Meat Intake, Cooking Methods, Doneness Preferences and Risk of Gastric Adenocarcinoma in the MCC-Spain Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection and Diet Assessment
2.3. Statistical Analyses
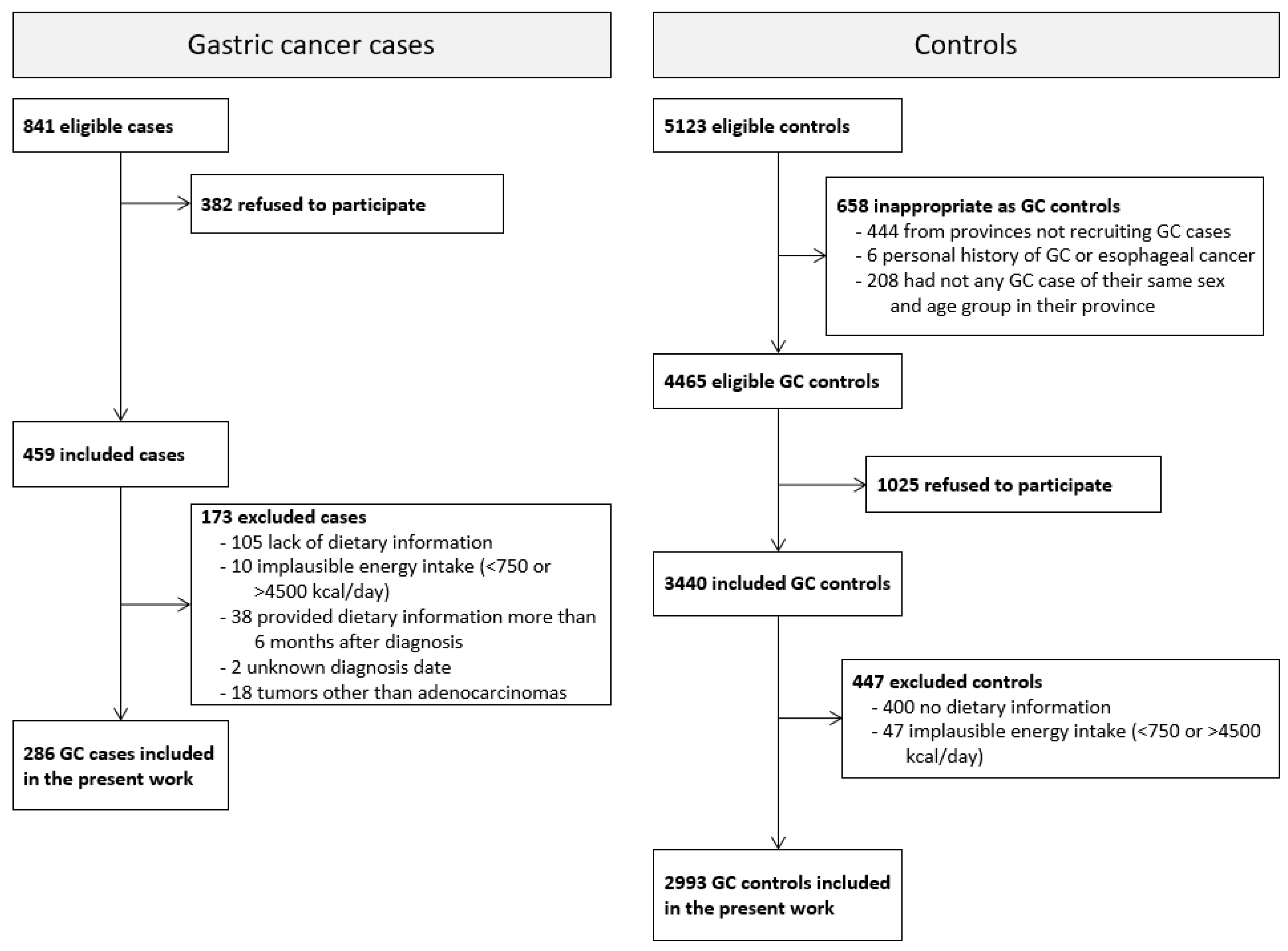
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global Surveillance of Trends in Cancer Survival 2000-14 (CONCORD-3): Analysis of Individual Records for 37 513 025 Patients Diagnosed with One of 18 Cancers from 322 Population-Based Registries in 71 Countries. Lancet Lond. Engl. 2018, 391, 1023–1075. [Google Scholar] [CrossRef]
- Guevara, M.; Molinuevo, A.; Salmerón, D.; Marcos-Gragera, R.; Carulla, M.; Chirlaque, M.-D.; Rodríguez Camblor, M.; Alemán, A.; Rojas, D.; Vizcaíno Batllés, A.; et al. Cancer Survival in Adults in Spain: A Population-Based Study of the Spanish Network of Cancer Registries (REDECAN). Cancers 2022, 14, 2441. [Google Scholar] [CrossRef]
- Arnold, M.; Park, J.Y.; Camargo, M.C.; Lunet, N.; Forman, D.; Soerjomataram, I. Is Gastric Cancer Becoming a Rare Disease? A Global Assessment of Predicted Incidence Trends to 2035. Gut 2020, 69, 823–829. [Google Scholar] [CrossRef]
- Aragonés, N.; Pérez-Gómez, B.; Pollán, M.; Ramis, R.; Vidal, E.; Lope, V.; García-Pérez, J.; Boldo, E.; López-Abente, G. The Striking Geographical Pattern of Gastric Cancer Mortality in Spain: Environmental Hypotheses Revisited. BMC Cancer 2009, 9, 316. [Google Scholar] [CrossRef] [PubMed]
- Martín-Richard, M.; Carmona-Bayonas, A.; Custodio, A.B.; Gallego, J.; Jiménez-Fonseca, P.; Reina, J.J.; Richart, P.; Rivera, F.; Alsina, M.; Sastre, J. SEOM Clinical Guideline for the Diagnosis and Treatment of Gastric Cancer (GC) and Gastroesophageal Junction Adenocarcinoma (GEJA) (2019). Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2020, 22, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Instituto de Salud Carlos III Mortalidad de Cáncer En España—Tabla de Datos. Available online: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesCronicas/Paginas/Tablas-de-datos.aspx (accessed on 8 November 2022).
