Mapping the Literature on Diet and Multiple Sclerosis: A Data-Driven Approach
Highlights
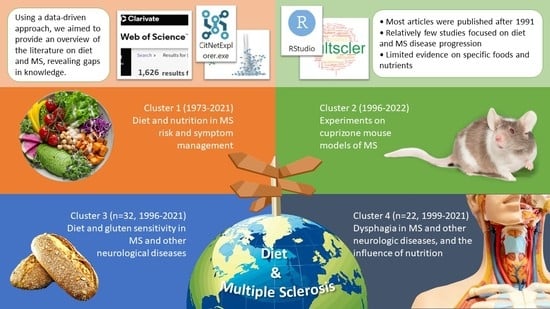
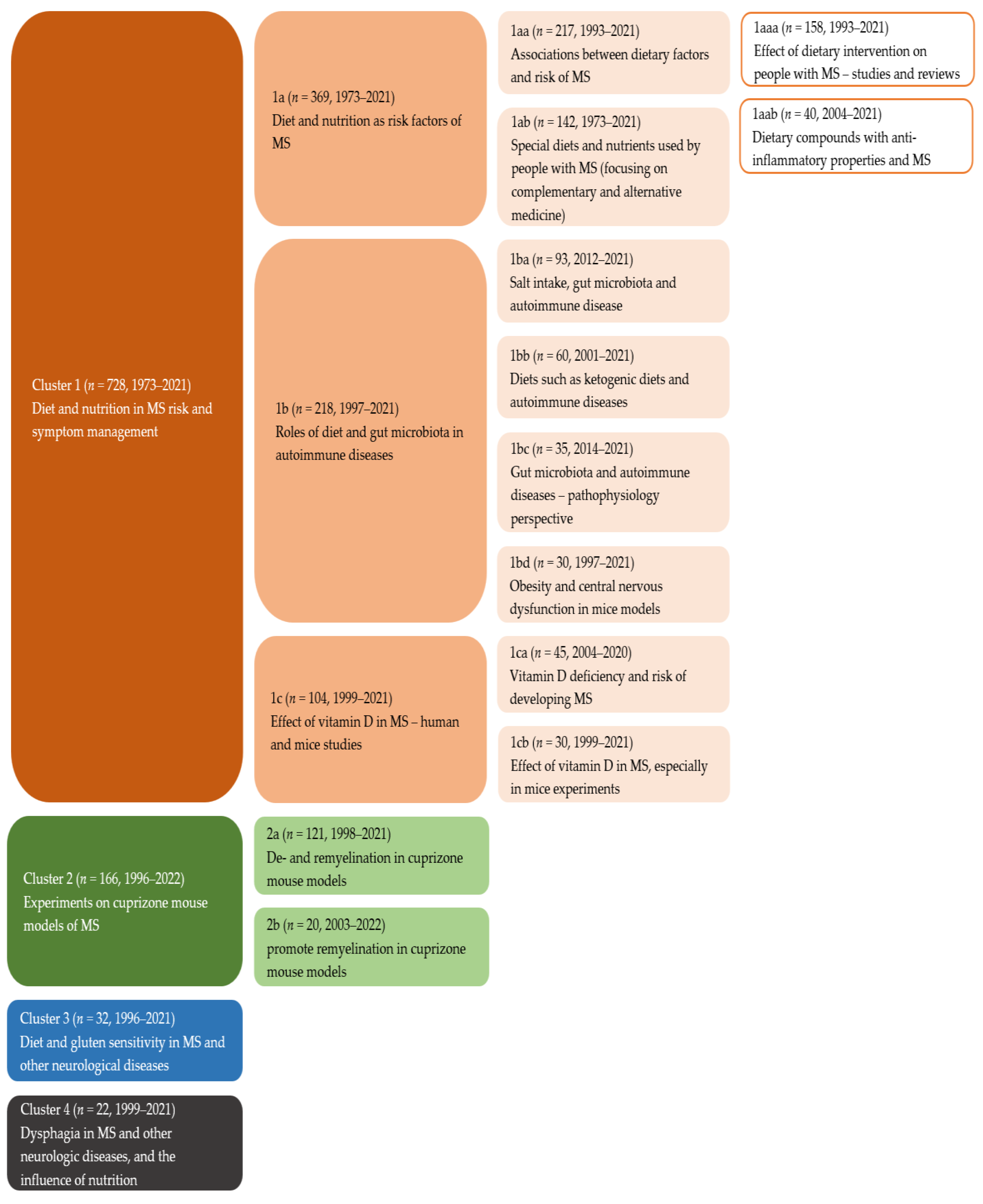
- Four main clusters were identified in publications on diet and multiple sclerosis (MS): MS risk and symptom management, mouse models of MS, gluten sensitivity, and dysphagia.
- Most studies focused on MS risk and symptoms, with limited exploration of the disease’s longitudinal progression over extended periods.
