Association between Blood Manganese Levels and Visceral Adipose Tissue in the United States: A Population-Based Study
Abstract
1. Introduction
2. Methods
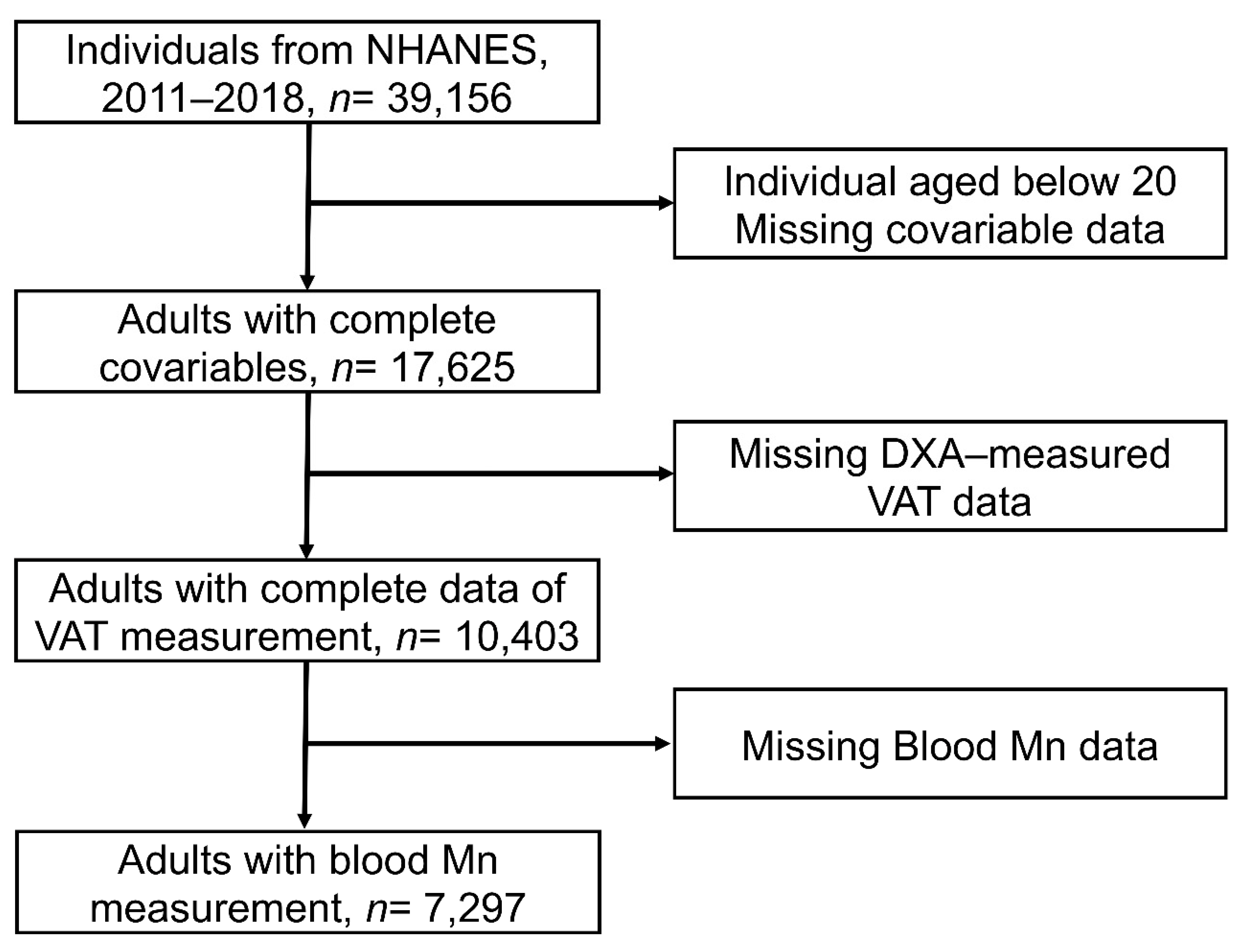
2.1. Study Population
2.2. Exposure Assessments
2.3. Outcomes
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Demographic Characteristics
3.2. Blood Mn Levels and VAT Mass
3.3. Associations between Blood Mn Levels and VAT Mass
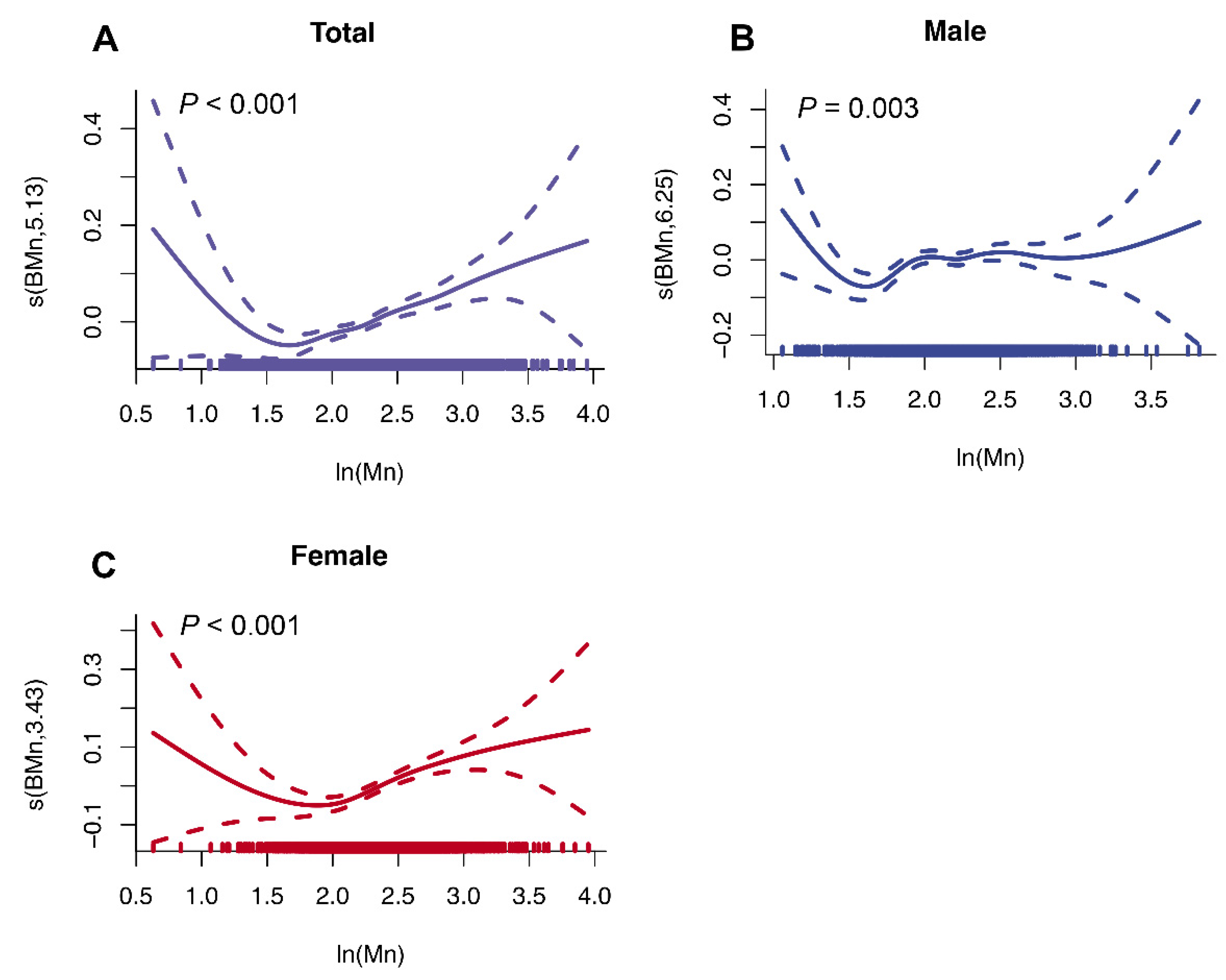
3.4. Non-Linear Associations between Blood Mn and VAT Mass
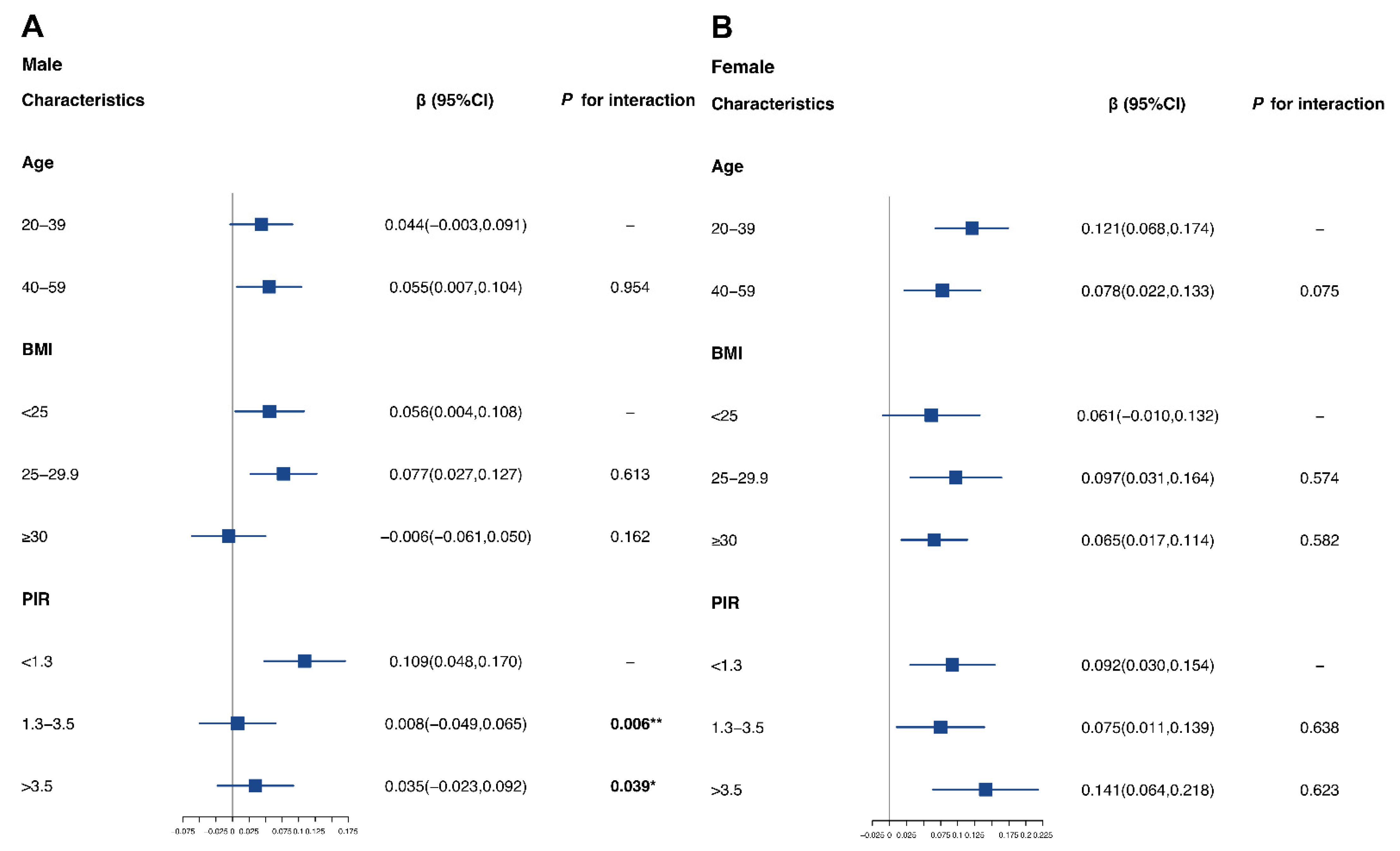
3.5. Sensitivity Analysis and Stratified Analysis
3.6. Associations between Mn Exposure and Visceral Obesity Risk
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Associate of Arts |
| GED | General Educational development |
| NCHS | National Center for Health Statistics |
| BMI | Body mass index |
| VAT | Visceral adipose tissue |
| Mn | Manganese |
| NHANES | National Health and Nutrition Examination Survey |
| DXA | Dual-energy X-ray absorptiometry |
| GAM | Generalized additive model |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| ICP-DRC-MS | Inductively coupled plasma mass spectrometry with dynamic reaction cell |
| PIR | Family income-to-poverty ratio |
| OR | Odds ratio |
References
