Effects of an Intermittent Fasting 5:2 Plus Program on Body Weight in Chinese Adults with Overweight or Obesity: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Characteristics
2.2. Outcomes
2.3. Data Collection
2.4. Statistical Analysis
3. Results
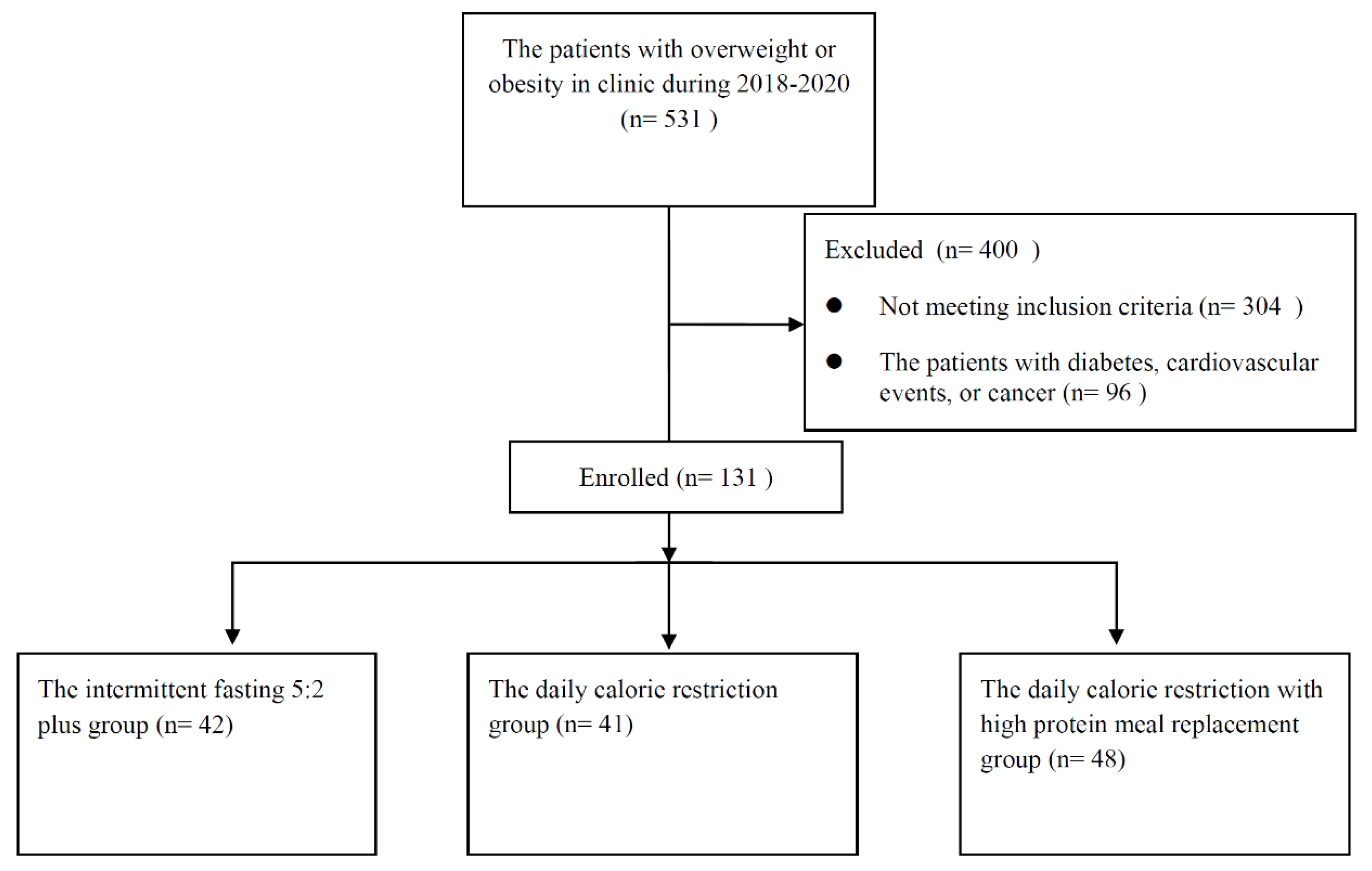
3.1. Patient Characteristics
3.2. The Differences among Groups
3.3. The Differences within Groups
3.4. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, Q.; Yang, Y.; Zheng, S.; Wang, Y.; Lu, W. The prevalence and increasing trends of overweight, general obesity, and abdominal obesity among Chinese adults: A repeated cross-sectional study. BMC Public Health 2019, 19, 1293. [Google Scholar] [CrossRef] [PubMed]
- Lean, M.E.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): An open-label, cluster-randomised trial. Lancet 2018, 391, 541–551. [Google Scholar] [CrossRef]
- Brun, J.F.; Myzia, J.; Varlet-Marie, E.; Raynaud de Mauverger, E.; Mercier, J. Beyond the Calorie Paradigm: Taking into Account in Practice the Balance of Fat and Carbohydrate Oxidation during Exercise? Nutrients 2022, 14, 1605. [Google Scholar] [CrossRef] [PubMed]
- Sayón-Orea, C.; Razquin, C.; Bulló, M.; Corella, D.; Fitó, M.; Romaguera, D.; Vioque, J.; Alonso-Gómez, Á.M.; Wärnberg, J.; Martínez, J.A.; et al. Effect of a Nutritional and Behavioral Intervention on Energy-Reduced Mediterranean Diet Adherence Among Patients with Metabolic Syndrome: Interim Analysis of the PREDIMED-Plus Randomized Clinical Trial. JAMA 2019, 322, 1486–1499. [Google Scholar] [CrossRef]
- Welton, S.; Minty, R.; O’Driscoll, T.; Willms, H.; Poirier, D.; Madden, S.; Kelly, L. Intermittent fasting and weight loss: Systematic review. Can. Fam. Physician 2020, 66, 117–125. [Google Scholar]
- Roman, Y.M.; Dominguez, M.C.; Easow, T.M.; Pasupuleti, V.; White, C.M.; Hernandez, A.V. Effects of intermittent versus continuous dieting on weight and body composition in obese and overweight people: A systematic review and meta-analysis of randomized controlled trials. Int. J. Obes. 2019, 43, 2017–2027. [Google Scholar] [CrossRef]
