Higher Intake of Total Dietary Essential Amino Acids Is Associated with a Lower Prevalence of Metabolic Syndrome among Korean Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Demographic and Lifestyle Information
2.3. Dietary Amino Acid and Total EAAS Calculation
2.4. Anthropometric and Metabolic Risk Factors
2.5. Statistical Analysis
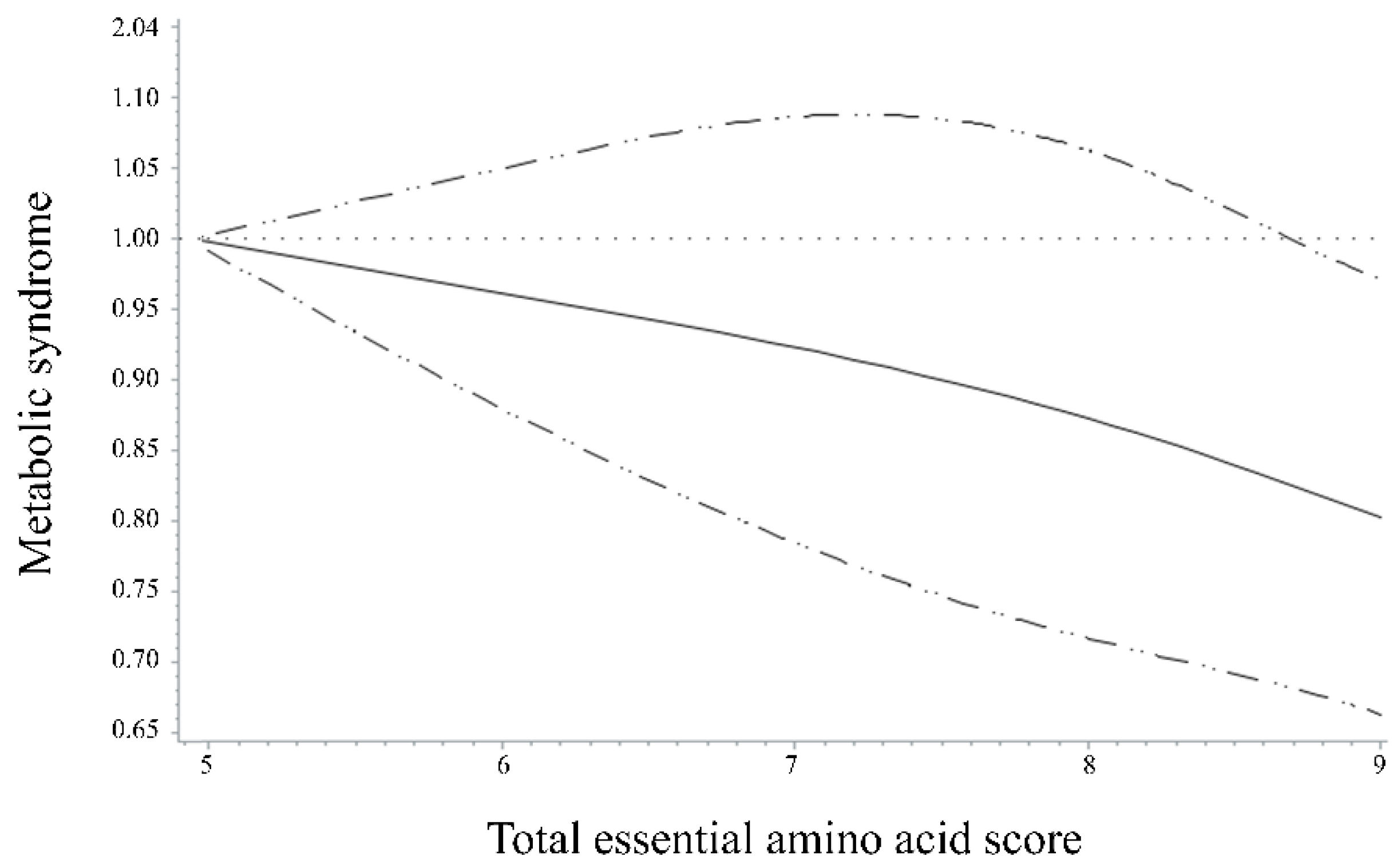
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Grundy, S.M. Metabolic syndrome: A multiplex cardiovascular risk factor. J. Clin. Endocrinol. Metab. 2007, 92, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Korean Society of Cardiometabolic Syndrome. Metabolic Syndrome Fact Sheet in Korea 2021. Available online: http://www.kscms.org/ (accessed on 3 September 2021).
