Triterpenoids and Polysaccharides from Ganoderma lucidum Improve the Histomorphology and Function of Testes in Middle-Aged Male Mice by Alleviating Oxidative Stress and Cellular Apoptosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Materials
2.3. Animal Mating Experiment
2.4. Measurements of Body Weight and Weight of Testis
2.5. Detection of Serum Testosterone
2.6. Pathologic Analysis
2.7. Aging-Related Molecular Assays of Tissue
2.8. Detection of Cellular Apoptosis and Proliferation
2.9. Detection of Oxidative Stress Molecules in Testes
2.10. Electron Microscopy Examination
2.11. Statistical Analysis
3. Results
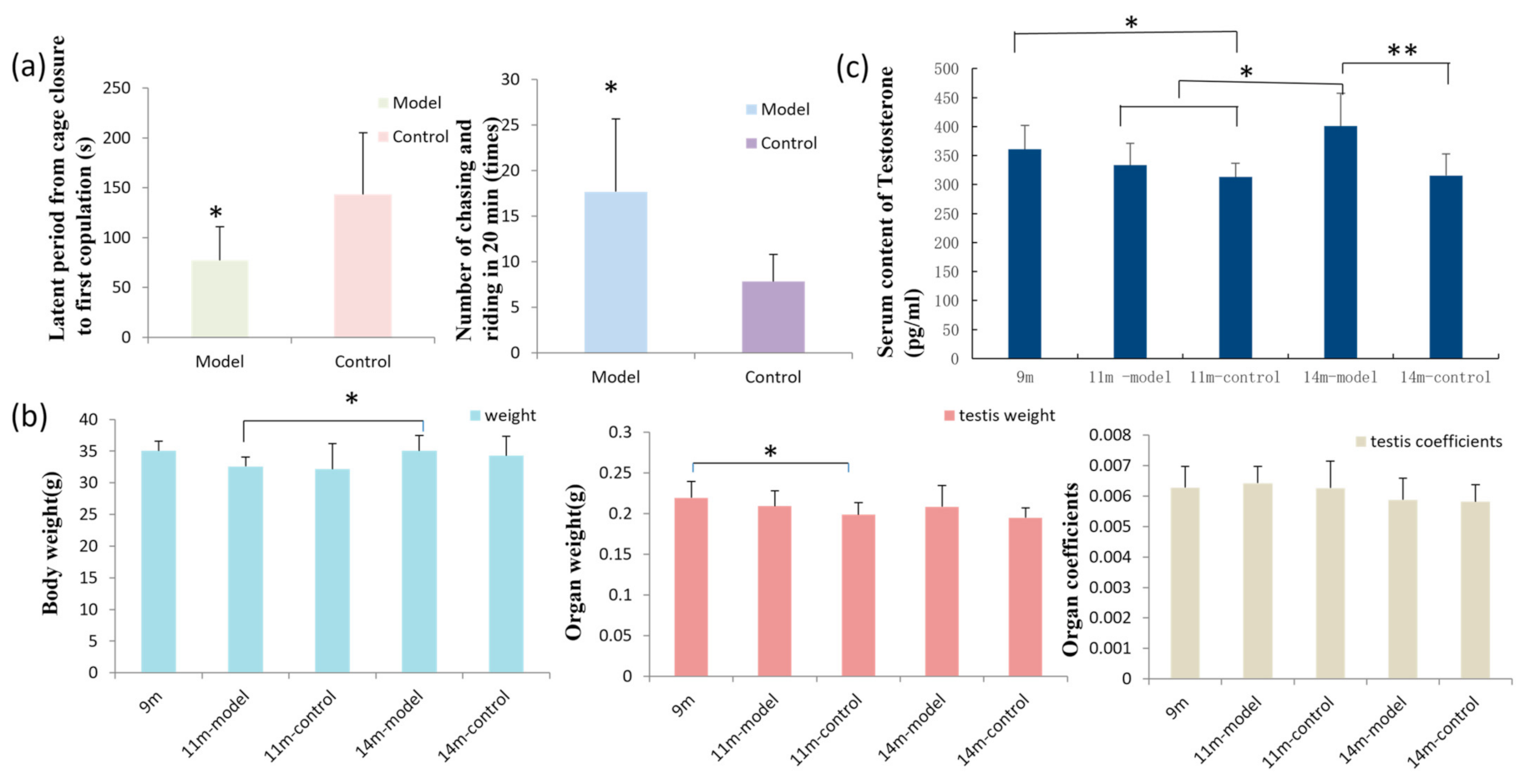
3.1. GL Preparation Improves Sexual Function in Male Mice
3.2. Results of Body Weight, Organ Weight, and Organ Coefficient
3.3. GL Preparation Increases Serum Testosterone Levels in Middle-Aged Male Mice
3.4. Pathological Morphology and Electron Microscopy
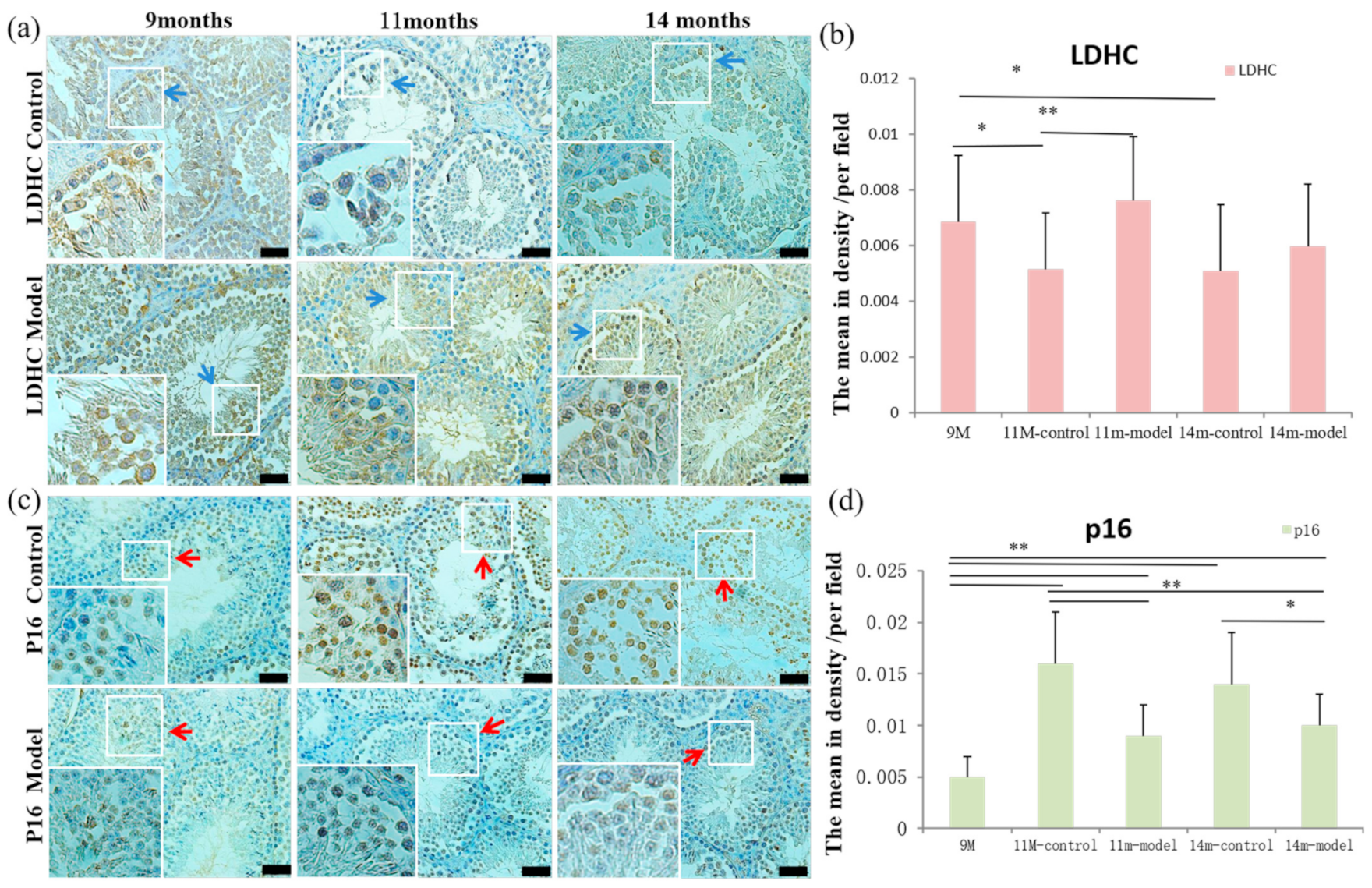
3.5. Detection of LDH-C4 and p16 in Testis Tissue
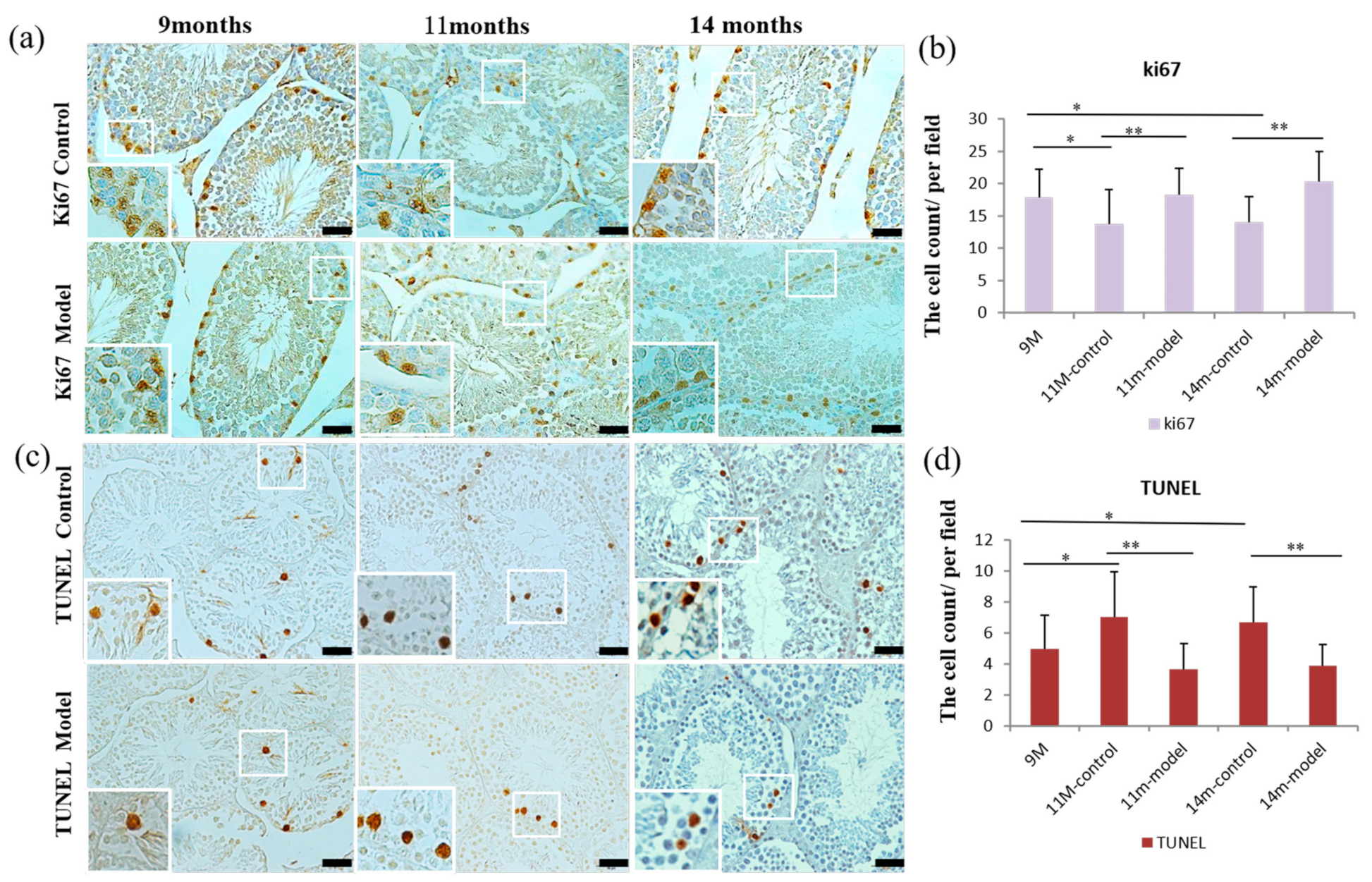
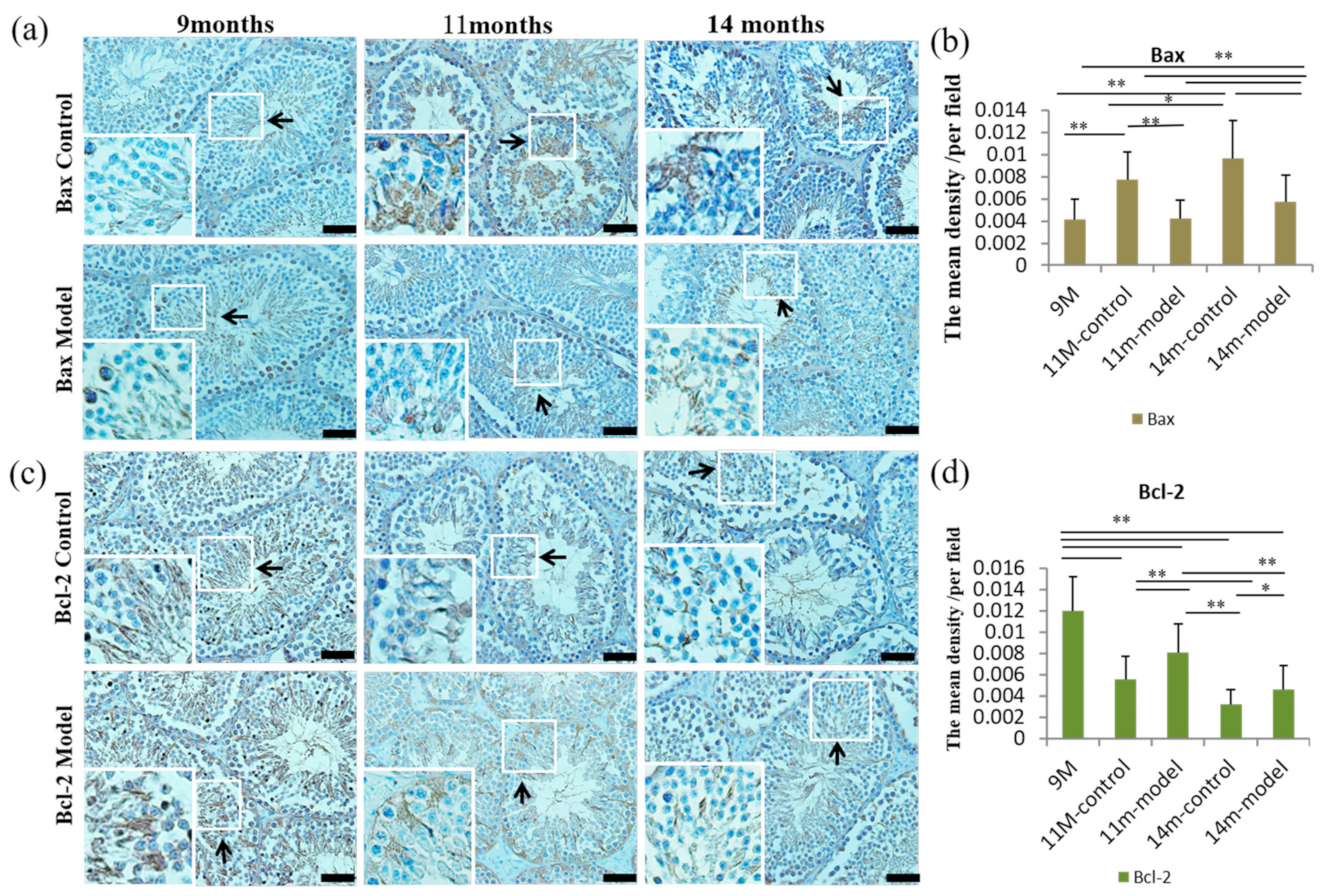
3.6. Detection of Cell Proliferation and Apoptosis in Testicular Tissue
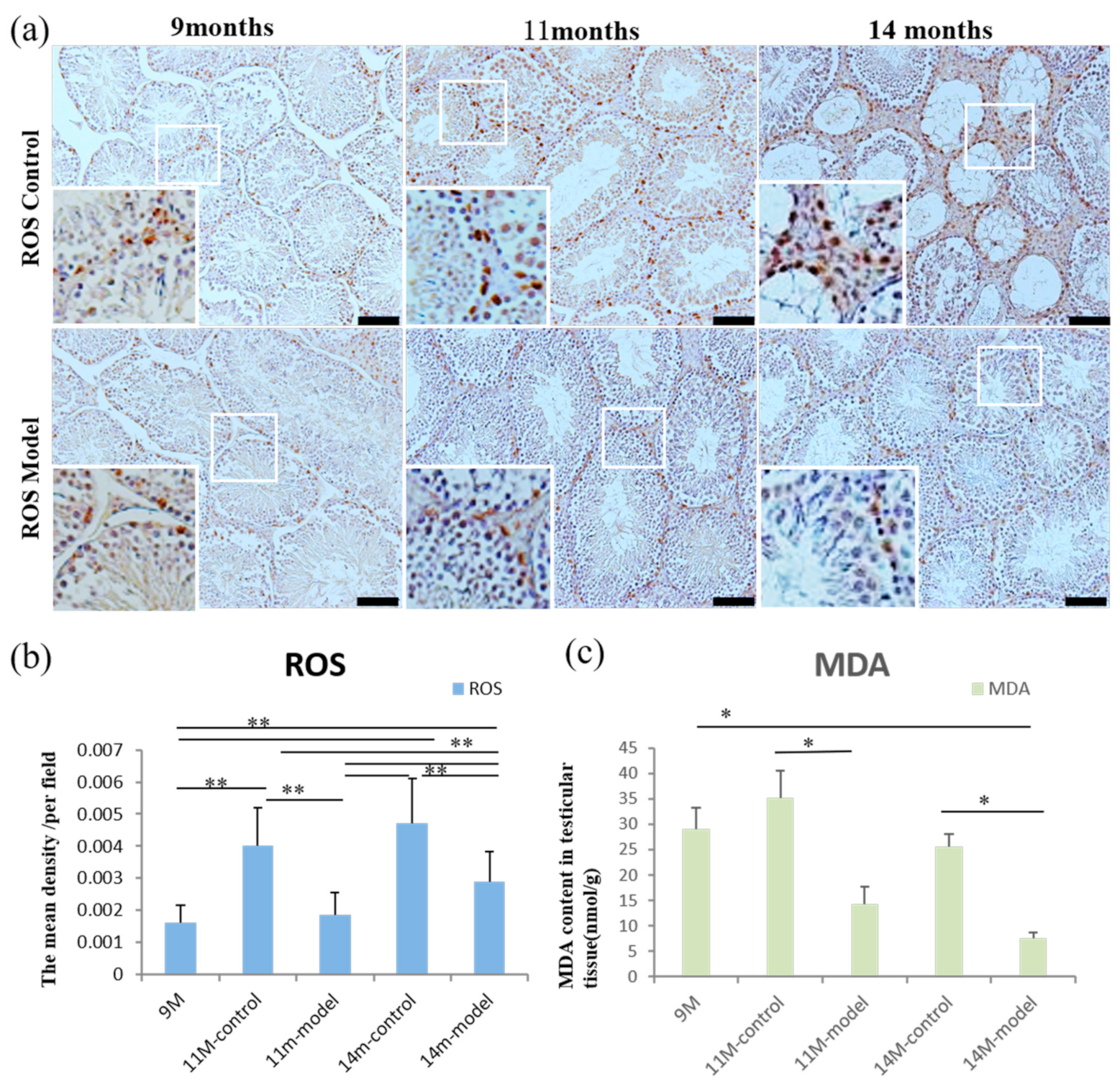
3.7. ROS and MDA Levels in Testicular Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Cao, B.; Zhao, H.; Feng, J. Emerging roles of Ganoderma lucidum in anti-aging. Aging Dis. 2017, 8, 691–707. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Ageing: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Gunes, S.; Hekim, G.N.; Arslan, M.A.; Asci, R. Effects of aging on the male reproductive system. J. Assist. Reprod. Genet. 2016, 33, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.; Barthold, S.; Davisson, M.; Newcomer, C.; Quimby, F.; Smit, A. The Mouse in Biomedical Research; American College Laboratory Animal Medicine (Elsevier): San Diego, CA, USA, 2006. [Google Scholar]