- Limited evidence was available for many foods and nutrients, such as antioxidants and anti-inflammatory foods, in relation to MS.
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
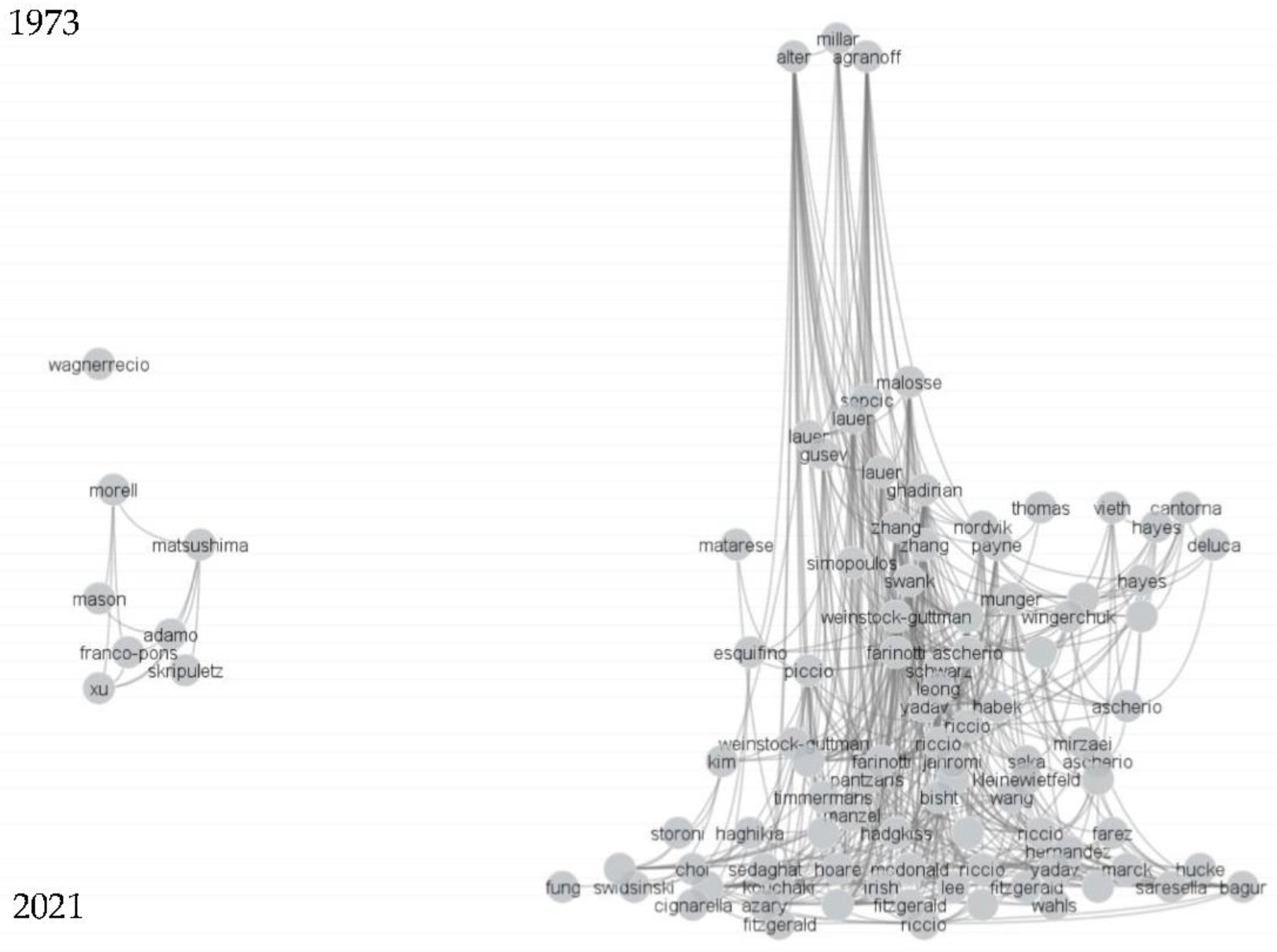
2.2. Citation Network Analysis and Clustering
2.3. Cluster Characteristics
3. Results
3.1. Literature Clustering
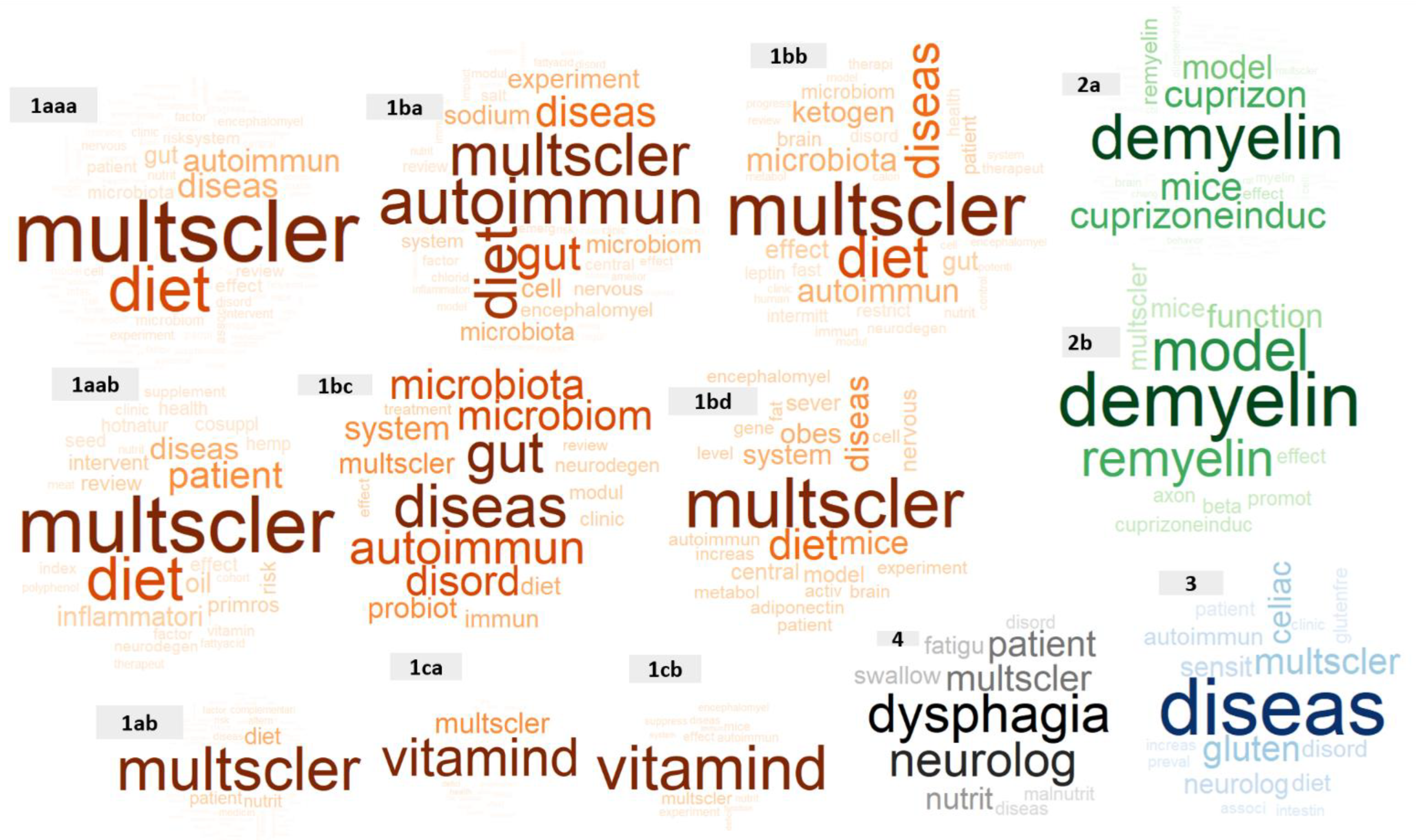
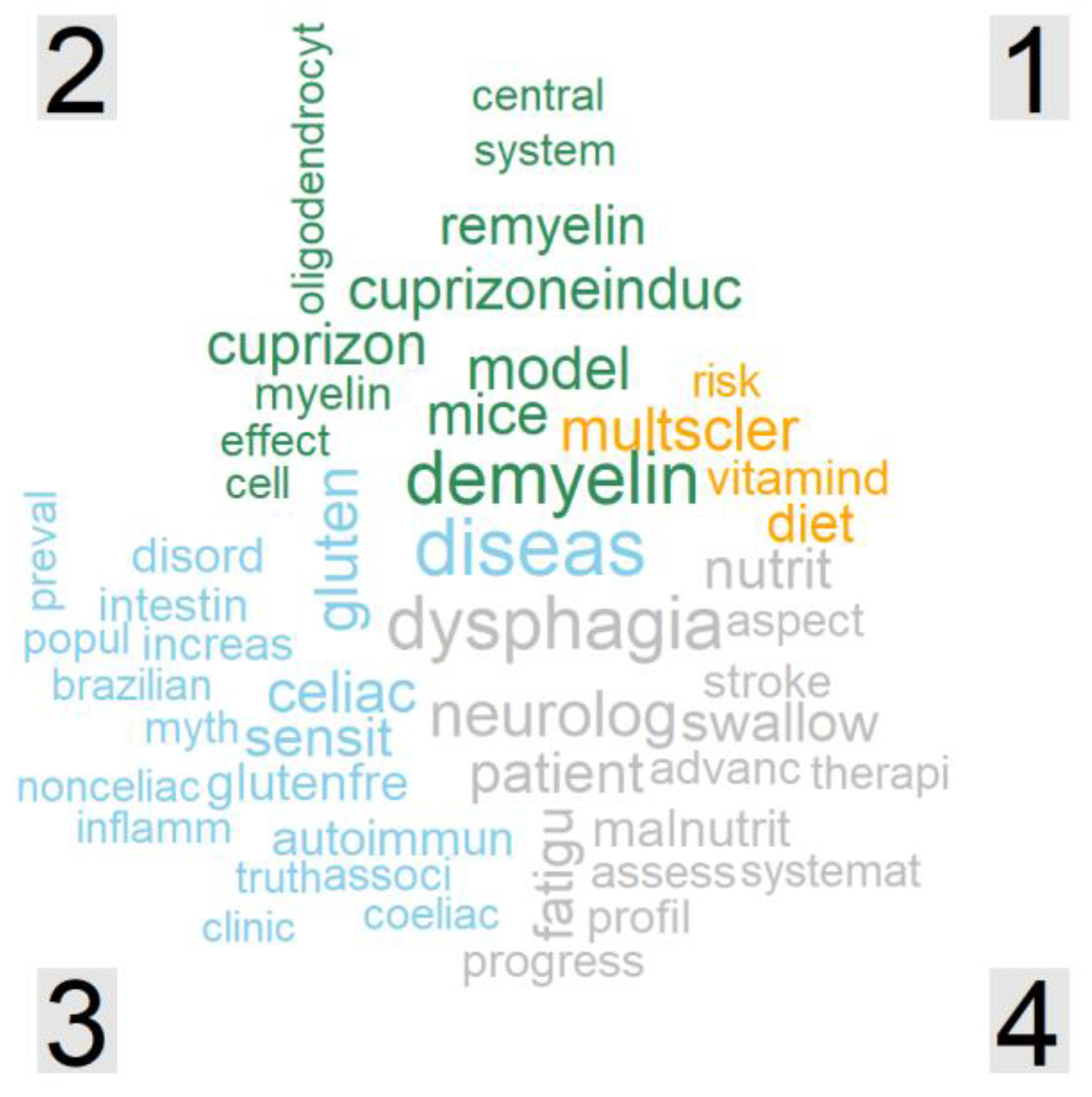
3.2. Frequent Words within Titles
3.3. Unique Words in Each Cluster
3.4. Topic Analysis
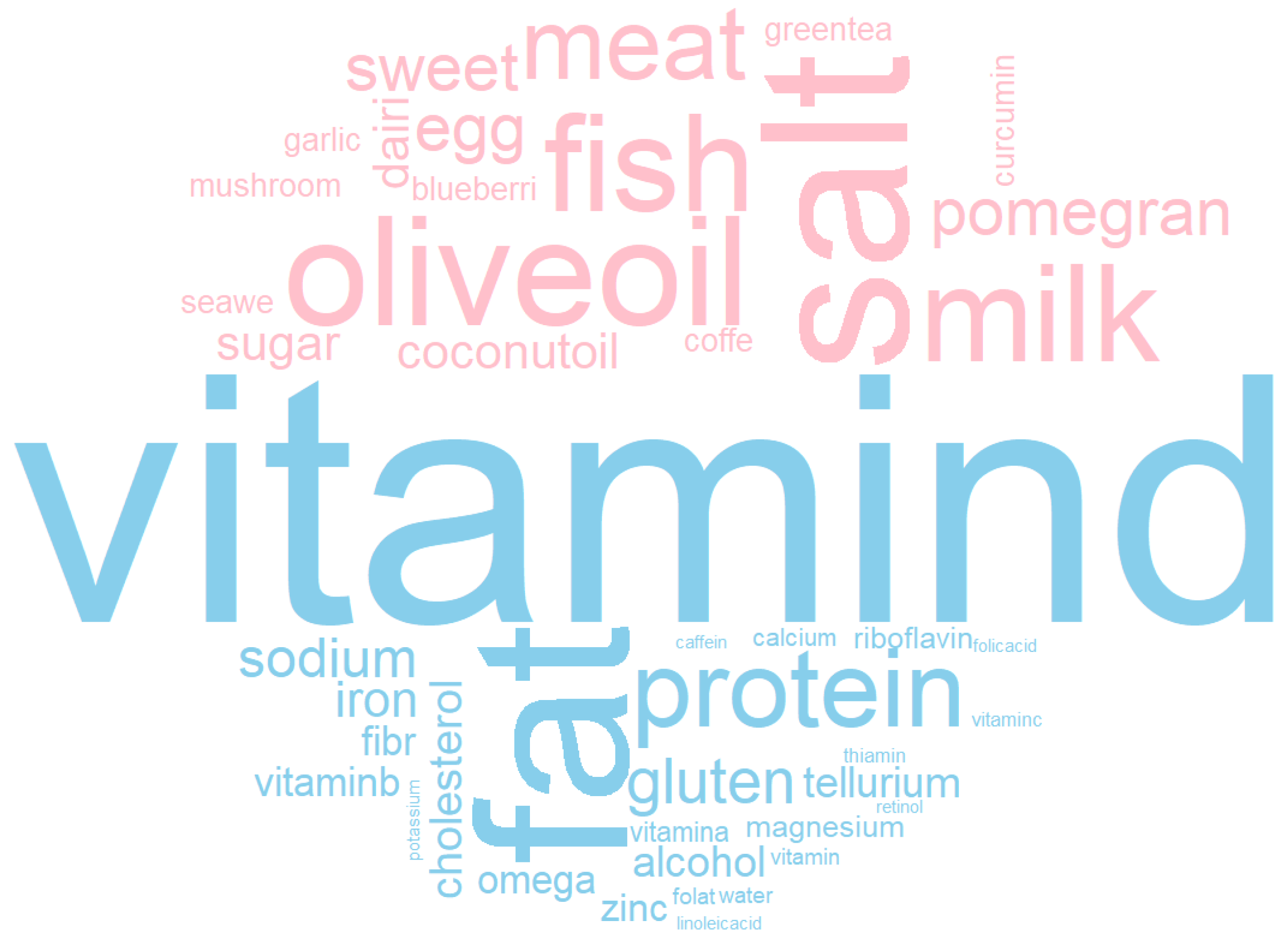
3.5. Most Studied Foods and Nutrients
3.6. Omitted Articles
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reich, D.S.; Lucchinetti, C.F.; Calabresi, P.A. Multiple Sclerosis. N. Engl. J. Med. 2018, 378, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Katz Sand, I. Classification, diagnosis, and differential diagnosis of multiple sclerosis. Curr. Opin. Neurol. 2015, 28, 193–205. [Google Scholar] [CrossRef]