- Isomaa, B.; Almgren, P.; Tuomi, T.; Forsén, B.; Lahti, K.; Nissén, M.; Taskinen, M.R.; Groop, L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 2001, 24, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, M.; Liu, J.; Bahsas, F.B.; Menotti, A. Syndrome X and mortality: A population-based study. Am. J. Epidemiol. 1998, 148, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Sucher, S.; Markova, M.; Hornemann, S.; Pivovarova, O.; Rudovich, N.; Thomann, R.; Schneeweiss, R.; Rohn, S.; Pfeiffer, A.F.H. Comparison of the effects of diets high in animal or plant protein on metabolic and cardiovascular markers in type 2 diabetes: A randomized clinical trial. Diabetes Obes. Metab. 2017, 19, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, F.; Maleki, V.; Kheirouri, S.; Alizadeh, M. The Effects of Taurine Supplementation on Metabolic Profiles, Pentosidine, Soluble Receptor of Advanced Glycation End Products and Methylglyoxal in Adults With Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Can. J. Diabetes 2021, 45, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, K.R.; Milton, J.E.; Packard, D.P.; Shuler, L.A.; Short, R.A. Dietary amino acids and blood pressure: A cohort study of patients with cardiovascular disease. Am. J. Kidney Dis. 2012, 59, 803–809. [Google Scholar] [CrossRef]
- Altorf-van der Kuil, W.; Engberink, M.F.; De Neve, M.; van Rooij, F.J.A.; Hofman, A.; van’t Veer, P.; Witteman, J.C.M.; Franco, O.H.; Geleijnse, J.M. Dietary amino acids and the risk of hypertension in a Dutch older population: The Rotterdam Study. Am. J. Clin. Nutr. 2013, 97, 403–410. [Google Scholar] [CrossRef]
- Teymoori, F.; Asghari, G.; Farhadnejad, H.; Mirmiran, P.; Azizi, F. Do dietary amino acid ratios predict risk of incident hypertension among adults? Int. J. Food Sci. Nutr. 2019, 70, 387–395. [Google Scholar] [CrossRef]
- Okekunle, A.P.; Wu, X.; Feng, R.; Li, Y.; Sun, C. Higher intakes of energy-adjusted dietary amino acids are inversely associated with obesity risk. Amino Acids 2019, 51, 373–382. [Google Scholar] [CrossRef]
- Korea Health Industry Development Institute. National Nutrition Statistics; Korea Health Industry Development Institute: Osong, Korea, 2018; Available online: http://www.khidi.or.kr/kps/dhraStat/result5?menuId=MENU01656&year= (accessed on 6 November 2021).
- Korea Disease Control and Prevention Agency. The seventh Korea National Health and Nutrition Examination Survey (KNHANES VIII-1). Available online: https://knhanes.kdca.go.kr/knhanes/sub03/sub03_02_05.do (accessed on 12 July 2021).
- Ainsworth, B.E.; Haskell, W.L.; Leon, A.S.; Jacobs, D.R.; Montoye, H.J.; Sallis, J.F.; Paffenbarger, R.S. Compendium of physical activities: Classification of energy costs of human physical activities. Med. Sci. Sport. Exerc. 1993, 25, 71–80. [Google Scholar] [CrossRef]
- Chae, M.; Park, H.; Park, K. Estimation of dietary amino acid intake and independent correlates of skeletal muscle mass index among Korean adults. Nutrients 2020, 12, 1043. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Agricultural Sciences. 9th Revision Korean Food Composition Table. Available online: http://koreanfood.rda.go.kr/kfi/fct/fctIntro/list?menuId=PS03562 (accessed on 10 May 2021).
- The Korean Nutrition Society. Computer Aided Nutritional Analysis Program. Available online: http://canpro5.kns.or.kr/EgovContent.do (accessed on 10 May 2021).
