Effects of Enzamin, a Microbial Product, on Alterations of Intestinal Microbiota Induced by a High-Fat Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animal Experiments
2.3. Isolation of DNA from Stool
2.4. Sequencing of 16S rRNA Gene and Microbiome Analysis
2.5. Blood Analyses
2.6. Immunoblotting
2.7. Statistics
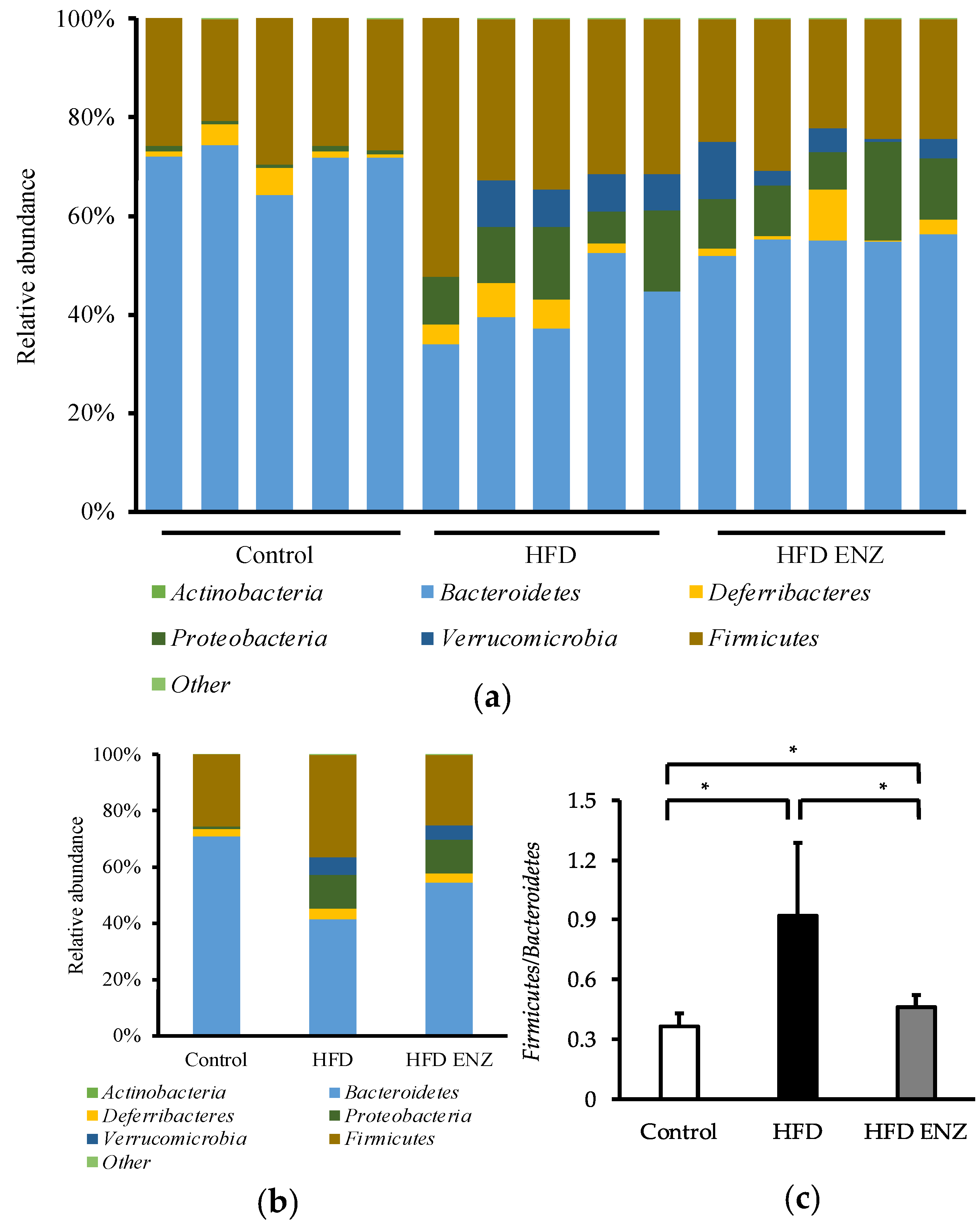
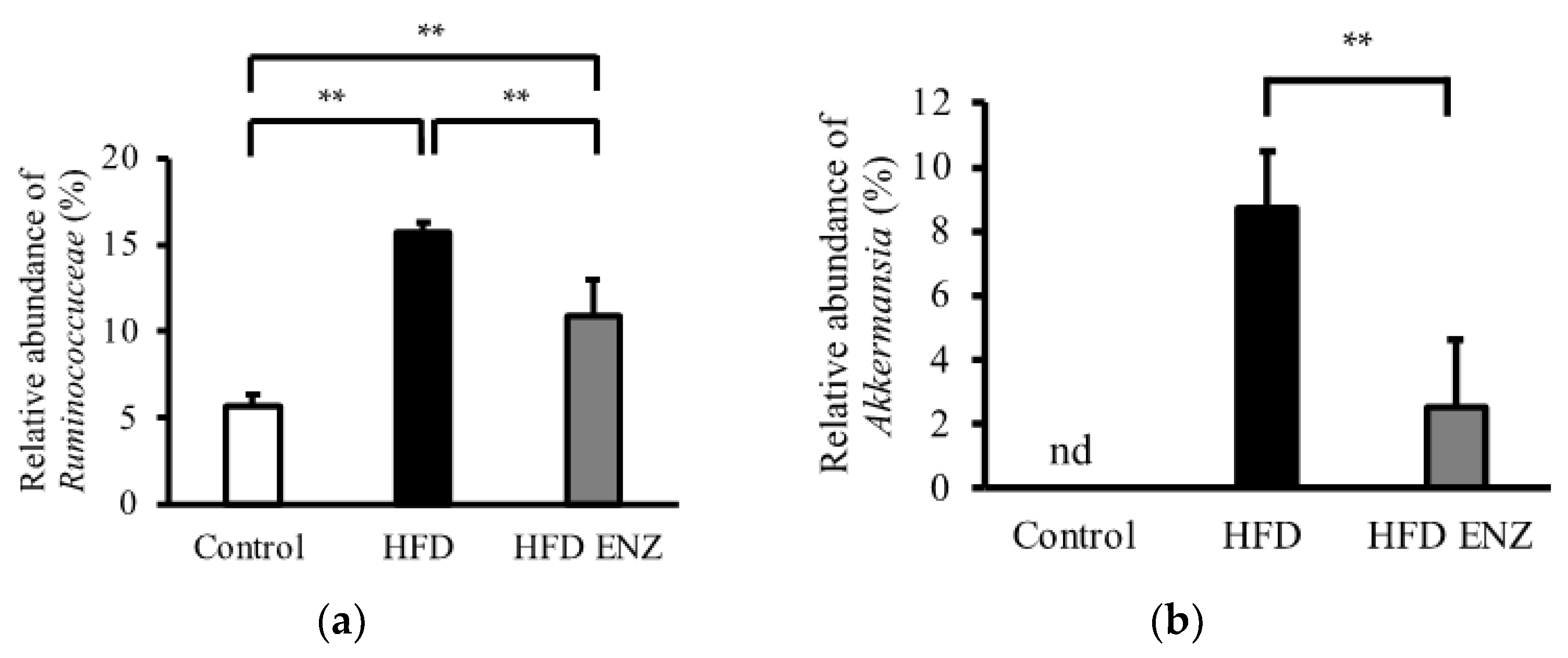
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, M.; Shi, B. Gut microbiota as a potential target of metabolic syndrome: The role of probiotics and prebiotics. Cell Biosci. 2017, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Bäckhed, F.; Fulton, L.; Gordon, J.I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 2008, 3, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Schneeberger, M.; Everard, A.; Gómez-Valadés, A.G.; Matamoros, S.; Ramírez, S.; Delzenne, N.M.; Gomis, R.; Claret, M.; Cani, P.D. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci. Rep. 2015, 5, 16643. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.N.; Amand, A.L.S.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; McKenzie, C.I.; Hijikata, A.; Wong, C.; et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.-L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti–PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef]
- Candore, G.; Caruso, C.; Jirillo, E.; Magrone, T.; Vasto, S. Low grade inflammation as a common pathogenetic denominator in age-related diseases: Novel drug targets for anti-ageing strategies and successful ageing achievement. Curr. Pharm. Des. 2010, 16, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Rurangwa, E.; Laranja, J.L.; Van Houdt, R.; Delaedt, Y.; Geraylou, Z.; van de Wiele, T.; van Loo, J.; van Craeyveld, V.; Courtin, C.M.; Delcour, J.A.; et al. Selected nondigestible carbohydrates and prebiotics support the growth of probiotic fish bacteria monocultures in vitro. J. Appl. Microbiol. 