Variability in Skeletal Muscle Protein Synthesis Rates in Critically Ill Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Protocol
2.2.1. Fractional Synthesis Rates (FSRs)
2.2.2. Muscle Biopsies
2.2.3. Measurement of FSR
2.2.4. Muscle Morphology
2.3. Statistical Analyses
3. Results
3.1. Patient Characteristics
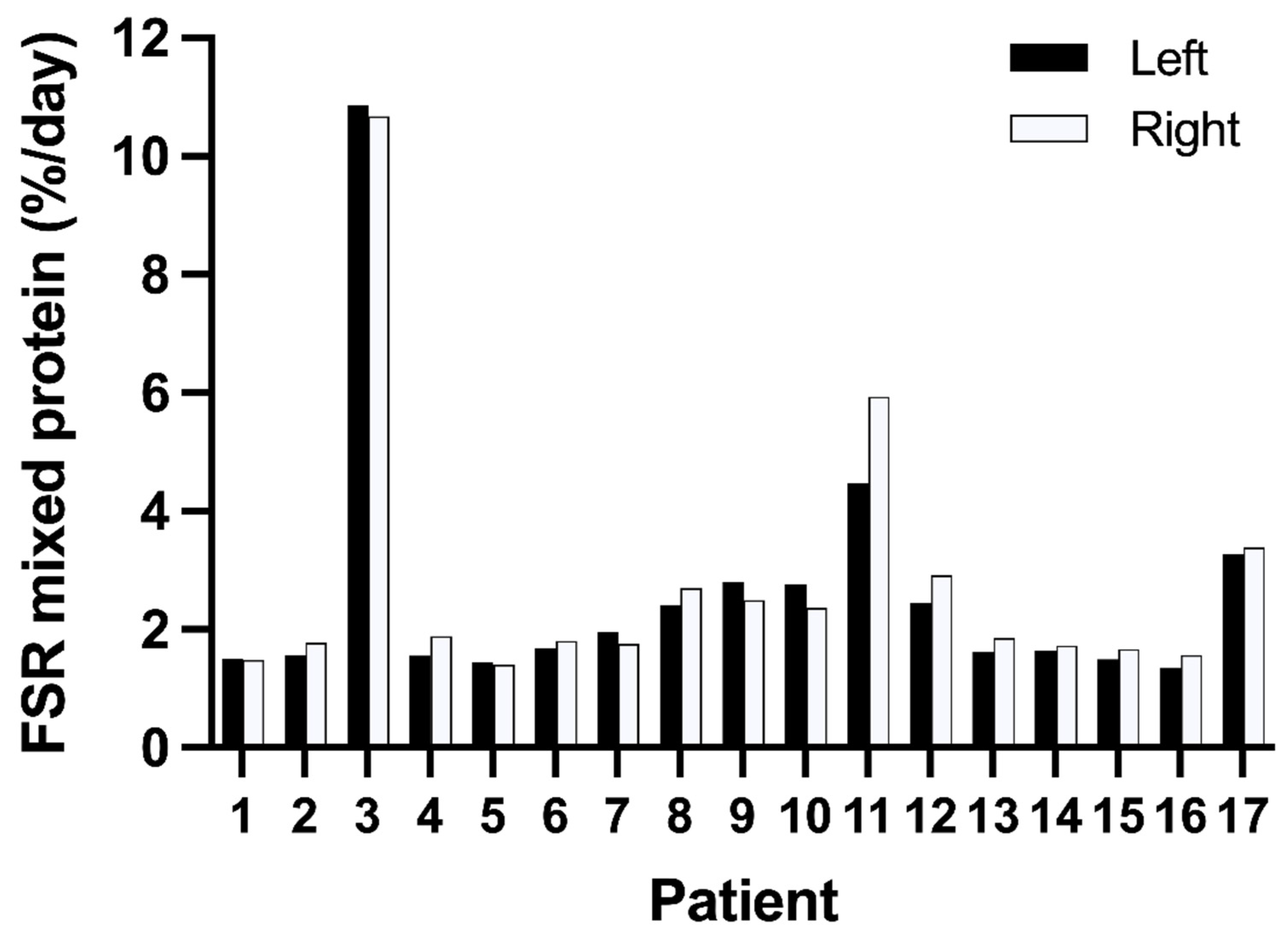
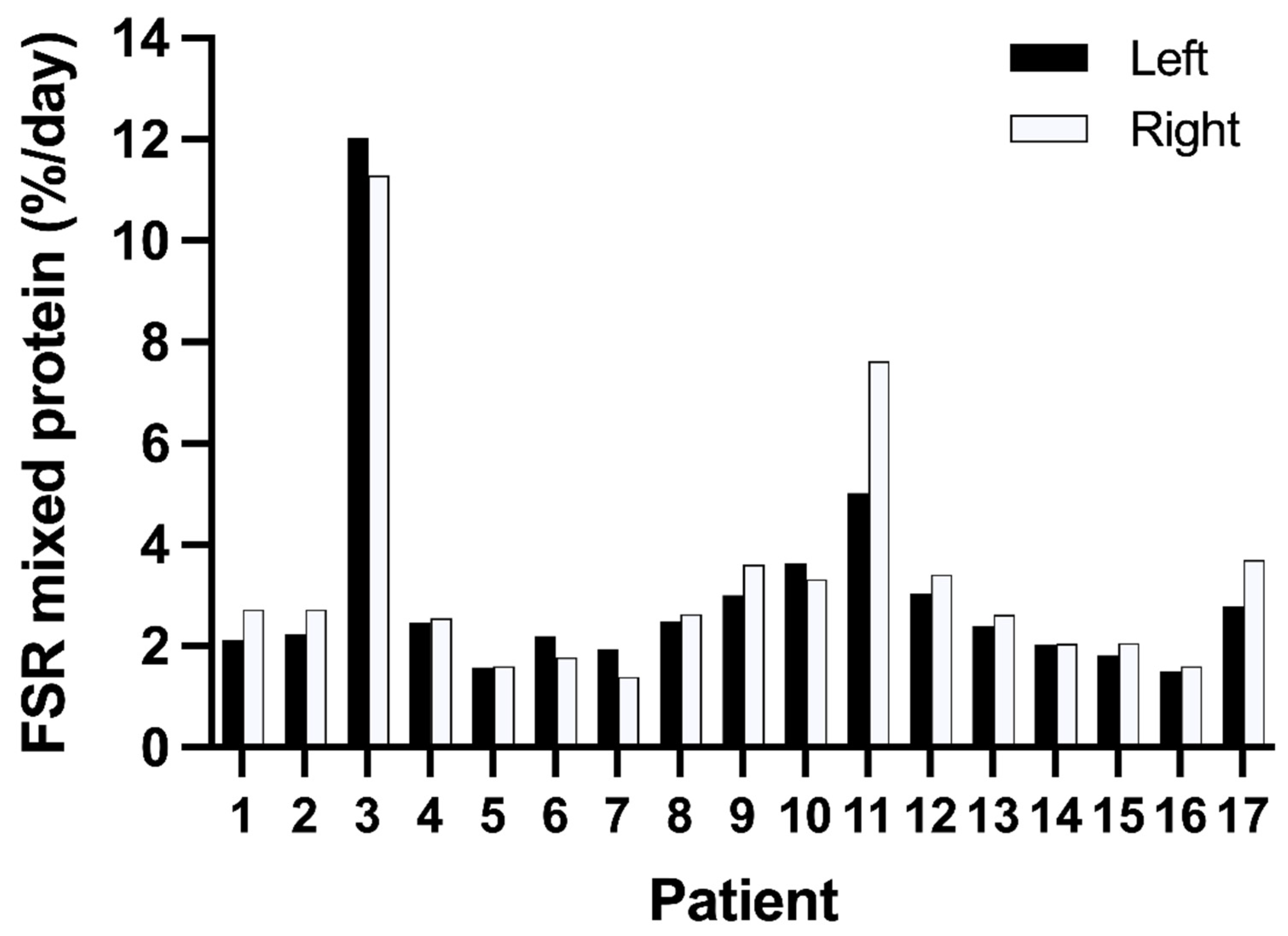
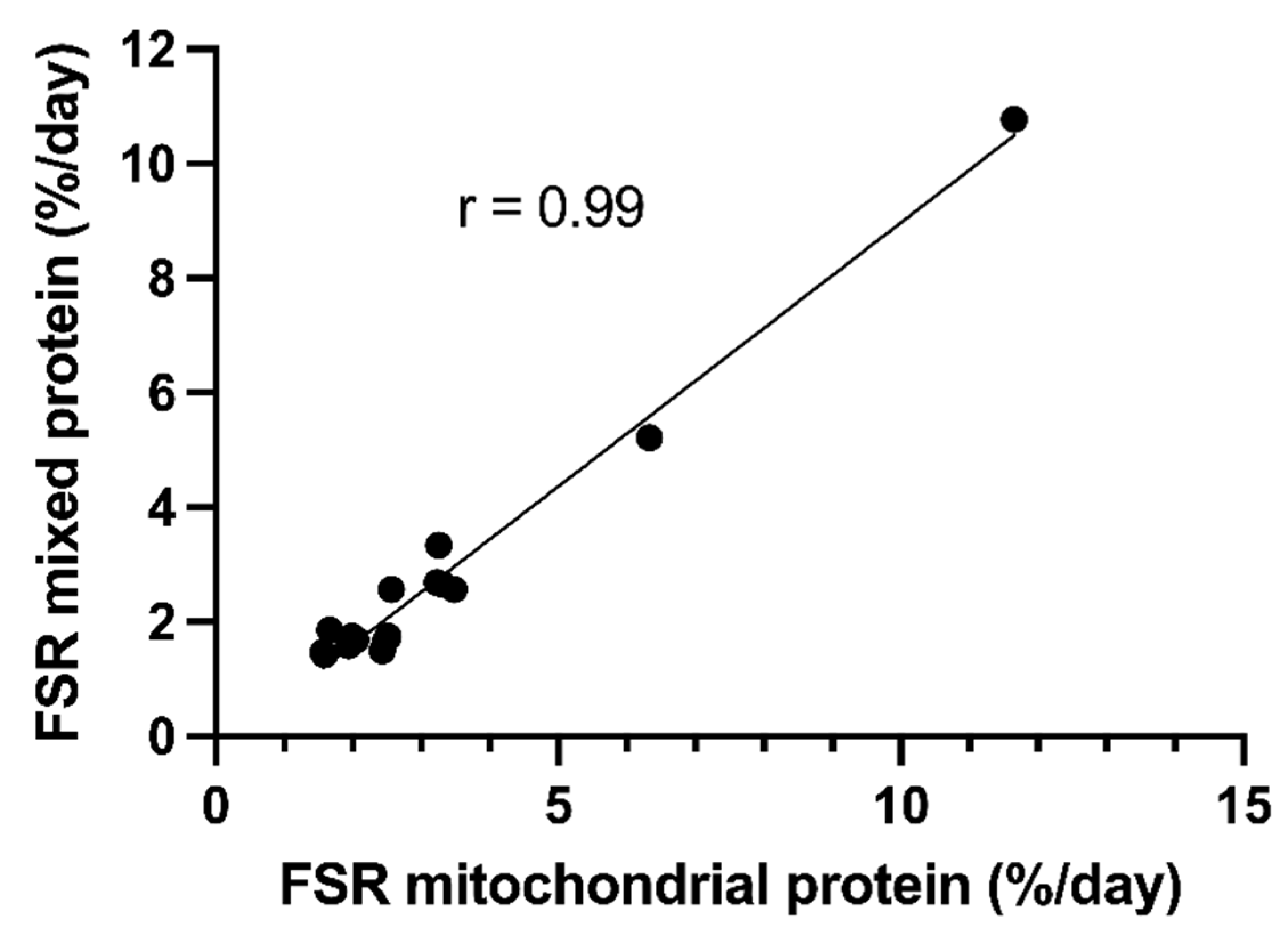
3.2. Variation in Muscle Protein Synthesis Rates
3.3. Morphology
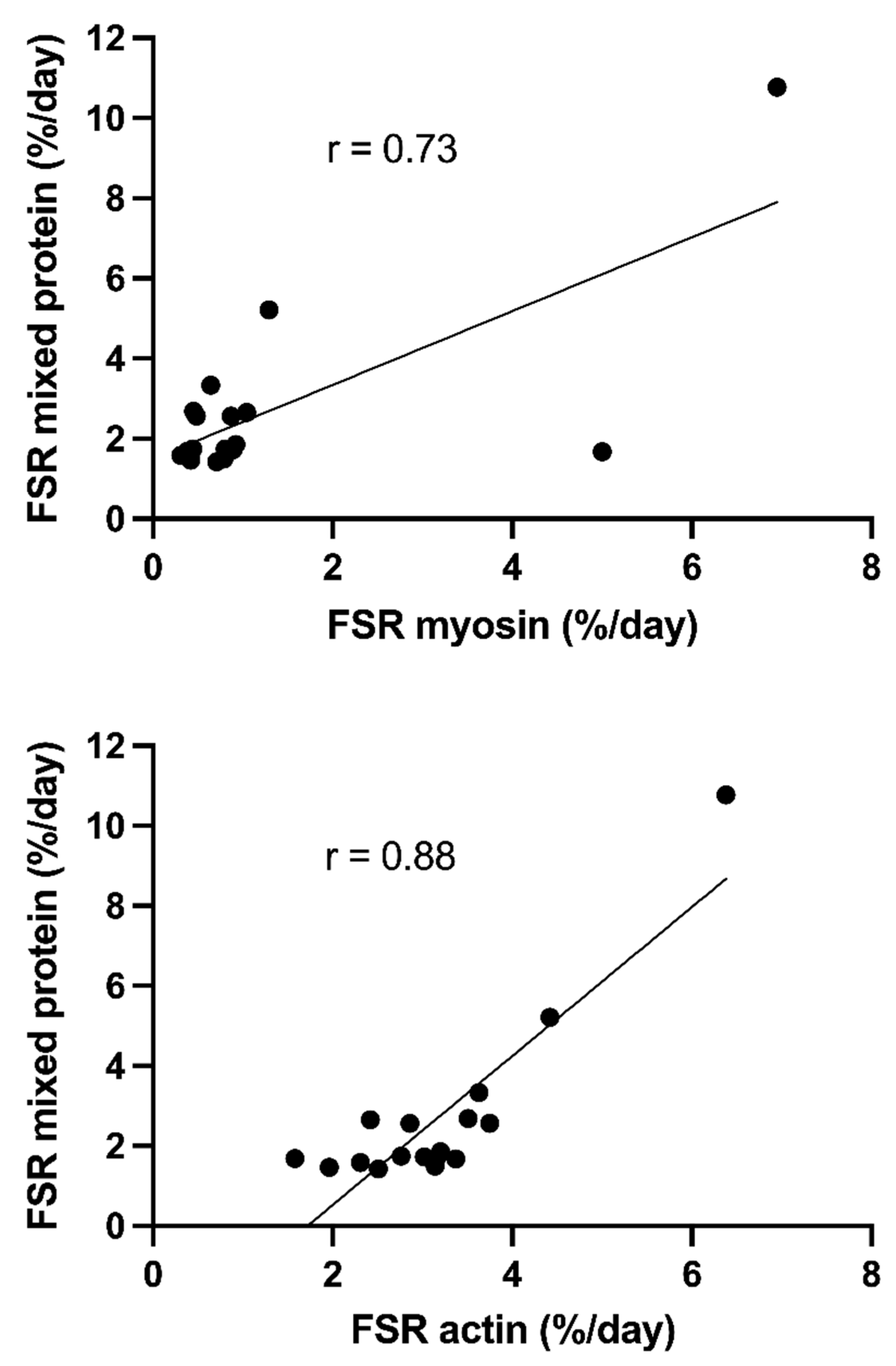
3.4. Synthesis Rates of Actin and Myosin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Puthucheary, Z.A.; Rawal, J.; McPhail, M.; Connolly, B.; Ratnayake, G.; Chan, P.; Hopkinson, N.S.; Phadke, R.; Dew, T.; Sidhu, P.S.; et al. Acute skeletal muscle wasting in critical illness. JAMA 2013, 310, 1591–1600. [Google Scholar] [CrossRef] [PubMed]
- Faisy, C.; Rabbat, A.; Kouchakji, B.; Laaban, J.P. Bioelectrical impedance analysis in estimating nutritional status and outcome of patients with chronic obstructive pulmonary disease and acute respiratory failure. Intensive Care Med. 2000, 26, 518–525. [Google Scholar] [CrossRef]
- Weijs, P.J.; Looijaard, W.G.; Dekker, I.M.; Stapel, S.N.; Girbes, A.R.; Oudemans-van Straaten, H.M.; Beishuizen, A. Low skeletal muscle area is a risk factor for mortality in mechanically ventilated critically ill patients. Crit. Care 2014, 18, R12. [Google Scholar] [CrossRef]