- Ministry of Health and Welfare of Korea and Korean Nutrition Society. Dietary Reference Intakes for Korean 2020: Energy and Macronutrients; Ministry of Health and Welfare and Korean Nutrition Society: Sejong, Korea, 2020; Available online: http://www.kns.or.kr/FileRoom/FileRoom_view.asp?mode=mod&restring=%252FFileRoom%252FFileRoom.asp%253Fxsearch%253D0%253D%253Dxrow%253D10%253D%253DBoardID%253DKdr%253D%253Dpage%253D1&idx=108&page=1&BoardID=Kdr&xsearch=1&cn_search= (accessed on 15 June 2021).
- Korea Disease Control and Prevention Agency. Guidelines for the 8th (2020) Korea National Health and Nutrition Examination Survey. Available online: https://knhanes.kdca.go.kr/knhanes/sub04/sub04_02_02.do?classType=4 (accessed on 6 January 2022).
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The Asia-Pacific Redefining Obesity and Its Treatment. Available online: https://apps.who.int/iris/bitstream/handle/10665/206936/0957708211_eng.pdf?sequence=1&tnqh_x0026;isAllowed=y (accessed on 30 September 2021).
- Hajihashemi, P.; Hassannejad, R.; Haghighatdoost, F.; Mohammadifard, N.; Sadeghi, M.; Roohafza, H.; Sajjadi, F.; Sarrafzadegan, N. The long-term association of different dietary protein sources with metabolic syndrome. Sci. Rep. 2021, 11, 19394. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpour-Niazi, S.; Tahmasebinejad, Z.; Esfandiar, Z.; Bakhshi, B.; Mirmiran, P.; Azizi, F. Weight gain, but not macronutrient intake, modifies the effect of dietary branch chain amino acids on the risk of metabolic syndrome. Diabetes Res. Clin. Pract. 2020, 161, 108039. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef]
- Bifari, F.; Ruocco, C.; Decimo, I.; Fumagalli, G.; Valerio, A.; Nisoli, E. Amino acid supplements and metabolic health: A potential interplay between intestinal microbiota and systems control. Genes Nutr. 2017, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.X.; Guo, F.F. Branched chain amino acids and metabolic regulation. Chin. Sci. Bull. 2013, 58, 1228–1235. [Google Scholar] [CrossRef]
- Kilberg, M.S.; Pan, Y.X.; Chen, H.; Leung-Pineda, V. Nutritional control of gene expression: How mammalian cells respond to amino acid limitation. Annu. Rev. Nutr. 2005, 25, 59–85. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.J. Role of leucine in the regulation of mTOR by amino acids: Revelations from structure-activity studies. J. Nutr. 2001, 131, 861s–865s. [Google Scholar] [CrossRef] [PubMed]
- Cota, D.; Proulx, K.; Smith, K.A.; Kozma, S.C.; Thomas, G.; Woods, S.C.; Seeley, R.J. Hypothalamic mTOR signaling regulates food intake. Science 2006, 312, 927–930. [Google Scholar] [CrossRef] [PubMed]
- Masuoka, H.C.; Townes, T.M. Targeted disruption of the activating transcription factor 4 gene results in severe fetal anemia in mice. Blood 2002, 99, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.A.; Yao, W.; Fioravante, D.; Smolen, P.D.; Byrne, J.H. cAMP-response elements in Aplysia creb1, creb2, and Ap-uch promoters: Implications for feedback loops modulating long term memory. J. Biol. Chem. 2005, 280, 27035–27043. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Matsuda, K.; Bialek, P.; Jacquot, S.; Masuoka, H.C.; Schinke, T.; Li, L.; Brancorsini, S.; Sassone-Corsi, P.; Townes, T.M.; et al. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin-Lowry Syndrome. Cell 2004, 117, 387–398. [Google Scholar] [CrossRef]