2009, 106, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.W.; Wang, S.L. Recent advances in exopolysaccharides from Paenibacillus spp.: Production, Isolation, Structure, and Bioactivities. Mar. Drugs 2015, 13, 1847–1863. [Google Scholar] [CrossRef] [PubMed]
- Daud, N.S.; Rosli, M.A.; Azam, Z.M.; Othman, N.Z.; Sarmidi, M.R. Paenibacillus polymyxa bioactive compounds for agricultural and biotechnological applications. Biocatal. Agric. Biotechnol. 2019, 18, 101092. [Google Scholar] [CrossRef]
- Ishii, M.; Matsumoto, Y.; Sekimizu, K. Bacterial polysaccharides inhibit sucrose-induced hyperglycemia in silkworms. Drug Discov. Ther. 2018, 12, 185–188. [Google Scholar] [CrossRef]
- Tamura, Y.; Okada, K.; Kawao, N.; Yano, M.; Ueshima, S.; Nagai, N.; Matsuo, O. Profibrinolytic effect of Enzamin, an extract of metabolic products from Bacillus subtilis AK and Lactobacillus. J. Thromb. Thrombolysis 2011, 32, 195–200. [Google Scholar] [CrossRef]
- Tamura, Y.; Yano, M.; Kawao, N.; Okumoto, K.; Ueshima, S.; Kaji, H.; Matsuo, O. Enzamin ameliorates adipose tissue inflammation with impaired adipocytokine expression and insulin resistance in db/db mice. J. Nutr. Sci. 2013, 2, e37. [Google Scholar] [CrossRef]
- Abujamel, T.S.; Al-Otaibi, N.M.; Abuaish, S.; AlHarbi, R.H.; Assas, M.B.; Alzahrani, S.A.; Alotaibi, S.M.; El-Ansary, A.; Aabed, K. Different Alterations in Gut Microbiota between Bifidobacterium longum and Fecal Microbiota Transplantation Treatments in Propionic Acid Rat Model of Autism. Nutrients 2022, 14, 608. [Google Scholar] [CrossRef]