- Bermel, R.A.; Rae-Grant, A.D.; Fox, R.J. Diagnosing multiple sclerosis at a later age: More than just progressive myelop-athy. Mult. Scler. 2010, 16, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Sand, I.K. The Role of Diet in Multiple Sclerosis: Mechanistic Connections and Current Evidence. Curr. Nutr. Rep. 2018, 7, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.; Martin, C.; Sherriff, J.; Mori, T.A.; Pereira, G.; Lucas, R.M.; Ponsonby, A.-L.; Taylor, B.; van der Mei, I.; Black, L.J. Omega-3 Index, fish consumption, use of fish oil supplements and first clinical diagnosis of central nervous system demyelination. Mult. Scler. Relat. Disord. 2021, 55, 103210. [Google Scholar] [CrossRef]
- Simpson-Yap, S.; Oddy, W.H.; Taylor, B.; Lucas, R.M.; Black, L.J.; Ponsonby, A.-L.; Blizzard, L.; van der Mei, I.; Dear, K.; Dwyer, T.; et al. High Prudent diet factor score predicts lower relapse hazard in early multiple sclerosis. Mult. Scler. 2020, 27, 1112–1124. [Google Scholar] [CrossRef]
- Beckett, J.M.; Bird, M.-L.; Pittaway, J.K.; Ahuja, K.D. Diet and Multiple Sclerosis: Scoping Review of Web-Based Recommendations. Interact. J. Med. Res. 2019, 8, e10050. [Google Scholar] [CrossRef]
- Riccio, P.; Rossano, R. Diet, Gut Microbiota, and Vitamins D + A in Multiple Sclerosis. Neurotherapeutics 2018, 15, 75–91. [Google Scholar] [CrossRef]
- Miller, E.D.; Dziedzic, A.; Saluk-Bijak, J.; Bijak, M. A Review of Various Antioxidant Compounds and their Potential Utility as Complementary Therapy in Multiple Sclerosis. Nutrients 2019, 11, 1528. [Google Scholar] [CrossRef]
- Walsh, E.I.; Cherbuin, N. Mapping the literature on nutritional interventions in cognitive health: A data-driven approach. Nutrients 2019, 11, 38. [Google Scholar] [CrossRef]
- Mehta, L.R.; Dworkin, R.H.; Schwid, S.R. Polyunsaturated fatty acids and their potential therapeutic role in multiple sclerosis. Nat. Clin. Prac. Neurol. 2009, 5, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Parks, N.E.; Jackson-Tarlton, C.S.; Vacchi, L.; Merdad, R.; Johnston, B.C. Dietary interventions for multiple sclerosis-related outcomes. Cochrane Database Syst. Rev. 2020, 5, CD004192. [Google Scholar] [CrossRef] [PubMed]
- Tredinnick, A.R.; Probst, Y.C. Evaluating the Effects of Dietary Interventions on Disease Progression and Symptoms of Adults with Multiple Sclerosis: An Umbrella Review. Adv. Nutr. 2020, 11, 1603–1615. [Google Scholar] [CrossRef] [PubMed]
- Tarozzi, A.; Angeloni, C.; Malaguti, M.; Morroni, F.; Hrelia, S.; Hrelia, P. Sulforaphane as a Potential Protective Phytochemical against Neurodegenerative Diseases. Oxidative Med. Cell Longev. 2013, 2013, 415078. [Google Scholar] [CrossRef] [PubMed]
- van Eck, N.J.; Waltman, L. Citation-based clustering of publications using CitNetExplorer and VOSviewer. Scientometrics 2017, 111, 1053–1070. [Google Scholar] [CrossRef] [PubMed]
- Waltman, L.; van Eck, N.J. A new methodology for constructing a publication-level classification system of science. J. Soc. Inf. Sci. Technol. 2012, 63, 2378–2392. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2021. Available online: https://www.R-project.org/ (accessed on 5 October 2022).