- Cao, J.; Dai, D.L.; Yao, L.; Yu, H.H.; Ning, B.; Zhang, Q.; Chen, J.; Cheng, W.H.; Shen, W.; Yang, Z.X. Saturated fatty acid induction of endoplasmic reticulum stress and apoptosis in human liver cells via the PERK/ATF4/CHOP signaling pathway. Mol. Cell. Biochem. 2012, 364, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Ohoka, N.; Yoshii, S.; Hattori, T.; Onozaki, K.; Hayashi, H. TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J. 2005, 24, 1243–1255. [Google Scholar] [CrossRef]
- Seo, J.; Fortuno, E.S., 3rd; Suh, J.M.; Stenesen, D.; Tang, W.; Parks, E.J.; Adams, C.M.; Townes, T.; Graff, J.M. Atf4 regulates obesity, glucose homeostasis, and energy expenditure. Diabetes 2009, 58, 2565–2573. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef]
- Hardie, D.G.; Sakamoto, K. AMPK: A key sensor of fuel and energy status in skeletal muscle. Physiology 2006, 21, 48–60. [Google Scholar] [CrossRef]
- Jakobsen, S.N.; Hardie, D.G.; Morrice, N.; Tornqvist, H.E. 5′-AMP-activated protein kinase phosphorylates IRS-1 on Ser-789 in mouse C2C12 myotubes in response to 5-aminoimidazole-4-carboxamide riboside. J. Biol. Chem. 2001, 276, 46912–46916. [Google Scholar] [CrossRef]
- Inoki, K.; Zhu, T.; Guan, K.L. TSC2 mediates cellular energy response to control cell growth and survival. Cell 2003, 115, 577–590. [Google Scholar] [CrossRef]
- Ha, K.; Sakaki, J.R.; Chun, O.K. Nutrient Adequacy Is Associated with Reduced Mortality in US Adults. J. Nutr. 2021, 151, 3214–3222. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P.; Bahadoran, Z.; Ghasemi, A.; Azizi, F. Contribution of dietary amino acids composition to incidence of cardiovascular outcomes: A prospective population-based study. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Ha, K.; Kim, K.; Chun, O.K.; Joung, H.; Song, Y. Differential association of dietary carbohydrate intake with metabolic syndrome in the US and Korean adults: Data from the 2007–2012 NHANES and KNHANES. Eur. J. Clin. Nutr. 2018, 72, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, K.; LeBlanc, R.E.; Loh, D.; Schwartz, G.J.; Yu, Y.H. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes 2007, 56, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Randolph, A.C.; Markofski, M.M.; Rasmussen, B.B.; Volpi, E. Effect of essential amino acid supplementation and aerobic exercise on insulin sensitivity in healthy older adults: A randomized clinical trial. Clin. Nutr. 2020, 39, 1371–1378. [Google Scholar] [CrossRef] [PubMed]
- Thalacker-Mercer, A.E.; Gheller, M.E. Benefits and Adverse Effects of Histidine Supplementation. J. Nutr. 2020, 150, 2588s–2592s. [Google Scholar] [CrossRef]
- Friedman, M. Absorption and Utilization of Amino Acids Volume III, 1st ed.; Boca Raton: Boca Raton, FL, USA, 1989; Volume 3. [Google Scholar]