- Yun, T.K. Update from Asia. Asian studies on cancer chemoprevention. Ann. N. Y. Acad. Sci. 1999, 889, 157–192. [Google Scholar] [CrossRef]
- Li, W.J.; Nie, S.P.; Peng, X.P.; Liu, X.Z.; Li, C.; Chen, Y.; Li, J.E.; Song, W.R.; Xie, M.Y. Ganoderma lucidum polysaccharide improves age-related oxidative stress and immune impairment in mice. J. Agric. Food Chem. 2012, 60, 1413–1418. [Google Scholar] [CrossRef]
- Zhu, K.X.; Nie, S.P.; Li, C.; Gong, D.; Xie, M.Y. Ganoderma lucidum polysaccharide improves aortic relaxation in diabetic rats via PI3K/Akt pathway. Carbohydr. Polym. 2014, 103, 520–527. [Google Scholar] [CrossRef]
- Doğan, G.; İpe, H. The protective effect of Ganoderma lucidum on testicular torsion/detorsion-induced ischemia-reperfusion (I/R) injury. Acta Cir. Bras. 2020, 35, e202000103. [Google Scholar] [CrossRef]
- Ghajari, G.; Nabiuni, M.; Amini, E. The association between testicular toxicity induced by Li2Co3 and protective effect of Ganoderma lucidum: Alteration of Bax & c-Kit genes expression. Tissue Cell 2021, 72, 101552. [Google Scholar]
- Bin-Jumah, M.N.; Nadeem, M.S.; Gilani, S.J.; Imam, S.S.; Alshehri, S.; Kazmi, I. Novelkaraya gum micro-particles loaded Ganoderma lucidum polysaccharide regulate sex hormones, oxidative stress and inflammatory cytokine levels in cadmium induced testicular toxicity in experimental animals. Int. J. Biol. Macromol. 2022, 194, 338–346. [Google Scholar] [CrossRef]
- Ahmad, M.F. Ganoderma lucidum: Persuasive biologically active constituents and their health endorsement. Biomed Pharmacother. 2018, 107, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Srivastava, M.; Pal, M.; Sharma, Y.K.; Bhattacharya, S.; Tulsawani, R.; Sugadev, R.; Misra, K. Screening of indian Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (Agaricomycetes): A UPC2-SQD-MS approach. Int. J. Med. Mushrooms 2016, 18, 177–189. [Google Scholar] [CrossRef]
