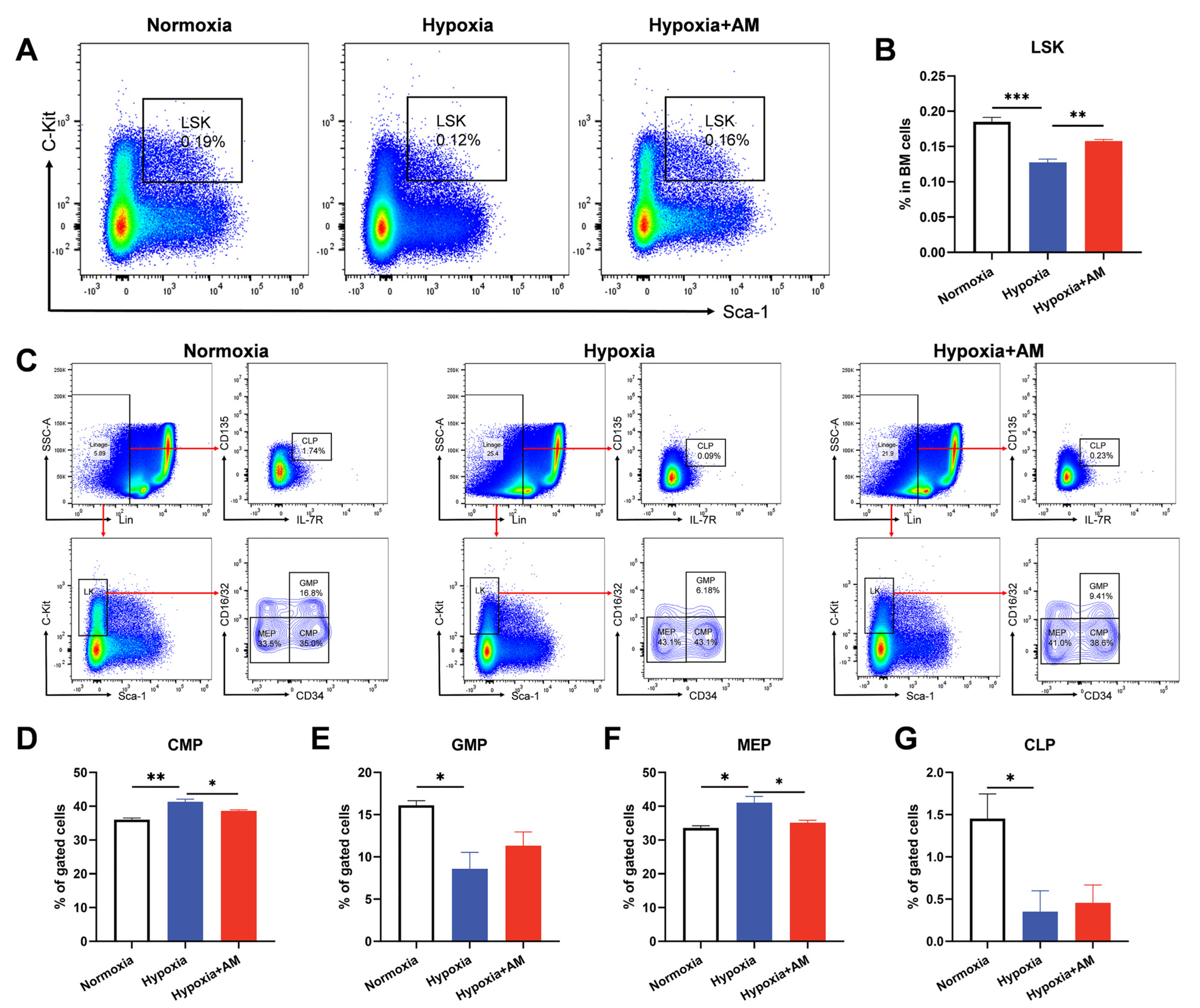
2.7. Cell Preparation and Flow Cytometry
2.8. Quantitative Real-Time PCR (qRT-PCR)
2.9. Statistical Analysis
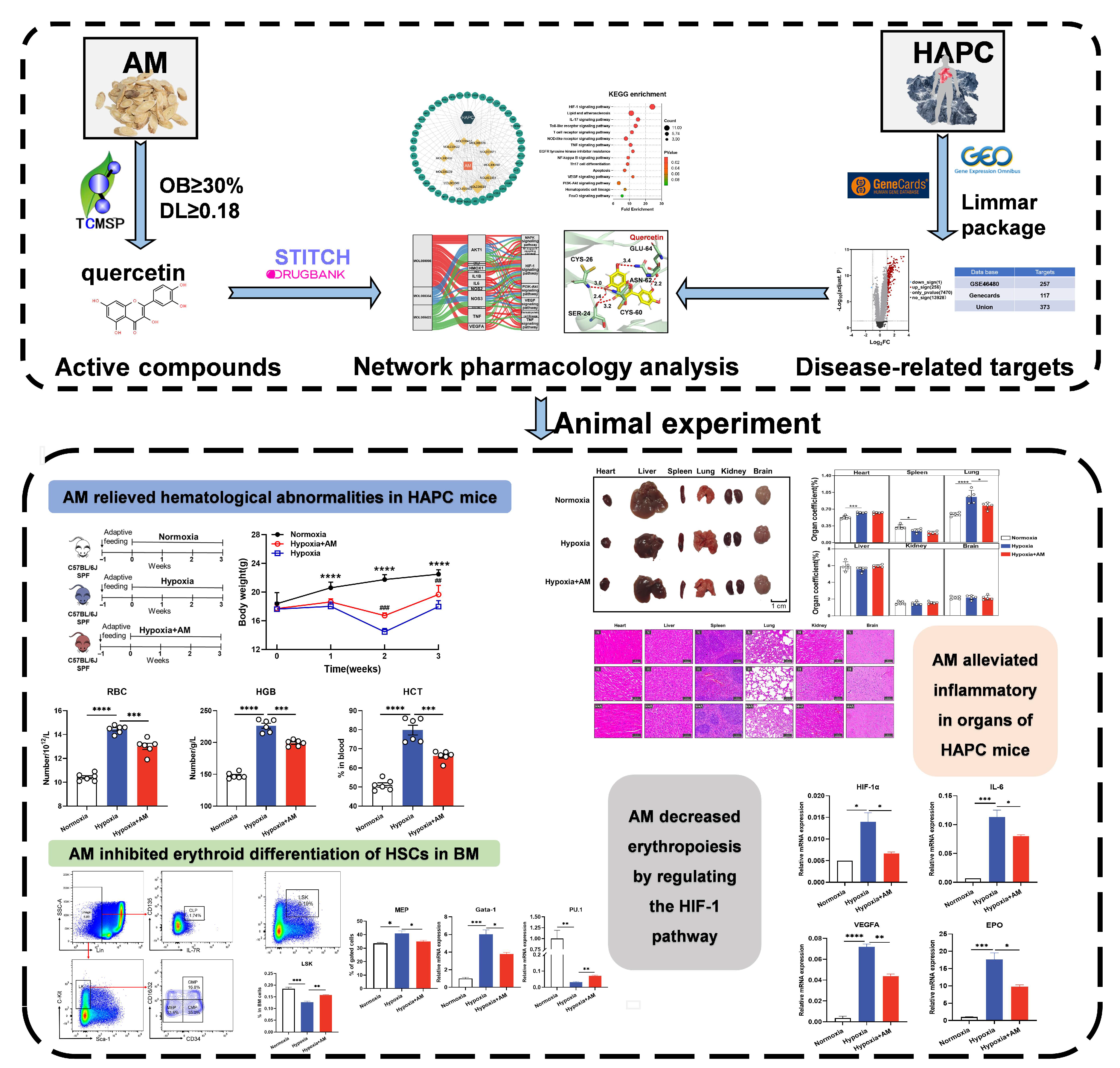
3. Results
3.1. Identification of Components in AM and Target Prediction
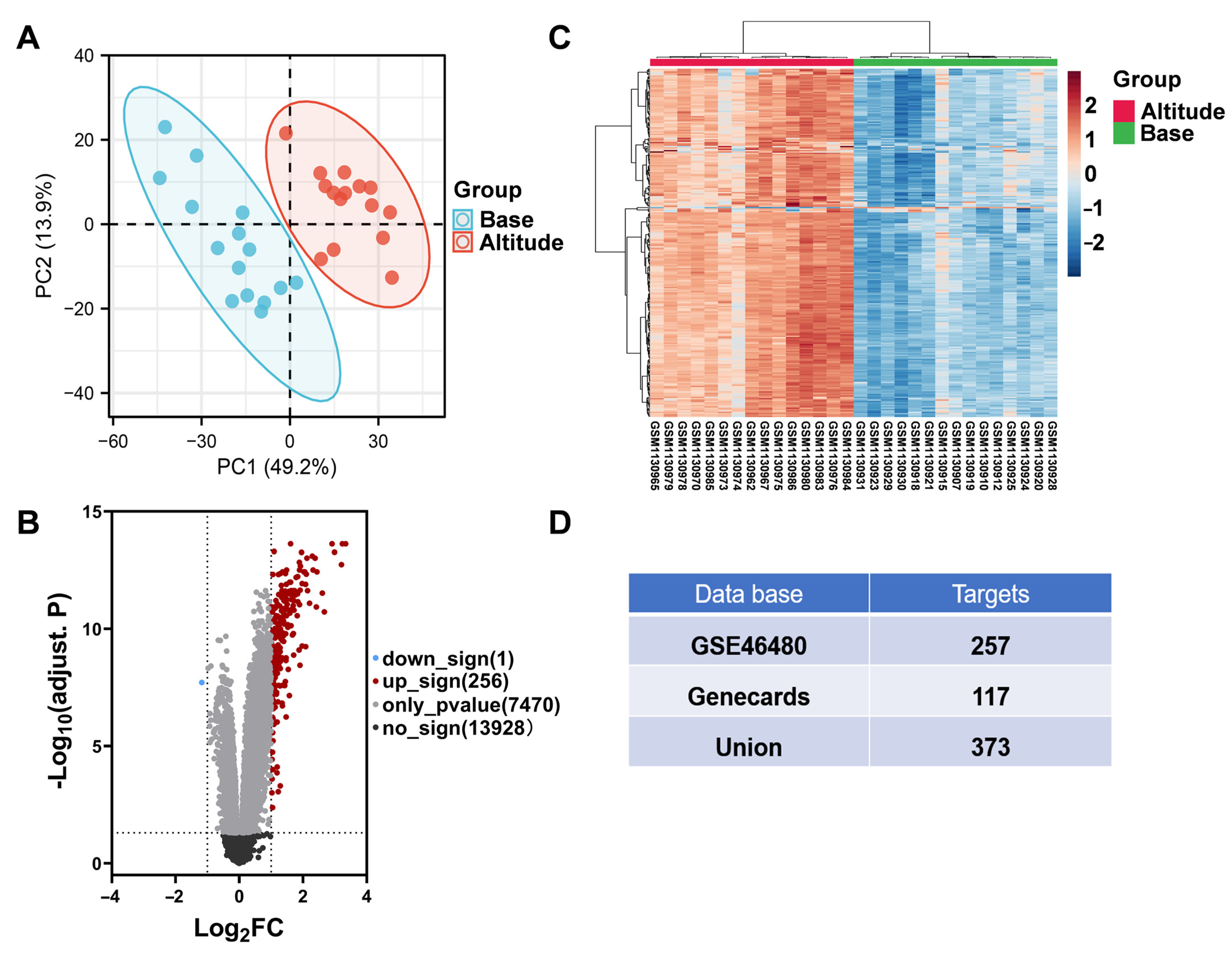
3.2. Screened Targets Related HAPC
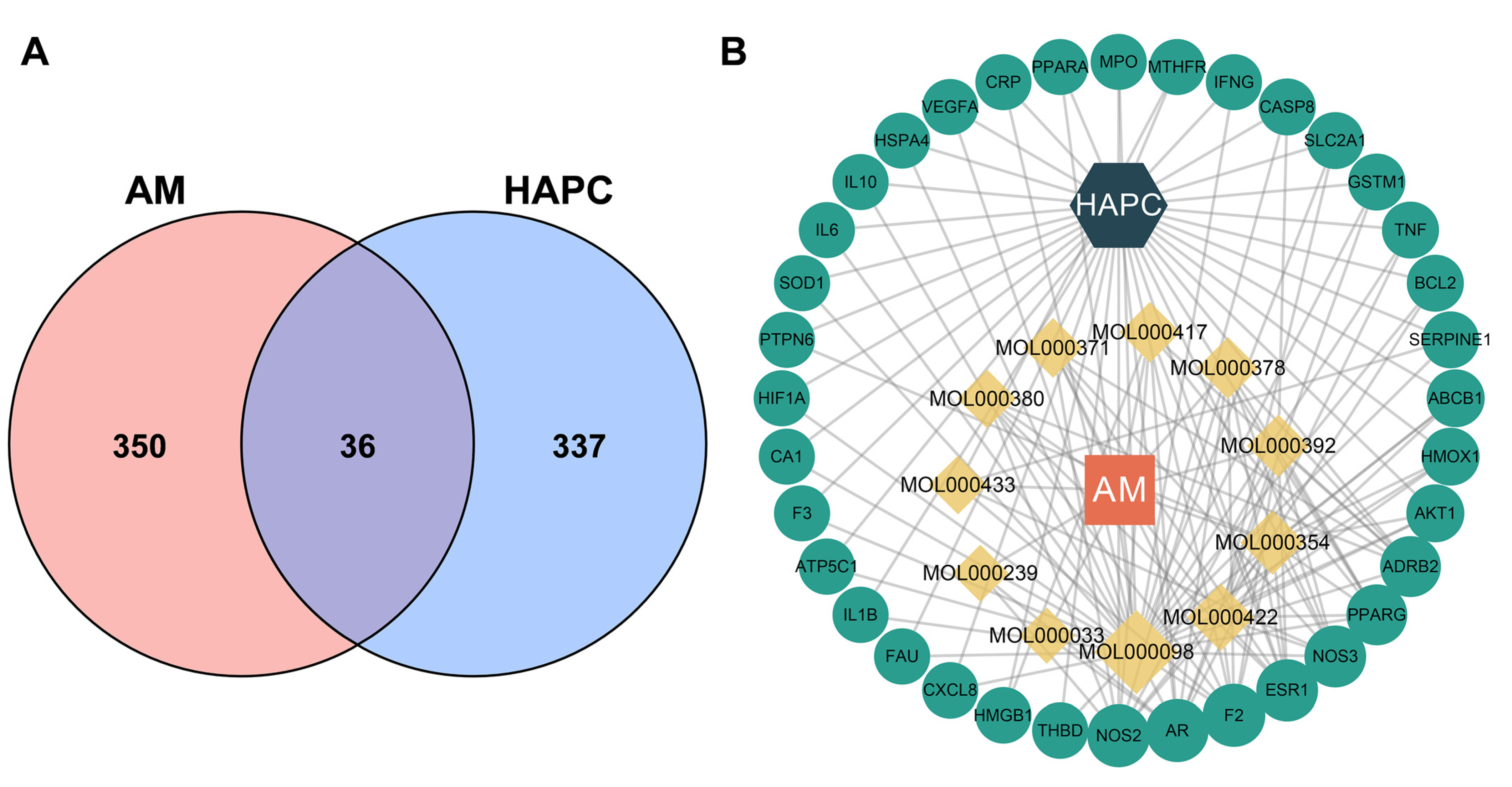
3.3. Identified 36 Common Targets between AM and HAPC
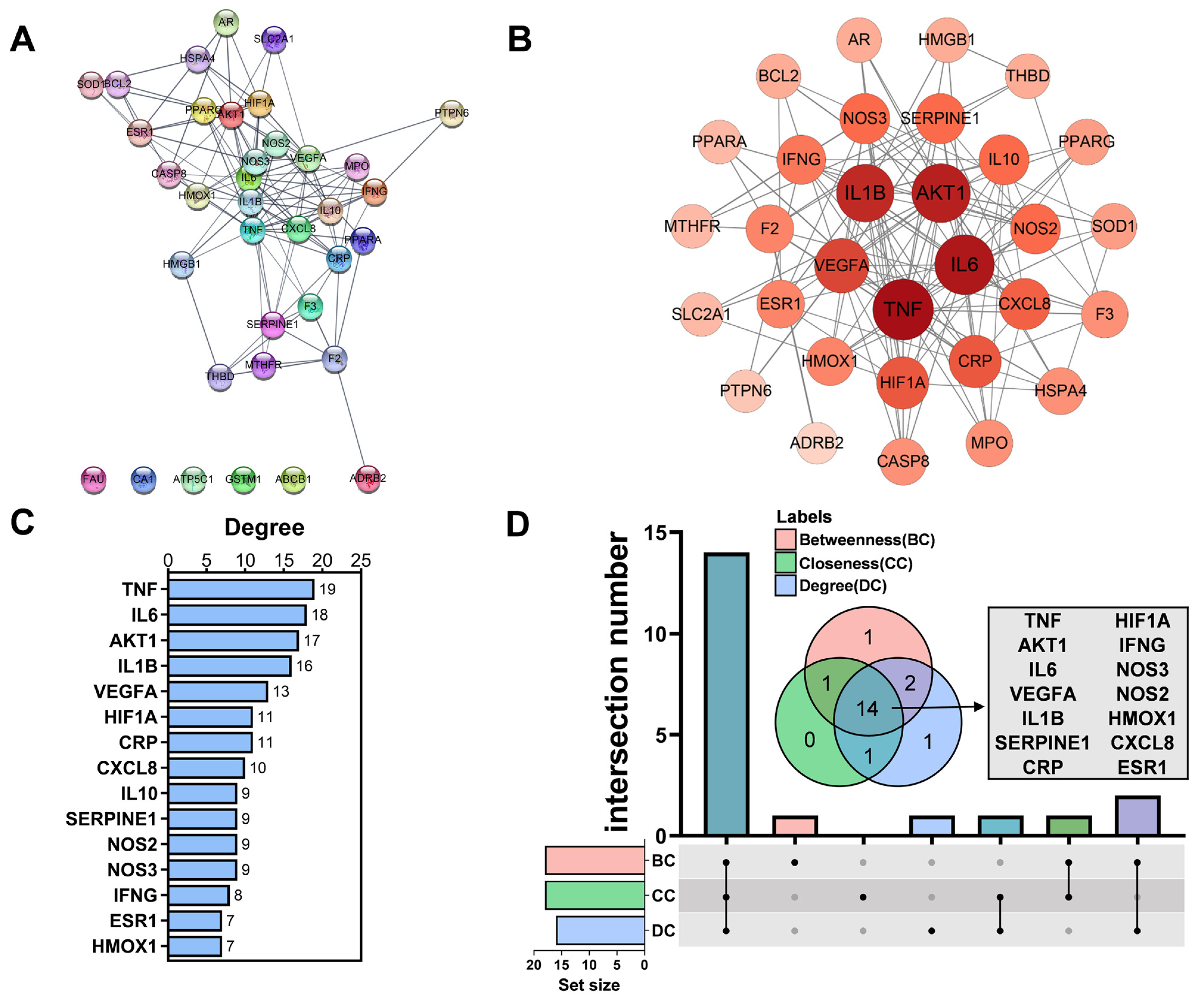
3.4. Analyzed Core Targets of AM on HAPC
3.5. Enriched Pathway Analysis of Common Targets and Core Targets
3.6. Molecular Docking Verified the Binding Mode of Compounds to Targets
3.7. AM Relieved Hematological Abnormalities in HAPC Mice
3.8. AM Alleviated Hypoxia-Induced Organ Damage
3.9. AM Inhibited Erythroid Differentiation of HSCs in BM
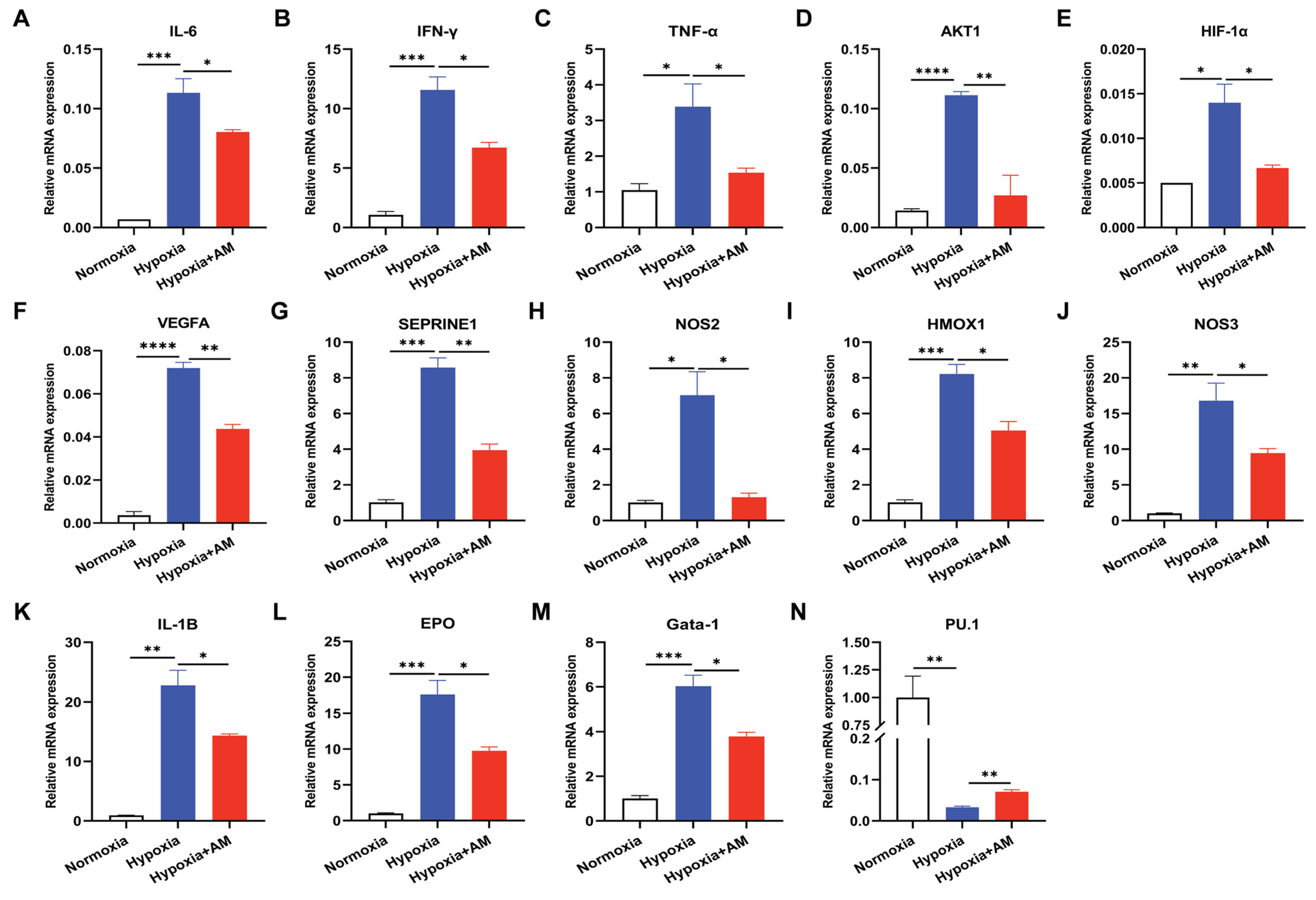
3.10. The Effect of AM on the HIF-1 Signaling Pathway in HAPC Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Tremblay, J.C.; Ainslie, P.N. Global and country-level estimates of human population at high altitude. Proc. Natl. Acad. Sci. USA 2021, 118, e2102463118. [Google Scholar] [CrossRef] [PubMed]
- West, J.B. Oxygen Conditioning: A New Technique for Improving Living and Working at High Altitude. Physiology 2016, 31, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhu, M.M.; Song, K.; Wuren, T.N.; Yan, J.; Ge, R.L.; Ji, L.H.; Cui, S. VHL gene methylation contributes to excessive erythrocytosis in chronic mountain sickness rat model by upregulating the HIF-2alpha/EPO pathway. Life Sci. 2021, 266, 118873. [Google Scholar] [CrossRef] [PubMed]
- Kasperska, A.; Zembron-Lacny, A. The effect of intermittent hypoxic exposure on erythropoietic response and hematological variables in elite athletes. Physiol. Res. 2020, 69, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tang, C.; Dang, Z.; Yong, A.; Liu, L.; Wang, S.; Zhao, M. Clinicopathological characteristics of high-altitude polycythemia-related kidney disease in Tibetan inhabitants. Kidney Int. 2022, 102, 196–206. [Google Scholar] [CrossRef]
- Chen, K.; Li, N.; Fan, F.; Geng, Z.; Zhao, K.; Wang, J.; Zhang, Y.; Tang, C.; Wang, X.; Meng, X. Tibetan Medicine Duoxuekang Capsule Ameliorates High-Altitude Polycythemia Accompanied by Brain Injury. Front. Pharmacol. 2021, 12, 680636. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, Z.; Li, X.; Li, C.; Hao, J.; Luo, Y.; Lei, M.; Li, J.; Liu, C.; He, K. Macitentan Attenuates Chronic Mountain Sickness in Rats by Regulating Arginine and Purine Metabolism. J. Proteome Res. 2020, 19, 3302–3314. [Google Scholar] [CrossRef]