- Batt, J.; dos Santos, C.C.; Cameron, J.I.; Herridge, M.S. Intensive care unit-acquired weakness: Clinical phenotypes and molecular mechanisms. Am. J. Respir. Crit. Care Med. 2013, 187, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, C.; Hussain, S.N.; Mathur, S.; Picard, M.; Herridge, M.; Correa, J.; Bain, A.; Guo, Y.; Advani, A.; Advani, S.L.; et al. Mechanisms of Chronic Muscle Wasting and Dysfunction after an Intensive Care Unit Stay. A Pilot Study. Am. J. Respir. Crit. Care Med. 2016, 194, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Jolley, S.E.; Bunnell, A.E.; Hough, C.L. ICU-Acquired Weakness. Chest 2016, 150, 1129–1140. [Google Scholar] [CrossRef]
- Schefold, J.C.; Bierbrauer, J.; Weber-Carstens, S. Intensive care unit-acquired weakness (ICUAW) and muscle wasting in critically ill patients with severe sepsis and septic shock. J. Cachexia Sarcopenia Muscle 2010, 1, 147–157. [Google Scholar] [CrossRef]
- Berger, D.; Bloechlinger, S.; von Haehling, S.; Doehner, W.; Takala, J.; Z’Graggen, W.J.; Schefold, J.C. Dysfunction of respiratory muscles in critically ill patients on the intensive care unit. J. Cachexia Sarcopenia Muscle 2016, 7, 403–412. [Google Scholar] [CrossRef]