- Choi, I.J.; Kim, C.G.; Lee, J.Y.; Kim, Y.-I.; Kook, M.-C.; Park, B.; Joo, J. Family History of Gastric Cancer and Helicobacter Pylori Treatment. N. Engl. J. Med. 2020, 382, 427–436. [Google Scholar] [CrossRef]
- Poorolajal, J.; Moradi, L.; Mohammadi, Y.; Cheraghi, Z.; Gohari-Ensaf, F. Risk Factors for Stomach Cancer: A Systematic Review and Meta-Analysis. Epidemiol. Health 2020, 42, e2020004. [Google Scholar] [CrossRef]
- Karimi, P.; Islami, F.; Anandasabapathy, S.; Freedman, N.D.; Kamangar, F. Gastric Cancer: Descriptive Epidemiology, Risk Factors, Screening, and Prevention. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2014, 23, 700–713. [Google Scholar] [CrossRef]
- Lauren, P. The Two Histological Main Types of Gastric Carcinoma: Diffuse and so-Called Intestinal-Type Carcinoma, an Attempt at a Histo-Clinical Classification. Acta Pathol. Microbiol. Scand. 1965, 64, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wu, X.; Li, S.; Li, C.; Guo, Z. Impact of Environmental Factors on Gastric Cancer: A Review of the Scientific Evidence, Human Prevention and Adaptation. J. Environ. Sci. Chin. 2020, 89, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; El Hajj, N.; Sittler, S.; Lammert, N.; Barnes, R.; Meloni-Ehrig, A. Gastric Cancer: Classification, Histology and Application of Molecular Pathology. J. Gastrointest. Oncol. 2012, 3, 251–261. [Google Scholar] [CrossRef]
- Ottavia, C.; Serena, S.; Chiara, M.; Alberto, B.; Antonio, N.; Gioacchino, L.; Tiziana, M.; de’ Angelis, G.L.; Francesco, D.M. Epidemiology of Gastric Cancer and Risk Factors. Acta Bio. Med. Atenei Parm. 2018, 89, 82–87. [Google Scholar] [CrossRef]
- Abdi, E.; Latifi-Navid, S.; Zahri, S.; Yazdanbod, A.; Pourfarzi, F. Risk Factors Predisposing to Cardia Gastric Adenocarcinoma: Insights and New Perspectives. Cancer Med. 2019, 8, 6114–6126. [Google Scholar] [CrossRef] [PubMed]
- Lagergren, F.; Xie, S.-H.; Mattsson, F.; Lagergren, J. Updated Incidence Trends in Cardia and Non-Cardia Gastric Adenocarcinoma in Sweden. Acta Oncol. Stockh. Swed. 2018, 57, 1173–1178. [Google Scholar] [CrossRef]
- Vahid, F.; Davoodi, S.H. Nutritional Factors Involved in the Etiology of Gastric Cancer: A Systematic Review. Nutr. Cancer 2021, 73, 376–390. [Google Scholar] [CrossRef]
- Maddineni, G.; Xie, J.J.; Brahmbhatt, B.; Mutha, P. Diet and Carcinogenesis of Gastric Cancer. Curr. Opin. Gastroenterol. 2022, 38, 588–591. [Google Scholar] [CrossRef]
- Fang, X.; Wei, J.; He, X.; An, P.; Wang, H.; Jiang, L.; Shao, D.; Liang, H.; Li, Y.; Wang, F.; et al. Landscape of Dietary Factors Associated with Risk of Gastric Cancer: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Eur. J. Cancer Oxf. Engl. 1990 2015, 51, 2820–2832. [Google Scholar] [CrossRef]
- Castelló, A.; Fernández de Larrea, N.; Martín, V.; Dávila-Batista, V.; Boldo, E.; Guevara, M.; Moreno, V.; Castaño-Vinyals, G.; Gómez-Acebo, I.; Fernández-Tardón, G.; et al. High Adherence to the Western, Prudent, and Mediterranean Dietary Patterns and Risk of Gastric Adenocarcinoma: MCC-Spain Study. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2018, 21, 372–382. [Google Scholar] [CrossRef]
- Daniel, C.R.; Cross, A.J.; Koebnick, C.; Sinha, R. Trends in Meat Consumption in the United States. Public Health Nutr. 2011, 14, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Kim, K.; Lee, S.A.; Kwon, S.O.; Lee, J.-K.; Keum, N.; Park, S.M. Effect of Red, Processed, and White Meat Consumption on the Risk of Gastric Cancer: An Overall and Dose−Response Meta-Analysis. Nutrients 2019, 11, 826. [Google Scholar] [CrossRef] [PubMed]
- González, C.A.; Jakszyn, P.; Pera, G.; Agudo, A.; Bingham, S.; Palli, D.; Ferrari, P.; Boeing, H.; del Giudice, G.; Plebani, M.; et al. Meat Intake and Risk of Stomach and Esophageal Adenocarcinoma within the European Prospective Investigation into Cancer and Nutrition (EPIC). J. Natl. Cancer Inst. 2006, 98, 345–354. [Google Scholar] [CrossRef]
- Zhao, Z.; Yin, Z.; Zhao, Q. Red and Processed Meat Consumption and Gastric Cancer Risk: A Systematic Review and Meta-Analysis. Oncotarget 2017, 8, 30563–30575. [Google Scholar] [CrossRef] [PubMed]