- Kim, K.A.; Gu, W.; Lee, I.A.; Joh, E.-H.; Kim, D.-H. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS ONE 2012, 7, e47713. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Feng, Y.; Huang, Y.; Wang, Y.; Wang, P.; Song, H.; Wang, F. Antibiotics induced intestinal tight junction barrier dysfunction is associated with microbiota dysbiosis, activated NLRP3 inflammasome and autophagy. PLoS ONE 2019, 14, e0218384. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Henke, M.T.; Kenny, D.J.; Cassilly, C.D.; Clardy, J. Ruminococcus gnavus, a member of the human gut microbiome associated with Crohn’s disease, produces an inflammatory polysaccharide. Proc. Natl. Acad. Sci. USA 2019, 116, 12672–12677. [Google Scholar] [CrossRef]
- Ganesh, B.P.; Klopfleisch, R.; Loh, G.; Blaut, M. Commensal Akkermansia muciniphila exacerbates gut inflammation in Salmonella Typhimurium-infected gnotobiotic mice. PLoS ONE 2013, 8, e74963. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.; Phillipson, M.; Petersson, J.; Velcich, A.; Holm, L.; Hansson, G.C. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 15064–15069. [Google Scholar] [CrossRef]
- Sato, J.; Kanazawa, A.; Ikeda, F.; Yoshihara, T.; Goto, H.; Abe, H.; Komiya, K.; Kawaguchi, M.; Shimizu, T.; Ogihara, T.; et al. Gut dysbiosis and detection of “live gut bacteria” in blood of Japanese patients with type 2 diabetes. Diabetes Care 2014, 37, 2343–2350. [Google Scholar] [CrossRef]
- Cani, P.D.; Osto, M.; Geurts, L.; Everard, A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes 2012, 3, 279–288. [Google Scholar] [CrossRef]
- Amar, J.; Chabo, C.; Waget, A.; Klopp, P.; Vachoux, C.; Bermúdez-Humarán, L.G.; Smirnova, N.; Bergé, M.; Sulpice, T.; Lahtinen, S.; et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: Molecular mechanisms and probiotic treatment. EMBO Mol. Med. 2011, 3, 559–572. [Google Scholar] [CrossRef]