- de Cabo, R.; Mattson, M.P. Effects of Intermittent Fasting on Health, Aging, and Disease. N. Engl. J. Med. 2019, 381, 2541–2551, Erratum in 2020, 382, 298. Erratum in 2020, 382, 978. [Google Scholar] [CrossRef]
- Patterson, R.E.; Sears, D.D. Metabolic Effects of Intermittent Fasting. Annu. Rev. Nutr. 2017, 37, 371–393. [Google Scholar] [CrossRef]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef]
- Headland, M.; Clifton, P.M.; Carter, S.; Keogh, J.B. Weight-Loss Outcomes: A Systematic Review and Meta-Analysis of Intermittent Energy Restriction Trials Lasting a Minimum of 6 Months. Nutrients 2016, 8, 354. [Google Scholar] [CrossRef] [PubMed]
- Sundfør, T.M.; Svendsen, M.; Tonstad, S. Effect of intermittent versus continuous energy restriction on weight loss, maintenance and cardiometabolic risk: A randomized 1-year trial. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 698–706. [Google Scholar] [CrossRef]
- Headland, M.L.; Clifton, P.M.; Keogh, J.B. Effect of intermittent compared to continuous energy restriction on weight loss and weight maintenance after 12 months in healthy overweight or obese adults. Int. J. Obes. 2019, 43, 2028–2036, Erratum in 2019, 43, 942. [Google Scholar] [CrossRef] [PubMed]
- Cienfuegos, S.; Gabel, K.; Kalam, F.; Ezpeleta, M.; Wiseman, E.; Pavlou, V.; Lin, S.; Oliveira, M.L.; Varady, K.A. Effects of 4- and 6-h Time-Restricted Feeding on Weight and Cardiometabolic Health: A Randomized Controlled Trial in Adults with Obesity. Cell Metab. 2020, 32, 366.e3–378.e3. [Google Scholar] [CrossRef] [PubMed]
- Trepanowski, J.F.; Kroeger, C.M.; Barnosky, A.; Klempel, M.C.; Bhutani, S.; Hoddy, K.K.; Gabel, K.; Freels, S.; Rigdon, J.; Rood, J.; et al. Effect of Alternate-Day Fasting on Weight Loss, Weight Maintenance, and Cardioprotection Among Metabolically Healthy Obese Adults: A Randomized Clinical Trial. JAMA Intern. Med. 2017, 177, 930–938. [Google Scholar] [CrossRef]