- Ferro, A.; Rosato, V.; Rota, M.; Costa, A.R.; Morais, S.; Pelucchi, C.; Johnson, K.C.; Hu, J.; Palli, D.; Ferraroni, M.; et al. Meat Intake and Risk of Gastric Cancer in the Stomach Cancer Pooling (StoP) Project. Int. J. Cancer 2020, 147, 45–55. [Google Scholar] [CrossRef] [PubMed]
- IARC. Red Meat and Processed Meat; IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2018; Volume 114, ISBN 978-92-832-0152-6. [Google Scholar]
- Huang, Y.; Cao, D.; Chen, Z.; Chen, B.; Li, J.; Guo, J.; Dong, Q.; Liu, L.; Wei, Q. Red and Processed Meat Consumption and Cancer Outcomes: Umbrella Review. Food Chem. 2021, 356, 129697. [Google Scholar] [CrossRef] [PubMed]
- WCRF. Diet, Nutrition, Physical Activity and Stomach Cancer. In Continuous Update Project Report; World Cancer Research Fund/American Institute for Cancer Research: London, UK, 2016; Revised 2018. [Google Scholar]
- Di Maso, M.; Talamini, R.; Bosetti, C.; Montella, M.; Zucchetto, A.; Libra, M.; Negri, E.; Levi, F.; La Vecchia, C.; Franceschi, S.; et al. Red Meat and Cancer Risk in a Network of Case-Control Studies Focusing on Cooking Practices. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013, 24, 3107–3112. [Google Scholar] [CrossRef]
- Song, P.; Lu, M.; Yin, Q.; Wu, L.; Zhang, D.; Fu, B.; Wang, B.; Zhao, Q. Red Meat Consumption and Stomach Cancer Risk: A Meta-Analysis. J. Cancer Res. Clin. Oncol. 2014, 140, 979–992. [Google Scholar] [CrossRef]
- Castano-Vinyals, G.; Aragones, N.; Perez-Gomez, B.; Martin, V.; Llorca, J.; Moreno, V.; Altzibar, J.M.; Ardanaz, E.; de Sanjose, S.; Jimenez-Moleon, J.J.; et al. Population-Based Multicase-Control Study in Common Tumors in Spain (MCC-Spain): Rationale and Study Design. Gac. Sanit. 2015, 29, 308–315. [Google Scholar] [CrossRef]
- García-Closas, R.; García-Closas, M.; Kogevinas, M.; Malats, N.; Silverman, D.; Serra, C.; Tardón, A.; Carrato, A.; Castaño-Vinyals, G.; Dosemeci, M.; et al. Food, Nutrient and Heterocyclic Amine Intake and the Risk of Bladder Cancer. Eur. J. Cancer Oxf. Engl. 1990 2007, 43, 1731–1740. [Google Scholar] [CrossRef]
- Zamani, N.; Hajifaraji, M.; Fazel-tabar Malekshah, A.; Keshtkar, A.A.; Esmaillzadeh, A.; Malekzadeh, R. A Case-Control Study of the Relationship between Gastric Cancer and Meat Consumption in Iran. Arch. Iran. Med. 2013, 16, 324–329. [Google Scholar] [PubMed]
- Buiatti, E.; Palli, D.; Bianchi, S.; Decarli, A.; Amadori, D.; Avellini, C.; Cipriani, F.; Cocco, P.; Giacosa, A.; Lorenzini, L. A Case-Control Study of Gastric Cancer and Diet in Italy. III. Risk Patterns by Histologic Type. Int. J. Cancer 1991, 48, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Mertens, C.; Tomat, E.; Brüne, B. Iron as a Central Player and Promising Target in Cancer Progression. Int. J. Mol. Sci. 2019, 20, 273. [Google Scholar] [CrossRef]
- Torti, S.V.; Torti, F.M. Iron and Cancer: More Ore to Be Mined. Nat. Rev. Cancer 2013, 13, 342–355. [Google Scholar] [CrossRef]
- Ward, M.H.; Cross, A.J.; Abnet, C.C.; Sinha, R.; Markin, R.S.; Weisenburger, D.D. Heme Iron from Meat and Risk of Adenocarcinoma of the Esophagus and Stomach. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. ECP 2012, 21, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Jakszyn, P.; Agudo, A.; Lujan-Barroso, L.; Bueno-de-Mesquita, H.B.; Jenab, M.; Navarro, C.; Palli, D.; Boeing, H.; Manjer, J.; Numans, M.E.; et al. Dietary Intake of Heme Iron and Risk of Gastric Cancer in the European Prospective Investigation into Cancer and Nutrition Study. Int. J. Cancer 2012, 130, 2654–2663. [Google Scholar] [CrossRef]
- Amieva, M.; Peek, R.M. Pathobiology of Helicobacter Pylori-Induced Gastric Cancer. Gastroenterology 2016, 150, 64–78. [Google Scholar] [CrossRef]
- Shimizu, T.; Marusawa, H.; Watanabe, N.; Chiba, T. Molecular Pathogenesis of Helicobacter Pylori-Related Gastric Cancer. Gastroenterol. Clin. North Am. 2015, 44, 625–638. [Google Scholar] [CrossRef]
- Rohrmann, S.; Linseisen, J. Processed Meat: The Real Villain? Proc. Nutr. Soc. 2016, 75, 233–241. [Google Scholar] [CrossRef]
- Patrad, E.; Khalighfard, S.; Amiriani, T.; Khori, V.; Alizadeh, A.M. Molecular Mechanisms Underlying the Action of Carcinogens in Gastric Cancer with a Glimpse into Targeted Therapy. Cell. Oncol. Dordr. 2022. [Google Scholar] [CrossRef]