- Gamrin, L.; Essén, P.; Hultman, E.; McNurlan, M.A.; Garlick, P.J.; Wernerman, J. Protein-sparing effect in skeletal muscle of growth hormone treatment in critically ill patients. Ann. Surg. 2000, 231, 577–586. [Google Scholar] [CrossRef]
- Tjader, I.; Rooyackers, O.; Forsberg, A.M.; Vesali, R.F.; Garlick, P.J.; Wernerman, J. Effects on skeletal muscle of intravenous glutamine supplementation to ICU patients. Intensive Care Med. 2004, 30, 266–275. [Google Scholar] [CrossRef]
- Klaude, M.; Mori, M.; Tjader, I.; Gustafsson, T.; Wernerman, J.; Rooyackers, O. Protein metabolism and gene expression in skeletal muscle of critically ill patients with sepsis. Clin. Sci. 2012, 122, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Gamrin-Gripenberg, L.; Sundstrom-Rehal, M.; Olsson, D.; Grip, J.; Wernerman, J.; Rooyackers, O. An attenuated rate of leg muscle protein depletion and leg free amino acid efflux over time is seen in ICU long-stayers. Crit. Care 2018, 22, 13. [Google Scholar] [CrossRef]
- McNurlan, M.A.; Essén, P.; Heys, S.D.; Buchan, V.; Garlick, P.J.; Wernerman, J. Measurement of protein synthesis in human skeletal muscle: Further investigation of the flooding technique. Clin. Sci. 1991, 81, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Essén, P.; McNurlan, M.A.; Gamrin, L.; Hunter, K.; Calder, G.; Garlick, P.J.; Wernerman, J. Tissue protein synthesis rates in critically ill patients. Crit. Care Med. 1998, 26, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, K.; Tjader, I.; Keller, P.; Petrovic, N.; Ahlman, B.; Scheele, C.; Wernerman, J.; Timmons, J.A.; Rooyackers, O. Dysregulation of mitochondrial dynamics and the muscle transcriptome in ICU patients suffering from sepsis induced multiple organ failure. PLoS ONE 2008, 3, e3686. [Google Scholar] [CrossRef]
- Klaude, M.; Fredriksson, K.; Tjader, I.; Hammarqvist, F.; Ahlman, B.; Rooyackers, O.; Wernerman, J. Proteasome proteolytic activity in skeletal muscle is increased in patients with sepsis. Clin. Sci. 2007, 112, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, J. Muscle electrolytes in man. Scand. J. Clin. Lab. Investig. 1962, 168, 11–13. [Google Scholar]
- Zangarelli, A.; Walrand, S.; Guillet, C.; Gachon, P.; Rousset, P.; Giraudet, C.; Picard, B.; Boirie, Y. Centrifugation-based isolation of myosin for measurement of its synthesis rate in small muscle samples. Anal. Biochem. 2004, 327, 55–60. [Google Scholar] [CrossRef]
- Calder, A.G.; Anderson, S.E.; Grant, I.; McNurlan, M.A.; Garlick, P.J. The determination of low d5-phenylalanine enrichment (0.002-0.09 atom perent excess), after conversion to phenylethylamine, in relation to protein turnover studies by gas chromatography/electron ionization mass spectrometry. Rap. Com. Mass Spec. 1992, 6, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Dahlberg, G. Statistical Methods for Medical and Biological Students; George Allen and Unwin: London, UK, 1940; pp. 122–132. [Google Scholar]