- Liu, P.; Zou, D.; Yi, L.; Chen, M.L.; Gao, Y.X.; Zhou, R.; Zhang, Q.Y.; Zhou, Y.; Zhu, J.D.; Chen, K.; et al. Quercetin ameliorates hypobaric hypoxia-induced memory impairment through mitochondrial and neuron function adaptation via the PGC-1alpha pathway. Restor. Neurol. Neurosci. 2015, 33, 143–157. [Google Scholar]
- Bao, H.H.; Li, R.Y.; He, M.L.; Kang, D.J.; Zhao, L.L. DTI Study on Brain Structure and Cognitive Function in Patients with Chronic Mountain Sickness. Sci. Rep. 2019, 9, 19334. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, H.Q.; Jiang, Y.Y. Methazolamide in high-altitude illnesses. Eur. J. Pharm. Sci. 2020, 148, 105326. [Google Scholar] [CrossRef]
- Xie, H.; Xu, G.; Aa, J.; Gu, S.; Gao, Y. Modulation of Perturbed Cardiac Metabolism in Rats Under High-Altitude Hypoxia by Combination Treatment With L-carnitine and Trimetazidine. Front. Physiol. 2021, 12, 671161. [Google Scholar] [CrossRef]
- Rivera-Ch, M.; Leon-Velarde, F.; Huicho, L. Treatment of chronic mountain sickness: Critical reappraisal of an old problem. Respir. Physiol. Neurobiol. 2007, 158, 251–265. [Google Scholar] [CrossRef]
- Jiang, S.; Jiao, G.; Chen, Y.; Han, M.; Wang, X.; Liu, W. Astragaloside IV attenuates chronic intermittent hypoxia-induced myocardial injury by modulating Ca2+ homeostasis. Cell Biochem. Funct. 2020, 38, 710–720. [Google Scholar] [CrossRef]
- Zheng, K.Y.; Choi, R.C.; Xie, H.Q.; Cheung, A.W.; Guo, A.J.; Leung, K.W.; Chen, V.P.; Bi, C.W.; Zhu, K.Y.; Chan, G.K.; et al. The expression of erythropoietin triggered by danggui buxue tang, a Chinese herbal decoction prepared from radix Astragali and radix Angelicae Sinensis, is mediated by the hypoxia-inducible factor in cultured HEK293T cells. J. Ethnopharmacol. 2010, 132, 259–267. [Google Scholar] [CrossRef]
- Chen, J.K.; Guo, M.K.; Bai, X.H.; Chen, L.Q.; Su, S.M.; Li, L.; Li, J.Q. Astragaloside IV ameliorates intermittent hypoxia-induced inflammatory dysfunction by suppressing MAPK/NF-kappaB signalling pathways in Beas-2B cells. Sleep Breath. 2020, 24, 1237–1245. [Google Scholar] [CrossRef]
- Fu, J.; Wang, Z.H.; Huang, L.F.; Zheng, S.H.; Wang, D.M.; Chen, S.L.; Zhang, H.T.; Yang, S.H. Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (Huangqi). Phytother. Res. 2014, 28, 1275–1283. [Google Scholar] [CrossRef]
- Zheng, Y.J.; Ren, W.Y.; Zhang, L.N.; Zhang, Y.M.; Liu, D.L.; Liu, Y.Q. A Review of the Pharmacological Action of Astragalus Polysaccharide. Front. Pharmacol. 2020, 11, 349. [Google Scholar] [CrossRef]
- Auyeung, K.K.; Han, Q.B.; Ko, J.K. Astragalus membranaceus: A Review of Its Protection Against Inflammation and Gastrointestinal Cancers. Am. J. Chin. Med. 2016, 44, 1–22. [Google Scholar] [CrossRef]
- Li, M.; Han, B.; Zhao, H.; Xu, C.Y.; Xu, D.K.; Sieniawska, E.; Lin, X.M.; Kai, G.Y. Biological active ingredients of Astragali Radix and its mechanisms in treating cardiovascular and cerebrovascular diseases. Phytomedicine 2022, 98, 153918. [Google Scholar] [CrossRef]
