Nutraceutical Approaches to Dyslipidaemia: The Main Formulative Issues Preventing Efficacy
Abstract
1. Introduction
2. Materials and Methods
3. Results
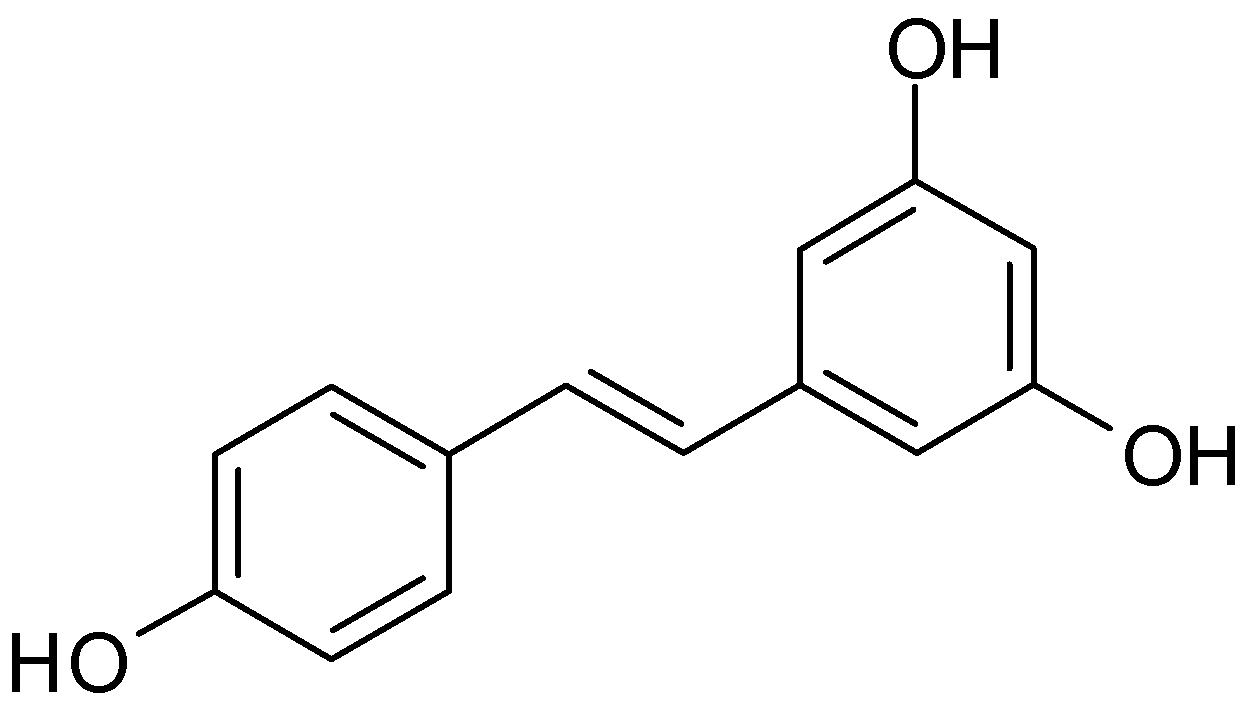
3.1. Trans-Resveratrol
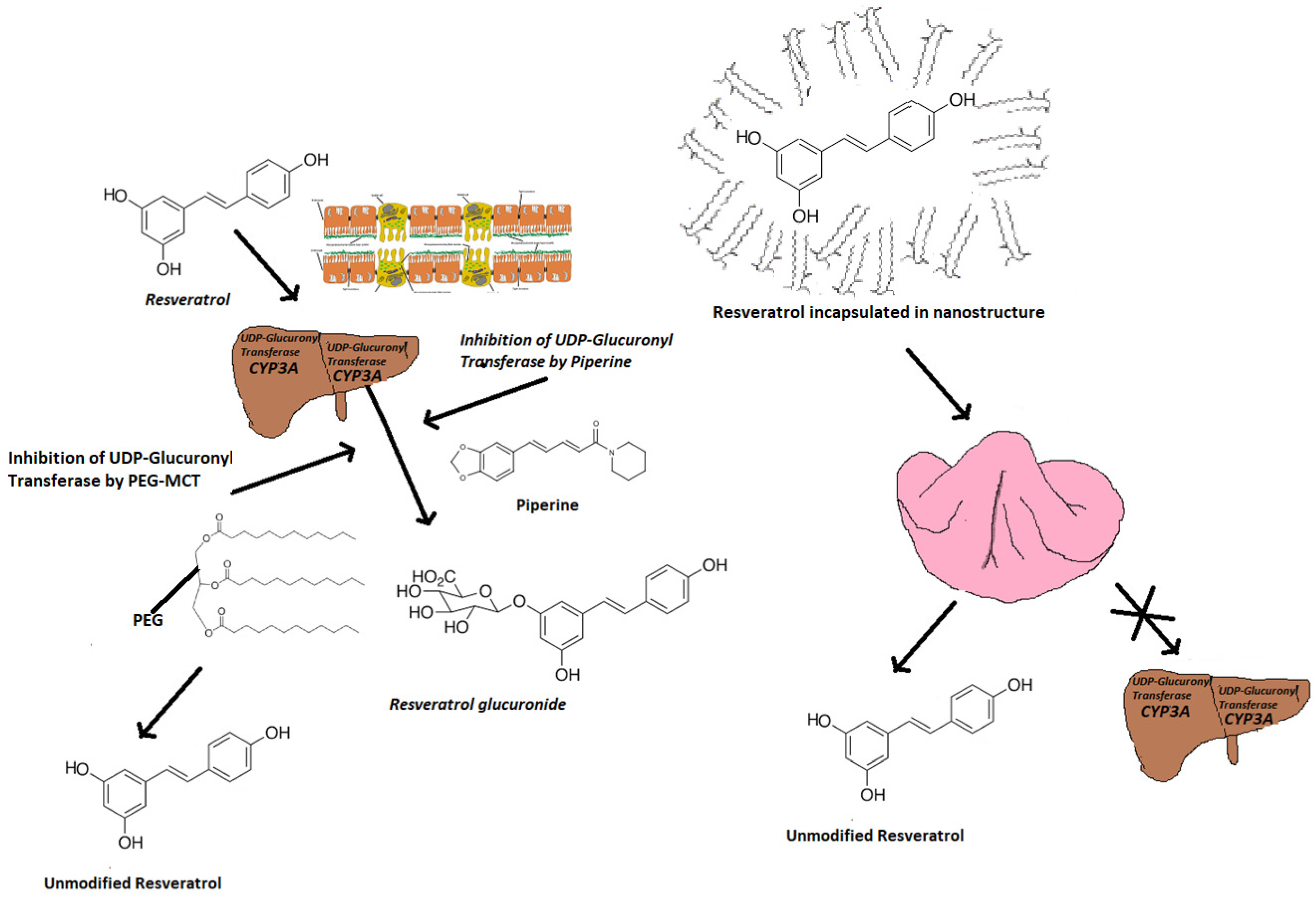
3.1.1. Bioavailability
3.1.2. Bionutraceutical Strategies to Improve Bioavailability of t-Res
3.1.3. Pharmacodynamics
3.1.4. Clinically Proven Effects
3.1.5. Safety Profile
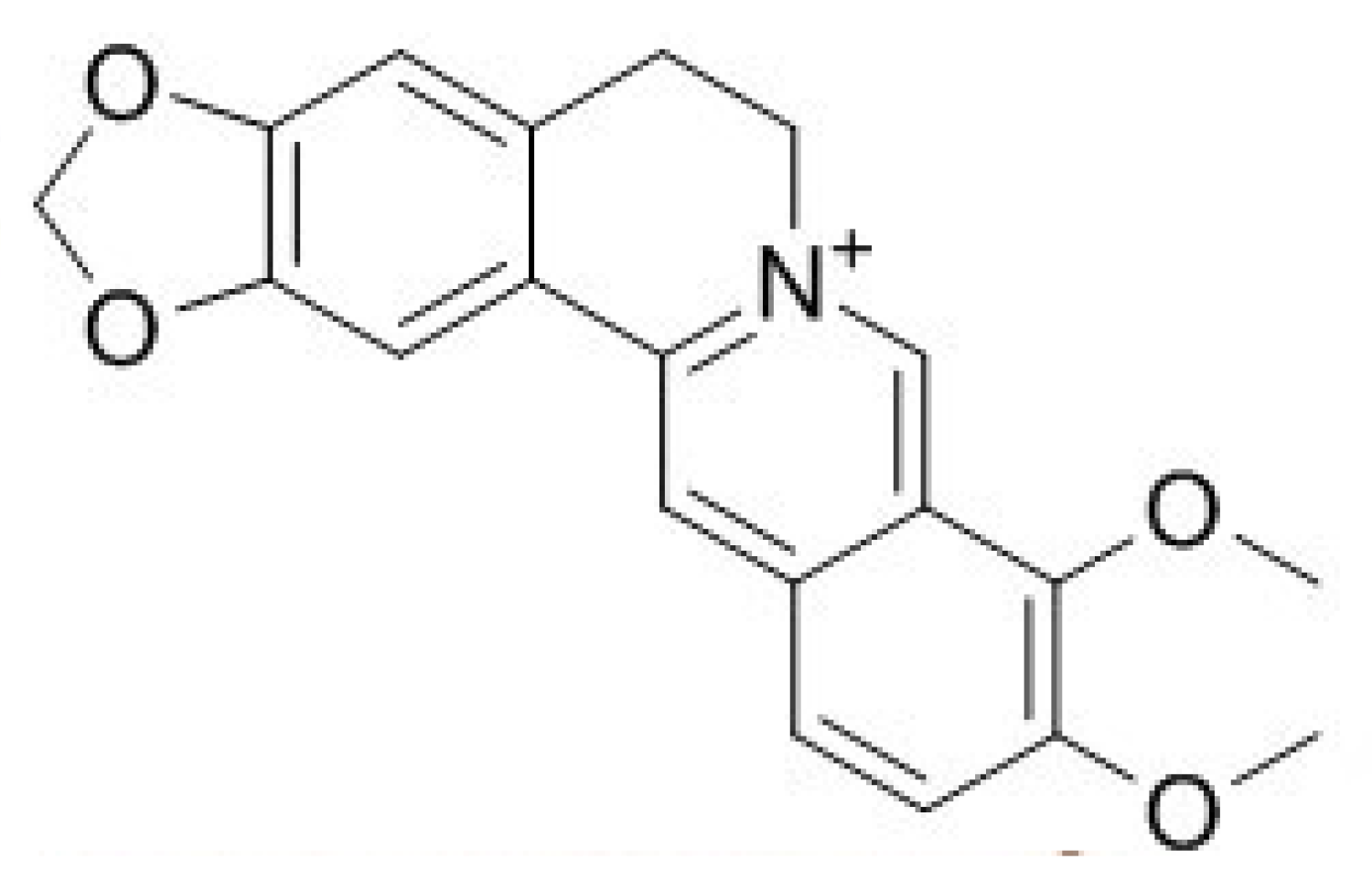
3.2. Berberine
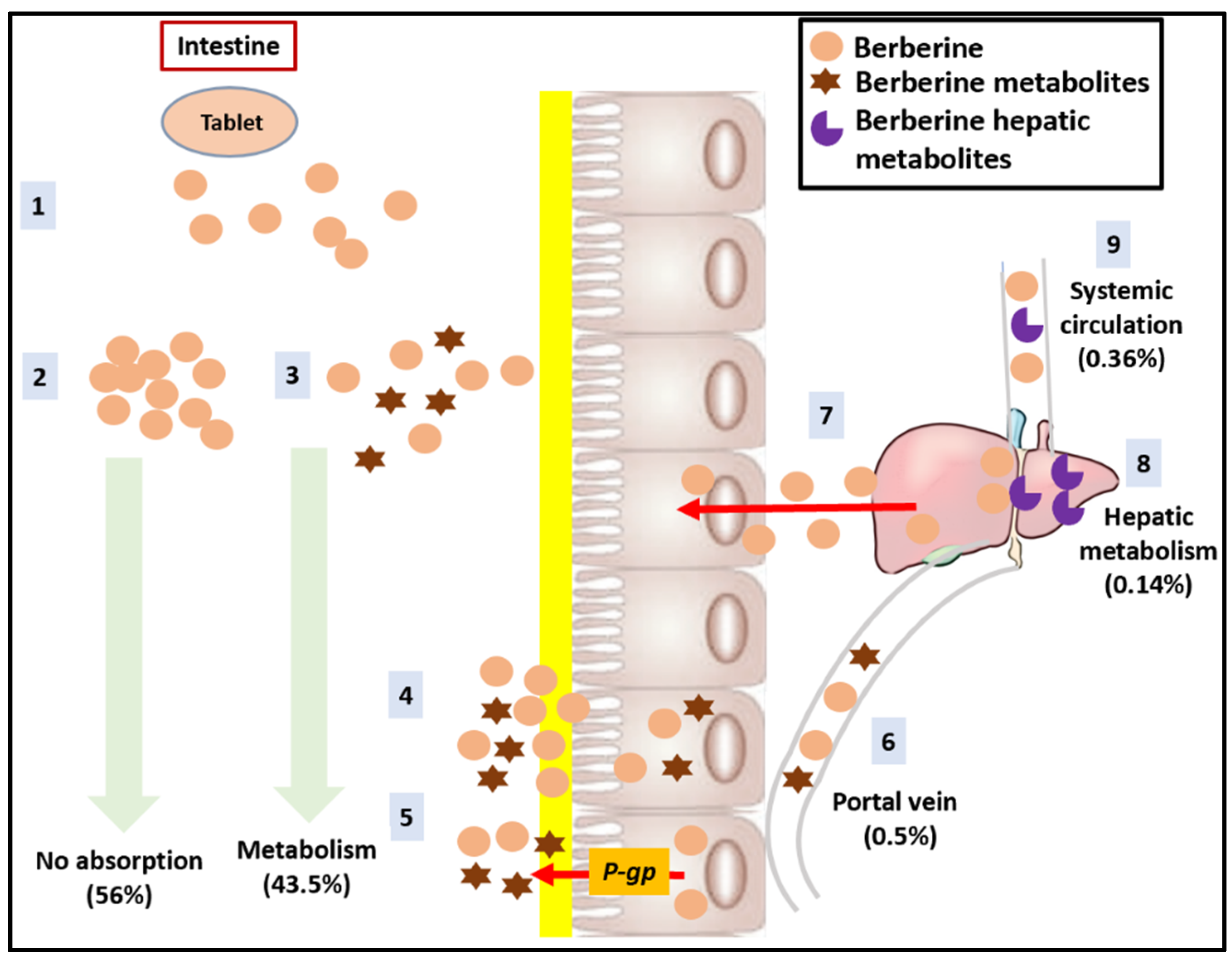
3.2.1. Bioavailability
3.2.2. Biopharmaceutical Strategies to Improve Bioavailability of BBR
3.2.3. Pharmacodynamics
3.2.4. Clinically Proven Effects
3.2.5. Safety Profile
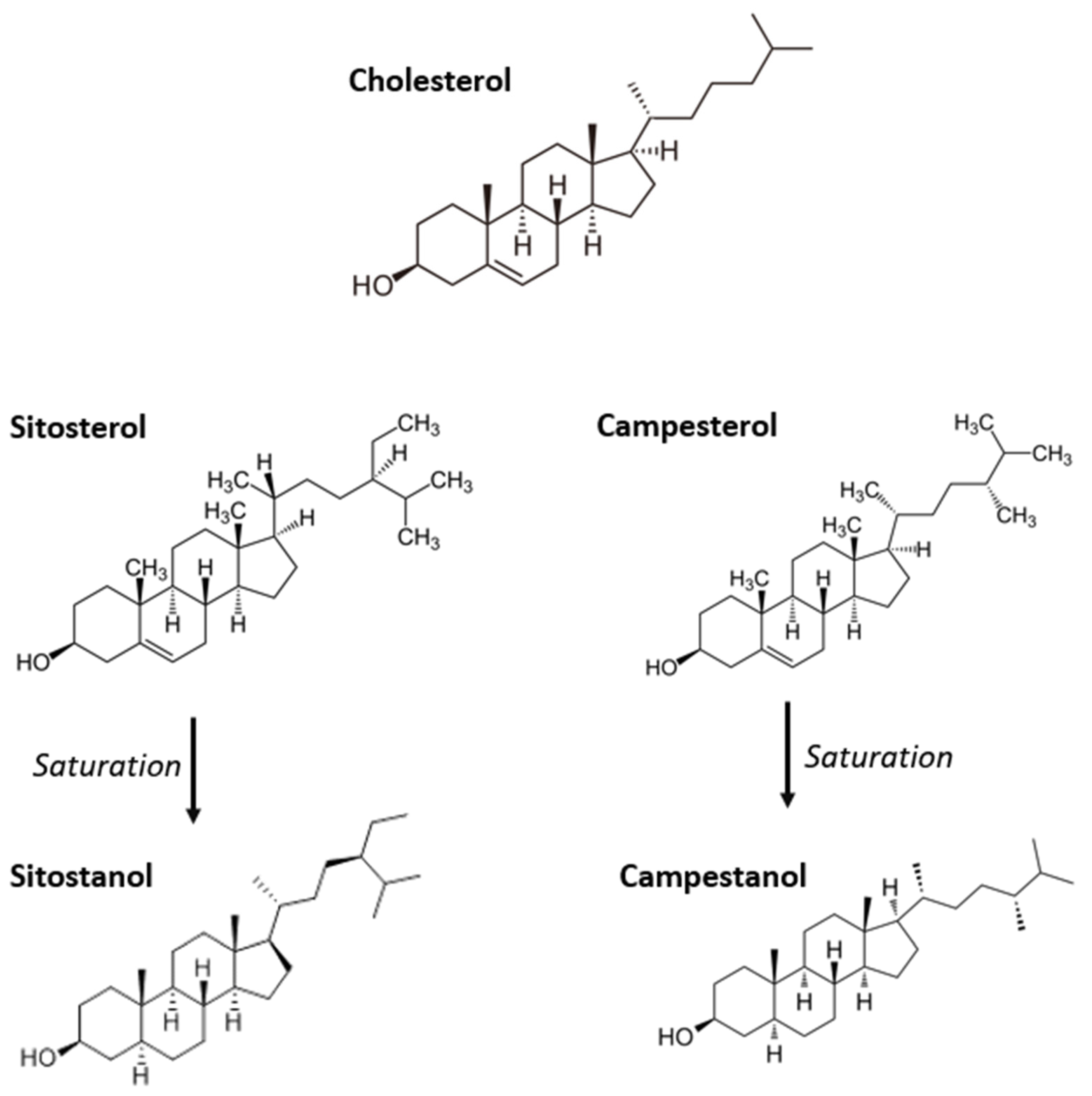
3.3. Plant Sterols and Stanols
3.3.1. Pharmacodynamics
3.3.2. Bioavailability
3.3.3. Clinically Proven Effects
3.3.4. Safety Profile
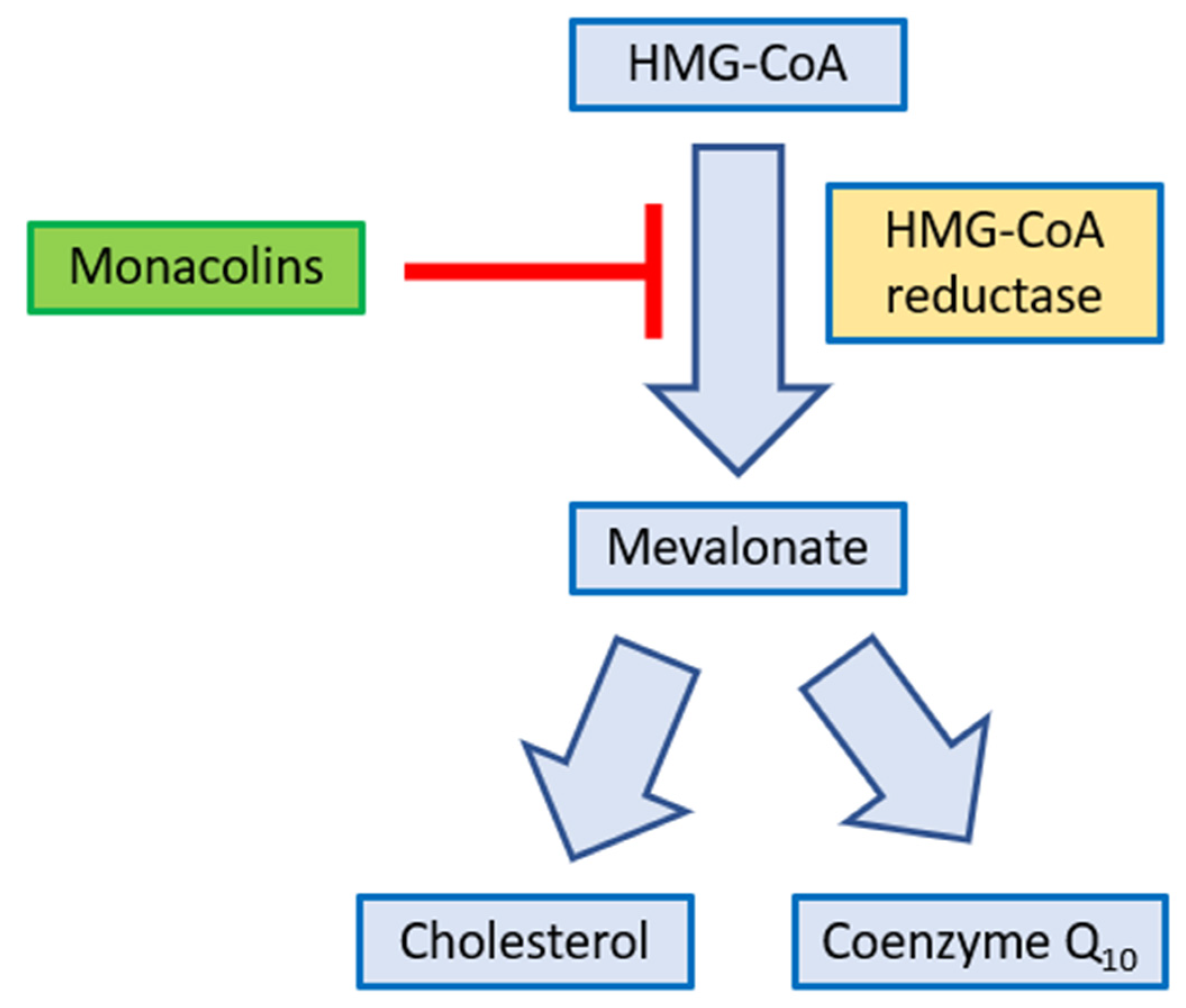
3.4. Monacolins from Red Yeast Rice (RYR)
3.4.1. Bioavailability
- -
- The presence of other monacolins within the phytocomplex that may work synergistically with lovastatin
- -
- The higher dissolution rate of monacolin K within the phytocomplex compared to the pure molecule
- -
3.4.2. Biopharmaceutical Strategies to Improve the Bioavailability of Monacolin K
3.4.3. Pharmacodynamics
3.4.4. Clinically Proven Effects
3.4.5. Safety Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sachdeva, V.; Roy, A.; Bharadvaja, N. Current Prospects of Nutraceuticals: A Review. Curr. Pharm. Biotechnol. 2020, 21, 10. [Google Scholar] [CrossRef] [PubMed]
- Das, L.; Bhaumik, E.; Raychaudhuri, U.; Chakraborty, R. Role of nutraceuticals in human health. J. Food Sci. Technol. 2012, 49, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Nasri, H.; Baradaran, A.; Shirzad, H.; Rafieian-Kopaei, M. New concepts in nutraceuticals as alternative for pharmaceuticals. Int. J. Prev. Med. 2014, 5, 1487–1499. [Google Scholar] [PubMed]
- Cicero, A.F.G.; Colletti, A.; von Haehling, S.; Vinereanu, D.; Bielecka-Dabrowa, A.; Sahebkar, A.; Toth, P.P.; Reiner, Ž.; Wong, N.D.; Mikhailidis, D.P.; et al. Nutraceutical Support in Heart Failure: A Position Paper of the International Lipid Expert Panel (ILEP). Nutr. Res. Rev. 2020, 16, 1–25. [Google Scholar] [CrossRef]
- Hang, L.; Basil, A.H.; Lim, K.L. Nutraceuticals in Parkinson’s Disease. Neuromol. Med. 2016, 18, 306–321. [Google Scholar] [CrossRef]
- Santini, A.; Novellino, E. Nutraceuticals in hypercholesterolaemia: An overview. Br. J. Pharmacol. 2017, 174, 1450–1463. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Colletti, A.; Bajraktari, G.; Descampas, O.; Djuric, D.M.; Ezhov, M.; Fras, Z.; Katsiki, N.; Langlois, M.; Latkovskis, G.; et al. Lipid-lowering Nutraceuticals in Clinical Practice: Position Paper From an International Lipid Expert Panel. Nutr. Rev. 2017, 75, 731–767. [Google Scholar] [CrossRef]
- McClements, D.J.; Li, F.; Xiao, H. The Nutraceutical Bioavailability Classification Scheme: Classifying Nutraceuticals According to Factors Limiting their Oral Bioavailability. Annu. Rev. Food Sci. Technol. 2015, 6, 299–327. [Google Scholar] [CrossRef]
- Fiod Riccio, B.V.; Fonseca-Santos, B.; Colerato Ferrari, P.; Chorilli, M. Characteristics, Biological Properties and Analytical Methods of Trans.-Resveratrol: A Review. Crit. Rev. Anal. Chem. 2019, 50, 339–358. [Google Scholar] [CrossRef]
- Teimouri, M.; Homayouni-Tabrizi, M.; Rajabian, A.; Amiri, H.; Hosseini, H. Anti-inflammatory effects of resveratrol in patients with cardiovascular disease: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2022, 70, 102863. [Google Scholar] [CrossRef]
- Jeyaraman, M.M.; Al-Yousif, N.S.H.; Singh Mann, A.; Dolinsky, V.W.; Rabbani, R.; Zarychanski, R.; Abou-Setta, A.M. Resveratrol for adults with type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2020, 1, CD011919. [Google Scholar] [CrossRef] [PubMed]
- Mohammadipoor, N.; Shafiee, F.; Rostami, A.; Kahrizi, M.S.; Soleimanpour, H.; Ghodsi, M.; Ansari, M.J.; Bokov, D.O.; Jannat, B.; Mosharkesh, E.; et al. Resveratrol supplementation efficiently improves endothelial health: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2022, 36, 3529–3539. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Liao, W.; Xia, H.; Wang, S.; Sun, G. The Effect of Resveratrol on Blood Lipid Profile: A Dose-Response Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 3755. [Google Scholar] [CrossRef] [PubMed]
- Leo, L.; Surico, D.; Deambrogio, F.; Scatuzzi, A.; Marzullo, P.; Tinelli, R.; Molinari, C.; Surico, N. Preliminary data on the effectiveness of resveratrol in a new formulation in treatment of hot flushes. Minerva. Ginecol. 2015, 67, 475–483. [Google Scholar] [PubMed]
- Tseng, P.C.; Hou, S.M.; Chen, R.J.; Peng, H.W.; Hsieh, C.F.; Kuo, M.L.; Yen, M.L. Resveratrol promotes osteogenesis of human mesenchymal stem cells by upregulating RUNX2 gene expression via the SIRT1/FOXO3A axis. J. Bone Miner. Res. 2011, 26, 2552–2563. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, K.; Ikeda, K.; Kawai, Y.; Yamori, Y. Resveratrol Stimulates the Proliferation and Differentiation of Osteoblastic MC3T3-E1 Cells. Biochem. Biophys. Res. Commun. 1998, 253, 859–863. [Google Scholar] [CrossRef]
- Rayalam, S.; Della-Fera, M.A.; Baile, C.A. Synergism between resveratrol and other phytochemicals: Implications for obesity and osteoporosis. Mol. Nutr. Food Res. 2011, 55, 1177–1185. [Google Scholar] [CrossRef]
- Aires, V.; Limagne, E.; Cotte, A.K.; Latruffe, N.; Ghiringhelli, F.; Delmas, D. Resveratrol metabolites inhibit human metastatic colon cancer cells progression and synergize with chemotherapeutic drugs to induce cell death. Mol. Nutr. Food Res. 2013, 57, 1170–1181. [Google Scholar] [CrossRef]