- Larsson, L.; Li, X.; Edstrom, L.; Eriksson, L.I.; Zackrisson, H.; Argentini, C.; Schiaffino, S. Acute quadriplegia and loss of muscle myosin in patients treated with nondepolarizing neuromuscular blocking agents and corticosteroids: Mechanisms at the cellular and molecular levels. Crit. Care Med. 2000, 28, 34–45. [Google Scholar] [CrossRef]
- Biolo, G.; Fleming, R.Y.; Maggi, S.P.; Nguyen, T.T.; Herndon, D.N.; Wolfe, R.R. Inverse regulation of protein turnover and amino acid transport in skeletal muscle of hypercatabolic patients. J. Clin. Endocrinol. Metab. 2002, 87, 3378–3384. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, J.G.; Nedergaard, A.; Reitelseder, S.; Mikkelsen, U.R.; Dideriksen, K.J.; Agergaard, J.; Kreiner, F.; Pott, F.C.; Schjerling, P.; Kjaer, M. Activated protein synthesis and suppressed protein breakdown signaling in skeletal muscle of critically ill patients. PLoS ONE 2011, 6, e18090. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gamrin, L.; Essen, P.; Hultman, E.; Hammarkvist, F.; McNurlan, M.A.; Garlick, P.J.; Werneman, J. Effects on skeletal muscle metabolism by glutamine and alfa-ketoglutarate supplementation in critically ill patients. Ph.D. Thesis, Karolinska Institutet, Solna, Sweden, 2000. [Google Scholar]
