The Synergic Effect of a Nutraceutical Supplementation Associated to a Mediterranean Hypocaloric Diet in a Population of Overweight/Obese Adults with NAFLD
Abstract
1. Introduction
2. Materials and Methods
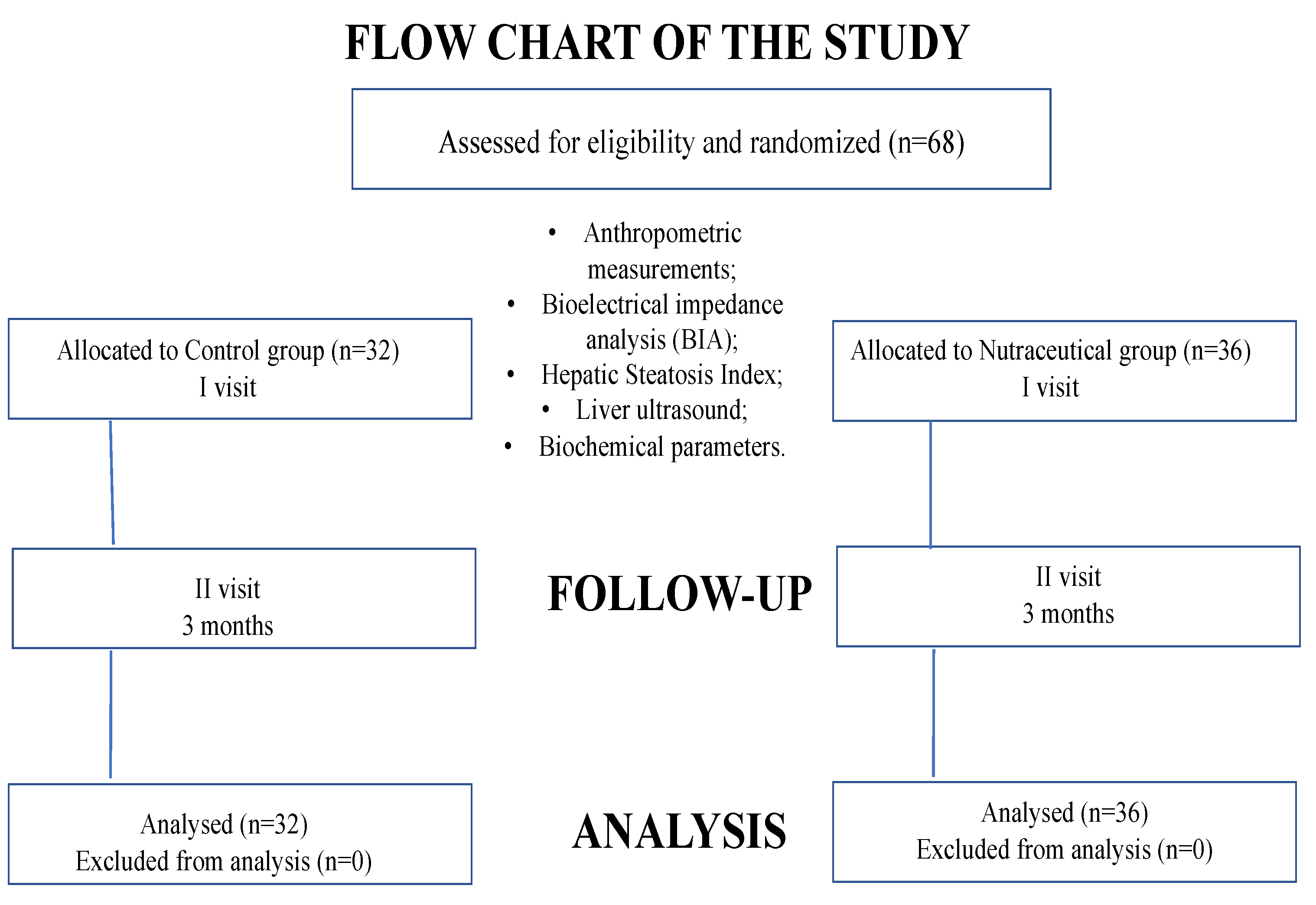
2.1. Study Design
2.2. Study Protocol
2.3. Dietary Treatment and Compliance
2.4. Statistical Analysis
3. Results
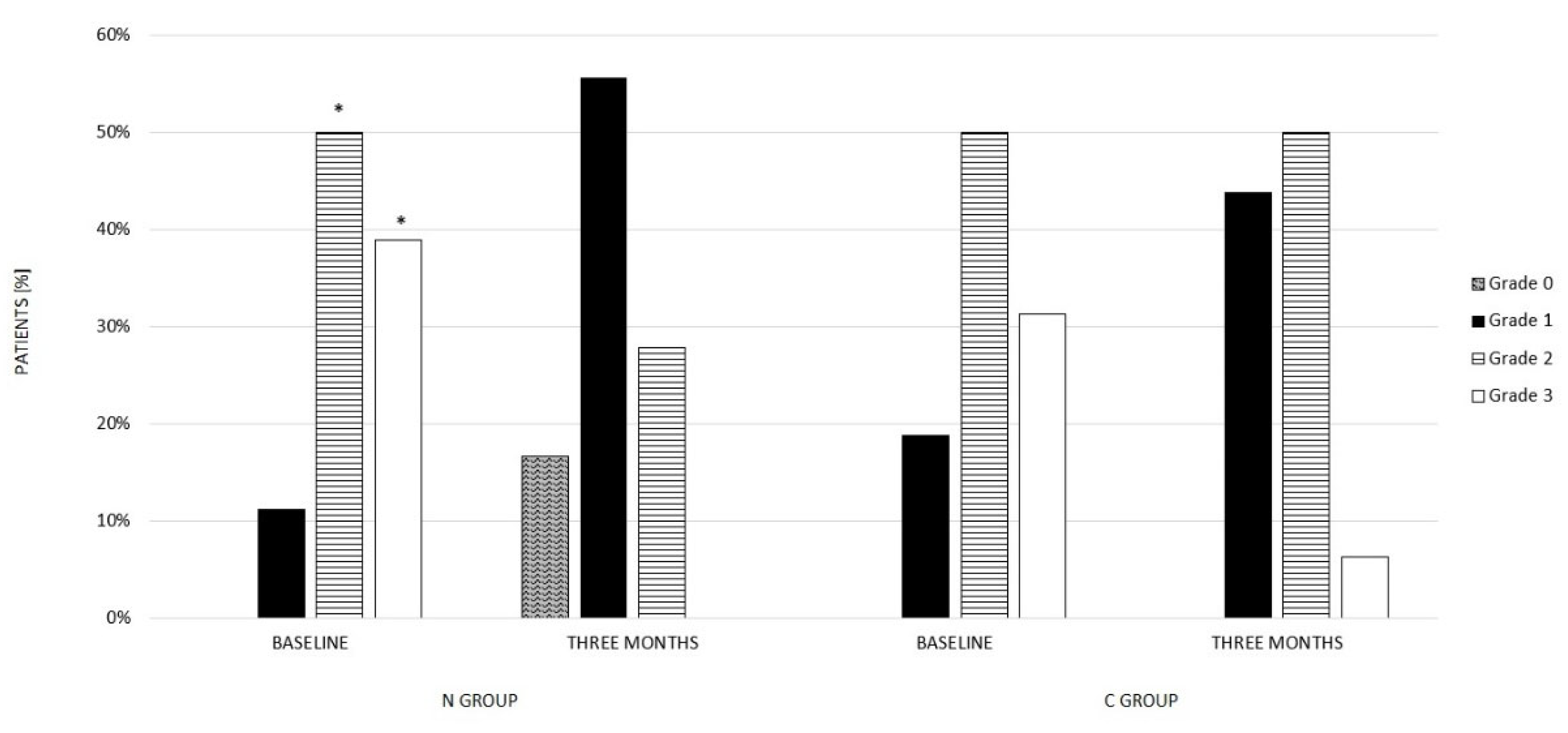
3.1. Nutraceutical Group (N Group)
3.2. Control Group (C Group)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, R.J.; Ahmed, A. Obesity and non-alcoholic fatty liver disease: Disparate associations among Asian populations. World J. Hepatol. 2014, 6, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Fukusato, T. Histopathology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J. Gastroenterol. 2014, 20, 15539–15548. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, P.; Hellerbrand, C. Non-alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Pr. Res. Clin. Gastroenterol. 2014, 28, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Flisiak-Jackiewicz, M.; Bobrus-Chociej, A.; Wasilewska, N.; Lebensztejn, D.M. From Nonalcoholic Fatty Liver Disease (NAFLD) to Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD)-New Terminology in Pediatric Patients as a Step in Good Scientific Direction? J. Clin. Med. 2021, 10, 924. [Google Scholar] [CrossRef]
- Godoy-Matos, A.F.; Silva Júnior, W.S.; Valerio, C.M. NAFLD as a continuum: From obesity to metabolic syndrome and diabetes. Diabetol. Metab. Syndr. 2020, 12, 60. [Google Scholar] [CrossRef]
- Yu, J.; Marsh, S.; Hu, J.; Feng, W.; Wu, C. The Pathogenesis of Nonalcoholic Fatty Liver Disease: Interplay between Diet, Gut Microbiota, and Genetic Background. Gastroenterol. Res. Pract. 2016, 2016, 2862173. [Google Scholar] [CrossRef]
- Bashir, A.; Duseja, A.; De, A.; Mehta, M.; Tiwari, P. Non-alcoholic fatty liver disease development: A multifactorial pathogenic phenomena. Liver Res. 2022, 6, 72–83. [Google Scholar] [CrossRef]