- Li, C.; Zhu, H.; Zhang, S.; Meng, F.; Li, S.; Li, G.; Zha, J.; Wu, S.; Zhu, L.; Dai, A. Astragaloside IV ameliorates pulmonary vascular remodeling in hypoxia-induced pulmonary hypertension by restraining the T follicular helper cell response and expanding T follicular regulatory cell response. Phytomedicine 2022, 102, 154171. [Google Scholar] [CrossRef]
- Zhao, F.; Meng, Y.; Wang, Y.; Fan, S.; Liu, Y.; Zhang, X.; Ran, C.; Wang, H.; Lu, M. Protective effect of Astragaloside IV on chronic intermittent hypoxia-induced vascular endothelial dysfunction through the calpain-1/SIRT1/AMPK signaling pathway. Front. Pharmacol. 2022, 13, 920977. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Liu, T.L.; Tao, W.D.; Li, M.X.; Li, X.L.; Yan, L. Effect of aqueous extract of Astragalus membranaceus on behavioral cognition of rats living at high altitude. J. Tradit. Chin. Med. 2022, 42, 58–64. [Google Scholar] [PubMed]
- Yoo, S.; Kim, K.; Nam, H.; Lee, D. Discovering Health Benefits of Phytochemicals with Integrated Analysis of the Molecular Network, Chemical Properties and Ethnopharmacological Evidence. Nutrients 2018, 10, 1042. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zheng, W.; Yang, J.; Ali, A.; Qin, H. Mechanism of Astragalus membranaceus Alleviating Acquired Hyperlipidemia Induced by High-Fat Diet through Regulating Lipid Metabolism. Nutrients 2022, 14, 954. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.L.; Li, P.; Wang, J.A.; Zhou, W.; Li, B.H.; Huang, C.; Li, P.D.; Guo, Z.H.; Tao, W.Y.; Yang, Y.F.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef]
- Hou, F.F.; Yu, Z.Y.; Cheng, Y.; Liu, Y.; Liang, S.; Zhang, F. Deciphering the pharmacological mechanisms of Scutellaria baicalensis Georgi on oral leukoplakia by combining network pharmacology, molecular docking and experimental evaluations. Phytomedicine 2022, 103, 154195. [Google Scholar] [CrossRef]
- Li, P.B.; Nie, H.J.; Liu, W.; Deng, B.N.; Zhu, H.L.; Duan, R.F.; Chen, Z.L.; Wang, H. A rat model of high altitude polycythemia rapidly established by hypobaric hypoxia exposure. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2014, 30, 526–531. [Google Scholar]
- Liu, Y.; Liu, F.; Yang, Y.; Li, D.; Lv, J.; Ou, Y.; Sun, F.; Chen, J.; Shi, Y.; Xia, P. Astragalus polysaccharide ameliorates ionizing radiation-induced oxidative stress in mice. Int. J. Biol. Macromol. 2014, 68, 209–214. [Google Scholar] [CrossRef]
- Pinzi, L.; Rastelli, G. Molecular Docking: Shifting Paradigms in Drug Discovery. Int. J. Mol. Sci. 2019, 20, 4331. [Google Scholar] [CrossRef]