- Wu, X.; Chen, L.; Cheng, J.; Qian, J.; Fang, Z.; Wu, J. Effect of Dietary Salt Intake on Risk of Gastric Cancer: A Systematic Review and Meta-Analysis of Case-Control Studies. Nutrients 2022, 14, 4260. [Google Scholar] [CrossRef] [PubMed]
- Bingham, S.A.; Hughes, R.; Cross, A.J. Effect of White versus Red Meat on Endogenous N-Nitrosation in the Human Colon and Further Evidence of a Dose Response. J. Nutr. 2002, 132, 3522S–3525S. [Google Scholar] [CrossRef] [PubMed]
- Koumbi, L. Dietary Factors Can Protect against Liver Cancer Development. World J. Hepatol. 2017, 9, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Daniel, C.R.; Cross, A.J.; Graubard, B.I.; Hollenbeck, A.R.; Park, Y.; Sinha, R. Prospective Investigation of Poultry and Fish Intake in Relation to Cancer Risk. Cancer Prev. Res. Phila. 2011, 4, 1903–1911. [Google Scholar] [CrossRef]
- Fung, T.T.; McCullough, M.L.; Newby, P.K.; Manson, J.E.; Meigs, J.B.; Rifai, N.; Willett, W.C.; Hu, F.B. Diet-Quality Scores and Plasma Concentrations of Markers of Inflammation and Endothelial Dysfunction. Am. J. Clin. Nutr. 2005, 82, 163–173. [Google Scholar] [CrossRef]
- Castelló, A.; Pollán, M.; Buijsse, B.; Ruiz, A.; Casas, A.M.; Baena-Cañada, J.M.; Lope, V.; Antolín, S.; Ramos, M.; Muñoz, M.; et al. Spanish Mediterranean Diet and Other Dietary Patterns and Breast Cancer Risk: Case-Control EpiGEICAM Study. Br. J. Cancer 2014, 111, 1454–1462. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, X.; Ma, Y.; Zhao, J.; Tang, Z. Concentrations and Distributions of Polycyclic Aromatic Hydrocarbon in Vegetables and Animal-Based Foods before and after Grilling: Implication for Human Exposure. Sci. Total Environ. 2019, 690, 965–972. [Google Scholar] [CrossRef]
- Ahmad Kamal, N.H.; Selamat, J.; Sanny, M. Simultaneous Formation of Polycyclic Aromatic Hydrocarbons (PAHs) and Heterocyclic Aromatic Amines (HCAs) in Gas-Grilled Beef Satay at Different Temperatures. Food Addit. Contam. Part Chem. Anal. Control Expo. Risk Assess. 2018, 35, 848–869. [Google Scholar] [CrossRef]
- Sugimura, T. Nutrition and Dietary Carcinogens. Carcinogenesis 2000, 21, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Rothman, N.; Brown, E.D.; Salmon, C.P.; Knize, M.G.; Swanson, C.A.; Rossi, S.C.; Mark, S.D.; Levander, O.A.; Felton, J.S. High Concentrations of the Carcinogen 2-Amino-1-Methyl-6-Phenylimidazo-[4,5-b]Pyridine (PhIP) Occur in Chicken but Are Dependent on the Cooking Method. Cancer Res. 1995, 55, 4516–4519. [Google Scholar]
- Solyakov, A.; Skog, K. Screening for Heterocyclic Amines in Chicken Cooked in Various Ways. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2002, 40, 1205–1211. [Google Scholar] [CrossRef]
- Sinha, R.; Knize, M.G.; Salmon, C.P.; Brown, E.D.; Rhodes, D.; Felton, J.S.; Levander, O.A.; Rothman, N. Heterocyclic Amine Content of Pork Products Cooked by Different Methods and to Varying Degrees of Doneness. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 1998, 36, 289–297. [Google Scholar] [CrossRef]
- de Batlle, J.; Gracia-Lavedan, E.; Romaguera, D.; Mendez, M.; Castaño-Vinyals, G.; Martín, V.; Aragonés, N.; Gómez-Acebo, I.; Olmedo-Requena, R.; Jimenez-Moleon, J.J.; et al. Meat Intake, Cooking Methods and Doneness and Risk of Colorectal Tumours in the Spanish Multicase-Control Study (MCC-Spain). Eur. J. Nutr. 2018, 57, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Helicobacter and Cancer Collaborative Group. Gastric Cancer and Helicobacter Pylori: A Combined Analysis of 12 Case Control Studies Nested within Prospective Cohorts. Gut 2001, 49, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, J.; Tsugawa, H.; Suzuki, H. Precision Medicine Approaches to Prevent Gastric Cancer. Gut Liver 2021, 15, 3–12. [Google Scholar] [CrossRef]
- Lorenzo, I.; Fernández-de-Larrea, N.; Michel, A.; Romero, B.; Lope, V.; Bessa, X.; Moreno, V.; Martín, V.; Amiano, P.; Castilla, J.; et al. Helicobacter Pylori Seroprevalence in Spain: Influence of Adult and Childhood Sociodemographic Factors. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. ECP 2019, 28, 294–303. [Google Scholar] [CrossRef]
- Calvert, C.; Cade, J.; Barrett, J.H.; Woodhouse, A. Using Cross-Check Questions to Address the Problem of Mis-Reporting of Specific Food Groups on Food Frequency Questionnaires. UKWCS Steering Group. United Kingdom Women’s Cohort Study Steering Group. Eur. J. Clin. Nutr. 1997, 51, 708–712. [Google Scholar] [CrossRef]