- Park, B. Amino Acid Imbalance-Biochemical Mechanism and Nutritional Aspects. Asian-Australas. J. Anim. Sci. 2006, 19, 1361–1368. [Google Scholar] [CrossRef]
- Im, J. Association between Dietary Essential Amino Acid Intake and Metabolic Syndrome among Korean Adults; Thesis Paper; Yeungnam University: Gyeongsan, Gyeongbuk, Korea, 2022; pp. 66–68. [Google Scholar]
- Friedman, M. Nutritional value of proteins from different food sources. A Review. J. Agric. Food Chem. 1996, 44, 6–29. [Google Scholar] [CrossRef]
| Total EAAS | p 1 | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| n = 6446 | n = 6447 | n = 6447 | n = 6447 | ||
| Score, Median (range) | 6.3 (0.5–7.4) | 8.0 (7.5–8.2) | 8.6 (8.3–8.8) | 9.0 (8.9–9.0) | |
| Age (years) | 54.03 ± 0.16 | 51.56 ± 0.16 | 49.28 ± 0.16 | 48.05 ± 0.16 | <0.001 |
| Sex | <0.001 | ||||
| Men | 2313 (35.88) | 2504 (38.84) | 2750 (42.66) | 3073 (47.67) | |
| Women | 4133 (64.12) | 3943 (61.16) | 3697 (57.34) | 3374 (52.33) | |
| Education level | <0.001 | ||||
| Lower than high school education | 2708 (44.50) | 2083 (34.09) | 1574 (25.59) | 1290 (20.85) | |
| High school educated or higher | 3377 (55.50) | 4028 (65.91) | 4578 (74.41) | 4898 (79.15) | |
| Household income | <0.001 | ||||
| Lower or Mid-low | 3319 (52.19) | 2657 (41.52) | 2321 (36.28) | 2150 (33.63) | |
| Mid-high or Higher | 3041 (47.81) | 3742 (58.48) | 4077 (63.72) | 4243 (66.37) | |
| Alcohol consumption | <0.001 | ||||
| Drinkers | 4254 (68.23) | 4470 (71.22) | 4760 (76.00) | 4922 (78.15) | |
| Non-drinkers | 1981 (31.77) | 1806 (28.78) | 1503 (24.00) | 1376 (21.85) | |
| Smoking status | <0.001 | ||||
| Smokers | 1160 (18.60) | 1113 (17.72) | 1259 (20.08) | 1417 (22.47) | |
| Non-smokers | 5078 (81.40) | 5168 (82.28) | 5012 (79.92) | 4888 (77.53) | |
| Body mass index (kg/m2) | 23.36 ± 0.04 | 23.40 ± 0.04 | 23.35 ± 0.04 | 23.50 ± 0.04 | 0.08 |
| Physical activity 2 | <0.001 | ||||
| Low | 2154 (35.35) | 2036 (33.27) | 1998 (32.47) | 1926 (31.12) | |
| Mid | 1989 (32.64) | 2080 (33.99) | 2078 (33.77) | 2110 (34.10) | |
| High | 1950 (32.00) | 2004 (32.75) | 2077 (33.76) | 2152 (34.78) | |
| Total EAAS | p for Trend | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| n = 6446 | n = 6447 | n = 6447 | n = 6447 | ||
| Score, median (range) | 6.3 (0.5–7.4) | 8.0 (7.5–8.2) | 8.6 (8.3–8.8) | 9.0 (8.9–9.0) | |
| Metabolic biomarkers | |||||
| Body mass index (kg/m2) | 23.63 ± 0.09 | 23.58 ± 0.08 | 23.46 ± 0.08 | 23.44 ± 0.09 | 0.07 |
| Waist circumference (cm) | 81.46 ± 0.16 | 81.46 ± 0.13 | 81.11 ± 0.13 | 81.03 ± 0.16 | 0.09 |
| Triglyceride (mg/dL) | 144.55 ± 2.35 | 139.92 ± 1.72 | 134.03 ± 1.60 | 133.99 ± 2.33 | 0.002 |
| Total cholesterol (mg/dL) | 195.77 ± 0.62 | 195.97 ± 0.55 | 195.32 ± 0.53 | 196.25 ± 0.63 | 0.6 |
| HDL-cholesterol (mg/dL) | 49.99 ± 0.20 | 50.11 ± 0.18 | 50.53 ± 0.18 | 50.57 ± 0.21 | 0.1 |
| LDL-cholesterol (mg/dL) | 119.42 ± 0.98 | 118.79 ± 0.84 | 118.74 ± 0.86 | 119.23 ± 0.98 | 0.9 |
| Systolic blood pressure (mm Hg) | 118.26 ± 0.24 | 117.17 ± 0.23 | 116.64 ± 0.22 | 116.04 ± 0.25 | <0.001 |
| Diastolic blood pressure (mm Hg) | 76.80 ± 0.17 | 76.33 ± 0.16 | 76.14 ± 0.15 | 75.64 ± 0.18 | <0.001 |