- Bluher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Du, Y.; Wu, Y.; Snetselaar, L.G.; Wallace, R.B.; Bao, W. Trends in obesity and adiposity measures by race or ethnicity among adults in the United States 2011–18: Population based study. BMJ 2021, 372, n365. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Schoufour, J.; Wang, D.D.; Dhana, K.; Pan, A.; Liu, X.; Song, M.; Liu, G.; Shin, H.J.; Sun, Q.; et al. Healthy lifestyle and life expectancy free of cancer, cardiovascular disease, and type 2 diabetes: Prospective cohort study. BMJ 2020, 368, l6669. [Google Scholar] [CrossRef] [PubMed]
- Despres, J.P.; Carpentier, A.C.; Tchernof, A.; Neeland, I.J.; Poirier, P. Management of Obesity in Cardiovascular Practice: JACC Focus Seminar. J. Am. Coll. Cardiol. 2021, 78, 513–531. [Google Scholar] [CrossRef]
- Tchernof, A.; Despres, J.P. Pathophysiology of human visceral obesity: An update. Physiol. Rev. 2013, 93, 359–404. [Google Scholar] [CrossRef]
- Stefan, N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol. 2020, 8, 616–627. [Google Scholar] [CrossRef]
- Karlsson, T.; Rask-Andersen, M.; Pan, G.; Hoglund, J.; Wadelius, C.; Ek, W.E.; Johansson, A. Contribution of genetics to visceral adiposity and its relation to cardiovascular and metabolic disease. Nat. Med. 2019, 25, 1390–1395. [Google Scholar] [CrossRef]
- Broberg, K.; Taj, T.; Guazzetti, S.; Peli, M.; Cagna, G.; Pineda, D.; Placidi, D.; Wright, R.O.; Smith, D.R.; Lucchini, R.G.; et al. Manganese transporter genetics and sex modify the association between environmental manganese exposure and neurobehavioral outcomes in children. Environ. Int. 2019, 130, 104908. [Google Scholar] [CrossRef]