- Gerges, S.H.; Wahdan, S.A.; Elsherbiny, D.A.; El-Demerdash, E. Non-alcoholic fatty liver disease: An overview of risk factors, pathophysiological mechanisms, diagnostic procedures, and therapeutic interventions. Life Sci. 2021, 271, 119220. [Google Scholar] [CrossRef]
- Abd El-Kader, S.M.; El-Den Ashmawy, E.M. Non-alcoholic fatty liver disease: The diagnosis and management. World J. Hepatol. 2015, 7, 846–858. [Google Scholar] [CrossRef]
- Petroni, M.L.; Brodosi, L.; Marchignoli, F.; Musio, A.; Marchesini, G. Moderate Alcohol Intake in Non-Alcoholic Fatty Liver Disease: To Drink or Not to Drink? Nutrients 2019, 11, 3048. [Google Scholar] [CrossRef]
- Naveed, S.; Forrest, E.; Preiss, D. Non-alcoholic fatty liver disease. BMJ 2014, 349, g4596. [Google Scholar] [CrossRef]
- Oh, R.C.; Hustead, T.R.; Ali, S.M.; Pantsari, M.W. Mildly Elevated Liver Transaminase Levels: Causes and Evaluation. Am. Fam. Physician 2017, 96, 709–715. [Google Scholar]
- Mumtaz, S.; Schomaker, N.; von Roenn, N. Noninvasive Imaging Has Replaced Biopsy as the Gold Standard in the Evaluation of Nonalcoholic Fatty Liver Disease. Clin. Liver Dis. 2019, 13, 111–113. [Google Scholar] [CrossRef]
- Castera, L. Non-invasive tests for liver fibrosis in NAFLD: Creating pathways between primary healthcare and liver clinics. Liver Int. 2020, 40, 77–81. [Google Scholar] [CrossRef]
- Carneros, D.; López-Lluch, G.; Bustos, M. Physiopathology of Lifestyle Interventions in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients 2020, 12, 3472. [Google Scholar] [CrossRef]
- Fernández, T.; Viñuela, M.; Vidal, C.; Barrera, F. Lifestyle changes in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0263931. [Google Scholar] [CrossRef]
- Abenavoli, L.; Greco, M.; Milic, N.; Accattato, F.; Foti, D.; Gulletta, E.; Luzza, F. Effect of Mediterranean Diet and Antioxidant Formulation in Non-Alcoholic Fatty Liver Disease: A Randomized Study. Nutrients 2017, 9, 870. [Google Scholar] [CrossRef]
- Sumida, Y.; Yoneda, M. Current and future pharmacological therapies for NAFLD/NASH. J. Gastroenterol. 2018, 53, 362–376. [Google Scholar] [CrossRef]