- Morell, P.; Barrett, C.V.; Mason, J.L.; Toews, A.D.; Hostettler, J.D.; Knapp, G.W.; Matsushima, G.K. Gene expression in brain during cuprizone-induced demyelination and remyelination. Mol. Cell Neurosci. 1998, 12, 220–227. [Google Scholar] [CrossRef]
- Liebetanz, D.; Merkler, D. Effects of commissural de- and remyelination on motor skill behaviour in the cuprizone mouse model of multiple sclerosis. Exp. Neurol. 2006, 202, 217–224. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, J.; Ma, W.; Zhang, W.; Xie, Y.; Chen, X.; Zhu, J.; Liu, Y.; Qin, X.; Lin, Y. The remyelination effect of DNA framework nucleic acids on demyelinating diseases. Appl. Mater. Today 2021, 24, 101098. [Google Scholar] [CrossRef]
- Jeyasingham, M.D.; Rooprai, H.K.; Dexter, D.; Pratt, O.E.; Komoly, S. Zinc supplementation does not prevent cuprizone toxicity in the brain of mice. Neurosci. Res. Comm 1998, 22, 181–187. [Google Scholar] [CrossRef]
- Pun, T.W.; Odrobina, E.; Xu, Q.G.; Lam, T.Y.; Munro, C.A.; Midha, R.; Stanisz, G.J. Histological and magnetic resonance analysis of sciatic nerves in the tellurium model of neuropathy. J. Peripher. Nerv. Syst. 2005, 10, 38–46. [Google Scholar] [CrossRef]
- Goodrum, J.F. Role of organotellurium species in tellurium neuropathy. Neurochem. Res. 1998, 23, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Salvati, S.; Sanchez, M.; Campeggi, L.M.; Suchanek, G.; Breitschop, H.; Lassmann, H. Accelerated myelinogenesis by dietary lipids in rat brain. J. Neurochem. 1996, 67, 1744–1750. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, G.K.; Morell, P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 2001, 11, 107–116. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Z.G.; Lu, M.; Wang, X.; Shang, X.; Elias, S.B.; Chopp, M. MiR-146a promotes remyelination in a cuprizone model of demyelinating injury. Neuroscience 2017, 348, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Duncan, I.D.; Brower, A.; Kondo, Y.; Curlee, J.F., Jr.; Schultz, R.D. Extensive remyelination of the CNS leads to functional recovery. Proc. Natl. Acad. Sci. USA 2009, 106, 6832–6836. [Google Scholar] [CrossRef]
- Crawford, D.K.; Mangiardi, M.; Xia, X.; López-Valdés, H.E.; Tiwari-Woodruff, S.K. Functional recovery of callosal axons following demyelination: A critical window. Neuroscience 2009, 164, 1407–1421. [Google Scholar] [CrossRef]
- Hadjivassiliou, M.; Gibson, A.; Davies-Jones, G.A.; Lobo, A.J.; Stephenson, T.J.; Milford-Ward, A. Does cryptic gluten sensitivity play a part in neurological illness? Lancet 1996, 347, 369–371. [Google Scholar] [CrossRef]
- Shor, D.B.; Barzilai, O.; Ram, M.; Izhaky, D.; Porat-Katz, B.S.; Chapman, J.; Blank, M.; Anaya, J.M.; Shoenfeld, Y. Gluten sensitivity in multiple sclerosis: Experimental myth or clinical truth? Ann. N. Y. Acad. Sci. 2009, 1173, 343–349. [Google Scholar] [CrossRef]
- Thomsen, H.L.; Jessen, E.B.; Passali, M.; Frederiksen, J.L. The role of gluten in multiple sclerosis: A systematic review. Mult Scler Relat. Disord. 2019, 27, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Passali, M.; Josefsen, K.; Frederiksen, J.L.; Antvorskov, J.C. Current Evidence on the Efficacy of Gluten-Free Diets in Multiple Sclerosis, Psoriasis, Type 1 Diabetes and Autoimmune Thyroid Diseases. Nutrients 2020, 12, 2316. [Google Scholar] [CrossRef] [PubMed]
- Calcagno, P.; Ruoppolo, G.; Grasso, M.G.; De Vincentiis, M.; Paolucci, S. Dysphagia in multiple sclerosis—Prevalence and prognostic factors. Acta Neurol. Scand. 2002, 105, 40–43. [Google Scholar] [CrossRef]