- Du, S.; Wu, X.; Han, T.; Duan, W.; Liu, L.; Qi, J.; Niu, Y.; Na, L.; Sun, C. Dietary manganese and type 2 diabetes mellitus: Two prospective cohort studies in China. Diabetologia 2018, 61, 1985–1995. [Google Scholar] [CrossRef]
- Zheng, F.; Goncalves, F.M.; Abiko, Y.; Li, H.; Kumagai, Y.; Aschner, M. Redox toxicology of environmental chemicals causing oxidative stress. Redox Biol. 2020, 34, 101475. [Google Scholar] [CrossRef]
- Pavilonis, B.T.; Lioy, P.J.; Guazzetti, S.; Bostick, B.C.; Donna, F.; Peli, M.; Zimmerman, N.J.; Bertrand, P.; Lucas, E.; Smith, D.R.; et al. Manganese concentrations in soil and settled dust in an area with historic ferroalloy production. J. Expo. Sci. Environ. Epidemiol. 2015, 25, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Erickson, M.L.; Elliott, S.M.; Brown, C.J.; Stackelberg, P.E.; Ransom, K.M.; Reddy, J.E.; Cravotta, C.A., 3rd. Machine-Learning Predictions of High Arsenic and High Manganese at Drinking Water Depths of the Glacial Aquifer System, Northern Continental United States. Environ. Sci. Technol. 2021, 55, 5791–5805. [Google Scholar] [CrossRef] [PubMed]