- Rha, C.S.; Jeong, H.W.; Park, S.; Lee, S.; Jung, Y.S.; Kim, D.O. Antioxidative, Anti-Inflammatory, and Anticancer Effects of Purified Flavonol Glycosides and Aglycones in Green Tea. Antioxidants 2019, 8, 278. [Google Scholar] [CrossRef]
- Curcio, A.; Romano, A.; Cuozzo, S.; Nicola, A.D.; Grassi, O.; Schiaroli, D.; Nocera, G.F.; Pironti, M. Silymarin in Combination with Vitamin, C.; Vitamin, E.; Coenzyme Q10 and Selenome-thionine to Improve Liver Enzymes and Blood Lipid Profile in NAFLD Patients. Medicina 2020, 56, 544. [Google Scholar] [CrossRef] [PubMed]
- Aller, R.; Izaola, O.; Gómez, S.; Tafur, C.; González, G.; Berroa, E.; Mora, N.; González, J.M.; de Luis, D.A. Effect of silymarin plus vitamin E in patients with non-alcoholic fatty liver disease. A randomized clinical pilot study. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3118–3124. [Google Scholar] [PubMed]
- Del Ben, M.; Polimeni, L.; Baratta, F.; Pastori, D.; Angelico, F. The role of nutraceuticals for the treatment of non-alcoholic fatty liver disease. Br. J. Clin. Pharmacol. 2017, 83, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Maboud, M.; Menshawy, A.; Menshawy, E.; Emara, A.; Alshandidy, M.; Eid, M. The efficacy of vitamin E in reducing non-alcoholic fatty liver disease: A systematic review, meta-analysis, and meta-regression. Ther. Adv. Gastroenterol. 2020, 13, 1756284820974917. [Google Scholar] [CrossRef] [PubMed]
- Weschawalit, S.; Thongthip, S.; Phutrakool, P.; Asawanonda, P. Glutathione and its antiaging and antimelanogenic effects. Clin. Cosmet. Investig. Dermatol. 2017, 10, 147–153. [Google Scholar] [CrossRef]
- Honda, Y.; Kessoku, T.; Sumida, Y.; Kobayashi, T.; Kato, T.; Ogawa, Y.; Tomeno, W.; Imajo, K.; Fujita, K.; Yoneda, M.; et al. Efficacy of glutathione for the treatment of nonalcoholic fatty liver disease: An open-label, single-arm, multicenter, pilot study. BMC Gastroenterol. 2017, 17, 96. [Google Scholar] [CrossRef]
- Kołota, A.; Głąbska, D. Dietary Intake of Milk Thistle Seeds as a Source of Silymarin and Its Influence on the Lipid Parameters in Nonalcoholic Fatty Liver Disease Patients. Appl. Sci. 2021, 11, 5836. [Google Scholar] [CrossRef]