- Willett, W. Recall of Remote Diet. In Nutritional Epidemiology; Oxford University Press: New York, NY, USA, 1998; ISBN 978-0-19-512297-8. [Google Scholar]
- Draugalis, J.R.; Coons, S.J.; Plaza, C.M. Best Practices for Survey Research Reports: A Synopsis for Authors and Reviewers. Am. J. Pharm. Educ. 2008, 72, 11. [Google Scholar] [CrossRef]
- Spanish Statistical Office—INE. ¿Cuánta Carne Se Consume Cada Año En España? Gráficos y Evolución. Available online: https://www.epdata.es/datos/carne-consume-ano-espana-graficos-evolucion/605 (accessed on 7 April 2022).
| Controls | Cases | p-Value | |
|---|---|---|---|
| n = 2993 | n = 286 | ||
| Age (years), mean (SD) | 63.94 (11.42) | 66.31 (12.40) | 0.001 |
| Age (10-year periods), n (%) | <0.001 | ||
| 26–35 | 28 (1%) | 6 (2%) | |
| 36–45 | 195 (7%) | 10 (4%) | |
| 46–55 | 462 (15%) | 44 (15%) | |
| 56–65 | 837 (28%) | 64 (22%) | |
| 66–75 | 975 (33%) | 84 (29%) | |
| 76–85 | 496 (17%) | 778 (27%) | |
| Education, n (%) | <0.001 | ||
| No formal Education | 537 (18%) | 80 (28%) | |
| Primary School | 1007 (34%) | 110 (38%) | |
| Secondary School | 837 (28%) | 65 (23%) | |
| University or higher | 612 (20%) | 31 (11%) | |
| Sex, n (%) | <0.001 | ||
| Male | 1669 (56%) | 200 (70%) | |
| Female | 1324 (44%) | 86 (30%) | |
| Energy (kcal/day),mean (SD) | 1912.50 (571.94) | 2095.19 (651.32) | <0.001 |
| Alcohol(g/day),median (IQI) | 7.57 (0.00;24.72) | 12.92 (1.41;40.42) | <0.001 |
| Salty fish (g/day),median (IQI) | 0.00 (0.00;4.59) | 0.00 (0.00;4.59) | 0.973 |
| Olives (g/day),median (IQI) | 1.97 (0.00;6.42) | 1.97 (0.00;6.42) | 0.534 |
| Fruits(g/day),median (IQI) | 330.21 (195.32;460.65) | 332.53 (203.02;456.81) | 0.947 |
| BMI a (kg/m2),median (IQI) | 26.68 (4.33) | 27.15 (3.86) | 0.084 |
| Physical activity (METs/week) a, n (%) | 0.001 | ||
| 0 | 1169 (40%) | 141 (49%) | |
| 0.1–8 | 406 (14%) | 33 (12%) | |
| 8.1–16 | 351 (12%) | 15 (5%) | |
| >16 | 1029 (35%) | 97 (34%) | |
| Smoking a, n (%) | 0.708 | ||
| Never Smoker | 1313 (44%) | 120 (42%) | |
| Former Smoker | 1076 (36%) | 103 (36%) | |
| Current Smoker | 592 (20%) | 62 (22%) | |
| Family history of GC, n (%) | <0.001 | ||
| No | 2661 (89%) | 223 (78%) | |
| 2nd degree | 139 (5%) | 14 (5%) | |
| One of 1st degree | 181 (6%) | 42 (15%) | |
| More than one of 1st degree | 12 (0%) | 7 (2%) | |
| Serology against H. pylori a, n (%) | 0.117 | ||
| Negative | 218 (11%) | 13 (7%) | |
| Positive | 1701 (89%) | 161 (93%) |
| Controls | Cases | p-Value | |
|---|---|---|---|
| n = 2993 | n = 286 | ||
| Daily Intake | |||
| Total Meat | |||
| Non-Consumers, n (%) | 7 (0.23%) | 0 (0.00%) | |
| Intake (g/day), median a (IQI) | 76.11 (53.92;105.57) | 89.83 (66.38;129.64) | <0.001 |
| White Meat | |||
| Non-Consumers, n (%) | 115 (3.84%) | 2 (0.70%) | |
| Intake (g/day), median a (IQI) | 19.22 (12.94;27.23) | 21.32 (15.37;32.05) | <0.001 |
| Red Meat | |||
| Non-Consumers, n (%) | 83 (2.77%) | 2 (0.70%) | |
| Intake (g/day), median a (IQI) | 32.21 (19.05;51.01) | 40.97 (25.85;61.20) | <0.001 |
| Processed Meat | |||
| Non-Consumers, n (%) | 82 (2.74%) | 5 (1.75%) | |
| Intake (g/day), median a (IQI) | 18.51 (9.91;30.35) | 23.29 (13.29;40.68) | <0.001 |
| Doneness Preference, n (%) | |||
| White Meat b | 0.006 | ||
| Rare | 196 (7.32%) | 10 (3.89%) | |
| Medium | 1629 (60.87%) | 143 (55.64%) | |
| Well-done | 851 (31.80%) | 104 (40.47%) | |
| Red Meat b | 0.012 | ||
| Rare | 286 (10.42%) | 28 (10.53%) | |
| Medium | 1894 (69.02%) | 163 (61.28%) | |
| Well-done | 564 (20.55%) | 75 (28.20%) | |
| Cooking Method, median a (IQI) | |||
| White Meat (g/day) | |||
| Griddle-grilled/BBQ | 3.99 (0.00;8.96) | 3.77 (0.00;7.83) | 0.246 |
| Pan-Fried/breaded-coated fried | 1.90 (0.00;5.73) | 2.61 (0.00;6.51) | 0.088 |
| Stewed | 3.42 (0.00;7.22) | 4.97 (1.85;9.37) | <0.001 |
| Oven-baked | 1.58 (0.00;3.88) | 2.40 (0.00;4.74) | 0.001 |
| Other/Unknown | 0.00 (0.00;0.00) | 0.00 (0.00;0.00) | 0.287 |
| Red Meat (g/day) | |||
| Griddle-grilled/BBQ | 11.44 (3.63;21.75) | 11.71 (3.71;22.44) | 0.726 |
| Pan-Fried/breaded-coated fried | 3.31 (0.00;11.20) | 5.68 (0.00;14.03) | 0.002 |