- Ghaisas, S.; Harischandra, D.S.; Palanisamy, B.; Proctor, A.; Jin, H.; Dutta, S.; Sarkar, S.; Langley, M.; Zenitsky, G.; Anantharam, V.; et al. Chronic Manganese Exposure and the Enteric Nervous System: An In Vitro and Mouse In Vivo Study. Environ. Health Perspect. 2021, 129, 87005. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, S.; Marsland, A.L.; Kinnee, E.J.; Tunno, B.J.; Manuck, S.B.; Gianaros, P.J.; Clougherty, J.E. Long-Term Ambient Air Pollution Exposures and Circulating and Stimulated Inflammatory Mediators in a Cohort of Midlife Adults. Environ. Health Perspect. 2021, 129, 57007. [Google Scholar] [CrossRef]
- Liu, X.; Shen, H.; Chen, M.; Shao, J. Clinical relevance of environmental manganese exposure with liver stiffness and steatosis detected by transient elastography in adults. Environ. Sci. Pollut. Res. Int. 2022, 29, 16624–16632. [Google Scholar] [CrossRef]
- Ngu, Y.J.; Skalny, A.V.; Tinkov, A.A.; Tsai, C.S.; Chang, C.C.; Chuang, Y.K.; Nikolenko, V.N.; Zotkin, D.A.; Chiu, C.F.; Chang, J.S. Association between Essential and Non-essential Metals, Body Composition, and Metabolic Syndrome in Adults. Biol. Trace Elem. Res. 2022, 200, 4903–4915. [Google Scholar] [CrossRef]
- Lo, K.; Yang, J.L.; Chen, C.L.; Liu, L.; Huang, Y.Q.; Feng, Y.Q.; Yang, A.M. Associations between blood and urinary manganese with metabolic syndrome and its components: Cross-sectional analysis of National Health and Nutrition Examination Survey 2011–2016. Sci. Total Environ. 2021, 780, 146527. [Google Scholar] [CrossRef]
- NHANES, US Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/nchs/nhanes/ (accessed on 2 November 2022).