- Wang, R.R.; Yuan, T.Y.; Chen, D.; Chen, Y.C.; Sun, S.C.; Wang, S.B.; Kong, L.L.; Fang, L.H.; Du, G.H. Dan-Shen-Yin Granules Prevent Hypoxia-Induced Pulmonary Hypertension via STAT3/HIF-1alpha/VEGF and FAK/AKT Signaling Pathways. Front. Pharmacol. 2022, 13, 844400. [Google Scholar] [CrossRef]
- Brewster, L.M.; Bain, A.R.; Garcia, V.P.; Fandl, H.K.; Stone, R.; DeSouza, N.M.; Greiner, J.J.; Tymko, M.M.; Vizcardo-Galindo, G.A.; Figueroa-Mujica, R.J.; et al. Global REACH 2018: Dysfunctional extracellular microvesicles in Andean highlander males with excessive erythrocytosis. Am. J. Physiol. Heart Circ Physiol. 2021, 320, H1851–H1861. [Google Scholar] [CrossRef]
- Liu, Y.S.; Huang, H.; Zhou, S.M.; Tian, H.J.; Li, P. Excessive Iron Availability Caused by Disorders of Interleukin-10 and Interleukin-22 Contributes to High Altitude Polycythemia. Front. Physiol. 2018, 9, 548. [Google Scholar] [CrossRef]
- Wang, Z.K.; Liu, F.J.; Ye, S.L.; Jiang, P.; Yu, X.C.; Xu, J.; Du, X.; Ma, L.; Cao, H.J.; Yuan, C.; et al. Plasma proteome profiling of high-altitude polycythemia using TMT-based quantitative proteomics approach. J. Proteom. 2019, 194, 60–69. [Google Scholar] [CrossRef]
- Adesso, S.; Russo, R.; Quaroni, A.; Autore, G.; Marzocco, S. Astragalus membranaceus Extract Attenuates Inflammation and Oxidative Stress in Intestinal Epithelial Cells via NF-kappaB Activation and Nrf2 Response. Int. J. Mol. Sci. 2018, 19, 800. [Google Scholar] [CrossRef]
- Liu, T.L.; Zhang, M.J.; Niu, H.Y.; Liu, J.; Ruilian, M.A.; Wang, Y.; Xiao, Y.F.; Xiao, Z.B.; Sun, J.J.; Dong, Y.; et al. Astragalus polysaccharide from Astragalus Melittin ameliorates inflammation via suppressing the activation of TLR-4/NF-kappaB p65 signal pathway and protects mice from CVB3-induced virus myocarditis. Int. J. Biol. Macromol. 2019, 126, 179–186. [Google Scholar] [CrossRef]
- Patir, H.; Sarada, S.K.; Singh, S.; Mathew, T.; Singh, B.; Bansal, A. Quercetin as a prophylactic measure against high altitude cerebral edema. Free Radic. Biol. Med. 2012, 53, 659–668. [Google Scholar] [CrossRef]
- Akiyama, M.; Mizokami, T.; Miyamoto, S.; Ikeda, Y. Kaempferol increases intracellular ATP content in C2C12 myotubes under hypoxic conditions by suppressing the HIF-1alpha stabilization and/or by enhancing the mitochondrial complex IV activity. J. Nutr. Biochem. 2022, 103, 108949. [Google Scholar] [CrossRef]
- Seo, S.; Seo, K.; Ki, S.H.; Shin, S.M. Isorhamnetin Inhibits Reactive Oxygen Species-Dependent Hypoxia Inducible Factor (HIF)-1alpha Accumulation. Biol. Pharm. Bull. 2016, 39, 1830–1838. [Google Scholar] [CrossRef]
- Hermankova, E.; Zatloukalova, M.; Biler, M.; Sokolova, R.; Bancirova, M.; Tzakos, A.G.; Kren, V.; Kuzma, M.; Trouillas, P.; Vacek, J. Redox properties of individual quercetin moieties. Free Radic. Biol. Med. 2019, 143, 240–251. [Google Scholar] [CrossRef]