- Mahamid, M.; Mahroum, N.; Bragazzi, N.L.; Shalaata, K.; Yavne, Y.; Adawi, M.; Amital, H.; Watad, A. Folate and B12 Levels Correlate with Histological Severity in NASH Patients. Nutrients 2018, 10, 440. [Google Scholar] [CrossRef]
- Pacana, T.; Cazanave, S.; Verdianelli, A.; Patel, V.; Min, H.K.; Mirshahi, F.; Quinlivan, E.; Sanyal, A.J. Dysregulated Hepatic Methionine Metabolism Drives Homocysteine Elevation in Diet-Induced Nonalcoholic Fatty Liver Disease. PLoS ONE 2015, 10, e0136822. [Google Scholar] [CrossRef]
- Mardinoglu, A.; Ural, D.; Zeybel, M.; Yuksel, H.H.; Uhlén, M.; Borén, J. The Potential Use of Metabolic Cofactors in Treatment of NAFLD. Nutrients 2019, 11, 1578. [Google Scholar] [CrossRef]
- Mourad, A.M.; de Carvalho Pincinato, E.; Mazzola, P.G.; Sabha, M.; Moriel, P. Influence of soy lecithin administration on hypercholesterolemia. Cholesterol 2010, 2010, 824813. [Google Scholar] [CrossRef]
- Siervo, M.; Stephan, B.C.; Nasti, G.; Colantuoni, A. Ageing, adiposity indexes and low muscle mass in a clinical sample of overweight and obese women. Obes. Res. Clin. Pr. 2012, 6, e1–e90. [Google Scholar] [CrossRef]
- Piccoli, A. Patterns of bioelectrical impedance vector analysis: Learning from electrocardiography and forgetting electric circuit models. Nutrition 2002, 18, 520–521. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, D.; Kim, H.J.; Lee, C.H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.H.; Cho, S.H.; Sung, M.W.; et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef]
- Han, A.L.; Lee, H.K. Comparison of the Diagnostic Performance of Steatosis Indices for Discrimination of CT-Diagnosed Metabolic Dysfunction-Associated Fatty Liver Disease. Metabolites 2022, 12, 664. [Google Scholar] [CrossRef]
- SINU. LARN—Livelli di Assunzione di Riferimento di Nutrienti ed Energia per la Popolazione Italiana—IV Revisione. 2014. Available online: https://sinu.it/tabelle-larn-2014/ (accessed on 5 November 2022).