- Scholtens, E.L.; Krebs, J.D.; Corley, B.T.; Hall, R.M. Intermittent fasting 5:2 diet: What is the macronutrient and micronutrient intake and composition? Clin. Nutr. 2020, 39, 3354–3360. [Google Scholar] [CrossRef]
- Hutchison, A.T.; Liu, B.; Wood, R.E.; Vincent, A.D.; Thompson, C.H.; O’Callaghan, N.J.; Wittert, G.A.; Heilbronn, L.K. Effects of Intermittent Versus Continuous Energy Intakes on Insulin Sensitivity and Metabolic Risk in Women with Overweight. Obesity 2019, 27, 50–58. [Google Scholar] [CrossRef]
- Bowen, J.; Brindal, E.; James-Martin, G.; Noakes, M. Randomized Trial of a High Protein, Partial Meal Replacement Program with or without Alternate Day Fasting: Similar Effects on Weight Loss, Retention Status, Nutritional, Metabolic, and Behavioral Outcomes. Nutrients 2018, 10, 1145. [Google Scholar] [CrossRef]
- Kalam, F.; Gabel, K.; Cienfuegos, S.; Ezpeleta, M.; Wiseman, E.; Varady, K.A. Alternate Day Fasting Combined with a Low Carbohydrate Diet: Effect on Sleep Quality, Duration, Insomnia Severity and Risk of Obstructive Sleep Apnea in Adults with Obesity. Nutrients 2021, 13, 211. [Google Scholar] [CrossRef]
- Astbury, N.M.; Aveyard, P.; Nickless, A.; Hood, K.; Corfield, K.; Lowe, R.; Jebb, S.A. Doctor Referral of Overweight People to Low Energy total diet replacement Treatment (DROPLET): Pragmatic randomised controlled trial. BMJ 2018, 362, k3760. [Google Scholar] [CrossRef]
- Ard, J.D.; Lewis, K.H.; Rothberg, A.; Auriemma, A.; Coburn, S.L.; Cohen, S.S.; Loper, J.; Matarese, L.; Pories, W.J.; Periman, S. Effectiveness of a Total Meal Replacement Program (OPTIFAST Program) on Weight Loss: Results from the OPTIWIN Study. Obesity 2019, 27, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.; Hamilton, S.; Azevedo, L.B.; Olajide, J.; De Brún, C.; Waller, G.; Whittaker, V.; Sharp, T.; Lean, M.; Hankey, C.; et al. Intermittent fasting interventions for treatment of overweight and obesity in adults: A systematic review and meta-analysis. JBI Database Syst. Rev. Implement. Rep. 2018, 16, 507–547. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.; McGarty, A.; Hutchison, L.; Ells, L.; Hankey, C. Short-term intermittent energy restriction interventions for weight management: A systematic review and meta-analysis. Obes Rev. 2018, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Allaf, M.; Elghazaly, H.; Mohamed, O.G.; Fareen, M.F.K.; Zaman, S.; Salmasi, A.M.; Tsilidis, K.; Dehghan, A. Intermittent fasting for the prevention of cardiovascular disease. Cochrane Database Syst Rev. 2021, 1, CD013496. [Google Scholar] [CrossRef]
- Horne, B.D.; Grajower, M.M.; Anderson, J.L. Limited Evidence for the Health Effects and Safety of Intermittent Fasting Among Patients with Type 2 Diabetes. JAMA 2020, 324, 341–342. [Google Scholar] [CrossRef]
- Rajpal, A.; Ismail-Beigi, F. Intermittent fasting and ‘metabolic switch’: Effects on metabolic syndrome, prediabetes and type 2 diabetes. Diabetes Obes Metab. 2020, 22, 1496–1510. [Google Scholar] [CrossRef]
- Grajower, M.M.; Horne, B.D. Clinical Management of Intermittent Fasting in Patients with Diabetes Mellitus. Nutrients 2019, 11, 873. [Google Scholar] [CrossRef]
- Wilkinson, M.J.; Manoogian, E.N.C.; Zadourian, A.; Lo, H.; Fakhouri, S.; Shoghi, A.; Wang, X.; Fleischer, J.G.; Navlakha, S.; Panda, S.; et al. Ten-Hour Time-Restricted Eating Reduces Weight, Blood Pressure, and Atherogenic Lipids in Patients with Metabolic Syndrome. Cell Metab. 2020, 31, 92.e5–104.e5. [Google Scholar] [CrossRef]
- Gabel, K.; Hoddy, K.K.; Haggerty, N.; Song, J.; Kroeger, C.M.; Trepanowski, J.F.; Panda, S.; Varady, K.A. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: A pilot study. Nutr. Healthy Aging 2018, 4, 345–353. [Google Scholar] [CrossRef]
- Trepanowski, J.F.; Kroeger, C.M.; Barnosky, A.; Klempel, M.; Bhutani, S.; Hoddy, K.K.; Rood, J.; Ravussin, E.; Varady, K.A. Effects of alternate-day fasting or daily calorie restriction on body composition, fat distribution, and circulating adipokines: Secondary analysis of a randomized controlled trial. Clin Nutr. 2018, 37, 1871–1878. [Google Scholar] [CrossRef]