- Cruz-Zuniga, J.M.; Soto-Valdez, H.; Peralta, E.; Mendoza-Wilson, A.M.; Robles-Burgueno, M.R.; Auras, R.; Gamez-Meza, N. Development of an antioxidant biomaterial by promoting the deglycosylation of rutin to isoquercetin and quercetin. Food Chem. 2016, 204, 420–426. [Google Scholar] [CrossRef]
- Corrado, C.; Fontana, S. Hypoxia and HIF Signaling: One Axis with Divergent Effects. Int. J. Mol. Sci. 2020, 21, 5611. [Google Scholar] [CrossRef] [PubMed]
- Gossage, L.; Eisen, T.; Maher, E.R. VHL, the story of a tumour suppressor gene. Nat. Rev. Cancer 2015, 15, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.G.; Hausenloy, D.J. Hypoxia-inducible factor as a therapeutic target for cardioprotection. Pharmacol. Ther. 2012, 136, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.K.; Llewellyn, O.P.; Bates, D.O.; Nicholson, L.B.; Dick, A.D. IL-10 regulation of macrophage VEGF production is dependent on macrophage polarisation and hypoxia. Immunobiology 2010, 215, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Jelkmann, W. Erythropoietin. J. Endocrinol. Investig. 2003, 26, 832–837. [Google Scholar] [CrossRef]
- Takeda, N.; O’Dea, E.L.; Doedens, A.; Kim, J.W.; Weidemann, A.; Stockmann, C.; Asagiri, M.; Simon, M.C.; Hoffmann, A.; Johnson, R.S. Differential activation and antagonistic function of HIF-{alpha}cc isoforms in macrophages are essential for NO homeostasis. Genes Dev. 2010, 24, 491–501. [Google Scholar] [CrossRef]
- LaGory, E.L.; Giaccia, A.J. The ever-expanding role of HIF in tumour and stromal biology. Nat. Cell Biol. 2016, 18, 356–365. [Google Scholar] [CrossRef]
- Tymko, M.M.; Lawley, J.S.; Ainslie, P.N.; Hansen, A.B.; Hofstaetter, F.; Rainer, S.; Amin, S.; Moralez, G.; Gasho, C.; Vizcardo-Galindo, G.; et al. Global Reach 2018 Heightened Alpha-Adrenergic Signaling Impairs Endothelial Function during Chronic Exposure to Hypobaric Hypoxia. Circ. Res. 2020, 127, e1–e13. [Google Scholar] [CrossRef]
- Salama, S.A.; Omar, H.A.; Maghrabi, I.A.; AlSaeed, M.S.; El-Tarras, A.E. Iron supplementation at high altitudes induces inflammation and oxidative injury to lung tissues in rats. Toxicol. Appl. Pharmacol. 2014, 274, 1–6. [Google Scholar] [CrossRef]
- Li, P.; Huang, J.; Tian, H.J.; Huang, Q.Y.; Jiang, C.H.; Gao, Y.Q. Regulation of bone marrow hematopoietic stem cell is involved in high-altitude erythrocytosis. Exp. Hematol. 2011, 39, 37–46. [Google Scholar] [CrossRef]
- Willcockson, M.A.; Taylor, S.J.; Ghosh, S.; Healton, S.E.; Wheat, J.C.; Wilson, T.J.; Steidl, U.; Skoultchi, A.I. Runx1 promotes murine erythroid progenitor proliferation and inhibits differentiation by preventing Pu.1 downregulation. Proc. Natl. Acad. Sci. USA 2019, 116, 17841–17847. [Google Scholar] [CrossRef]