- Zhou, J.; Meng, X.; Deng, L.; Liu, N. Non-linear associations between metabolic syndrome and four typical heavy metals: Data from NHANES 2011–2018. Chemosphere 2022, 291 Pt 2, 132953. [Google Scholar] [CrossRef]
- Shetty, S.; Kapoor, N.; Thomas, N.; Paul, T.V. DXA Measured Visceral Adipose Tissue, Total Fat, Anthropometric Indices and its Association with Cardiometabolic Risk Factors in Mother-Daughter Pairs from India. J. Clin. Densitom. 2021, 24, 146–155. [Google Scholar] [CrossRef]
- Zhao, L.; Ogden, C.L.; Yang, Q.; Jackson, S.L.; Loria, C.M.; Galuska, D.A.; Wiltz, J.L.; Merritt, R.; Cogswell, M.E. Association of Usual Sodium Intake with Obesity among US Children and Adolescents, NHANES 2009–2016. Obesity 2021, 29, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Guo, Y.; Hu, F.B.; Liu, L.; Qi, Q. Association of Low-Carbohydrate and Low-Fat Diets with Mortality among US Adults. JAMA Intern. Med. 2020, 180, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, X.; Wang, Z.; Bancks, M.P.; Carnethon, M.R.; Greenland, P.; Feng, Y.Q.; Wang, H.; Zhong, V.W. Trends in Prevalence of Diabetes and Control of Risk Factors in Diabetes among US Adults, 1999–2018. JAMA 2021, 326, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Trepanowski, J.F.; Kroeger, C.M.; Barnosky, A.; Klempel, M.; Bhutani, S.; Hoddy, K.K.; Rood, J.; Ravussin, E.; Varady, K.A. Effects of alternate-day fasting or daily calorie restriction on body composition, fat distribution, and circulating adipokines: Secondary analysis of a randomized controlled trial. Clin. Nutr. 2018, 37, 1871–1878. [Google Scholar] [CrossRef] [PubMed]
- Meisinger, C.; Rospleszcz, S.; Wintermeyer, E.; Lorbeer, R.; Thorand, B.; Bamberg, F.; Peters, A.; Schlett, C.L.; Linseisen, J. Isocaloric Substitution of Dietary Carbohydrate Intake with Fat Intake and MRI-Determined Total Volumes of Visceral, Subcutaneous and Hepatic Fat Content in Middle-Aged Adults. Nutrients 2019, 11, 1151. [Google Scholar] [CrossRef]
- FDNN. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group (accessed on 28 September 2022).
- Kolnes, K.J.; Petersen, M.H.; Lien-Iversen, T.; Hojlund, K.; Jensen, J. Effect of Exercise Training on Fat Loss-Energetic Perspectives and the Role of Improved Adipose Tissue Function and Body Fat Distribution. Front. Physiol. 2021, 12, 737709. [Google Scholar] [CrossRef]
- Luo, K.; Liu, J.; Wang, Y.; Aimuzi, R.; Luo, F.; Ao, J.; Zhang, J. Associations between organophosphate esters and sex hormones among 6–19-year old children and adolescents in NHANES 2013–2014. Environ. Int. 2020, 136, 105461. [Google Scholar] [CrossRef]
- Lazarevic, N.; Barnett, A.G.; Sly, P.D.; Knibbs, L.D. Statistical Methodology in Studies of Prenatal Exposure to Mixtures of Endocrine-Disrupting Chemicals: A Review of Existing Approaches and New Alternatives. Environ. Health Perspect. 2019, 127, 26001. [Google Scholar] [CrossRef]
- Golub, J.S.; Brickman, A.M.; Ciarleglio, A.J.; Schupf, N.; Luchsinger, J.A. Association of Subclinical Hearing Loss with Cognitive Performance. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 57–67. [Google Scholar] [CrossRef]
- Bouzid, M.; Colon-Gonzalez, F.J.; Lung, T.; Lake, I.R.; Hunter, P.R. Climate change and the emergence of vector-borne diseases in Europe: Case study of dengue fever. BMC Public Health 2014, 14, 781. [Google Scholar] [CrossRef]
- Ravindra, K.; Rattan, P.; Mor, S.; Aggarwal, A.N. Generalized additive models: Building evidence of air pollution, climate change and human health. Environ. Int. 2019, 132, 104987. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, T.; Hu, W.; Wang, X.; Xu, B.; Lin, Z.; Hofer, T.; Stefanoff, P.; Chen, Y.; Wang, X.; et al. Association between exposure to a mixture of phenols, pesticides, and phthalates and obesity: Comparison of three statistical models. Environ. Int. 2019, 123, 325–336. [Google Scholar] [CrossRef]
- NHANES, US Centers for Disease Control and Prevention. Module 4: Variance Estimation. Available online: https://wwwn.cdc.gov/nchs/nhanes/tutorials/module4.aspx (accessed on 28 September 2022).