- Derde, S.; Hermans, G.; Derese, I.; Guiza, F.; Hedstrom, Y.; Wouters, P.J.; Bruyninckx, F.; D’Hoore, A.; Larsson, L.; Van den Berghe, G.; et al. Muscle atrophy and preferential loss of myosin in prolonged critically ill patients. Crit. Care Med. 2012, 40, 79–89. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tjäder, I.; Klaude, M.; Hssain, A.A.; Guillet, C.; Nennesmo, I.; Wernerman, J.; Rooyackers, O. Variability in Skeletal Muscle Protein Synthesis Rates in Critically Ill Patients. Nutrients 2022, 14, 3733. https://doi.org/10.3390/nu14183733
Tjäder I, Klaude M, Hssain AA, Guillet C, Nennesmo I, Wernerman J, Rooyackers O. Variability in Skeletal Muscle Protein Synthesis Rates in Critically Ill Patients. Nutrients. 2022; 14(18):3733. https://doi.org/10.3390/nu14183733
Chicago/Turabian StyleTjäder, Inga, Maria Klaude, Ali Ait Hssain, Christelle Guillet, Inger Nennesmo, Jan Wernerman, and Olav Rooyackers. 2022. "Variability in Skeletal Muscle Protein Synthesis Rates in Critically Ill Patients" Nutrients 14, no. 18: 3733. https://doi.org/10.3390/nu14183733
APA StyleTjäder, I., Klaude, M., Hssain, A. A., Guillet, C., Nennesmo, I., Wernerman, J., & Rooyackers, O. (2022). Variability in Skeletal Muscle Protein Synthesis Rates in Critically Ill Patients. Nutrients, 14(18), 3733. https://doi.org/10.3390/nu14183733