- Smina, T.P.; De, S.; Devasagayam, T.P.; Adhikari, S.; Janardhanan, K.K. Ganoderma lucidum total triterpenes prevent radiation-induced DNA damage and apoptosis in splenic lymphocytes in vitro. Mutat. Res. 2011, 726, 188–194. [Google Scholar] [CrossRef] [PubMed]
- You, Y.H.; Lin, Z.B. Antioxidant effect of Ganoderma polysaccharide peptide. Yao Xue Xue Bao 2003, 38, 85–88. [Google Scholar] [PubMed]
- Sudheesh, N.P.; Ajith, T.A.; Janardhanan, K.K. Ganoderma lucidum (Fr.) P. Karst enhances activities of heart mitochondrial enzymes and respiratory chain complexes in the aged rat. Biogerontology 2009, 10, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Sudheesh, N.P.; Ajith, T.A.; Ramnath, V.; Janardhanan, K.K. Therapeutic potential of Ganoderma lucidum (Fr.) P. Karst. against the declined antioxidant status in the mitochondria of post-mitotic tissues of aged mice. Clin. Nutr. 2010, 29, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Wu, S.Q.; Chen, B.S.; Wu, X.X.; Qu, K.Y.; Liu, J.M.; Zhang, G.F.; Xu, Y.F.; Shu, S.; Sun, L.; et al. Pathological changes in APP/PS-1 transgenic mouse models of Alzheimer’s disease treated with Ganoderma lucidum preparation. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2017, 39, 552–561. [Google Scholar]
- Wei, W.; Wu, X.M.; Li, Y.J. Experiment Methodology of Pharmacology; People’s Health Publishing House: Beijng, China, 2020. [Google Scholar]
- Firman, R.C.; Young, F.J.; Rowe, D.C.; Duong, H.T.; Gasparini, C. Sexual rest and post-meiotic sperm ageing in house mice. J. Evol. Biol. 2015, 28, 1373–1382. [Google Scholar] [CrossRef]
- Xiong, R.; Hu, C.J.; Zhang, M.; Chen, Z.M.; Cui, Y.Y. Study on the effect of the crude and mutton-fat-progressed epimedii folium in Er’Xian decoction on freeze resistance capability and mating ability in model of kidney-yang asthenia. Asia Pac. Tradit. Med. 2015, 11, 5–7. [Google Scholar]