- Signes-Pastor, A.J.; Desai, G.; Garcia-Villarino, M.; Karagas, M.R.; Kordas, K. Exposure to a mixture of metals and growth indicators in 6–11-year-old children from the 2013–16 NHANES. Expo. Health 2021, 13, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Rotter, I.; Kosik-Bogacka, D.; Dolegowska, B.; Safranow, K.; Lubkowska, A.; Laszczynska, M. Relationship between the concentrations of heavy metals and bioelements in aging men with metabolic syndrome. Int. J. Environ. Res. Public Health 2015, 12, 3944–3961. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, X. The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxidative Med. Cell. Longev. 2018, 2018, 7580707. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, M.; Cui, J.; Du, Y.; Teng, X.; Zhang, Z. Heat shock proteins took part in oxidative stress-mediated inflammatory injury via NF-κB pathway in excess manganese-treated chicken livers. Ecotoxicol. Environ. Saf. 2021, 226, 112833. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Culbreth, M.; Aschner, M. Exposure, epidemiology, and mechanism of the environmental toxicant manganese. Environ. Sci. Pollut. Res. Int. 2016, 23, 13802–13810. [Google Scholar] [CrossRef]
- Jakubiak, G.K.; Osadnik, K.; Lejawa, M.; Kasperczyk, S.; Osadnik, T.; Pawlas, N. Oxidative Stress in Association with Metabolic Health and Obesity in Young Adults. Oxidative Med. Cell. Longev. 2021, 2021, 9987352. [Google Scholar] [CrossRef] [PubMed]
- de Mello, A.H.; Costa, A.B.; Engel, J.D.G.; Rezin, G.T. Mitochondrial dysfunction in obesity. Life Sci. 2018, 192, 26–32. [Google Scholar] [CrossRef]
- Gandhi, D.; Rudrashetti, A.P.; Rajasekaran, S. The impact of environmental and occupational exposures of manganese on pulmonary, hepatic, and renal functions. J. Appl. Toxicol. 2022, 42, 103–129. [Google Scholar] [CrossRef]
- Suzuki, T.; Tsukamoto, I. Manganese-induced apoptosis in hepatocytes after partial hepatectomy. Eur. J. Pharmacol. 2005, 525, 48–53. [Google Scholar] [CrossRef]
- Lv, W.H.; Zhao, T.; Pantopoulos, K.; Chen, G.H.; Wei, X.; Zhang, D.G.; Luo, Z. Manganese (Mn)-induced oxidative stress contributes to intestinal lipid deposition via the deacetylation of PPARγ at K339 by SIRT1. Antioxid. Redox Signal. 2022, 37, 417–436. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhou, Z.; Fu, J. Effect of manganese chloride exposure on liver and brain mitochondria function in rats. Environ. Res. 2003, 93, 149–157. [Google Scholar] [CrossRef]
- Dhanraj, P.; Venter, C.; Bester, M.J.; Oberholzer, H.M. Induction of hepatic portal fibrosis, mitochondria damage, and extracellular vesicle formation in Sprague-Dawley rats exposed to copper, manganese, and mercury, alone and in combination. Ultrastruct. Pathol. 2020, 44, 182–192. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, C.; Liu, C.; Yan, D.Y.; Tan, X.; Liu, K.; Jing, M.J.; Deng, Y.; Liu, W.; Xu, B. Manganese induces autophagy dysregulation: The role of S-nitrosylation in regulating autophagy related proteins in vivo and in vitro. Sci. Total Environ. 2020, 698, 134294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yan, J.; Bowman, A.B.; Bryan, M.R.; Singh, R.; Aschner, M. Dysregulation of TFEB contributes to manganese-induced autophagic failure and mitochondrial dysfunction in astrocytes. Autophagy 2020, 16, 1506–1523. [Google Scholar] [CrossRef]
- Chi, L.; Gao, B.; Bian, X.; Tu, P.; Ru, H.; Lu, K. Manganese-induced sex-specific gut microbiome perturbations in C57BL/6 mice. Toxicol. Appl. Pharmacol. 2017, 331, 142–153. [Google Scholar] [CrossRef]