- Singh, C.K.; Ndiaye, M.A.; Ahmad, N. Resveratrol and cancer: Challenges for clinical translation. Biochim. Biophys. Acta. 2015, 1855, 1178–1185. [Google Scholar] [CrossRef]
- Bishayee, A. Cancer prevention and treatment with resveratrol: From rodent studies to clinical trials. Cancer Prev. Res. 2009, 2, 409–418. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, E.; Somoza, V. Metabolism and bioavailability of trans-resveratrol. Mol. Nutr. Food Res. 2005, 49, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Cottart, C.H.; Nivet-Antoine, V.; Laguillier-Morizot, C.; Beaudeux, J.L. Resveratrol bioavailability and toxicity in humans. Mol. Nutr. Food Res. 2010, 54, 7–16. [Google Scholar] [CrossRef]
- Sergides, C.; Chirilă, M.; Silvestro, L.; Pitta, D.; Pittas, A. Bioavailability and safety study of resveratrol 500 mg tablets in healthy male and female volunteers. Exp. Ther. Med. 2016, 11, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Boocock, D.J.; Faust, G.E.; Patel, K.R.; Schinas, A.M.; Brown, V.A.; Ducharme, M.P.; Booth, T.D.; Crowell, J.A.; Perloff, M.; Gescher, A.J.; et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1246–1252. [Google Scholar] [CrossRef]
- Almeida, L.; Vaz-da-Silva, M.; Falcão, A.; Soares, E.; Costa, R.; Loureiro, A.L.; Fernandes-Lopes, C.; Rocha, J.F.; Nunes, T.; Wright, L.; et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol. Nutr. Food Res. 2009, 53 (Suppl. 1), S7–S15. [Google Scholar] [CrossRef]
- Amri, A.; Chaumeil, J.C.; Sfar, S.; Charrueau, C. Administration of resveratrol: What formulation solutions to bioavailability limitations? J. Control Release 2012, 158, 182–193. [Google Scholar] [CrossRef]
- Sessa, M.; Balestrieri, M.L.; Ferrari, G.; Servillo, L.; Castaldo, D.; D’Onofrio, N.; Donsì, F.; Tsao, R. Bioavailability of encapsulated resveratrol into nanoemulsion-based delivery systems. Food Chem. 2014, 147, 42–50. [Google Scholar] [CrossRef]
- Summerlin, N.; Soo, E.; Thakur, S.; Qu, Z.; Jambhrunkar, S.; Popat, A. Resveratrol nanoformulations: Challenges and opportunities. Int. J. Pharm. 2015, 479, 282–290. [Google Scholar] [CrossRef]
- Gambini, J. Properties of resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxid. Med. Cell. Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef] [PubMed]
- Satia, M.C.; Mukim, A.G.; Tibrewala, K.D.; Bhavsar, M.S. A randomized two way cross over study for comparison of absorption of vitamin D3 buccal spray and soft gelatin capsule formulation in healthy subjects and in patients with intestinal malabsorption. Nutr. J. 2015, 14, 114. [Google Scholar] [CrossRef] [PubMed]
- Fratter, A.; Biagi, D.; Cicero, A.F.G. Sublingual Delivery of Astaxanthin through a Novel Ascorbyl Palmitate-Based Nanoemulsion: Preliminary Data. Mar. Drugs. 2019, 17, 508. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, M.; Yang, F.F.; Liu, C.Y.; Pan, R.L.; Chang, Q.; Liu, X.M.; Liao, Y.H. Involvement of the inhibition of intestinal glucuronidation in enhancing the oral bioavailability of resveratrol by labrasol containing nanoemulsions. Mol. Pharm. 2015, 12, 1084–1095. [Google Scholar] [CrossRef]
- Dong, D.; Quan, E.; Yuan, X.; Xie, Q.; Li, Z.; Wu, B. Sodium Oleate-Based Nanoemulsion Enhances Oral Absorption of Chrysin through Inhibition of UGT-Mediated Metabolism. Mol. Pharm. 2017, 14, 2864–2874. [Google Scholar] [CrossRef]
- Wightman, E.L.; Reay, J.L.; Haskell, C.F.; Williamson, G.; Dew, T.P.; Kennedy, D.O. Effects of resveratrol alone or in combination with piperine on cerebral blood flow parameters and cognitive performance in human subjects: A randomised, double-blind, placebo-controlled, cross-over investigation. Br. J. Nutr. 2014, 112, 203–213. [Google Scholar] [CrossRef]
- Johnson, J.J.; Nihal, M.; Siddiqui, I.A.; Scarlett, C.O.; Bailey, H.H.; Mukhtar, H.; Ahmad, N. Enhancing the bioavailability of resveratrol by combining it with piperine. Mol. Nutr. Food Res. 2011, 55, 1169–1176. [Google Scholar] [CrossRef]
- Calvo-Castro, L.A.; Schiborr, C.; David, F.; Ehrt, H.; Voggel, J.; Sus, N.; Behnam, D.; Bosy-Westphal, A.; Frank, J. The Oral Bioavailability of Trans-Resveratrol from a Grapevine-Shoot Extract in Healthy Humans is Significantly Increased by Micellar Solubilization. Mol. Nutr. Food Res. 2018, 62, e1701057. [Google Scholar] [CrossRef]
- Basheer, L.; Kerem, Z. Interactions between CYP3A4 and Dietary Polyphenols. Oxid. Med. Cell. Longev. 2015, 2015, 854015. [Google Scholar] [CrossRef]
- Itai, S. Development of Novel Functional Formulations Based on Pharmaceutical Technologies. Yaguku Zasshi J. Pharm. Soc. Jpn. 2019, 139, 419–435. [Google Scholar] [CrossRef]
- Kurangi, B.; Jalalpure, S.; Jagwani, S. A Validated StabilityIndicating HPLC Method for Simultaneous Estimation of Resveratrol and Piperine in Cubosome and Human Plasma. J. Chromatogr. B 2019, 1122–1123, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Pai, R.S. Trans-Resveratrol Self-Nano-Emulsifying Drug Delivery System (SNEDDS) with Enhanced Bioavailability Potential: Optimization, Pharmacokinetics and in situ Single Pass Intestinal Perfusio Studies. Drug Deliv. 2015, 22, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.J.H.; Ullah, R.S.; Haroon, M.; Fahad, S.; Liu, J.; Shaarani, T.; Khan, R.U.; Nazir, A. Recent Progress in Electron Paramagnetic Resonance Study of Polymers. Polymer Chem. 2018, 9, 3306–3335. [Google Scholar] [CrossRef]
- Bowers, J.L.; Tyulmenkov, V.V.; Jernigan, S.C.; Klinge, C.M. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors alpha and beta. Endocrinology 2000, 141, 3657–3667. [Google Scholar] [CrossRef]
- Klinge, C.M.; Blankenship, K.A.; Risinger, K.E.; Bhatnagar, S.; Noisin, E.L.; Sumanasekera, W.K.; Zhao, L.; Brey, D.M.; Keynton, R.S. Resveratrol and estradiol rapidly activate MAPK signaling through estrogen receptors alpha and beta in endothelial cells. J. Biol. Chem. 2005, 280, 7460–7468. [Google Scholar] [CrossRef] [PubMed]