- Xia, X.; Wang, L.; Yang, X.; Hu, Y.; Liu, Q. Acute damage to the sperm quality and spermatogenesis in male mice exposed to curcumin-loaded nanoparticles. Int. J. Nanomed. 2020, 15, 1853–1862. [Google Scholar] [CrossRef]
- Rydze, R.T.; Patton, B.K.; Briley, S.M.; Salazar Torralba, H.; Gipson, G.; James, R.; Rajkovic, A.; Thompson, T.; Pangas, S.A. Deletion of Gremlin-2 alters estrous cyclicity and disrupts female fertility in mice. Biol. Reprod. 2021, 105, 1205–1220. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Chen, Y.; Hu, M.; Lin, Y.; Zhang, S.; Kong, L.; Chen, Y. Diagnostic and prognostic value of the cancer-testis antigen lactate dehydrogenase C4 in breast cancer. Clin. Chim. Acta 2020, 503, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Ding, H.; Liang, M.; Chen, X.; Yan, Y.; Wan, N.; Chen, Q.; Zhang, J.; Cao, J. Curcumin induces ferroptosis innon-small-cell lung cancer via activating autophagy. Thorac. Cancer 2021, 12, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Loo, D.T. In situ detection of apoptosis by the TUNEL assay: An overview of techniques. Methods Mol. Biol. 2011, 682, 3–13. [Google Scholar]
- Chen, Y.; Zhang, D.; Xin, N.; Xiong, Y.; Chen, P.; Li, B.; Tu, X.; Lan, F. Construction of sperm-specific lactate dehydrogenase DNA vaccine and experimental study of its immunocontraceptive effect on mice. Sci. China C Life Sci. 2008, 51, 308–316. [Google Scholar] [CrossRef]
- Aliabadi, E.; Karimi, F.; Rasti, M.; Akmali, M.; Esmaeilpour, T. Effects of L-carnitine and pentoxifylline on the activity of lactate dehydrogenase C4 isozyme and motility of testicular spermatozoa in mice. J. Reprod. Infertil. 2013, 14, 56–61. [Google Scholar]
- Odet, F.; Duan, C.; Willis, W.D.; Goulding, E.H.; Kung, A.; Eddy, E.M. Goldberg, E.l. Expression of the gene for mouse lactate dehydrogenase C (Ldhc) is required for male fertility. Biol. Reprod. 2008, 79, 26–34. [Google Scholar] [CrossRef]