- Gabel, K.; Kroeger, C.M.; Trepanowski, J.F.; Hoddy, K.K.; Cienfuegos, S.; Kalam, F.; Varady, K.A. Differential Effects of Alternate-Day Fasting Versus Daily Calorie Restriction on Insulin Resistance. Obesity 2019, 27, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Redman, L.M.; Smith, S.R.; Burton, J.H.; Martin, C.K.; Il’yasova, D.; Ravussin, E. Metabolic Slowing and Reduced Oxidative Damage with Sustained Caloric Restriction Support the Rate of Living and Oxidative Damage Theories of Aging. Cell Metab. 2018, 27, 805.e4–815.e4. [Google Scholar] [CrossRef] [PubMed]
- Kraus, W.E.; Bhapkar, M.; Huffman, K.M.; Pieper, C.F.; Krupa Das, S.; Redman, L.M.; Villareal, D.T.; Rochon, J.; Roberts, S.B.; Ravussin, E.; et al. 2 years of calorie restriction and cardiometabolic risk (CALERIE): Exploratory outcomes of a multicentre, phase 2, randomised controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 673–683. [Google Scholar] [CrossRef]
- Trevizol, A.P.; Brietzke, E.; Grigolon, R.B.; Subramaniapillai, M.; McIntyre, R.S.; Mansur, R.B. Peripheral interleukin-6 levels and working memory in non-obese adults: A post-hoc analysis from the CALERIE study. Nutrition 2019, 58, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Hartmann-Boyce, J.; Theodoulou, A.; Oke, J.L.; Butler, A.R.; Scarborough, P.; Bastounis, A.; Dunnigan, A.; Byadya, R.; Hobbs, F.R.; Sniehotta, F.F.; et al. Association between characteristics of behavioural weight loss programmes and weight change after programme end: Systematic review and meta-analysis. BMJ 2021, 374, n1840. [Google Scholar] [CrossRef]
- Han, K.; Singh, K.; Rodman, M.J.; Hassanzadeh, S.; Wu, K.; Nguyen, A.; Huffstutler, R.D.; Seifuddin, F.; Dagur, P.K.; Saxena, A.; et al. Fasting-induced FOXO4 blunts human CD4+ T helper cell responsiveness. Nat. Metab. 2021, 3, 318–326. [Google Scholar] [CrossRef]