- Tomé-Carneiro, J. Consumption of a grape extract supplement containing resveratrol decreases oxidized LDL and ApoB in patients undergoing primary prevention of cardiovascular disease: A triple-blind, 6-month follow-up, placebo-controlled, randomized trial. Mol. Nutr. Food Res. 2012, 56, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Zern, T.L.; Wood, R.J.; Greene, C.; West, K.L.; Liu, Y.; Aggarwal, D.; Shachter, N.S.; Fernandez, M.L. Grape polyphenols exert a cardioprotective effect in pre- and postmenopausal women by lowering plasma lipids and reducing oxidative stress. J. Nutr. 2005, 135, 1911–1917. [Google Scholar] [CrossRef]
- Cho, I.J.; Ahn, J.Y.; Kim, S.; Choi, M.S.; Ha, T.Y. Resveratrol attenuates the expression of HMG-CoA reductase mRNA in hamsters. Biochem. Biophys. Res. Commun. 2008, 367, 190–194. [Google Scholar] [CrossRef]
- Yashiro, T.; Nanmoku, M.; Shimizu, M.; Inoue, J.; Sato, R. Resveratrol increases the expression and activity of the low density lipoprotein receptor in hepatocytes by the proteolytic activation of the sterol regulatory element-binding proteins. Atherosclerosis 2012, 220, 369–374. [Google Scholar] [CrossRef]
- Witztum, J.L.; Steinberg, D. Role of oxidized low density lipoprotein in atherogenesis. J. Clin. Investig. 1991, 88, 1785–1792. [Google Scholar] [CrossRef]
- El-Mowafy, A.M.; Alkhalaf, M.; El-Kashef, H.A. Resveratrol reverses hydrogen peroxide-induced proliferative effects in human coronary smooth muscle cells: A novel signaling mechanism. Arch. Med. Res. 2008, 39, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Haskó, G.; Pacher, P. Endothelial Nrf2 activation: A new target for resveratrol? Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H10–H12. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.H.; Alex, D.; Huang, H.Q.; Wang, N.; Yu, N.; Wang, Y.T.; Leung, G.P.; Lee, S.M. Inhibition of TNF-α-mediated endothelial cell-monocyte cell adhesion and adhesion molecules expression by the resveratrol derivative, trans-3,5,4′-trimethoxystilbene. Phytother. Res. 2011, 25, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Haghighatdoost, F.; Hariri, M. Effect of resveratrol on lipid profile: An updated systematic review and meta-analysis on randomized clinical trials. Pharmacol. Res. 2018, 129, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, A.; Namazi, G.; Farrokhian, A.; Reiner, Ž.; Aghadavod, E.; Bahmani, F.; Asemi, Z. The effects of resveratrol on metabolic status in patients with type 2 diabetes mellitus and coronary heart disease. Food Funct. 2019, 10, 6042–6051. [Google Scholar] [CrossRef]
- Hausenblas, H.A.; Schoulda, J.A.; Smoliga, J.M. Resveratrol treatment as an adjunct to pharmacological management in type 2 diabetes mellitus--systematic review and meta-analysis. Mol. Nutr. Food Res. 2015, 59, 147–159. [Google Scholar] [CrossRef]
- Cicero, A.F.; Morbini, M.; Parini, A.; Urso, R.; Rosticci, M.; Grandi, E.; Borghi, C. Effect of red yeast rice combined with antioxidants on lipid pattern, hs-CRP level, and endothelial function in moderately hypercholesterolemic subjects. Ther. Clin. Risk Manag. 2016, 12, 281–286. [Google Scholar] [CrossRef]
- Brown, A.; Patel, K.R.; Viskaduraki, M.; Crowell, J.A.; Perloff, M.; Booth, T.D.; Vasilinin, G.; Sen, A.; Schinas, A.M.; Piccirilli, G.; et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: Safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010, 70, 9003–9011. [Google Scholar] [CrossRef]
- Zarei, A.; Changizi-Ashtiyani, S.; Taheri, S.; Ramezani, M. A quick overview on some aspects of endocrinological and therapeutic effects of Berberis vulgaris L. Avicenna J. Phytomed. 2015, 5, 485–497. [Google Scholar]
- Yang, Y.; Ye, X.L.; Li, X.G.; Zhen, J.; Zhang, B.S.; Yuan, L.J. Synthesis and Antimicrobial Activity of 8-alkyl Berberine Derivatives with a Long Aliphatic Chain. Planta Med. 2007, 73, 602–604. [Google Scholar] [CrossRef]
- Kaboli, P.J.; Rahmat, A.; Ismail, P.; Ling, K.H. Targets and mechanisms of berberine, a natural drug with potential to treat cancer with special focus on breast cancer. Eur. J. Pharmacol. 2014, 740, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Maffioli, P.; Cicero, A.F. Berberine on metabolic and cardiovascular risk factors: An analysis from preclinical evidences to clinical trials. Expert. Opin. Biol. Ther. 2012, 12, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.T.; Hao, H.P.; Xie, H.G.; Lai, L.; Wang, Q.; Liu, C.X. Extensive intestinal firstpass elimination and predominant hepatic distribution of berberine explain its low plasma levels in rats. Drug Metab. Dispos. 2010, 38, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Miao, Y.Q.; Fan, D.J.; Yang, S.S.; Lin, X.; Meng, L.K.; Tang, X. Bioavailability study of berberine and the enhancing effects of TPGS on intestinal absorption in rats. AAPS Pharm. Sci. Tech. 2011, 12, 705–711. [Google Scholar] [CrossRef]
- Hua, W.; Ding, L.; Chen, Y.; Gong, B.; He, J.; Xu, G. Determination of berberine in human plasma by liquid chromatography–electrospray ionization–mass spectrometry. J. Pharm. Biomed. 2007, 44, 931–937. [Google Scholar] [CrossRef]
- Spinozzi, S.; Colliva, C.; Camborata, C.; Roberti, M.; Ianni, C.; Neri, F. Berberine and its metabolites: Relationship between physicochemical properties and plasma levels after administration to human subjects. J. Nat. Prod. 2014, 77, 766–772. [Google Scholar] [CrossRef]
- Tsai, P.L.; Tsai, T.H. Hepatobiliary excretion of berberine. Drug Metab. Dispos. 2004, 32, 405–412. [Google Scholar] [CrossRef]
- Battu, S.K.; Repka, M.A.; Maddineni, S.; Chittiboyina, A.G.; Avery, M.A.; Majumdar, S. Physicochemical characterization of berberine chloride: A perspective in the development of a solution dosage form for oral delivery. AAPS Pharm. Sci. Tech. 2010, 11, 1466–1475. [Google Scholar] [CrossRef]