| Stewed | 8.27 (3.03;15.17) | 10.91 (4.85;21.02) | <0.001 |
| Oven-baked | 0.00 (0.00;3.19) | 0.00 (0.00;3.32) | 0.617 |
| Other/Unknown | 0.00 (0.00;1.64) | 0.00 (0.00;2.05) | <0.001 |
| All | Males | Females | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sv/Week a | Controls | Cases | OR b | LL b | UL b | Sv/Week a | Controls | Cases | OR b | LL b | UL b | Sv/Week a | Controls | Cases | OR b | LL b | UL b | p–Het b | |
| Weekly Intake | n = 2821 | n = 271 | n = 1587 | n = 196 | n = 1234 | n = 75 | |||||||||||||
| Total Meat c | |||||||||||||||||||
| Q1 d | <3.0 | 707 | 38 | 1 | <3.44 | 396 | 27 | 1 | <2.61 | 314 | 12 | 1 | 0.004 | ||||||
| Q2 d | 3.0–4.3 | 700 | 55 | 1.28 | 0.82 | 1.99 | 3.44–4.81 | 400 | 39 | 1.16 | 0.68 | 1.98 | 2.61–3.61 | 305 | 16 | 1.37 | 0.63 | 2.99 | |
| Q3 d | 4.3–5.9 | 716 | 75 | 1.58 | 1.02 | 2.42 | 4.81–6.55 | 403 | 43 | 1.37 | 0.81 | 2.32 | 3.61–5.03 | 309 | 33 | 2.67 | 1.33 | 5.36 | |
| Q4 d | ≥5.9 | 698 | 103 | 1.73 | 1.10 | 2.71 | ≥6.55 | 388 | 87 | 2.39 | 1.44 | 3.98 | ≥5.03 | 306 | 14 | 1.06 | 0.47 | 2.40 | |
| p–trend | 0.012 | <0.001 | 0.409 | ||||||||||||||||
| 1 serving/week increase | 1.11 | 1.05 | 1.17 | 1.13 | 1.06 | 1.20 | 1.02 | 0.91 | 1.14 | 0.095 | |||||||||
| White Meat e | |||||||||||||||||||
| Q1 d | <0.7 | 709 | 49 | 1 | <0.72 | 400 | 34 | 1 | <0.72 | 308 | 15 | 1 | 0.926 | ||||||
| Q2 d | 0.7–1.1 | 716 | 62 | 1.24 | 0.82 | 1.88 | 0.72–1.19 | 399 | 54 | 1.28 | 0.79 | 2.07 | 0.72–0.95 | 321 | 21 | 1.25 | 0.62 | 2.54 | |
| Q3 d | 1.1–1.5 | 698 | 72 | 1.05 | 0.70 | 1.57 | 1.19–1.56 | 391 | 43 | 0.97 | 0.59 | 1.60 | 0.95–1.39 | 306 | 17 | 0.95 | 0.45 | 1.99 | |
| Q4 d | ≥1.5 | 698 | 88 | 1.38 | 0.93 | 2.04 | ≥1.56 | 397 | 65 | 1.43 | 0.89 | 2.29 | ≥1.39 | 299 | 22 | 1.31 | 0.65 | 2.64 | |
| p–trend | 0.195 | 0.269 | 0.641 | ||||||||||||||||
| 1 serving/week increase | 1.11 | 0.98 | 1.26 | 1.14 | 0.99 | 1.31 | 1.01 | 0.76 | 1.35 | 0.475 | |||||||||
| Red Meat e | |||||||||||||||||||
| Q1 d | <1.1 | 710 | 39 | 1 | <1.25 | 404 | 31 | 1 | <0.91 | 309 | 14 | 1 | 0.371 | ||||||
| Q2 d | 1.1–1.8 | 700 | 58 | 1.39 | 0.89 | 2.15 | 1.25–2.07 | 387 | 35 | 1.04 | 0.61 | 1.76 | 0.91–1.50 | 308 | 17 | 1.09 | 0.52 | 2.31 | |
| Q3 d | 1.8–2.9 | 699 | 76 | 1.73 | 1.12 | 2.66 | 2.07–3.18 | 399 | 58 | 1.65 | 1.01 | 2.70 | 1.50–2.44 | 311 | 25 | 1.78 | 0.89 | 3.57 | |
| Q4 d | ≥2.9 | 712 | 98 | 1.76 | 1.14 | 2.72 | ≥3.18 | 397 | 72 | 1.72 | 1.06 | 2.81 | ≥2.44 | 306 | 19 | 1.24 | 0.59 | 2.60 | |
| p–trend | 0.009 | 0.008 | 0.339 | ||||||||||||||||
| 1 serving/week increase | 1.11 | 1.02 | 1.20 | 1.12 | 1.03 | 1.22 | 1.02 | 0.84 | 1.23 | 0.337 | |||||||||
| Processed Meat e | |||||||||||||||||||
| Q1 d | <1.4 | 700 | 43 | 1 | <1.72 | 398 | 34 | 1 | <1.15 | 307 | 12 | 1 | 0.300 | ||||||
| Q2 d | 1.4–2.6 | 706 | 59 | 1.25 | 0.82 | 1.92 | 1.72–3.13 | 398 | 41 | 1.04 | 0.63 | 1.72 | 1.15–2.09 | 316 | 22 | 1.63 | 0.78 | 3.40 | |
| Q3 d | 2.6–4.3 | 718 | 67 | 1.22 | 0.80 | 1.87 | 3.13–5.03 | 401 | 47 | 1.18 | 0.72 | 1.93 | 2.09–3.28 | 305 | 19 | 1.40 | 0.66 | 3.00 | |
| Q4 d | ≥4.3 | 697 | 102 | 1.48 | 0.97 | 2.28 | ≥5.03 | 390 | 74 | 1.67 | 1.02 | 2.72 | ≥3.28 | 306 | 22 | 1.27 | 0.60 | 2.69 | |
| p–trend | 0.095 | 0.026 | 0.771 | ||||||||||||||||
| 1 serving/week increase | 1.04 | 1.00 | 1.08 | 1.06 | 1.01 | 1.11 | 0.99 | 0.91 | 1.08 | 0.148 |
| Tumor Subsite | Tumor Morphology | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls n = 2821 | Cardia n = 65 | Non-Cardia n = 199 | Intestinal n = 106 | Diffuse n = 66 | ||||||||||||||||
| Sv/Week a | n | n | RRR b | LL b | UL b | n | RRR b | LL b | UL b | p-Het b | n | RRR b | LL b | UL b | n | RRR b | LL b | UL b | p-Het b | |
| Weekly Intake | ||||||||||||||||||||
| Total Meat c | ||||||||||||||||||||
| Q1 d | <3.0 | 707 | 9 | 1 | 28 | 1 | 0.444 | 12 | 1 | 11 | 1 | 0.119 | ||||||||
| Q2 d | 3.0–4.3 | 700 | 8 | 0.66 | 0.24 | 1.77 | 45 | 1.49 | 0.90 | 2.46 | 22 | 1.74 | 0.83 | 3.66 | 17 | 1.36 | 0.62 | 2.99 | ||
| Q3 d | 4.3–5.9 | 716 | 20 | 1.20 | 0.52 | 2.78 | 54 | 1.70 | 1.04 | 2.80 | 33 | 2.80 | 1.37 | 5.70 | 13 | 0.92 | 0.39 | 2.13 | ||
| Q4 d | ≥5.9 | 698 | 28 | 1.04 | 0.43 | 2.48 | 72 | 1.95 | 1.16 | 3.27 | 39 | 3.23 | 1.53 | 6.84 | 25 | 1.29 | 0.56 | 2.95 | ||
| p-trend | 0.576 | 0.013 | 0.001 | 0.773 | ||||||||||||||||