- Caesar, R.; Reigstad, S.C.; Bäckhed, H.K.; Reinhardt, C.; Ketonen, M.; Lundén, G.Ö.; Cani, P.D.; Bäckhed, F. Gut-derived lipopolysaccharide augments adipose macrophage accumulation but is not essential for impaired glucose or insulin tolerance in mice. Gut 2012, 61, 1701–1707. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, J.; Liu, Z.; Li, Z.; Teng, L.; Wang, D. Inonotus hispidus Protects against Hyperlipidemia by Inhibiting Oxidative Stress and Inflammation through Nrf2/NF-κB Signaling in High Fat Diet Fed Mice. Nutrients 2022, 14, 3477. [Google Scholar] [CrossRef]

| Control | HFD | HFD ENZ | |
|---|---|---|---|
| Initial body weight (g) | 14.6 ± 1.1 | 14.5 ± 0.9 | 14.3 ± 1.0 |
| Body weight after 4 weeks (g) | 23.6 ± 1.1 | 30.0 ± 2.4 ** | 29.9 ± 3.6 ** |
| Control | HFD | HFD ENZ | |
|---|---|---|---|
| LPS (ng/mL) | 22.3 ± 3.6 | 63.3 ± 12.4 ** | 46.0 ± 6.6 **,# |
| TG (mg/dL) | 65.9 ± 9.3 | 125.0 ± 8.4 ** | 95.1 ± 9.4 **,## |
| TC (mg/dL) | 79.0 ± 7.3 | 131.5 ± 6.8 ** | 108.4 ± 12.9 **,## |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasuzawa, T.; Nishi, R.; Ishitani, S.; Matsuo, O.; Ueshima, S. Effects of Enzamin, a Microbial Product, on Alterations of Intestinal Microbiota Induced by a High-Fat Diet. Nutrients 2022, 14, 4743. https://doi.org/10.3390/nu14224743
Yasuzawa T, Nishi R, Ishitani S, Matsuo O, Ueshima S. Effects of Enzamin, a Microbial Product, on Alterations of Intestinal Microbiota Induced by a High-Fat Diet. Nutrients. 2022; 14(22):4743. https://doi.org/10.3390/nu14224743
Chicago/Turabian StyleYasuzawa, Toshinori, Ryota Nishi, Satono Ishitani, Osamu Matsuo, and Shigeru Ueshima. 2022. "Effects of Enzamin, a Microbial Product, on Alterations of Intestinal Microbiota Induced by a High-Fat Diet" Nutrients 14, no. 22: 4743. https://doi.org/10.3390/nu14224743
APA StyleYasuzawa, T., Nishi, R., Ishitani, S., Matsuo, O., & Ueshima, S. (2022). Effects of Enzamin, a Microbial Product, on Alterations of Intestinal Microbiota Induced by a High-Fat Diet. Nutrients, 14(22), 4743. https://doi.org/10.3390/nu14224743