- Kaufman, J.M.; Lapauw, B.; Mahmoud, A.; T’Sjoen, G.; Huhtaniemi, I.T. Aging and the male reproductive system. Endocr. Rev. 2019, 40, 906–972. [Google Scholar] [CrossRef]
- Bassas Arnau, L. Exploration of testicular function. Endocrinol. Nutr. 2009, 56, 18–31. [Google Scholar] [CrossRef]
- Romagosa, C.; Simonetti, S.; López-Vicente, L.; Mazo, A.; Lleonart, M.E.; Castellvi, J.; Ramony Cajal, S. p16(Ink4a) overexpression in cancer: A tumor suppressor gene associated with senescence and high-grade tumors. Oncogene 2011, 30, 2087–2097. [Google Scholar] [CrossRef]
- Aitken, R.J.; Curry, B.J. Redox regulation of human sperm function: From the physiological control of sperm capacitation to the etiology of infertility and DNA damage in the germ line. Antioxid. Redox Signal 2011, 14, 367–381. [Google Scholar] [CrossRef] [PubMed]
- De Lamirande, E.; Lamothe, G. Reactive oxygen-induced reactive oxygen formation during human sperm capacitation. Free Radic. Biol. Med. 2009, 46, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Koksal, I.T.; Usta, M.; Orhan, I.; Abbasoglu, S.; Kadioglu, A. Potential role of reactive oxygen species on testicular pathology associated with infertility. Asian J. Androl. 2003, 5, 95–99. [Google Scholar] [PubMed]
- Liochev, S.I. Reactive oxygen species and the free radical theory of aging. Free Radic. Biol. Med. 2013, 60, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Molteni, C.G.; Principi, N.; Esposito, S. Reactive oxygen and nitrogen species during viral infections. Free Radic. Res. 2014, 48, 1163–1169. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Victor, V.M.; Rocha, M.; De la Fuente, M. Immune cells: Free radicals and antioxidants in sepsis. Int. Immunopharmacol. 2004, 4, 327–347. [Google Scholar] [CrossRef]
- Durackova, Z. Some current insights into oxidative stress. Physiol. Res. 2010, 59, 459. [Google Scholar] [CrossRef]