- Fang, H.; Berg, E.; Cheng, X.; Shen, W. How to best assess abdominal obesity. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 360–365. [Google Scholar] [CrossRef]
- Lu, Y.; Tang, H.; Huang, P.; Wang, J.; Deng, P.; Li, Y.; Zheng, J.; Weng, L. Assessment of causal effects of visceral adipose tissue on risk of cancers: A Mendelian randomization study. Int. J. Epidemiol. 2022, 51, 1204–1218. [Google Scholar] [CrossRef]
- Ofenheimer, A.; Breyer-Kohansal, R.; Hartl, S.; Burghuber, O.C.; Krach, F.; Schrott, A.; Wouters, E.F.M.; Franssen, F.M.E.; Breyer, M.K. Reference values of body composition parameters and visceral adipose tissue (VAT) by DXA in adults aged 18–81 years-results from the LEAD cohort. Eur. J. Clin. Nutr. 2020, 74, 1181–1191. [Google Scholar] [CrossRef]
- Zhou, B.; Su, X.; Su, D.; Zeng, F.; Wang, M.H.; Huang, L.; Huang, E.; Zhu, Y.; Zhao, D.; He, D.; et al. Dietary intake of manganese and the risk of the metabolic syndrome in a Chinese population. Br. J. Nutr. 2016, 116, 853–863. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, Z. Cross-Sectional Studies: Strengths, Weaknesses, and Recommendations. Chest 2020, 158, S65–S71. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.L.G.; Araujo, C.F.S.; Dos Santos, N.R.; Bandeira, M.J.; Anjos, A.L.S.; Carvalho, C.F.; Lima, C.S.; Abreu, J.N.S.; Mergler, D.; Menezes-Filho, J.A. Airborne manganese exposure and neurobehavior in school-aged children living near a ferro-manganese alloy plant. Environ. Res. 2018, 167, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Mair, K.M.; Harvey, K.Y.; Henry, A.D.; Hillyard, D.Z.; Nilsen, M.; MacLean, M.R. Obesity alters oestrogen metabolism and contributes to pulmonary arterial hypertension. Eur. Respir. J. 2019, 53, 1801524. [Google Scholar] [CrossRef] [PubMed]

| Characteristic a | Quantile of Blood Mn Levels | p-Value b | |||
|---|---|---|---|---|---|
| Quantile 1 (1.88–7.59) n = 1827 | Quantile 2 (7.60–9.44) n = 1823 | Quantile 3 (9.45–11.86) n = 1823 | Quantile 4 (11.87–52.00) n = 1824 | ||
| Age (mean (SD)), year | 40.20 (11.60) | 39.25 (11.89) | 39.54 (11.61) | 38.78 (11.00) | 0.002 |
| BMI (mean (SD)), kg/m2 | 28.68 (6.66) | 28.98 (6.78) | 29.55 (7.10) | 29.13 (7.28) | 0.002 |
| PIR (mean (SD)) | 2.54 (1.66) | 2.55 (1.67) | 2.50 (1.65) | 2.49 (1.67) | 0.624 |
| 24 h energy intake (mean (SD)), kcal | 2364.94 (1100.27) | 2308.76 (1065.27) | 2223.33 (1010.60) | 2125.35 (950.04) | <0.001 |
| 24 h fat intake (mean (SD)), g | 91.92 (52.34) | 87.85 (50.36) | 85.58 (47.83) | 80.49 (45.05) | <0.001 |
| Gender | |||||
| Male | 1161 (63.5) | 1019 (55.9) | 896 (49.1) | 627 (34.4) | <0.001 |
| Female | 666 (36.5) | 804 (44.1) | 927 (50.9) | 1197 (65.6) | |
| Race | |||||
| Mexican American | 164 (9.0) | 198 (10.9) | 266 (14.6) | 332 (18.2) | <0.001 |
| Other Hispanic | 144 (7.9) | 181 (9.9) | 182 (10.0) | 182 (10.0) | |
| Non-Hispanic White | 737 (40.3) | 744 (40.8) | 704 (38.6) | 509 (27.9) | |
| Non-Hispanic Black | 640 (35.0) | 451 (24.7) | 308 (16.9) | 223 (12.2) | |
| Non-Hispanic Asian | 59 (3.2) | 153 (8.4) | 284 (15.6) | 501 (27.5) | |
| Other race—including multi-racial | 83 (4.5) | 96 (5.3) | 79 (4.3) | 77 (4.2) | |
| Educational level | |||||
| Less than 9th grade | 76 (4.2) | 93 (5.1) | 89 (4.9) | 124 (6.8) | <0.001 |
| 9–11th grade (includes 12th grade with no diploma) | 199 (10.9) | 199 (10.9) | 228 (12.5) | 205 (11.2) | |
| High school graduate/GED or equivalent | 447 (24.5) | 397 (21.8) | 375 (20.6) | 366 (20.1) | |
| College or AA degree | 657 (36.0) | 637 (34.9) | 594 (32.6) | 556 (30.5) | |