- Bansal, T.; Mishra, G.; Jaggi, M.; Khar, R.K.; Talegaonkar, S. Effect of P-glycoprotein inhibitor, verapamil, on oral bioavailability and pharmacokinetics of irinotecan in rats. Eur. J. Pharm. Sci. 2009, 36, 580–590. [Google Scholar] [CrossRef]
- Bardelmeijer, H.A.; Ouwehand, M.; Buckle, T.; Huisman, M.T.; Schellens, J.H.; Beijnen, J.H. Low systemic exposure of oral docetaxel in mice resulting from extensive first-pass metabolism is boosted by ritonavir. Cancer Res. 2002, 62, 6158–6164. [Google Scholar]
- Wilkinson, G.R. Drug metabolism and variability among patients in drug response. N. Engl. J. Med. 2005, 352, 2211–2221. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, F.; Ma, X.; Cheng, X.; Zhou, H.; Klaassen, C.D. CYP2D plays a major role in berberine metabolism in liver of mice and humans. Xenobiotica 2011, 41, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Zuo, F.; Nakamura, N.; Akao, T.; Hattori, M. Pharmacokinetics of berberine and its main metabolites in conventional and pseudo germ-free rats determined by liquid chromatography/ion trap mass spectrometry. Drug Metab. Dispos. 2006, 34, 2064–2072. [Google Scholar] [CrossRef]
- Li, Y.; Ren, G.; Wang, Y.; Kong, W.; Yang, P.; Wang, Y.; Jiang, J.D. Bioactivities of berberine metabolites after transformation through CYP450 isoenzymes. J. Transl. Med. 2011, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Feng, X.; Chai, L.; Cao, S.; Qiu, F. The Metabolism of Berberine and Its Contribution to the Pharmacological Effects. Drug Metab. Rev. 2017, 49, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.S.; Zheng, Y.R.; Zhang, Y.F.; Long, X.Y. Research progress on berberine with a special focus on its oral bioavailability. Fitoterapia 2016, 109, 274–282. [Google Scholar] [CrossRef]
- Maher, S.; Leonard, T.W.; Jacobsen, J.; Brayden, D.J. Safety and efficacy of sodium caprate in promoting oral drug absorption: From in vitro to the clinic. Adv. Drug Deliv. Rev. 2009, 61, 1427–1449. [Google Scholar] [CrossRef]
- Anderberg, E.K.; Lindmark, T.; Artursson, P. Sodium caprate elicits dilatations in human intestinal tight junctions and enhances drug absorption by the paracellular route. Pharm. Res. 1993, 10, 857–864. [Google Scholar] [CrossRef]
- Fan, D.; Wu, X.; Dong, W.; Sun, W.; Li, J.; Tang, X. Enhancement by sodium caprate and sodium deoxycholate of the gastrointestinal absorption of berberine chloride in rats. Drug Dev. Ind. Pharm. 2013, 39, 447–1456. [Google Scholar] [CrossRef]
- Lv, X.; Li, J.; Zhang, M.; Wang, C.; Fan, Z.; Wang, C. Enhancement of sodium caprate on intestine absorption and antidiabetic action of berberine. AAPS Pharm. Sci. Technol. 2010, 11, 372–382. [Google Scholar] [CrossRef]
- Chen, W.; Fan, D.; Meng, L.; Miao, Y.; Yang, S.; Weng, Y. Enhancing effects of chitosan and chitosan hydrochloride on intestinal absorption of berberine in rats. Drug Dev. Ind. Pharm. 2012, 38, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Cano-Cebrian, M.J.; Zornoza, T.; Granero, L.; Polache, A. Intestinal absorption enhancement via the paracellular route by fatty acids, chitosans and others: A target for drug delivery. Curr. Drug Deliv. 2005, 2, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Mourya, V.K.; Inamdar, N.N. Trimethyl chitosan and its applications in drug delivery. J. Mater. Sci. Mater. Med. 2009, 20, 1057–1079. [Google Scholar] [CrossRef] [PubMed]
- Fratter, A.; Servi, B. New Oral Delivery System to Improve Absorption of Berberine: Likely Interaction of Cationized Chitosan with PG-P Pump. Int. J. Drug Deliv. Technol. 2014, 5, 33–42. [Google Scholar]
- Zhou, S.; Lim, L.Y.; Chowbay, B. Herbal modulation of P-glycoprotein. Drug Metab. Rev. 2004, 36, 57–104. [Google Scholar] [CrossRef]
- Chakraborty, S.; Shukla, D.; Mishra, B.; Singh, S. Lipid—An emerging platform for oral deliveryof drugs with poor bioavailability. Eur. J.Pharm. Biopharm. 2009, 73, 1–15. [Google Scholar] [CrossRef]
- Zhu, J.; Tang, D.; Feng, L.; Zheng, Z.; Wang, R.; Wu, A.; Zhu, Q. Development of selfmicroemulsifying drug delivery system for oral bioavailability enhancement of berberine hydrochloride. Drug Dev. Ind. Pharm. 2013, 39, 499–506. [Google Scholar] [CrossRef]
- Li, H.; Dong, B.; Park, S.W.; Lee, H.S.; Chen, W.; Liu, J. Hepatocyte nuclear factor 1alpha plays a critical role in PCSK9 gene transcription and regulation by the natural hypocholesterolemic compound berberine. J. Biol. Chem. 2009, 284, 28885–28895. [Google Scholar] [CrossRef]
- Abidi, P.; Zhou, Y.; Jiang, J.D.; Liu, J. Extracellular signal-regulated kinase-dependent stabilization of hepatic low-density lipoprotein receptor mRNA by herbal medicine berberine. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2170–2176. [Google Scholar] [CrossRef]
- Feng, X.; Sureda, A.; Jafari, S.; Memariani, Z.; Tewari, D.; Annunziata, G.; Barrea, L.; Hassan, S.T.S. Berberine in Cardiovascular and Metabolic Diseases: From Mechanisms to Therapeutics. Theranostics 2019, 9, 1923–1951. [Google Scholar] [CrossRef]
- Lan, J.; Zhao, Y.; Dong, F.; Yan, Z.; Zheng, W.; Fan, J.; Sun, G. Meta-analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia and hypertension. J. Ethnopharmacol. 2015, 161, 69–81. [Google Scholar] [CrossRef]
- Cao, C.; Su, M. Effects of berberine on glucose-lipid metabolism, inflammatory factors and insulin resistance in patients with metabolic syndrome. Exp. Ther. Med. 2019, 17, 3009–3014. [Google Scholar] [CrossRef]