| 1 serving/week increase | 1.08 | 0.98 | 1.19 | 1.11 | 1.04 | 1.18 | 0.651 | 1.17 | 1.08 | 1.28 | 0.99 | 0.89 | 1.10 | 0.011 | ||||||
| White Meat e | ||||||||||||||||||||
| Q1 d | <0.7 | 709 | 10 | 1 | 38 | 1 | 0.578 | 22 | 1 | 7 | 1 | 0.057 | ||||||||
| Q2 d | 0.7–1.1 | 716 | 12 | 1.41 | 0.58 | 3.41 | 49 | 1.19 | 0.75 | 1.88 | 28 | 1.08 | 0.58 | 2.02 | 23 | 3.06 | 1.26 | 7.41 | ||
| Q3 d | 1.1–1.5 | 698 | 23 | 1.38 | 0.63 | 3.04 | 46 | 0.89 | 0.56 | 1.42 | 23 | 0.55 | 0.29 | 1.05 | 19 | 2.55 | 1.02 | 6.37 | ||
| Q4 d | ≥1.5 | 698 | 20 | 1.32 | 0.59 | 2.98 | 66 | 1.39 | 0.89 | 2.16 | 33 | 0.89 | 0.48 | 1.65 | 17 | 2.34 | 0.93 | 5.91 | ||
| p-trend | 0.592 | 0.262 | 0.424 | 0.212 | ||||||||||||||||
| 1 serving/week increase | 1.03 | 0.81 | 1.31 | 1.14 | 0.99 | 1.32 | 0.438 | 1.05 | 0.84 | 1.32 | 1.13 | 0.93 | 1.38 | 0.626 | ||||||
| Red Meat e | ||||||||||||||||||||
| Q1 d | <1.1 | 710 | 6 | 1 | 32 | 1.00 | 0.908 | 10 | 1 | 16 | 1 | <0.001 | ||||||||
| Q2 d | 1.1–1.8 | 700 | 14 | 1.89 | 0.70 | 5.08 | 43 | 1.29 | 0.79 | 2.11 | 26 | 2.77 | 1.28 | 6.00 | 12 | 0.60 | 0.27 | 1.32 | ||
| Q3 d | 1.8–2.9 | 699 | 18 | 2.02 | 0.76 | 5.35 | 57 | 1.72 | 1.06 | 2.78 | 28 | 3.62 | 1.66 | 7.90 | 22 | 0.91 | 0.45 | 1.85 | ||
| Q4 d | ≥2.9 | 712 | 27 | 2.05 | 0.78 | 5.37 | 67 | 1.69 | 1.04 | 2.76 | 42 | 5.60 | 2.58 | 12.13 | 16 | 0.54 | 0.25 | 1.19 | ||
| p-trend | 0.223 | 0.023 | <0.001 | 0.310 | ||||||||||||||||
| 1 serving/week increase | 1.11 | 0.97 | 1.26 | 1.10 | 1.00 | 1.20 | 0.938 | 1.26 | 1.12 | 1.42 | 0.89 | 0.74 | 1.07 | 0.002 | ||||||
| Processed Meat e | ||||||||||||||||||||
| Q1 d | <1.4 | 700 | 8 | 1 | 32 | 1.00 | 0.767 | 21 | 1 | 10 | 1 | 0.805 | ||||||||
| Q2 d | 1.4–2.6 | 706 | 14 | 1.50 | 0.61 | 3.72 | 45 | 1.34 | 0.82 | 2.17 | 22 | 0.89 | 0.47 | 1.72 | 13 | 1.14 | 0.48 | 2.67 | ||
| Q3 d | 2.6–4.3 | 718 | 16 | 1.23 | 0.50 | 3.02 | 49 | 1.35 | 0.83 | 2.19 | 26 | 0.96 | 0.51 | 1.81 | 18 | 1.58 | 0.70 | 3.55 | ||
| Q4 d | ≥4.3 | 697 | 27 | 1.29 | 0.53 | 3.16 | 73 | 1.79 | 1.10 | 2.92 | 37 | 1.21 | 0.63 | 2.31 | 25 | 1.78 | 0.77 | 4.09 | ||
| p-trend | 0.751 | 0.029 | 0.539 | 0.078 | ||||||||||||||||
| 1 serving/week increase | 1.03 | 0.95 | 1.11 | 1.05 | 1.00 | 1.10 | 0.605 | 1.05 | 0.99 | 1.12 | 1.02 | 0.96 | 1.10 | 0.589 | ||||||
| All | Males | Females | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls | Cases | OR a,b | LL a,b | UL a,b | Controls | Cases | OR a,c | LL a,c | UL a,c | Controls | Cases | OR a,c | LL a,c | UL a,c | p-Int a | |
| Doneness Preference | ||||||||||||||||
| White Meat | n= 2520 | n= 244 | n= 1394 | n= 173 | n= 1126 | n= 71 | ||||||||||
| Rare/Medium | 1740 | 145 | 1 | 960 | 98 | 1 | 780 | 47 | 1 | |||||||
| Well-done | 780 | 99 | 1.16 | 0.86 | 1.56 | 434 | 75 | 1.29 | 0.90 | 1.83 | 346 | 24 | 0.91 | 0.53 | 1.54 | 0.268 |
| Red Meat | n= 2582 | n= 253 | n= 1464 | n= 183 | n= 1118 | n= 70 | ||||||||||
| Rare/Medium | 2067 | 185 | 1 | 1169 | 132 | 1 | 898 | 53 | 1 | |||||||
| Well-done | 515 | 68 | 1.23 | 0.89 | 1.69 | 295 | 51 | 1.23 | 0.84 | 1.80 | 220 | 17 | 1.22 | 0.68 | 2.20 | 0.984 |
| Controls | Cases | OR a,d | LL a,d | UL a,d | Controls | Cases | OR a,e | LL a,e | UL a,e | Controls | Cases | OR a,e | LL a,e | UL a,e | p-Int a | |
| Cooking Methods | ||||||||||||||||
| White Meat | n= 2711 | n= 269 | n= 1516 | n= 194 | n= 1195 | n= 75 | ||||||||||
| Griddle/BBQ | 1961 | 185 | 1.49 | 1.08 | 2.07 | 1051 | 131 | 1.56 | 1.07 | 2.27 | 910 | 54 | 1.33 | 0.77 | 2.32 | 0.631 |
| Fried | 1625 | 172 | 1.30 | 0.98 | 1.74 | 929 | 124 | 1.27 | 0.90 | 1.79 | 696 | 48 | 1.38 | 0.84 | 2.28 | 0.777 |
| Stewed | 2072 | 228 | 1.71 | 1.19 | 2.47 | 1143 | 167 | 1.97 | 1.26 | 3.08 | 929 | 61 | 1.26 | 0.68 | 2.33 | 0.253 |
| Oven-Baked | 1654 | 179 | 1.62 | 1.20 | 2.20 | 918 | 136 | 2.03 | 1.41 | 2.93 | 736 | 43 | 1.01 | 0.61 | 1.66 | 0.022 |
| Red Meat | n= 2740 | n= 269 | n= 1546 | n= 195 | n= 1194 | n= 74 | ||||||||||
| Griddle/BBQ | 2395 | 237 | 1.59 | 1.03 | 2.45 | 1345 | 169 | 1.43 | 0.88 | 2.34 | 1050 | 68 | 2.15 | 0.90 | 5.17 | 0.409 |
| Fried | 1809 | 194 | 1.27 | 0.94 | 1.72 | 1039 | 141 | 1.23 | 0.86 | 1.77 | 770 | 53 | 1.36 | 0.79 | 2.34 | 0.761 |
| Stewed | 2392 | 247 | 1.62 | 1.01 | 2.60 | 1353 | 180 | 1.68 | 0.95 | 3.00 | 1039 | 67 | 1.49 | 0.66 | 3.35 | 0.805 |