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef]
- Amanullah, I.; Khan, Y.H.; Anwar, I.; Gulzar, A.; Mallhi, T.H.; Raja, A.A. Effect of vitamin E in non-alcoholic fatty liver disease: A systematic review and meta-analysis of randomised controlled trials. Postgrad. Med. J. 2019, 95, 601–611. [Google Scholar] [CrossRef]
- El Hadi, H.; Vettor, R.; Rossato, M. Vitamin E as a Treatment for Nonalcoholic Fatty Liver Disease: Reality or Myth? Antioxidants 2018, 7, 12. [Google Scholar] [CrossRef]
- Usman, M.; Bakhtawar, N. Vitamin E as an Adjuvant Treatment for Non-alcoholic Fatty Liver Disease in Adults: A Systematic Review of Randomized Controlled Trials. Cureus 2020, 12, e9018. [Google Scholar] [CrossRef]
- Nobili, V.; Alisi, A.; Mosca, A.; Crudele, A.; Zaffina, S.; Denaro, M.; Smeriglio, A.; Trombetta, D. The Antioxidant Effects of Hydroxytyrosol and Vitamin E on Pediatric Nonalcoholic Fatty Liver Disease, in a Clinical Trial: A New Treatment? Antioxid. Redox Signal. 2019, 31, 127–133. [Google Scholar] [CrossRef]
- Nobili, V.; Manco, M.; Devito, R.; Ciampalini, P.; Piemonte, F.; Marcellini, M. Effect of vitamin E on aminotransferase levels and insulin resistance in children with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2006, 24, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Mosca, A.; Crudele, A.; Smeriglio, A.; Braghini, M.R.; Panera, N.; Comparcola, D.; Alterio, A.; Sartorelli, M.R.; Tozzi, G.; Raponi, M.; et al. Antioxidant activity of Hydroxytyrosol and Vitamin E reduces systemic inflammation in children with paediatric NAFLD. Dig. Liver Dis. 2021, 53, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- D’Espessailles, A.; Campos, V.; Juretić, N.; Tapia, G.S.; Pettinelli, P. Hepatic retinaldehyde dehydrogenases are modulated by tocopherol supplementation in mice with hepatic steatosis. Nutrition 2022, 94, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Panera, N.; Braghini, M.R.; Crudele, A.; Smeriglio, A.; Bianchi, M.; Condorelli, A.G.; Nobili, R.; Conti, L.A.; De Stefanis, C.; Lioci, G.; et al. Combination Treatment with Hydroxytyrosol and Vitamin E Improves NAFLD-Related Fibrosis. Nutrients 2022, 14, 3791. [Google Scholar] [CrossRef] [PubMed]
- Irie, M.; Sohda, T.; Anan, A.; Fukunaga, A.; Takata, K.; Tanaka, T.; Yokoyama, K.; Morihara, D.; Takeyama, Y.; Shakado, S.; et al. Reduced Glutathione suppresses Oxidative Stress in Nonalcoholic Fatty Liver Disease. Euroasian J. Hepatogastroenterol. 2016, 6, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Marin, V.; Gazzin, S.; Gambaro, S.E.; Dal Ben, M.; Calligaris, S.; Anese, M.; Raseni, A.; Avellini, C.; Giraudi, P.J.; Tiribelli, C.; et al. Effects of Oral Administration of Silymarin in a Juvenile Murine Model of Non-alcoholic Steatohepatitis. Nutrients 2017, 9, 1006. [Google Scholar] [CrossRef]