- Pirro, M.; Mannarino, M.R.; Bianconi, V.; Simental-Mendía, L.E.; Bagaglia, F.; Mannarino, E.; Sahebkar, A. The effects of a nutraceutical combination on plasma lipids and glucose: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2016, 110, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Wang, L.S.; Huang, Z.Q.; Zhou, Q.; Sun, Y.G.; Cao, J.T.; Li, Y.G.; Wang, C.Q. Berberine ameliorates inflammation in patients with acute coronary syndrome following percutaneous coronary intervention. Clin. Exp. Pharmacol. Physiol. 2012, 39, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Li, M.F.; Zhou, X.M.; Li, X.L. The Effect of Berberine on Polycystic Ovary Syndrome Patients with Insulin Resistance (PCOS-IR): A Meta-Analysis and Systematic Review. Evid. Based Complement. Alternat. Med. 2018, 2018, 2532935. [Google Scholar] [CrossRef] [PubMed]
- Sosnowska, B.; Penson, P.; Banach, M. The role of nutraceuticals in the prevention of cardiovascular disease. Cardiovasc. Diagn. Ther. 2017, 7 (Suppl. 1), S21–S31. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.M.; Xia, M.F.; Wang, Y.; Chang, X.X.; Yao, X.Z.; Rao, S.X.; Zeng, M.S.; Tu, Y.F. Efficacy of Berberine in Patients with Non-Alcoholic Fatty Liver Disease. PLoS ONE 2015, 10, e0134172. [Google Scholar] [CrossRef] [PubMed]
- Bateman, J.; Chapman, R.D.; Simpson, D. Possible toxicity of herbal remedies. Scott. Med. J. 1998, 43, 7–15. [Google Scholar] [CrossRef]
- Chan, E. Displacement of bilirubin from albumin by berberine. Biol. Neonate. 1993, 63, 201–208. [Google Scholar] [CrossRef]
- Xin, H.W.; Wu, X.C.; Li, Q.; Yu, A.R.; Zhong, M.Y.; Liu, Y.Y. The Effects of Berberine on the Pharmacokinetics of Cyclosporin A in Healthy Volunteers. Methods Find. Exp. Clin. Pharmacol. 2006, 28, 25–29. [Google Scholar] [CrossRef]
- Pirro, M.; Francisci, D.; Bianconi, V.; Schiaroli, E.; Mannarino, M.R.; Barsotti, F.; Spinozzi, A.; Bagaglia, F. NUtraceutical TReatment for hYpercholesterolemia in HIV-infected patients: The NU-TRY(HIV) randomized cross-over trial. Atherosclerosis 2019, 280, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhao, M.; Qiu, F.; Sun, Y.; Zhao, L. Pharmacokinetic interactions and tolerability of berberine chloride with simvastatin and fenofibrate: An open-label, randomized, parallel study in healthy Chinese subjects. Drug Des. Dev. Ther. 2018, 13, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Banach, M.; Patti, A.M.; Giglio, R.V.; Cicero, A.F.G.; Atanasov, A.G.; Bajraktari, G.; Bruckert, E.; Descamps, O.; Djuric, D.M.; Ezhov, M.; et al. International Lipid Expert Panel (ILEP). The Role of Nutraceuticals in Statin Intolerant Patients. J. Am. Coll. Cardiol. 2018, 72, 96–118. [Google Scholar] [CrossRef] [PubMed]
- Moreau, R.A.; Nystrom, L.; Whitaker, B.D.; Winkler-Moser, J.K.; Baer, D.J.; Gebauer, S.K.; Hicks, K.B. Phytosterols and their derivatives: Structural diversity, distribution, metabolism, analysis, and health-promoting uses. Prog. Lipid Res. 2018, 70, 35–61. [Google Scholar] [CrossRef]
- Klingberg, S.; Andersson, H.; Mulligan, A.; Bhaniani, A.; Welch, A.; Bingham, S.; Ellegard, L. Food sources of plant sterols in the EPIC Norfolk population. Eur. J. Clin. Nutr. 2008, 62, 695–703. [Google Scholar] [CrossRef]
- Devaraj, S.; Jialal, I. The role of dietary supplementation with plant sterols and stanols in the prevention of cardiovascular disease. Nutr. Rev. 2006, 64, 348–354. [Google Scholar] [CrossRef]
- Law, M. Plant sterol and stanol margarines and health. Br. Med. J. 2000, 320, 861–864. [Google Scholar] [CrossRef]
- Ferguson, J.J.; Stojanovski, E.; MacDonald-Wicks, L.; Garg, L.A. Fat type in phytosterol products influence their cholesterol-lowering potential: A systematic review and meta-analysis of RCTs. Prog. Lipid Res. 2016, 64, 16–29. [Google Scholar] [CrossRef]
- Davis, H.R., Jr.; Zhu, L.J.; Hoos, L.M.; Tetzloff, G.; Maguire, M.; Liu, J. NiemannPick C1 Like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J. Biol. Chem. 2004, 279, 33586–33592. [Google Scholar] [CrossRef]
- Altmann, S.W.; Davis, H.R., Jr.; Zhu, L.J.; Yao, X.; Hoos, L.M.; Tetzloff, G. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science 2004, 303, 1201–1204. [Google Scholar] [CrossRef]
- Ras, R.T.; Hiemstra, H.; Lin, Y.; Vermeer, M.A.; Duchateau, G.M.S.J.E.; Trautwein, E.A. Consumption of plant sterol-enriched foods and effects on plasma plant sterol concentrations—A meta-analysis of randomized controlled studies. Atherosclerosis 2013, 230, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Inoue, I.; Takenaka, Y.; Ono, H.; Katayama, S.; Awata, T.; Murakoshi, T. Ezetimibe promotes brush border membrane-to-lumen cholesterol efflux in the small intestine. PLoS ONE 2016, 11, e0152207. [Google Scholar] [CrossRef] [PubMed]
- Escola-Gil, J.C.; Quesada, H.; Julve, J.; Martin-Campos, J.M.; Cedo, L.; BlancoVaca, F. Sitosterolemia: Diagnosis, investigation, and management. Curr. Atheroscler Rep. 2014, 16, 424. [Google Scholar] [CrossRef] [PubMed]
- Fumeron, F.; Bard, J.M.; Lecerf, J.M. Inter individual variability in the cholesterol lowering effect of supplementation with plant sterols or stanols. Nutr. Rev. 2017, 75, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Stellaard, F.; Lutjohann, D. Fractional cholesterol absorption measurements in humans: Determinants of the blood-based dual stable isotope tracer technique. J. Clin. Lipidol. 2015, 9, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, T.A.; Gylling, H.; Nissinen, M.J. The role of serum non-cholesterol sterols as surrogate markers of absolute cholesterol synthesis and absorption. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 765–769. [Google Scholar] [CrossRef]
- Ras, R.T.; Geleijnse, J.M.; Trautwein, E.A. LDL-cholesterol-lowering effect of plant sterols and stanols across different dose ranges: A meta-analysis of randomised controlled studies. Br. J. Nutr. 2014, 112, 214–219. [Google Scholar] [CrossRef]