- Grigorakaki, C.; Morceau, F.; Chateauvieux, S.; Dicato, M.; Diederich, M. Tumor necrosis factor alpha-mediated inhibition of erythropoiesis involves GATA-1/GATA-2 balance impairment and PU.1 over-expression. Biochem. Pharmacol. 2011, 82, 156–166. [Google Scholar] [CrossRef]
- Love, P.E.; Warzecha, C.; Li, L.Q. Ldb1 complexes: The new master regulators of erythroid gene transcription. Trends Genet. 2014, 30, 1–9. [Google Scholar] [CrossRef]



| Target Gene | Primer Sequence |
|---|---|
| β-actin | Forward 5′-GCTGTATTCCCCTCCATCGTG-3′ |
| Reverse 5′-CACGGTTGGCCTTAGGGTTCAG-3′ | |
| VEGFA | Forward 5′-ATCGAGTACATCTTCAAGCCAT-3′ |
| Reverse 5′-GTGAGGTTTGATCCGCATAATC-3′ | |
| HIF-1α | Forward 5′-TGACGGCGACAGGGTTTACA-3′ |
| Reverse 5′-AATATGGCCCGTGCAGTGAA-3′ | |
| TNF-α | Forward 5′-ATGTCTCAGCCTCTTCTCATTC-3′ |
| Reverse 5′-GCTTGTCACTCGAATTTTGAGA-3′ | |
| IL-6 | Forward 5′-TAGTCCTTCCTACCCCAATTTCC-3′ |
| Reverse 5′-TTGGTCCTTAGCCACTCCTTC-3′ | |
| IL-1B | Forward 5′-CACTACAGGCTCCGAGATGAACAAC-3′ |
| Reverse 5′-TGTCGTTGCTTGGTTCTCCTTGTAC-3′ | |
| Gata-1 | Forward 5′-GTCCTCACCATCAGATTCCACAG-3′ |
| Reverse 5′-GAGTGCCGTCTTGCCATAGG-3′ | |
| PU.1 | Forward 5′-AGGAGTCTTCTACGACCTGGA-3′ |
| Reverse 5′-GAAGGCTTCATAGGGAGCGAT-3′ | |
| IFN-γ | Forward 5′-GCCACGGCACAGTCATTGA-3′ |
| Reverse 5′-TGCTGATGGCCTGATTGTCTT-3′ | |
| AKT1 | Forward 5′-GCCCTCAAGTACTCATTCCAG-3′ |
| Reverse 5′-ACACAATCTCCGCACCATAG-3′ | |
| SEPRINE1 | Forward 5′-GGAATAAGGCGAGTTGGAAGAA-3′ |
| Reverse 5′ GGCCGTTCGATAATCGGTCTAT-3′ | |
| NOS2 | Forward 5′-GGAGTGACGGCAAACATGACT-3′ |
| Reverse 5′-TCGATGCACAACTGGGTGAAC-3′ | |
| HMOX1 | Forward 5′-GATAGAGCGCAACAAGCAGAA-3′ |
| Reverse 5′-CAGTGAGGCCCATACCAGAAG-3′ | |
| NOS3 | Forward 5′-TGTGACCCTCACCGCTACAA-3′ |
| Reverse 5′-GCACAATCCAGGCCCAATC-3′ | |
| EPO | Forward 5′-ACTCTCCTTGCTACTGATTCCT-3′ |
| Reverse 5′-ATCGTGACATTTTCTGCCTCC-3′ |
| Mol ID a | Mol Name b | OB c (%) | DL d | PubChem ID |
|---|---|---|---|---|
| MOL000098 | Quercetin | 46.43 | 0.28 | 5280343 |
| MOL000211 | Mairin | 55.38 | 0.78 | 64971 |
| MOL000239 | Kumatakenin | 50.83 | 0.3 | 5318869 |
| MOL000296 | Hederagenin | 36.91 | 0.75 | 73299 |
| MOL000354 | Isorhamnetin | 49.6 | 0.31 | 5281654 |
| MOL000387 | Bifendate | 31.1 | 0.67 | 108213 |
| MOL000392 | Formononetin | 69.67 | 0.21 | 5280378 |
| MOL000398 | Isoflavanone | 109.99 | 0.3 | 160767 |
| MOL000417 | Calycosin | 47.75 | 0.24 | 5280448 |
| MOL000422 | Kaempferol | 41.88 | 0.24 | 5280863 |
| MOL000433 | Folsaeure (FA) | 68.96 | 0.7057 | 6037 |
| MOL000442 | Sucrose | 39.05 | 0.48 | 5316760 |
| MOL000371 | 3,9-di-O-methylnissolin | 53.74 | 0.48 | 15689655 |
| MOL000374 | 5′-hydroxyiso-muronulatol-2′,5′-di-O-glucoside | 41.72 | 0.7 | NA |
| MOL000378 | 7-O-methylisomucronulatol | 74.69 | 0.3 | 15689652 |
| MOL000379 | Methylnissolin-3-O-Glucoside | 36.74 | 0.92 | 74977390 |