- Vajapey, R.; Rini, D.; Walston, J.; Abadir, P. The impact of age-related dysregulation of the angiotensin system on mitochondrial redox balance. Front. Physiol. 2014, 5, 439. [Google Scholar] [CrossRef]
- Jang, Y.C.; Van Remmen, H. The mitochondrial theory of aging: Insight from transgenic and knockout mouse models. Exp. Gerontol. 2009, 44, 256–260. [Google Scholar] [CrossRef]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Hekimi, S.; Lapointe, L.J.; Wen, Y. Taking a “good” look at free radicals in the aging process. Trends Cell Biol. 2011, 21, 569. [Google Scholar] [CrossRef] [PubMed]
- Conley, K.E.; Marcinek, D.J.; Villarin, J. Mitochondrial dysfunction and age. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.Y.; Chen, G.; Huang, X.; Yuan, Y.; Wu, X.; Wu, B.; Li, Z.; Shun, F.; Chen, H.; Shi, H. Effects of reactive oxygen species from activated leucocytes on human sperm motility, viability and morphology. Andrologi 2012, 44, 696–703. [Google Scholar] [CrossRef]
- Kao, S.H.; Chao, H.T.; Chen, H.W.; Hwang, T.I.S.; Liao, T.L.; Wei, Y.H. Increase of oxidative stress in human sperm with lower motility. Fertil. Steril. 2008, 89, 1183–1190. [Google Scholar] [CrossRef]
- Aitken, R.J.; Buckingham, D.; West, K.; Wu, F.C.; Zikopoulos, K. Richardson, D.W. Differential contribution of leucocytes and spermatozoa to the generation of reactive oxygen species in the ejaculates of oligozoospermic patients and fertile donors. J. Reprod. Fertil. 1992, 94, 451–462. [Google Scholar] [CrossRef]
- Durairajanayagam, D.; Agarwal, A.; Ong, C.; Prashast, P. Lycopene and male infertility. Asian J. Androl. 2014, 16, 420–425. [Google Scholar]
- Sharma, R.; Masaki, J.; Agarwal, A. Sperm DNA fragmentation analysis using the TUNEL assay. Methods Mol. Biol. 2013, 927, 121–136. [Google Scholar]