- Demonty, I.; Ras, R.T.; van der Knaap, H.C.; Meijer, L.; Zock, P.L.; Jeleijnse, J.M.; Trautwein, E.A. The effect of plant sterols on serum triglyceride concentrations is dependent on baseline concentrations: A pooled analysis of 12 randomised controlled trials. Eur. J. Nutr. 2013, 52, 153–160. [Google Scholar] [CrossRef]
- Athyros, V.G.; Kakafika, A.I.; Papageorgiou, A.A.; Tziomalos, K.; Peletidou, A.; Vosikis, C.; Karagiannis, A.; Mikhailidis, D.P. Effect of a plant stanol ester-containing spread, placebo spread, or Mediterranean diet on estimated cardiovascular risk and lipid, inflammatory and haemostatic factors. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 213–221. [Google Scholar] [CrossRef]
- Scholle, J.M.; Baker, W.L.; Talati, R.; Coleman, C.I. The effect of adding plant sterols or stanols to statin therapy in hypercholesterolemic patients: Systematic review and meta-Analysis. J. Am. Coll. Nutr. 2009, 28, 517–524. [Google Scholar] [CrossRef]
- Lin, X.; Racette, S.B.; Lefevre, M.; Ma, L.; Spearie, C.A.; Steger-May, K.; Ostlund, R.E. Combined Effects of Ezetimibe and Phytosterols on Cholesterol Metabolism: A Randomized, Controlled Feeding Study in Humans. Circulation 2011, 124, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Jakulj, L.; Trip, M.D.; Sudhop, T.; von Bergmann, K.; Kastelein, J.J.P.; Vissers, M.N. Inhibition of cholesterol absorption by the combination of dietary plant sterols and ezetimibe: Effects on plasma lipid levels. J. Lipid Res. 2005, 46, 2692–2698. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.R.; Tershakovec, A.M.; Tomassini, J.E.; Musliner, T. Intestinal sterol transporters and cholesterol absorption inhibition. Curr. Opin Lipidol. 2011, 22, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Salen, G.; von Bergmann, K.; Lütjohann, D.; Kwiterovich, P.; Kane, J.; Patel, S.B.; Musliner, T.; Stein, P.; Musser, B. Multicenter Sitosterolemia Study Group. Ezetimibe Effectively Reduces Plasma Plant Sterols in Patients With Sitosterolemia. Circulation 2004, 109, 966–971. [Google Scholar] [CrossRef]
- Tsubakio-Yamamoto, K.; Nishida, M.; Nakagawa-Toyama, Y.; Masuda, D.; Ohama, T.; Yamashita, S. Current therapy for patients with sitosterolemia--effect of ezetimibe on plant sterol metabolism. Atheroscler. Thromb. Z 2010, 17, 891–900. [Google Scholar] [CrossRef]
- Ras, R.T.; Fuchs, D.; Koppenol, W.P.; Garczarek, U.; Greyling, A.; Trautwein, E.A. The effect of a low-fat spread with added plant sterols on vascular function markers: Results of the Investigating Vascular Function Effects of Plant Sterols (INVEST) study. Am. J. Clin. Nutr. 2015, 101, 733–741. [Google Scholar] [CrossRef]
- Baumgartner, S.; Ras, R.T.; Trautwein, E.A.; Mensink, R.P.; Plat, J. Plasma Fat-Soluble Vitamin and Carotenoid Concentrations After Plant Sterol and Plant Stanol Consumption: A Meta-Analysis of Randomized Controlled Trials. Eur. J. Nutr. 2017, 56, 909–923. [Google Scholar] [CrossRef]
- Hendriks, H.F.J.; Weststrate, J.A.; van Vliet, T.; Meijer, G.W. Spreads enriched with three different concentrations of vegetable oil sterols and the degree of cholesterol lowering in normocholesterolemic and mildly hypercholesterolemic subjects. Eur. J. Clin. Nutr. 1999, 53, 319–327. [Google Scholar] [CrossRef]
- Miettinen, T.A. Phytosterolaemia, xanthomatosis and premature atherosclerotic arterial disease: A case with high plant sterol absorption, impaired sterol elimination and low cholesterol synthesis. Eur. J. Clin. Investig. 1980, 10, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, Y.; Ye, Q.; Li, J.; Hua, Y.; Ju, D.; Zhang, D.; Cooper, R.; Chang, M. Constituents of red yeast rice, a traditional Chinese food and medicine. Agric. Food Chem. 2000, 48, 5220–5225. [Google Scholar] [CrossRef]
- Burke, F.M. Red Yeast Rice for the Treatment of Dyslipidemia. Curr. Atheroscler. Rep. 2015, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Functional food red yeast rice (RYR) for metabolic syndrome amelioration: A review on pros and cons. World J. Microb. Biot. 2016, 32, 87. [Google Scholar] [CrossRef] [PubMed]
- Kalaivani, M.; Sabitha, R.; Kalaiselvan, V.; Rajasekaran, A. Biofortification of Indian rice (IR-532-E-576) with monacolin K by RSM optimisation using Monascus purpureus MTCC 1090. Food Bioprocess. Tech. 2009, 3, 333–339. [Google Scholar] [CrossRef]
- Mazzanti, G.; Moro, P.A.; Raschi, E.; Da Cas, R.; Menniti-Ippolito, F. Adverse reactions to dietary supplements containing red yeast rice: Assessment of cases from the Italian surveillance system. Br. J. Clin. Pharmacol. 2017, 83, 894–908 . [Google Scholar] [CrossRef]
- Zhu, B.; Qi, F.; Wu, J.; Yin, G.; Hua, J.; Zhang, Q.; Qin, L. Red Yeast Rice: A Systematic Review of the Traditional Uses, Chemistry, Pharmacology, and Quality Control of an Important Chinese Folk Medicine. Front. Pharmacol. 2019, 10, 1449. [Google Scholar] [CrossRef]
- Endo, A. Monacolin K, a new hypocholesterolemic agent produced by a Monascus species. J. Antibiot. 1979, 32, 852–854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ali, Z.; Khan, S.I.; Khan, I.A. Cytotoxic monacolins from red yeast rice, a Chinese medicine and food. Food Chem. 2016, 202, 262–268. [Google Scholar] [CrossRef]
- Zhou, W.B.; Guo, R.; Guo, W.L.; Hong, J.L.; Li, L.; Ni, L. Monascus yellow, red and orange pigments from red yeast rice ameliorate lipid metabolic disorders and gut microbiota dysbiosis in Wistar rats fed on a high- fat diet. Food Funct. 2019, 10, 1073–1084. [Google Scholar] [CrossRef]
- Hsu, Y.-W.; Hsu, L.-C.; Chang, C.-L.; Liang, Y.-H.; Kuo, Y.-H.; Pan, T.-M. New Anti-Inflammatory and Anti-Proliferative Constituents from Fermented Red Mold Rice Monascus purpureus NTU 568. Molecules 2010, 15, 7815–7824. [Google Scholar] [CrossRef]
- Cheng, M.J.; Wu, M.D.; Chen, I.S.; Chen, C.Y.; Lo, W.L.; Yuan, G.F. Secondary metabolites from the red mould rice of Monascus purpureus BCRC 38113. Nat. Prod. Res. 2010, 24, 1719–1725. [Google Scholar] [CrossRef]