- Patterson, R.E.; Laughlin, G.A.; Sears, D.D.; LaCroix, A.Z.; Marinac, C.; Gallo, L.C.; Hartman, S.J.; Natarajan, L.; Senger, C.M.; Martínez, M.E.; et al. Intermittent Fasting and Human Metabolic Health. J. Acad. Nutr. Diet. 2015, 115, 1203–1212. [Google Scholar] [CrossRef]
- Zhao, B.; Sun, S.; He, X.; Yang, J.; Ma, X.; Yan, B. Sleep fragmentation and the risk of obesity: The Sleep Heart Health Study. Obesity 2021, 29, 1387–1393. [Google Scholar] [CrossRef]
- Yang, Y.; Miao, Q.; Zhu, X.; Qin, L.; Gong, W.; Zhang, S.; Zhang, Q.; Lu, B.; Ye, H.; Li, Y. Sleeping Time, BMI, and Body Fat in Chinese Freshmen and Their Interrelation. Obes. Facts 2020, 13, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Zerón-Rugerio, M.F.; Hernáez, Á.; Porras-Loaiza, A.P.; Cambras, T.; Izquierdo-Pulido, M. Eating Jet Lag: A Marker of the Variability in Meal Timing and Its Association with Body Mass Index. Nutrients 2019, 11, 2980, Erratum in 2020, 12, 816. [Google Scholar] [CrossRef] [PubMed]
- Chastin, S.; McGregor, D.; Palarea-Albaladejo, J.; Diaz, K.M.; Hagströmer, M.; Hallal, P.C.; van Hees, V.T.; Hooker, S.; Howard, V.J.; Lee, I.M.; et al. Joint association between accelerometry-measured daily combination of time spent in physical activity, sedentary behaviour and sleep and all-cause mortality: A pooled analysis of six prospective cohorts using compositional analysis. Br. J. Sports Med. 2021, 55, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.P.; Dickison, M.; Coughlin, N.; Karan, A.; Mauer, E.; Truong, W.; Casper, A.; Emiliano, A.B.; Kumar, R.B.; Saunders, K.H.; et al. The impact of food order on postprandial glycaemic excursions in prediabetes. Diabetes Obes. Metab. 2019, 21, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Pureza, I.R.O.M.; Melo, I.S.V.; Macena, M.L.; Praxedes, D.R.S.; Vasconcelos, L.G.L.; Silva-Júnior, A.E.; Florencio, T.M.; Bueno, N.B. Acute effects of time-restricted feeding in low-income women with obesity placed on hypoenergetic diets: Randomized trial. Nutrition 2020, 77, 110796. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Maranhão Pureza, I.R.; da Silva Junior, A.E.; Silva Praxedes, D.R.; Lessa Vasconcelos, L.G.; de Lima Macena, M.; Vieira de Melo, I.S.; Florêncio, T.M.D.M.T.; Bueno, N.B. Effects of time-restricted feeding on body weight, body composition and vital signs in low-income women with obesity: A 12-month randomized clinical trial. Clin. Nutr. 2021, 40, 759–766. [Google Scholar] [CrossRef]
- Kang, J.; Ma, E. Long-term outcomes of dietary carbohydrate restriction for HbA1c reduction in type 2 diabetes mellitus are needed. Diabetologia 2022, 65, 1058–1059. [Google Scholar] [CrossRef] [PubMed]
- Templeman, I.; Smith, H.A.; Chowdhury, E.; Chen, Y.C.; Carroll, H.; Johnson-Bonson, D.; Hengist, A.; Smith, R.; Creighton, J.; Clayton, D.; et al. A randomized controlled trial to isolate the effects of fasting and energy restriction on weight loss and metabolic health in lean adults. Sci. Transl. Med. 2021, 13, eabd8034. [Google Scholar] [CrossRef]
| Characteristics | IF (n = 42) | CR (n = 41) | HP (n = 48) | p for Difference among Groups |
|---|---|---|---|---|
| Age, y, mean ± SD | 34.7 ± 9.8 | 37.5 ± 11.7 | 34.0 ± 8.9 | 0.25 |
| Range | 22–63 | 22–67 | 19–58 | |
| Sex (%) | 0.472 | |||
| Male | 9 (21.4) | 5 (12.2) | 10 (20.8) | |
| Female | 33 (78.6) | 36 (87.8) | 38 (79.2) | |
| Height, cm | 166.1 ± 9.3 | 165.4 ± 7.4 | 166.1 ± 7.4 | 0.896 |
| Weight, kg | 86.2 ± 18.6 | 82.8 ± 14.6 | 85.3 ± 16.0 | 0.624 |
| BMI, kg/m2 | 30.9 ± 4.9 | 30.2 ± 4.2 | 30.8 ± 5.1 | 0.772 |
| Basic energy expenditure, kcal | 1529.3 ± 298.0 | 1463.3 ± 156.7 | 1492.7 ± 196.6 | 0.473 |
| Characteristics | IF (n = 42) | CR (n = 41) | HP (n = 48) | p for Difference among Groups |
|---|---|---|---|---|
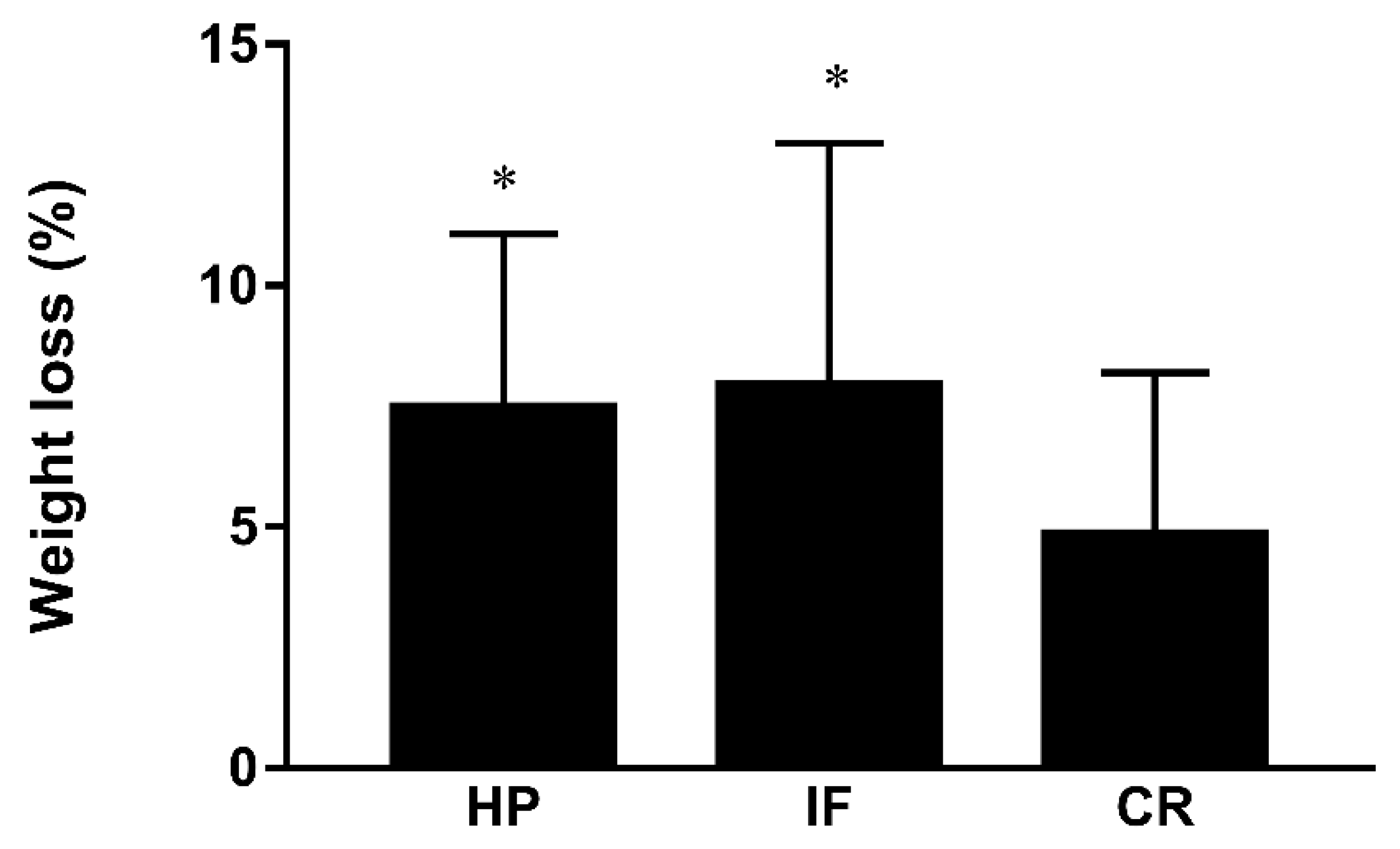
| Weight loss, kg, mean ± SD | 7.9 ± 5.0 | 4.7 ± 3.4 | 7.5 ± 3.6 | 0.001 |
| Percentage of weight loss, % | 9.0 ± 5.3 | 5.7 ± 3.7 | 8.6 ± 3.5 | <0.001 |