| Fasting blood glucose (mg/dL) | 97.04 ± 0.32 | 96.98 ± 0.25 | 96.45 ± 0.23 | 96.17 ± 0.30 | 0.2 |
| Total EAAS | p for Trend | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| n = 6446 | n = 6447 | n = 6447 | n = 6447 | ||
| Metabolic syndrome | |||||
| Case | 1648 | 1514 | 1335 | 1384 | |
| Model 1 | 1 | 0.92 (0.83–1.02) | 0.79 (0.72–0.87) | 0.82 (0.75–0.90) | <0.001 |
| Model 2 | 1 | 0.95 (0.86–1.05) | 0.85 (0.77–0.94) | 0.90 (0.81–0.99) | 0.003 |
| Model 3 | 1 | 0.97 (0.86–1.10) | 0.87 (0.76–0.998) | 0.86 (0.74–0.996) | 0.03 |
| Abdominal obesity | |||||
| Case | 2311 | 2178 | 1966 | 2015 | |
| Model 1 | 1 | 0.95 (0.86–1.03) | 0.80 (0.73–0.87) | 0.84 (0.77–0.92) | <0.001 |
| Model 2 | 1 | 0.996 (0.91–1.09) | 0.89 (0.81–0.97) | 0.99 (0.90–1.08) | 0.2 |
| Model 3 | 1 | 1.05 (0.90–1.23) | 0.93 (0.79–1.09) | 0.96 (0.79–1.16) | 0.5 |
| Hyperglycemia | |||||
| Case | 1696 | 1756 | 1639 | 1661 | |
| Model 1 | 1 | 1.12 (1.02–1.23) | 1.02 (0.93–1.12) | 0.99 (0.90–1.09) | 0.9 |
| Model 2 | 1 | 1.15 (1.04–1.26) | 1.07 (0.97–1.18) | 1.04 (0.94–1.15) | 0.3 |
| Model 3 | 1 | 1.19 (1.06–1.33) | 1.08 (0.95–1.22) | 0.95 (0.83–1.09) | 0.7 |
| High blood pressure | |||||
| Case | 1861 | 1689 | 1572 | 1568 | |
| Model 1 | 1 | 0.90 (0.82–0.99) | 0.87 (0.79–0.95) | 0.86 (0.78–0.94) | <0.001 |
| Model 2 | 1 | 0.93 (0.84–1.02) | 0.92 (0.83–1.01) | 0.91 (0.83–1.01) | 0.048 |
| Model 3 | 1 | 0.91 (0.82–1.02) | 0.91 (0.81–1.02) | 0.86 (0.75–0.98) | 0.03 |
| Hypo-HDL-cholesterolemia | |||||
| Case | 2607 | 2414 | 2257 | 2160 | |
| Model 1 | 1 | 0.91 (0.84–0.996) | 0.78 (0.72–0.85) | 0.75 (0.69–0.82) | <0.001 |
| Model 2 | 1 | 0.95 (0.87–1.04) | 0.85 (0.78–0.93) | 0.86 (0.79–0.94) | <0.001 |
| Model 3 | 1 | 1.00 (0.91–1.10) | 0.91 (0.82–1.01) | 0.96 (0.86–1.08) | 0.2 |
| Hypertriglyceridemia | |||||
| Case | 1961 | 1894 | 1871 | 1924 | |
| Model 1 | 1 | 0.98 (0.90–1.08) | 0.95 (0.87–1.04) | 0.98 (0.90–1.08) | 0.5 |
| Model 2 | 1 | 0.96 (0.88–1.06) | 0.92 (0.84–1.01) | 0.92 (0.84–1.01) | 0.04 |
| Model 3 | 1 | 0.96 (0.87–1.07) | 0.93 (0.83–1.04) | 0.86 (0.76–0.98) | 0.047 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Im, J.; Park, H.; Park, K. Higher Intake of Total Dietary Essential Amino Acids Is Associated with a Lower Prevalence of Metabolic Syndrome among Korean Adults. Nutrients 2022, 14, 4771. https://doi.org/10.3390/nu14224771
Im J, Park H, Park K. Higher Intake of Total Dietary Essential Amino Acids Is Associated with a Lower Prevalence of Metabolic Syndrome among Korean Adults. Nutrients. 2022; 14(22):4771. https://doi.org/10.3390/nu14224771
Chicago/Turabian StyleIm, Jihyun, Hyoungsu Park, and Kyong Park. 2022. "Higher Intake of Total Dietary Essential Amino Acids Is Associated with a Lower Prevalence of Metabolic Syndrome among Korean Adults" Nutrients 14, no. 22: 4771. https://doi.org/10.3390/nu14224771
APA StyleIm, J., Park, H., & Park, K. (2022). Higher Intake of Total Dietary Essential Amino Acids Is Associated with a Lower Prevalence of Metabolic Syndrome among Korean Adults. Nutrients, 14(22), 4771. https://doi.org/10.3390/nu14224771