- Wang, J.J.; Wang, H.Y.; Shih, C.D. Autonomic nervous system and nitric oxide in antihypertensive and cardiac inhibitory effects induced by Red Mold Rice in spontaneously hypertensive rats. J. Agric. Food Chem. 2010, 13, 7940–7948. [Google Scholar] [CrossRef]
- Li, Z.P.; Seeram, N.P.; Lee, R.; Thames, G.; Minutti, C.; Wang, H.J. Plasma clearance of lovastatin versus chinese red yeast rice in healthy volunteers. J. Altern. Complement. Med. 2005, 11, 1031–1038. [Google Scholar] [CrossRef]
- Hong, M.Y.; Seerarn, N.P.; Zhang, Y.; Heber, D. Anticancer effects of Chinese red yeast rice versus monacolin K alone on colon cancer cells. J. Nutr. Biochem. 2008, 19, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Serajuddin, A.T.; Ranadive, S.A.; Mahoney, E.M. Relative lipophilicities, solubilities, and structure-pharmacological considerations of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors pravastatin, lovastatin, mevastatin, and simvastatin. J. Pharm. Sci. 1991, 80, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Mireles, R.J.; Campbell, S.D.; Lin, J.; Mills, J.B.; Xu, J.J. Differential interaction of 3-hydroxy-3-methylglutaryl-coa reductase inhibitors with ABCB1, ABCC2, and OATP1B1. Drug Metab. Dispos. 2005, 33, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Neuvonen, P.J.; Backman, J.T.; Niemi, M. Pharmacokinetic comparison of the potential over-the-counter statins simvastatin, lovastatin, fluvastatin and pravastatin. Clin. Pharmacokinet. 2008, 47, 463–474. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Banach, M. Red Yeast Rice for Hypercholesterolemia. Methodist. Debakey Cardiovasc. J. 2019, 15, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Uang, Y.S.; Wang, S.T.; Yang, J.C.; Lin, C.J. Interaction between Red Yeast Rice and CYP450 Enzymes/P-Glycoprotein and its implication for the clinical pharmacokinetics of lovastatin. Evid. Based Complement. Alternat. Med. 2012, 2012, 127043. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Yang, J.C.; Uang, Y.S.; Lin, C.J. Improved dissolution rate and oral bioavailability of lovastatin in red yeast rice products. Int. J. Pharm. 2013, 444, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Heber, D.; Yip, I.; Ashley, J.M.; Elashoff, D.A.; Elashoff, R.M.; Go, V.L. Cholesterol-lowering effects of a proprietary Chinese red-yeast-rice dietary supplement. Am. J. Clin. Nutr. 1999, 69, 231–236. [Google Scholar] [CrossRef]
- Becker, D.J.; Gordon, R.Y.; Halbert, S.C.; French, B.; Morris, P.B.; Rader, D.J. Red yeast rice for dyslipidemia in statin-intolerant patients a randomized trial. Ann. Intern. Med. 2009, 150, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Kantola, T.; Kivistö, K.T.; Neuvonen, P.J. Grapefruit juice greatly increases serum concentrations of lovastatin and lovastatin acid. Clin. Pharmacol. Ther. 1998, 63, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, Z.; Zhi, Z.; Jiang, T.; Zhang, J.; Wang, S. Development of biodegradable porous starch foam for improving oral delivery of poorly watersoluble drugs. Int. J. Pharm. 2001, 403, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Pouton, C.W. Lipid formulations for oral administration of drugs: Non-emulsifying, self-emulsifying and ‘self-microemulsifying’ drug delivery systems. Eur. J. Pharm. Sci. 2000, 11, S93–S98. [Google Scholar] [CrossRef]
- Humberstone, A.J.; Charman, W.N. Lipid-based vehicles for the oral delivery of poorly water soluble drugs. Adv. Drug Deliv. Rev. 1997, 25, 103–128. [Google Scholar] [CrossRef]
- Wu, C.Y.; Benet, L.Z. Predicting drug disposition via application of BCS: Transport/absorption/elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm. Res. 2005, 22, 11–23. [Google Scholar] [CrossRef]
- Li, C.; Zhu, Y.; Wang, Y.; Zhu, J.S.; Chang, J.; Kritchevsky, D. Monascus purpureus-fermented rice (red yeast rice): A natural food product that lowers blood cholesterol in animal models of hypercholesterolemia. Nutr. Res. 1998, 18, 71–81. [Google Scholar] [CrossRef]
- Gerards, M.C.; Terlou, R.J.; Yu, H.; Koks, C.H.W.; Gerdes, V.E.A. Traditional Chinese lipid-lowering agent red yeast rice results in significant LDL reduction but safety is uncertain—A systematic review and meta-analysis. Atherosclerosis 2015, 240, 415–423. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Shi, Y.; Grimsgaard, S.; Alraek, T.; Fonnebo, V. Chinese red yeast rice (Monascus purpureus) for primary hyperlipidemia: A meta-analysis of randomized controlled trials. Chin. Med. 2006, 1, 4. [Google Scholar] [CrossRef]
- Lu, Z.; Kou, W.; Du, B.; Wu, Y.; Zhao, S.; Brusco, O.A.; Morgan, J.M.; Capuzzi, D.M. Effect of Xuezhikang, an extract from red yeast Chinese rice, on coronary events in a Chinese population with previous myocardial infarction. Am. J. Cardiol. 2008, 101, 1689–1693. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Lu, Z.L.; Kou, W.R.; Chen, Z.; Wu, Y.F.; Yu, X.H.; Zhao, Y.C. Chinese Coronary Secondary Prevention Study Group. Beneficial impact of Xuezhikang on cardiovascular events and mortality in elderly hypertensive patients with previous myocardial infarction from the China Coronary Secondary Prevention Study (CCSPS). J. Clin. Pharmacol. 2009, 49, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.P.; Liu, L.; Cheng, Y.C.; Li, Y.L. Effect of xuezhikang, a cholestin extract, on reflecting postprandial triglyceridemia after a high-fat meal in patients with coronary heart disease. Atherosclerosis 2003, 168, 375–380. [Google Scholar] [CrossRef]
- Polsani, V.R.; Jones, P.H.; Ballantyne, C.M.; Nambi, V. A case report of myopathy from consumption of red yeast rice. J. Clin. Lipidol. 2008, 2, 60–62. [Google Scholar] [CrossRef]