| Oven-Baked | 1418 | 136 | 0.95 | 0.72 | 1.25 | 824 | 103 | 0.99 | 0.72 | 1.38 | 594 | 33 | 0.86 | 0.52 | 1.40 | 0.616 |
| Tumor Subsite | Tumor Morphology | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls | Cardia | Non-Cardia | Intestinal | Diffuse | |||||||||||||||
| n | n | RRR a,b | LL a,b | UL a,b | n | RRR a,b | LL a,b | UL a,b | p-Het a | n | RRR a,b | LL a,b | UL a,b | n | RRR a,b | LL a,b | UL a,b | p-Het a | |
| Doneness Preference | |||||||||||||||||||
| White Meat | 2520 | 62 | 175 | 94 | 55 | ||||||||||||||
| Rare/Medium | 1740 | 41 | 1 | 99 | 1 | 0.233 | 51 | 1 | 37 | 1 | 0.185 | ||||||||
| Well-done | 780 | 21 | 1.08 | 0.62 | 1.86 | 76 | 1.57 | 1.14 | 2.16 | 43 | 1.69 | 1.10 | 2.59 | 18 | 1.05 | 0.59 | 1.87 | ||
| Red Meat | 2582 | 63 | 183 | 101 | 57 | ||||||||||||||
| Rare/Medium | 2067 | 48 | 1 | 132 | 1 | 0.842 | 70 | 1 | 48 | 1 | 0.051 | ||||||||
| Well-done | 515 | 15 | 1.33 | 0.72 | 2.44 | 51 | 1.42 | 1.00 | 2.02 | 31 | 1.61 | 1.02 | 2.53 | 9 | 0.70 | 0.34 | 1.44 | ||
| Controls | Cardia | Non-Cardia | Intestinal | Diffuse | |||||||||||||||
| n | n | RRR a,c | LL a,c | UL a,c | n | RRR a,c | LL a,c | UL a,c | p-Het a | n | RRR a,c | LL a,c | UL a,c | n | RRR a,c | LL a,c | UL a,c | p-Het a | |
| Cooking Methods | |||||||||||||||||||
| White Meat | 2711 | 65 | 197 | 104 | 66 | ||||||||||||||
| Griddle-grilled/BBQ | 1961 | 46 | 1.00 | 0.56 | 1.79 | 136 | 1.12 | 0.80 | 1.58 | 0.729 | 69 | 1.15 | 0.73 | 1.81 | 42 | 0.69 | 0.41 | 1.19 | 0.151 |
| Fried | 1625 | 46 | 1.45 | 0.81 | 2.59 | 121 | 1.07 | 0.78 | 1.48 | 0.357 | 69 | 1.44 | 0.92 | 2.26 | 41 | 0.97 | 0.57 | 1.65 | 0.251 |
| Stewed | 2072 | 53 | 1.20 | 0.62 | 2.30 | 168 | 1.73 | 1.14 | 2.63 | 0.340 | 92 | 2.40 | 1.28 | 4.49 | 52 | 1.02 | 0.55 | 1.88 | 0.051 |
| Oven-Baked | 1654 | 50 | 2.14 | 1.15 | 3.96 | 127 | 1.36 | 0.98 | 1.88 | 0.192 | 67 | 1.57 | 1.01 | 2.44 | 47 | 1.55 | 0.88 | 2.72 | 0.972 |
| Red Meat | 2740 | 65 | 197 | 106 | 64 | ||||||||||||||
| Griddle-grilled /BBQ | 2395 | 52 | 0.53 | 0.27 | 1.05 | 178 | 1.71 | 1.02 | 2.85 | 0.005 | 95 | 2.16 | 1.09 | 4.29 | 55 | 0.86 | 0.41 | 1.82 | 0.069 |
| Fried | 1809 | 51 | 1.57 | 0.84 | 2.93 | 138 | 1.09 | 0.79 | 1.52 | 0.305 | 76 | 1.25 | 0.79 | 1.97 | 45 | 0.98 | 0.56 | 1.73 | 0.511 |
| Stewed | 2392 | 61 | 1.63 | 0.58 | 4.61 | 179 | 1.32 | 0.79 | 2.21 | 0.721 | 98 | 1.82 | 0.86 | 3.86 | 59 | 1.39 | 0.54 | 3.57 | 0.655 |
| Oven-Baked | 1418 | 31 | 0.70 | 0.41 | 1.17 | 102 | 1.00 | 0.74 | 1.35 | 0.223 | 57 | 1.15 | 0.76 | 1.73 | 37 | 1.23 | 0.73 | 2.07 | 0.833 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boldo, E.; Fernández de Larrea, N.; Pollán, M.; Martín, V.; Obón-Santacana, M.; Guevara, M.; Castaño-Vinyals, G.; Canga, J.M.; Pérez-Gómez, B.; Gómez-Acebo, I.; et al. Meat Intake, Cooking Methods, Doneness Preferences and Risk of Gastric Adenocarcinoma in the MCC-Spain Study. Nutrients 2022, 14, 4852. https://doi.org/10.3390/nu14224852
Boldo E, Fernández de Larrea N, Pollán M, Martín V, Obón-Santacana M, Guevara M, Castaño-Vinyals G, Canga JM, Pérez-Gómez B, Gómez-Acebo I, et al. Meat Intake, Cooking Methods, Doneness Preferences and Risk of Gastric Adenocarcinoma in the MCC-Spain Study. Nutrients. 2022; 14(22):4852. https://doi.org/10.3390/nu14224852
Chicago/Turabian StyleBoldo, Elena, Nerea Fernández de Larrea, Marina Pollán, Vicente Martín, Mireia Obón-Santacana, Marcela Guevara, Gemma Castaño-Vinyals, Jose María Canga, Beatriz Pérez-Gómez, Inés Gómez-Acebo, and et al. 2022. "Meat Intake, Cooking Methods, Doneness Preferences and Risk of Gastric Adenocarcinoma in the MCC-Spain Study" Nutrients 14, no. 22: 4852. https://doi.org/10.3390/nu14224852
APA StyleBoldo, E., Fernández de Larrea, N., Pollán, M., Martín, V., Obón-Santacana, M., Guevara, M., Castaño-Vinyals, G., Canga, J. M., Pérez-Gómez, B., Gómez-Acebo, I., Fernández-Tardón, G., Vanaclocha-Espi, M., Olmedo-Requena, R., Alguacil, J., Chirlaque, M. D., Kogevinas, M., Aragonés, N., & Castelló, A., on behalf of the MCC-Spain Researchers. (2022). Meat Intake, Cooking Methods, Doneness Preferences and Risk of Gastric Adenocarcinoma in the MCC-Spain Study. Nutrients, 14(22), 4852. https://doi.org/10.3390/nu14224852