- Fatma, A.M.; Mahmoud, H.A.; Hanan, H.A.; Hashem, G.; Mahmoud, M.; Hesham, M. The Beneficial Effects of Silymarin in Treatment of Experimentally Induced Non-Alcoholic Fatty Liver Disease in Rats Med. J. Cairo Univ. 2018, 86, 1055–1064. [Google Scholar] [CrossRef]
- Mengesha, T.; Gnanasekaran, N.; Mehare, T. Hepatoprotective effect of silymarin on fructose induced nonalcoholic fatty liver disease in male albino wistar rats. BMC Complement. Med. Ther. 2021, 21, 104. [Google Scholar] [CrossRef]
- Gillessen, A.; Schmidt, H.H. Silymarin as Supportive Treatment in Liver Diseases: A Narrative Review. Adv. Ther. 2020, 37, 1279–1301. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Nutritional Information® | |
|---|---|
| Ingredients | Amount per Serving |
| Tocopherol (Vit. E) | 10 mg |
| Cyanocobalamin (Vit. B12) | 1 mg |
| L-Methionine | 100 mg |
| L-Cysteine | 100 mg |
| L-Glutathione | 50 mg |
| Soy Phospholipidis | 40 mg |
| Silybum marianum E.S. tit. min.70% in silimarina | 285.72 mg |
| Nutritional Assessment and Body Composition | ||
|---|---|---|
| Nutraceutical Group (n = 36) | Control Group (n = 32) | |
| Male, n° (%) | 16 (44.4) | 16 (50) |
| Age, years | 56.1 ± 9.9 | 59.8 ± 10.9 |
| Weight, kg | 100.3 ± 19.2 | 92.1 ± 17.8 |
| BMI, kg/m2 | 36.0 ± 8.6 | 33.9 ± 6.6 |
| Waist circumference, cm | 110.0 ± 15.4 | 104.1 ± 11.3 |
| Hip circumference, cm | 117.8 ± 13.9 | 113.1 ± 13.7 |
| TBW % | 46.9 ± 8.7 | 48.5 ± 7.8 |
| FM % | 36.9 ± 12.5 | 35.2 ± 11.4 |
| FFM % | 63.1 ± 12.5 | 62.7 ± 11.9 |
| Comorbidities | ||
| Nutraceutical Group (n = 36) | Control Group (n = 32) | |
| ULS grade | ||
| Patients, n° (%) | ||
| Mild | 4 (11.1) | 6 (18.8) |
| Moderate | 18 (50) | 16 (50) |
| Severe | 14 (38.9) | 10 (31.2) |
| Hepatic Steatosis Index | 49.3 ± 10.1 | 46.9 ± 7.6 |
| Diabetes n (%) | ||
| No prediabetes or diabetes | 24 (66.7) | 24 (75) |
| Prediabetes | 12 (33.3) | 8 (25) |
| Diabetes | 0 (0) | 0 (0) |
| Plasma Metabolic Parameteres | ||
| Nutraceutical Group (n = 36) | Control Group (n = 32) | |
| Glucose, mg/dL | 105.6 ± 25.9 | 101.1 ± 22.5 |
| Total Cholesterol, mg/dL | 207.9 ± 49.7 | 213.1 ± 42.3 |
| HDL Cholesterol, mg/dL | 40.9 ± 13.8 | 59.4 ± 19.6 * |
| LDL Cholesterol, mg/dL | 204.3 ± 56.9 | 180.5 ± 41.2 * |
| LDL/HDL ratio | 5.6 ± 3.1 | 3.4 ± 1.4 * |
| TG/HDL ratio | 4.3 ± 2.4 | 2.7 ± 1.8 * |
| Triglyceride, mg/dL | 159.4 ± 65.9 | 133.8 ± 53.0 |
| AST | 38.5 ± 15.6 | 36.9 ± 13.0 |
| ALT | 53.8 ± 28.8 | 54.0 ± 21.1 |
| Other Features | ||
| Nutraceutical Group (n =36) | Control Group (n = 32) | |
| Current smoking n (%) | 26 (72) | 22 (69) |
| Nutritional Assessment and Body Composition | ||||
| Nutraceutical Group (n = 36) | Control Group (n = 32) | |||
| T0 | T1 | T0 | T1 | |
| Body Weight, kg | 100.3 ± 19.2 | 92.8 ± 19.6 * | 92.1 ± 17.8 | 84.7 ± 12.2 * |
| BMI, kg/m2 | 36.7 ± 8.6 | 34.0 ± 8.4 * | 33.9 ± 6.6 | 31.8 ± 5.7 * |
| Waist circumference, cm | 110.0 ± 15.4 | 103.7 ± 14.9 * | 104.1 ± 11.3 | 97.5 ± 11.0 * |
| Hip circumference, cm | 117.8 ± 13.9 | 114.0 ± 12.1 * | 113.1 ± 13.7 | 110.3 ± 11.8 * |