| MOL000380 | Methylnissolin | 64.26 | 0.42 | 14077830 |
| MOL000438 | (R)-Isomucronulatol | 67.67 | 0.26 | 10380176 |
| MOL000439 | Isomucronulatol-7,2′-di-O-glucosiole | 49.28 | 0.62 | 15689653 |
| MOL000033 | (24S)-24-Propylcholesta-5-ene-3beta-ol | 36.23 | 0.78 | 15976101 |
| No. | Gene Name | Protein Name | PubChem ID |
|---|---|---|---|
| 1 | NOS2 | nitric oxide synthase 2 | P35228 |
| 2 | AR | androgen receptor | P10275 |
| 3 | ESR1 | estrogen receptor 1 | P03372 |
| 4 | PPARG | peroxisome proliferator activated receptor gamma | P37231 |
| 5 | PTPN6 | protein tyrosine phosphatase non-receptor type 6 | P29350 |
| 6 | F2 | coagulation factor II, thrombin | P00734 |
| 7 | NOS3 | nitric oxide synthase 3 | P29474 |
| 8 | ADRB2 | adrenoceptor beta 2 | P07550 |
| 9 | AKT1 | AKT serine/threonine kinase 1 | P31749 |
| 10 | BCL2 | BCL2 apoptosis regulator | P10415 |
| 11 | TNF | tumor necrosis factor | P01375 |
| 12 | HMOX1 | heme oxygenase 1 | P09601 |
| 13 | GSTM1 | glutathione S-transferase mu 1 | P09488 |
| 14 | VEGFA | vascular endothelial growth factor A | P15692 |
| 15 | IL10 | interleukin 10 | P22301 |
| 16 | IL6 | interleukin 6 | P05231 |
| 17 | CASP8 | caspase 8 | Q14790 |
| 18 | SOD1 | superoxide dismutase 1 | P00441 |
| 19 | HIF1A | hypoxia inducible factor 1 subunit alpha | Q16665 |
| 20 | F3 | coagulation factor III, tissue factor | P13726 |
| 21 | IL1B | interleukin 1 beta | P01584 |
| 22 | CXCL8 | C-X-C motif chemokine ligand 8 | P10145 |
| 23 | THBD | thrombomodulin | P07204 |
| 24 | SERPINE1 | serpin family E member 1 | P05121 |
| 25 | IFNG | interferon gamma | P01579 |
| 26 | MPO | myeloperoxidase | P05164 |
| 27 | PPARA | peroxisome proliferator activated receptor alpha | Q07869 |
| 28 | CRP | C-reactive protein | P02741 |
| 29 | ABCB1 | ATP binding cassette subfamily B member 1 | P08183 |
| 30 | SLC2A1 | solute carrier family 2 member 1 | P11166 |
| 31 | MTHFR | methylenetetrahydrofolate reductase | P42898 |
| 32 | HSPA4 | heat shock protein family A | P34932 |
| 33 | CA1 | carbonic anhydrase 1 | P00915 |
| 34 | ABCB1 | ATP-dependent translocase ABCB1 | P08183 |
| 35 | HMGB1 | high mobility group protein B1 | P09429 |
| 36 | SLC2A1 | solute carrier family 2, facilitated glucose transporter member 1 | P11166 |
| Gene Name | DC a | BC b | CC c |
|---|---|---|---|
| TNF | 19 | 133.5863 | 0.157658 |
| AKT1 | 17 | 128.2761 | 0.154185 |
| IL6 | 18 | 97.26547 | 0.156951 |
| VEGFA | 13 | 96.63991 | 0.153509 |
| IL1B | 16 | 72.26746 | 0.154867 |
| SERPINE1 | 9 | 59.16642 | 0.148936 |
| CRP | 11 | 54.56417 | 0.150862 |
| NOS3 | 9 | 39.74613 | 0.150215 |
| HIF1A | 11 | 34.52048 | 0.150215 |
| NOS2 | 9 | 18.6465 | 0.150215 |
| IFNG | 8 | 17.08651 | 0.145833 |