| 5% weight loss (%) | 36 (85.7) | 24 (58.5) | 39 (81.3) | 0.008 |
| 10% weight loss (%) | 19 (45.2) | 6 (14.6) | 20 (41.7) | 0.005 |
| Characteristics | IF (n = 42) | CR (n = 41) | HP (n = 48) | p for Difference among Groups |
|---|---|---|---|---|
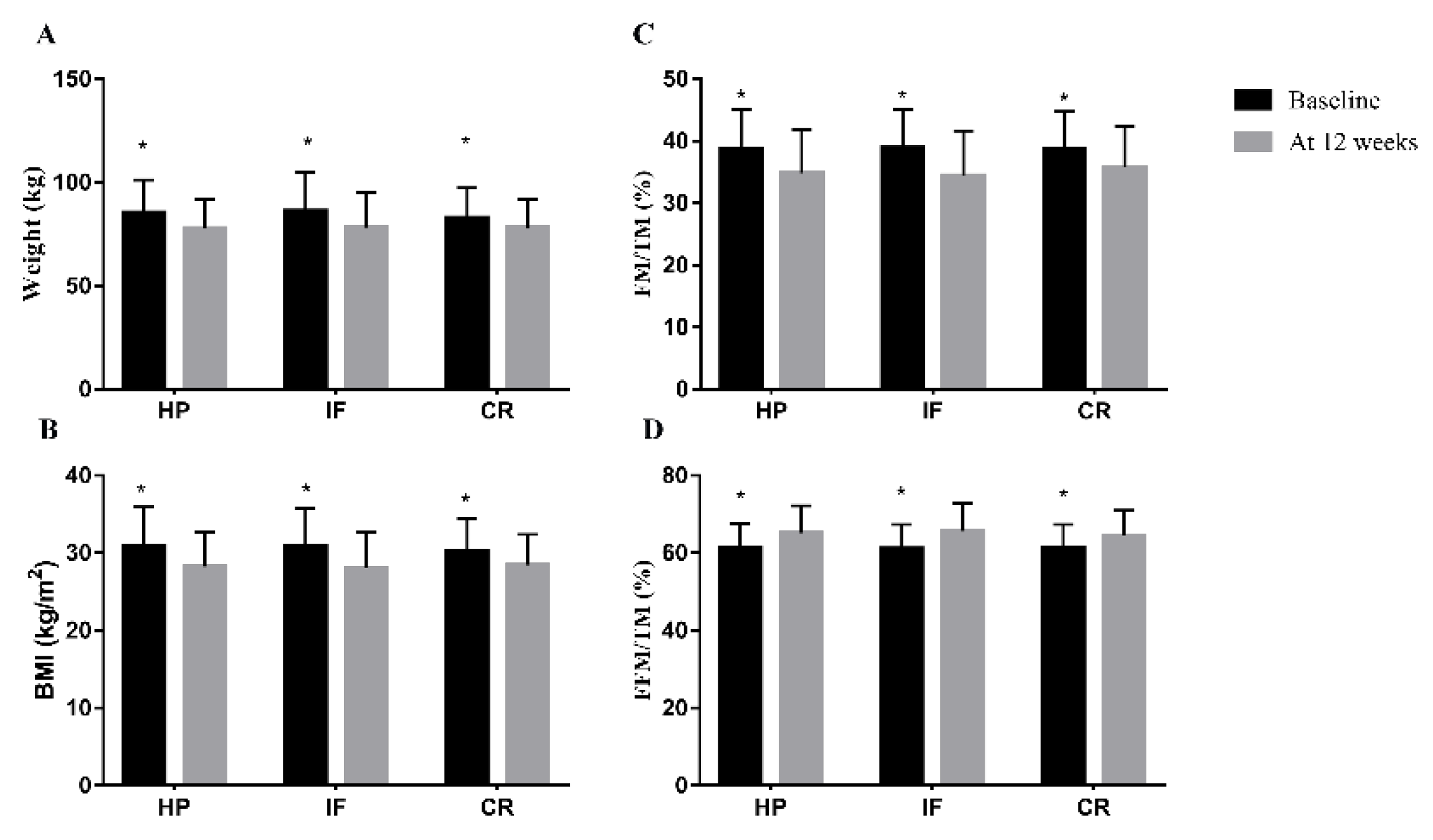
| Weight, kg, mean ± SD | ||||
| Baseline | 86.2 ± 18.6 | 82.8 ± 14.6 | 85.3 ± 16.0 | 0.624 |
| Week 12 | 78.2 ± 16.9 * | 78.1 ± 13.6 * | 77.8 ± 14.2 * | 0.99 |
| BMI, kg/m2 | ||||
| Baseline | 30.9 ± 4.9 | 30.2 ± 4.2 | 30.8 ± 5.1 | 0.772 |
| Week 12 | 28.1 ± 4.6 * | 28.4 ± 4.0 * | 28.2 ± 4.5 * | 0.934 |
| FM/TM, % | ||||
| Baseline | 38.9 ± 6.3 | 38.7 ± 6.1 | 38.8 ± 6.3 | 0.99 |
| Week 12 | 34.5 ± 7.2 * | 35.7 ± 6.7 * | 34.9 ± 7.0 * | 0.706 |
| FFM/TM, % | ||||
| Baseline | 61.1 ± 6.2 | 61.3 ± 6.2 | 61.2 ± 6.4 | 0.994 |
| Week 12 | 65.6 ± 7.2 * | 64.3 ± 6.6 * | 65.2 ± 7.0 * | 0.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.; Shi, X.; Fu, J.; Li, H.; Ma, E.; Chen, W. Effects of an Intermittent Fasting 5:2 Plus Program on Body Weight in Chinese Adults with Overweight or Obesity: A Pilot Study. Nutrients 2022, 14, 4734. https://doi.org/10.3390/nu14224734
Kang J, Shi X, Fu J, Li H, Ma E, Chen W. Effects of an Intermittent Fasting 5:2 Plus Program on Body Weight in Chinese Adults with Overweight or Obesity: A Pilot Study. Nutrients. 2022; 14(22):4734. https://doi.org/10.3390/nu14224734
Chicago/Turabian StyleKang, Junren, Xiaodong Shi, Ji Fu, Hailong Li, Enling Ma, and Wei Chen. 2022. "Effects of an Intermittent Fasting 5:2 Plus Program on Body Weight in Chinese Adults with Overweight or Obesity: A Pilot Study" Nutrients 14, no. 22: 4734. https://doi.org/10.3390/nu14224734
APA StyleKang, J., Shi, X., Fu, J., Li, H., Ma, E., & Chen, W. (2022). Effects of an Intermittent Fasting 5:2 Plus Program on Body Weight in Chinese Adults with Overweight or Obesity: A Pilot Study. Nutrients, 14(22), 4734. https://doi.org/10.3390/nu14224734