| College graduate or above | 448 (24.5) | 497 (27.3) | 537 (29.5) | 573 (31.4) | |
| BMI category, kg/m2 | |||||
| <25 | 573 (31.4) | 551 (30.2) | 531 (29.1) | 578 (31.7) | 0.046 |
| 25–29.9 | 608 (33.3) | 563 (30.9) | 552 (30.3) | 553 (30.3) | |
| ≥30 | 646 (35.4) | 709 (38.9) | 740 (40.6) | 693 (38.0) | |
| Marital status | |||||
| Married | 810 (44.3) | 822 (45.1) | 893 (49.0) | 933 (51.2) | <0.001 |
| Separated, divorced, or widowed | 286 (15.7) | 256 (14.0) | 268 (14.7) | 245 (13.4) | |
| Never married | 731 (40.0) | 745 (40.9) | 662 (36.3) | 646 (35.4) | |
| PIR | |||||
| <1.3 | 579 (31.7) | 589 (32.3) | 588 (32.3) | 614 (33.7) | 0.845 |
| 1.3–3.5 | 670 (36.7) | 649 (35.6) | 669 (36.7) | 636 (34.9) | |
| >3.5 | 578 (31.6) | 585 (32.1) | 566 (31.0) | 574 (31.5) | |
| 24 h alcohol consumption, g | |||||
| 0 | 1248 (68.3) | 1301 (71.4) | 1370 (75.2) | 1484 (81.4) | <0.001 |
| 0.1–4.9 | 16 (0.9) | 14 (0.8) | 10 (0.5) | 19 (1.0) | |
| 5–14.9 | 96 (5.3) | 95 (5.2) | 86 (4.7) | 50 (2.7) | |
| 15–29.9 | 131 (7.2) | 136 (7.5) | 105 (5.8) | 109 (6.0) | |
| ≥30 | 336 (18.4) | 277 (15.2) | 252 (13.8) | 162 (8.9) | |
| Smoking status | |||||
| Smoker | 824 (45.1) | 799 (43.8) | 683 (37.5) | 608 (33.3) | <0.001 |
| Non-smoker | 1003 (54.9) | 1024 (56.2) | 1140 (62.5) | 1216 (66.7) | |
| Physical activity, h/week | |||||
| 0 | 779 (42.6) | 809 (44.4) | 802 (44.0) | 869 (47.6) | 0.065 |
| >0–<1 | 63 (3.4) | 60 (3.3) | 67 (3.7) | 62 (3.4) | |
| ≥1–<3.5 | 428 (23.4) | 416 (22.8) | 451 (24.7) | 410 (22.5) | |
| ≥3.5–<6 | 225 (12.3) | 208 (11.4) | 228 (12.5) | 208 (11.4) | |
| ≥6 | 332 (18.2) | 330 (18.1) | 275 (15.1) | 275 (15.1) | |
| Characteristics | Minimally Adjusted Model a | Fully Adjusted Model b | ||
|---|---|---|---|---|
| β (95% CI) | p-Value | β (95% CI) | p-Value | |
| β per ln-unit change | ||||
| Total | 0.181 (0.144, 0.218) | <0.001 | 0.086 (0.059, 0.112) | <0.001 |
| Male | 0.102 (0.056, 0.148) | <0.001 | 0.049 (0.015, 0.082) | 0.005 |
| Female | 0.239 (0.183, 0.295) | <0.001 | 0.103 (0.064, 0.141) | <0.001 |
| β per quantile change | ||||
| Total | 0.055 (0.043, 0.066) | <0.001 | 0.025 (0.017, 0.033) | <0.001 |
| Male | 0.032 (0.018, 0.045) | <0.001 | 0.012 (0.002, 0.022) | 0.020 |
| Female | 0.076 (0.057, 0.094) | <0.001 | 0.036 (0.023, 0.048) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, C.; Huang, Y.; Huang, X.; Li, Z.; Fan, Y.; Zhang, Y.; Wan, T.; Lu, L.; Xu, Q.; Wu, W.; et al. Association between Blood Manganese Levels and Visceral Adipose Tissue in the United States: A Population-Based Study. Nutrients 2022, 14, 4770. https://doi.org/10.3390/nu14224770
Tao C, Huang Y, Huang X, Li Z, Fan Y, Zhang Y, Wan T, Lu L, Xu Q, Wu W, et al. Association between Blood Manganese Levels and Visceral Adipose Tissue in the United States: A Population-Based Study. Nutrients. 2022; 14(22):4770. https://doi.org/10.3390/nu14224770
Chicago/Turabian StyleTao, Chengzhe, Yuna Huang, Xuzhao Huang, Zhi Li, Yun Fan, Yan Zhang, Tingya Wan, Liyu Lu, Qiaoqiao Xu, Wei Wu, and et al. 2022. "Association between Blood Manganese Levels and Visceral Adipose Tissue in the United States: A Population-Based Study" Nutrients 14, no. 22: 4770. https://doi.org/10.3390/nu14224770
APA StyleTao, C., Huang, Y., Huang, X., Li, Z., Fan, Y., Zhang, Y., Wan, T., Lu, L., Xu, Q., Wu, W., Wu, D., Zhang, F., & Lu, C. (2022). Association between Blood Manganese Levels and Visceral Adipose Tissue in the United States: A Population-Based Study. Nutrients, 14(22), 4770. https://doi.org/10.3390/nu14224770