| HMOX1 | 7 | 13.84444 | 0.147059 |
| CXCL8 | 10 | 13.132 | 0.151515 |
| ESR1 | 7 | 12.39106 | 0.145228 |
| Core Targets | PDB ID a | Compounds | Affinity (kcal/mol) |
|---|---|---|---|
| TNF | 2E7A | Quercetin | −6.807 |
| Kaempferol | −6.581 | ||
| AKT1 | 1UNQ | Quercetin | −7.517 |
| Kaempferol | −7.351 | ||
| Isorhamnetin | −7.456 | ||
| IL6 | 1ALU | Quercetin | −7.495 |
| VEGFA | 1MKK | Quercetin | −7.804 |
| IL1B | 5R8Q | Quercetin | −7.277 |
| HIF1A | 4H6J | Quercetin | −7.090 |
| SERPINE1 | 1LJ5 | Quercetin | −7.512 |
| Folsaeure (FA) | −8.197 | ||
| CRP | 3PVN | Quercetin | −7.399 |
| CXCL8 | 4XDX | Quercetin | −7.367 |
| IFNG | 1FYH | Quercetin | −7.295 |
| NOS2 | 3E7G | Formononetin | −7.922 |
| Kumatakenin | −8.035 | ||
| Isorhamnetin | −7.665 | ||
| Calycosin | −8.134 | ||
| Kaempferol | −7.604 | ||
| 3,9-di-O-methylnissolin | −8.069 | ||
| 7-O-methylisomucronulatol | −8.097 | ||
| Methylnissolin | −7.966 | ||
| NOS3 | 4D1P | Formononetin | −7.696 |
| Quercetin | −7.719 | ||
| Isorhamnetin | −7.964 | ||
| Kaempferol | −7.461 | ||
| 3,9-di-O-methylnissolin | −7.877 | ||
| 7-O-methylisomucronulatol | −8.235 | ||
| Folsaeure (FA) | −9.103 | ||
| HMOX1 | 1N45 | Quercetin | −7.604 |
| Isorhamnetin | −7.509 | ||
| Kaempferol | −7.319 | ||
| ESR1 | 7NFB | Formononetin | −6.988 |
| Isorhamnetin | −7.148 | ||
| Calycosin | −8.170 | ||
| Kaempferol | −7.187 | ||
| 3,9-di-O-methylnissolin | −7.308 | ||
| 7-O-methylisomucronulatol | −7.375 | ||
| Methylnissolin | −6.975 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhang, H.; Yan, J.; Li, X.; Li, J.; Hu, J.; Shang, X.; Yang, H. Deciphering the Efficacy and Mechanism of Astragalus membranaceus on High Altitude Polycythemia by Integrating Network Pharmacology and In Vivo Experiments. Nutrients 2022, 14, 4968. https://doi.org/10.3390/nu14234968
Liu X, Zhang H, Yan J, Li X, Li J, Hu J, Shang X, Yang H. Deciphering the Efficacy and Mechanism of Astragalus membranaceus on High Altitude Polycythemia by Integrating Network Pharmacology and In Vivo Experiments. Nutrients. 2022; 14(23):4968. https://doi.org/10.3390/nu14234968
Chicago/Turabian StyleLiu, Xiru, Hao Zhang, Jinxiao Yan, Xiang Li, Jie Li, Jialu Hu, Xuequn Shang, and Hui Yang. 2022. "Deciphering the Efficacy and Mechanism of Astragalus membranaceus on High Altitude Polycythemia by Integrating Network Pharmacology and In Vivo Experiments" Nutrients 14, no. 23: 4968. https://doi.org/10.3390/nu14234968
APA StyleLiu, X., Zhang, H., Yan, J., Li, X., Li, J., Hu, J., Shang, X., & Yang, H. (2022). Deciphering the Efficacy and Mechanism of Astragalus membranaceus on High Altitude Polycythemia by Integrating Network Pharmacology and In Vivo Experiments. Nutrients, 14(23), 4968. https://doi.org/10.3390/nu14234968