- Solaro, C.; Cuccaro, A.; Gamberini, G.; Patti, F.; D’Amico, E.; Bergamaschi, R.; Berra, E.; Giusti, A.; Rezzani, C.; Messmer Uccelli, M.; et al. Prevalence of dysphagia in a consecutive cohort of subjects with MS using fibre-optic endoscopy. Neurol. Sci. 2020, 41, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Keage, M.; Delatycki, M.; Corben, L.; Vogel, A. A systematic review of self-reported swallowing assessments in progressive neurological disorders. Dysphagia 2015, 30, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Re, G.L.; Terranova, M.C.; Vernuccio, F.; Calafiore, C.; Picone, D.; Tudisca, C.; Salerno, S.; Lagalla, R. Swallowing impairment in neurologic disorders: The role of videofluorographic swallowing study. Pol. J. Radiol. 2018, 83, e394–e400. [Google Scholar] [CrossRef]
- Tarameshlu, M.; Ghelichi, L.; Azimi, A.R.; Ansari, N.N.; Khatoonabadi, A.R. The effect of traditional dysphagia therapy on the swallowing function in patients with Multiple Sclerosis: A pilot double-blinded randomized controlled trial. J. Bodyw. Mov. 2019, 23, 171–176. [Google Scholar] [CrossRef]
- Munger, K.L.; Zhang, S.M.; O’Reilly, E.; Hernán, M.A.; Olek, M.J.; Willett, W.C.; Ascherio, A. Vitamin D intake and incidence of multiple sclerosis. Neurology 2004, 62, 60–65. [Google Scholar] [CrossRef]
- Mirzaei, F.; Michels, K.B.; Munger, K.; O’Reilly, E.; Chitnis, T.; Forman, M.R.; Giovannucci, E.; Rosner, B.; Ascherio, A. Gestational vitamin D and the risk of multiple sclerosis in offspring. Ann. Neurol. 2011, 70, 30–40. [Google Scholar] [CrossRef]
- Sharif, K.; Watad, A.; Bragazzi, N.L.; Adawi, M.; Amital, H.; Shoenfeld, Y. Coffee and autoimmunity: More than a mere hot beverage! Autoimmun. Rev. 2017, 16, 712–721. [Google Scholar] [CrossRef]
- Barbalace, M.C.; Malaguti, M.; Giusti, L.; Lucacchini, A.; Hrelia, S.; Angeloni, C. Anti-Inflammatory Activities of Marine Algae in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 3061. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Valado, A. The Seaweed Diet in Prevention and Treatment of the Neurodegenerative Diseases. Mar. Drugs 2021, 19, 128. [Google Scholar] [CrossRef] [PubMed]
- Swank, R.L. Treatment of multiple sclerosis with low-fat diet results of five and one-half years’ experience. AMA Arch. Neurol. Psychiatry 1955, 73, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Swank, R.L. Multiple sclerosis: Twenty years on low fat diet. Arch. Neurol. 1970, 23, 460–474. [Google Scholar] [CrossRef]
- Swank, R.L.; Goodwin, J. Review of MS patient survival on a Swank low saturated fat diet. Nutrition 2003, 19, 161–162. [Google Scholar] [CrossRef]
- Swank, R.L. Multiple sclerosis: Fat-oil relationship. Nutrition 1991, 7, 368–376. [Google Scholar]
- Wahls, T.; Scott, M.O.; Alshare, Z.; Rubenstein, L.; Darling, W.; Carr, L.; Smith, K.; Chenard, C.A.; LaRocca, N.; Snetselaar, L. Dietary approaches to treat MS-related fatigue: Comparing the modified Paleolithc (Wahls Elimination) and low saturated fat (Swank) diets on perceived fatigue in persons with relapsing-remitting multiple sclerosis: Study protocol for a randomized controlled trial. Trials 2018, 19, 309. [Google Scholar] [CrossRef]
- Wahls, T.L.; Titcomb, T.J.; Bisht, B.; Eyck, P.T.; Rubenstein, L.M.; Carr, L.J.; Darling, W.G.; Hoth, K.F.; Kamholz, J.; Snetselaar, L.G. Impact of the Swank and Wahls elimination dietary interventions on fatigue and quality of life in relapsing-remitting multiple sclerosis: The WAVES randomized parallel-arm clinical trial. Mult. Scler. J. Exp. Transl. Clin. 2021, 7, 205521732110353–20552173211035399. [Google Scholar] [CrossRef]
- Zhang, S.M.; Hernán, M.A.; Olek, M.J.; Spiegelman, D.; Willett, W.C.; Ascherio, A. Intakes of carotenoids, vitamin C, and vitamin E and MS risk among two large cohorts of women. Neurology 2001, 10, 75–80. [Google Scholar] [CrossRef]
- Pantzaris, M.C.; Loukaides, G.N.; Ntzani, E.E.; Patrikios, I.S. A novel oral nutraceutical formula of omega-3 and omega-6 fatty acids with vitamins (PLP10) in relapsing remitting multiple sclerosis: A randomised, double-blind, placebo-controlled proof-of-concept clinical trial. BMJ Open 2013, 17, e002170. [Google Scholar] [CrossRef]