| TBW % | 46.9 ± 8.7 | 49.3 ± 9.2 * | 48.5 ± 7.8 | 50.5 ± 7.3 * |
| FM % | 36.9 ± 12.5 | 33.7 ± 13.6 * | 35.2 ± 11.4 | 32.6 ± 10.7 * |
| FFM % | 63.1 ± 12.5 | 66.0 ± 13.6 * | 62.7 ± 11.9 | 67.3 ± 10.8 * |
| Comorbidities | ||||
| Nutraceutical Group (n = 36) | Control Group (n = 32) | |||
| T0 | T1 | T0 | T1 | |
| ULS grade | ||||
| Patients, n° (%) | ||||
| Absent | 0 (0) | 6 (16.6) | 0 (0) | 0 (0) |
| Mild | 4 (11.1) | 20 (55.6) | 6 (18.8) | 14 (44) |
| Moderate | 18 (50) | 10 (27.8) | 16 (50) | 16 (50) |
| Severe | 14 (38.9) | 0 (0) | 10 (31.3) | 2 (6) |
| Hepatic Steatosis Index | 49.3 ± 10.1 | 43.3 ± 9.0 * | 46.9 ± 7.6 | 45.9 ± 6.9 |
| Plasma Metabolic Parameteres | ||||
| Nutraceutical Group (n = 36) | Control Group (n = 32) | |||
| T0 | T1 | T0 | T1 | |
| Glucose, mg/dL | 105.6 ± 25.9 | 93.0 ± 15.4 * | 101.1 ± 22.5 | 92.8 ± 13.8 * |
| Total Cholesterol, mg/dL | 207.9 ± 49.7 | 161.4 ± 31.4 * | 213.1 ± 42.3 | 187.7 ± 36.0 *° |
| HDL Cholesterol, mg/dL | 40.9 ± 13.8 | 42.9 ± 13.9 | 59.4 ± 19.6 | 58.6 ± 11.6 |
| LDLCholesterol, mg/dL | 204.3 ± 56.9 | 143.0 ± 37.1 * | 180.5 ± 41.2 | 150.2 ± 31.7 *° |
| LDL/HDL ratio | 5.6 ± 3.1 | 3.6 ± 1.6 * | 3.4 ± 1.4 | 2.6 ± 0.6 * |
| TG/HDL ratio | 4.3 ± 2.4 | 3.1 ± 1.6 * | 2.7 ± 1.8 | 1.8 ± 0.6 * |
| Triglyceride, mg/dL | 159.4 ± 65.9 | 122.9 ± 43.4 * | 133.8 ± 53.0 | 105.5 ± 35.4 * |
| AST | 38.5 ± 15.6 | 25.0 ± 9.4 * | 36.9 ± 13.0 | 24.6 ± 17.4 * |
| ALT | 53.8 ± 28.8 | 29.4 ± 9.7 * | 54.0 ± 21.1 | 38.3 ± 10.6 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiurazzi, M.; Cacciapuoti, N.; Di Lauro, M.; Nasti, G.; Ceparano, M.; Salomone, E.; Guida, B.; Lonardo, M.S. The Synergic Effect of a Nutraceutical Supplementation Associated to a Mediterranean Hypocaloric Diet in a Population of Overweight/Obese Adults with NAFLD. Nutrients 2022, 14, 4750. https://doi.org/10.3390/nu14224750
Chiurazzi M, Cacciapuoti N, Di Lauro M, Nasti G, Ceparano M, Salomone E, Guida B, Lonardo MS. The Synergic Effect of a Nutraceutical Supplementation Associated to a Mediterranean Hypocaloric Diet in a Population of Overweight/Obese Adults with NAFLD. Nutrients. 2022; 14(22):4750. https://doi.org/10.3390/nu14224750
Chicago/Turabian StyleChiurazzi, Martina, Nunzia Cacciapuoti, Mariastella Di Lauro, Gilda Nasti, Margherita Ceparano, Elisabetta Salomone, Bruna Guida, and Maria Serena Lonardo. 2022. "The Synergic Effect of a Nutraceutical Supplementation Associated to a Mediterranean Hypocaloric Diet in a Population of Overweight/Obese Adults with NAFLD" Nutrients 14, no. 22: 4750. https://doi.org/10.3390/nu14224750
APA StyleChiurazzi, M., Cacciapuoti, N., Di Lauro, M., Nasti, G., Ceparano, M., Salomone, E., Guida, B., & Lonardo, M. S. (2022). The Synergic Effect of a Nutraceutical Supplementation Associated to a Mediterranean Hypocaloric Diet in a Population of Overweight/Obese Adults with NAFLD. Nutrients, 14(22), 4750. https://doi.org/10.3390/nu14224750