- Tamburrino, L.; Marchiani, S.; Montoya, M.; Elia Marino, F.; Natali, I.; Cambi, M.; Forti, G.; Baldi, E.; Muratori, M. Mechanisms and clinical correlates of sperm DNA damage. Asian J. Androl. 2012, 14, 24–31. [Google Scholar] [CrossRef]
- Belloc, S.; Benkhalifa, M.; Cohen-Bacrie, M.; Dalleac, A.; Amar, E.; Zini, A. Sperm deoxyribonucleic acid damage in normozoospermic men is related to age and sperm progressive motility. Fertil. Steril. 2014, 101, 1588–1593. [Google Scholar] [CrossRef] [PubMed]
- Pastor, L.M.; Zuasti, A.; Ferrer, C.; Bernal-Mañas, C.M.; Morales, E.; Beltrán-Frutos, E.; Seco-Rovira, V. Proliferation and apoptosis in aged and photoregressed mammalian seminiferous epithelium, with particular attention to rodents and humans. Reprod. Domest. Anim. 2011, 46, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Itoh, N.; Takagi, S.; Sasao, T.; Takahashi, A.; Masumori, N.; Tsukamoto, T. Balance of apoptosis and proliferation of germ cells related to spermatogenesis in aged men. J. Androl. 2003, 24, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhu, W.J.; Li, J.; Chen, Q.J.; Liang, W.B.; Gu, Y.Q. Quantitative histological analysis and ultrastructure of the aging human testis. Int. Urol. Nephrol. 2014, 46, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, Y. Role of Bcl-2 family proteins in apoptosis: Apoptosomes or mitochondria? Genes Cells 1998, 3, 697–707. [Google Scholar] [CrossRef]
- Gross, A.; Jockel, J.; Wei, M.C.; Korsmeyer, S.J. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 1998, 17, 3878–3885. [Google Scholar] [CrossRef]

| Group | Model | Control |
|---|---|---|
| Birth ratio and litter size of the first litter | 5/6 (83.3%); 23 | 4/6 (66.7%); 17 |
| Birth ratio and litter size of the second litter | 3/6 (50%); 19 | 3/6 (50%); 10 |
| Birth ratio and litter size of the third litter | 2/6 (33.3%); 10 | 2/6 (33.3%); 4 |
| Birth ratio and litter size of the fourth litter | 2/6 (33.3%); 10 | 0; 0 |