- Roselle, H.; Ekatan, A.; Tzeng, J.; Sapienza, M.; Kocher, J. Symptomatic hepatitis associated with the use of herbal red yeast rice. Ann. Intern. Med. 2008, 149, 516–517. [Google Scholar] [CrossRef]
- Kumari, S.; Sherriff, J.M.; Spooner, D.; Beckett, R. Peripheral neuropathy induced by red yeast rice in a patient with a known small bowel gastrointestinal tumour. BMJ Case Rep. 2013, 2013, bcr2013009060. [Google Scholar] [CrossRef]
- Liu, Z.G.; Chen, P.J. A case of erectile dysfunction induced by red yeast rice in lipid-lowering therapy. Phytother. Res. 2018, 32, 953–954. [Google Scholar] [CrossRef]
- Fogacci, F.; Banach, M.; Mikhailidis, D.P.; Bruckert, E.; Toth, P.P.; Watts, G.F.; Reiner, Ž.; Mancini, J. Safety of red yeast rice supplementation: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2019, 143, 1–16. [Google Scholar] [CrossRef]
- Mornar, A.; Sertic, M.; Nigovic, B. Development of a rapid LC/DAD/FLD/MSn method for the simultaneous determination of monacolins and citrinin in red fermented rice products. J. Agric. Food Chem. 2013, 61, 1072–1080. [Google Scholar] [CrossRef]
- Heber, D.; Lembertas, A.; Lu, Q.Y.; Bowerman, S.; Go, V.L. An analysis of nine proprietary Chinese red yeast rice dietary supplements: Implications of variability in chemical profile and contents. J. Altern. Complement. Med. 2001, 7, 133–139. [Google Scholar] [CrossRef]
- Marley, E.; Brown, P.; Leeman, D.; Donnelly, C. Analysis of citrinin in cereals, red yeast rice dietary supplement, and animal feed by immunoaffinity column cleanup and LC with fluorescence detection. J. AOAC Int. 2016, 99, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cardiovascular Diseases (CVDs). 2015. Available online: http://www.who.int/mediacentre/factsheets/fs317/en/ (accessed on 28 July 2017).
- Perk, J.; De Backer, G.; Gohlke, H.; Graham, I.; Reiner, Z.; Verschuren, M.; Albus, C.; Benlian, P. European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG). European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur. Heart J. 2012, 33, 1635–1701. [Google Scholar] [CrossRef] [PubMed]
- Bloom, D.E.; Cafiero, E.T.; Jané-Llopis, E.; Abrahams-Gessel, S.; Bloom, L.R.; Fathima, S.; Feigl, A.B.; Gaziano, T.; Mowafi, M.; Pandya, A.; et al. The Global Economic Burden of Noncommunicable Diseases; World Economic Forum: Geneva, Switzerland, 2011. [Google Scholar]
- Catapano, A.L.; Graham, I.; De Backer, G.; Wiklund, O.; Chapman, M.J.; Drexel, H.; Hoes, A.W.; Jennings, C.S.; Landmesser, U.; Pedersen, T.R.; et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Developed with the special contribution of the European Assocciation for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis 2016, 253, 281–344. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare. High Blood Cholesterol; Australian Institute of Health & Welfare: Canberra, Australia, 2013.
- Colantonio, L.D.; Bittner, V.; Reynolds, K.; Levitan, E.B.; Rosenson, R.S.; Banach, M.; Kent, S.T.; Derose, S.F.; Zhou, H.; Safford, M.M.; et al. Association of Serum Lipids and Coronary Heart Disease in Contemporary Observational Studies. Circulation 2016, 133, 256–264. [Google Scholar] [CrossRef]
- Hobbs, F.D.; Banach, M.; Mikhailidis, D.P.; Malhotra, A.; Capewell, S. Is statin-modified reduction in lipids the most important preventive therapy for cardiovascular disease? A pro/con debate. BMC Med. 2016, 14, 4. [Google Scholar] [CrossRef]
- Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; Simes, J.; et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [CrossRef]
- Sahebkar, A.; Serban, M.C.; Gluba-Brzózka, A.; Mikhailidis, D.P.; Cicero, A.F.; Rysz, J.; Banach, M. Lipid-modifying effects of nutraceuticals: An evidence-based approach. Nutrition 2016, 32, 1179–1192. [Google Scholar] [CrossRef]
- Helal, N.A.; Eassa, H.A.; Amer, A.M.; Eltokhy, M.A.; Edafiogho, I.; Nounou, M.I. Nutraceuticals’ Novel Formulations: The Good, the Bad, the Unknown and Patents Involved. Recent Pat. Drug Deliv. Formul. 2019, 13, 105–156. [Google Scholar] [CrossRef]
- Dickinson, A. History and overview of DSHEA. Fitoterapia 2011, 82, 5–10. [Google Scholar] [CrossRef]
- Santini, A.; Novellino, E. Nutraceuticals—Shedding light on the grey area between pharmaceuticals and food. Expert Rev. Clin. Pharmacol. 2018, 11, 545–547. [Google Scholar] [CrossRef]


| Structures | Molecules | R |
|---|---|---|
 | Monacolin K (MK) | 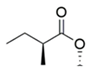 |
| Monacolin J (MJ) | -OH | |
| Monacolin L (ML) | -H | |
| Monacolin X (MX) | 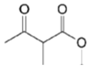 | |
| Monacolin M (MM) |  | |
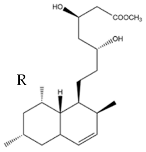 | MK Hydroxy acid | 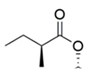 |
| MJ Hydroxy acid | -OH | |
| ML Hydroxy acid | -H | |
| MX Hydroxy acid | 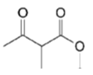 | |
| MM Hydroxy acid | 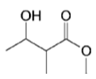 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colletti, A.; Fratter, A.; Pellizzato, M.; Cravotto, G. Nutraceutical Approaches to Dyslipidaemia: The Main Formulative Issues Preventing Efficacy. Nutrients 2022, 14, 4769. https://doi.org/10.3390/nu14224769
Colletti A, Fratter A, Pellizzato M, Cravotto G. Nutraceutical Approaches to Dyslipidaemia: The Main Formulative Issues Preventing Efficacy. Nutrients. 2022; 14(22):4769. https://doi.org/10.3390/nu14224769
Chicago/Turabian StyleColletti, Alessandro, Andrea Fratter, Marzia Pellizzato, and Giancarlo Cravotto. 2022. "Nutraceutical Approaches to Dyslipidaemia: The Main Formulative Issues Preventing Efficacy" Nutrients 14, no. 22: 4769. https://doi.org/10.3390/nu14224769
APA StyleColletti, A., Fratter, A., Pellizzato, M., & Cravotto, G. (2022). Nutraceutical Approaches to Dyslipidaemia: The Main Formulative Issues Preventing Efficacy. Nutrients, 14(22), 4769. https://doi.org/10.3390/nu14224769