- Mauriz, E.; Laliena, A.; Vallejo, D.; Tuñón, M.J.; Rodríguez-López, J.M.; Rodríguez-Pérez, R.; García-Fernández, M.C. Effects of a low-fat diet with antioxidant supplementation on biochemical markers of multiple sclerosis long-term care residents. Nutr. Hosp. 2013, 28, 2229–2235. [Google Scholar] [PubMed]
- Da Costa Silva, B.Y.; De Carvalho Sampaio, H.A.; Shivappa, N.; Hébert, J.; Silva Albuquerque, L.D.; Ferreira Carioca, A.A.; Costa D’Almeida, J.A.; Costa Maia, C.S.; Pereira De Melo, M.L. Interactions between dietary inflammatory index, nutritional state and Multiple Sclerosis clinical condition. Clin. Nutr. ESPEN 2018, 26, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Shinto, L.; Bourdette, D. Complementary and alternative medicine for the treatment of multiple sclerosis. Expert. Rev. Clin. Immunol. 2010, 6, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Libbey, J.E.; Sanchez, J.M.; Doty, D.J.; Sim, J.T.; Cusick, M.F.; Cox, J.E.; Fischer, K.F.; Round, J.L.; Fujinami, R.S. Variations in diet cause alterations in microbiota and metabolites that follow changes in disease severity in a multiple sclerosis model. Benef. Microbes. 2018, 9, 495–513. [Google Scholar] [CrossRef]
- Hucke, S.; Eschborn, M.; Liebmann, M.; Herold, M.; Freise, N.; Engbers, A.; Ehling, P.; Meuth, S.G.; Roth, J.; Kuhlmann, T.; et al. Sodium chloride promotes pro-inflammatory macrophage polarization thereby aggravating CNS autoimmunity. J. Autoimmun. 2016, 67, 90–101. [Google Scholar] [CrossRef]
- Bock, M.; Karber, M.; Kuhn, H. Ketogenic diets attenuate cyclooxygenase and lipoxygenase gene expression in multiple sclerosis. EBioMedicine 2018, 36, 293–303. [Google Scholar] [CrossRef]
- Morshedi, M.; Hashemi, R.; Moazzen, S.; Sahebkar, A.; Hosseinifard, E.S. Immunomodulatory and anti-inflammatory effects of probiotics in multiple sclerosis: A systematic review. J Neuroinflammation. 2019, 16, 231. [Google Scholar] [CrossRef]
- Langley, M.R.; Yoon, H.; Kim, H.N.; Choi, C.I.; Simon, W.; Kleppe, L.; Lanza, I.R.; LeBrasseur, N.K.; Matveyenko, A.; Scarisbrick, I.A. High fat diet consumption results in mitochondrial dysfunction, oxidative stress, and oligodendrocyte loss in the central nervous system. Biochim. Biophys. Acta Mol. Basis. Dis. 2020, 1866, 165630. [Google Scholar] [CrossRef]
- Wingerchuk, D.M.; Lesaux, J.; Rice, G.P.A.; Kremenchutzky, M.; Ebers, G.C. A pilot study of oral calcitriol (1,25-dihydroxyvitamin D3) for relapsing–remitting multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1294–1296. [Google Scholar] [CrossRef]
- DeLuca, H.F.; Plum, L.A. Vitamin D deficiency diminishes the severity and delays onset of experimental autoimmune encephalomyelitis. Arch. Biochem. Biophys. 2011, 513, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.J.; Wiles, C.M. Dysphagia and nutritional status in multiple sclerosis. J. Neurol. 1999, 246, 677–682. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, X.; Walsh, E.I.; Cherbuin, N.; Black, L.J. Mapping the Literature on Diet and Multiple Sclerosis: A Data-Driven Approach. Nutrients 2022, 14, 4820. https://doi.org/10.3390/nu14224820
Qu X, Walsh EI, Cherbuin N, Black LJ. Mapping the Literature on Diet and Multiple Sclerosis: A Data-Driven Approach. Nutrients. 2022; 14(22):4820. https://doi.org/10.3390/nu14224820
Chicago/Turabian StyleQu, Xiaochen, Erin I. Walsh, Nicolas Cherbuin, and Lucinda J. Black. 2022. "Mapping the Literature on Diet and Multiple Sclerosis: A Data-Driven Approach" Nutrients 14, no. 22: 4820. https://doi.org/10.3390/nu14224820
APA StyleQu, X., Walsh, E. I., Cherbuin, N., & Black, L. J. (2022). Mapping the Literature on Diet and Multiple Sclerosis: A Data-Driven Approach. Nutrients, 14(22), 4820. https://doi.org/10.3390/nu14224820