| Average litter size per litter | 4.67 | 3.4 |
| Group | Disordered Arrangement of Spermatogenic Cells | Spermatogenic Cell and Spermatozoa Decrease | Leydig Cell Reduction, Degeneration, and Lipid Deposition |
|---|---|---|---|
| 9-month | 18.3% | 15.4%; 16.9% | 14.3%; --; -- |
| 11-month control | 50.7% | 47.2%; 51.4% | 31.4%; 17.1%; 8.6% |
| 11-month model | 38.5% | 30.5%; 31.9% | 24.3%; 14.3%; 5.7% |
| 14-month control | 65.7% | 60%; 70% | 41.4%; 31.4%; 18.6% |
| 14-month model | 47.1% | 42.8%; 47.1% | 30%; 20%; 12.9% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Liang, W.; Han, Y.; Zhao, W.; Wang, S.; Qin, C. Triterpenoids and Polysaccharides from Ganoderma lucidum Improve the Histomorphology and Function of Testes in Middle-Aged Male Mice by Alleviating Oxidative Stress and Cellular Apoptosis. Nutrients 2022, 14, 4733. https://doi.org/10.3390/nu14224733
Li Y, Liang W, Han Y, Zhao W, Wang S, Qin C. Triterpenoids and Polysaccharides from Ganoderma lucidum Improve the Histomorphology and Function of Testes in Middle-Aged Male Mice by Alleviating Oxidative Stress and Cellular Apoptosis. Nutrients. 2022; 14(22):4733. https://doi.org/10.3390/nu14224733
Chicago/Turabian StyleLi, Yanhong, Wei Liang, Yunlin Han, Wenjie Zhao, Siyuan Wang, and Chuan Qin. 2022. "Triterpenoids and Polysaccharides from Ganoderma lucidum Improve the Histomorphology and Function of Testes in Middle-Aged Male Mice by Alleviating Oxidative Stress and Cellular Apoptosis" Nutrients 14, no. 22: 4733. https://doi.org/10.3390/nu14224733
APA StyleLi, Y., Liang, W., Han, Y., Zhao, W., Wang, S., & Qin, C. (2022). Triterpenoids and Polysaccharides from Ganoderma lucidum Improve the Histomorphology and Function of Testes in Middle-Aged Male Mice by Alleviating Oxidative Stress and Cellular Apoptosis. Nutrients, 14(22), 4733. https://doi.org/10.3390/nu14224733





