Mechanistic Insights into the Neuroprotective Potential of Sacred Ficus Trees
Abstract
1. Introduction
2. Phytochemicals and Therapeutic Properties
2.1. F. religiosa
2.2. F. benghalensis
3. Neuroprotective Effect of Ficus religiosa
3.1. Leaves
3.2. Root
3.3. Fruit
3.4. Bark
4. Neuroprotective Effect of Ficus benghalensis
4.1. Leaves
4.2. Bark
4.3. Root
5. Neuroprotection by Phytochemicals
5.1. Amyrin
5.2. Azelaic Acid
5.3. Bergapten (5-Methoxypsoralen)
5.4. Eudesmol
5.5. Eugenol
5.6. Kaempferol
5.7. Lanosterol
5.8. Leucoanthocyanins
5.9. Limonene
5.10. Linalool
5.11. Lupeol
5.12. Myo-Inositol (Vitamin B8)
5.13. Myricetin
5.14. Pinene
5.15. Psoralen
5.16. Quercetin
5.17. Rhein
5.18. Rutin
5.19. Stigmasterol
5.20. Synephrine
5.21. β-Caryophyllene
5.22. β-Sitosterol
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cowan, M.C.; Raymond, L.A. Selective neuronal degeneration in Huntington’s disease. Curr. Top. Dev. Biol. 2006, 75, 25–71. [Google Scholar]
- Erkkinen, M.G.; Kim, M.-O.; GeWschwind, M.D. Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2018, 10, a033118. [Google Scholar] [PubMed]
- Ragagnin, A.M.; Shadfar, S.; Vidal, M.; Jamali, M.S.; Atkin, J.D. Motor neuron susceptibility in ALS/FTD. Front. Neurosci. 2019, 13, 532. [Google Scholar] [CrossRef] [PubMed]
- Salvadores, N.; Gerónimo-Olvera, C.; Court, F.A. Axonal degeneration in AD: The contribution of Aβ and Tau. Front. Aging Neurosci. 2020, 12, 581767. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, J.T.; Heegaard, N.H. Analysis of protein aggregation in neurodegenerative disease. Anal. Chem. 2013, 85, 4215–4227. [Google Scholar] [CrossRef]
- Wang, B.; Zhong, Y.; Gao, C.; Li, J. Myricetin ameliorates scopolamine-induced memory impairment in mice via inhibiting acetylcholinesterase and down-regulating brain iron. Biochem. Biophys. Res. Commun. 2017, 490, 336–342. [Google Scholar] [CrossRef]
- Jarosińska, O.D.; Rüdiger, S.G. Molecular strategies to target protein aggregation in Huntington’s disease. Front. Mol. Biosci. 2021, 8, 769184. [Google Scholar] [CrossRef]
- Robinson, J.L.; Geser, F.; Stieber, A.; Umoh, M.; Kwong, L.K.; Van Deerlin, V.M.; Lee, V.M.-Y.; Trojanowski, J.Q. TDP-43 skeins show properties of amyloid in a subset of ALS cases. Acta Neuropathol. 2013, 125, 121–131. [Google Scholar] [CrossRef]
- Migliore, L.; Coppedè, F. Genetics, environmental factors and the emerging role of epigenetics in neurodegenerative diseases. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2009, 667, 82–97. [Google Scholar] [CrossRef]
- Tan, M.A.; Sharma, N.; An, S.S.A. Phyto-Carbazole Alkaloids from the Rutaceae Family as Potential Protective Agents against Neurodegenerative Diseases. Antioxidants 2022, 11, 493. [Google Scholar] [CrossRef]
- Bagyinszky, E.; Kang, M.J.; Pyun, J.; Van Giau, V.; An, S.S.A.; Kim, S. Early-onset Alzheimer’s disease patient with prion (PRNP) p. Val180Ile mutation. Neuropsychiatr. Dis. Treat. 2019, 15, 2003. [Google Scholar] [CrossRef] [PubMed]
- Giau, V.V.; Bagyinszky, E.; Yang, Y.S.; Youn, Y.C.; An, S.S.A.; Kim, S.Y. Genetic analyses of early-onset Alzheimer’s disease using next generation sequencing. Sci. Rep. 2019, 9, 8368. [Google Scholar] [CrossRef] [PubMed]
- Bagyinszky, E.; Yang, Y.; Van Giau, V.; Youn, Y.C.; An, S.S.A.; Kim, S. Novel prion mutation (p. Tyr225Cys) in a Korean patient with atypical Creutzfeldt–Jakob disease. Clin. Interv. Aging 2019, 14, 1387. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.J.; Yi, S.; Han, J.-y.; Park, S.Y.; Jang, J.-W.; Chun, I.K.; Kim, S.E.; Lee, B.S.; Kim, G.J.; Yu, J.S. Oligomeric forms of amyloid-β protein in plasma as a potential blood-based biomarker for Alzheimer’s disease. Alzheimer’s Res. Ther. 2017, 9, 98. [Google Scholar] [CrossRef]
- Van Giau, V.; An, S.S.A. Emergence of exosomal miRNAs as a diagnostic biomarker for Alzheimer’s disease. J. Neurol. Sci. 2016, 360, 141–152. [Google Scholar] [CrossRef]
- Hornykiewicz, O. A brief history of levodopa. J. Neurol. 2010, 257, 249–252. [Google Scholar] [CrossRef]
- Lahlou, M. The success of natural products in drug discovery. Pharmacol. Pharm. 2013, 4, 17–31. [Google Scholar] [CrossRef]
- Nawaz, H.; Waheed, R.; Nawaz, M. Phytochemical composition, antioxidant potential, and medicinal significance of Ficus. Mod. Fruit Ind. 2020, 1, 20. [Google Scholar]
- Rahman, A.; Khanom, A. Taxonomic and ethno-medicinal study of species from Moraceae (Mulberry) Family in Bangladesh Flora. Res. Plant Sci. 2013, 1, 53–57. [Google Scholar]
- Pierantoni, M.; Tenne, R.; Rephael, B.; Brumfeld, V.; van Casteren, A.; Kupczik, K.; Oron, D.; Addadi, L.; Weiner, S. Mineral deposits in Ficus leaves: Morphologies and locations in relation to function. Plant Physiol. 2018, 176, 1751–1763. [Google Scholar] [CrossRef]
- Gopukumar, S.; Praseetha, P. Ficus benghalensis Linn–the sacred Indian medicinal tree with potent pharmacological remedies. Int. J. Pharm. Sci. Rev. Res. 2015, 32, 223–227. [Google Scholar]
- The Holy Bible: New International Version; Zondervan: Grand Rapids, MI, USA, 1984.
- Chandrasekar, S.; Bhanumathy, M.; Pawar, A.; Somasundaram, T. Phytopharmacology of Ficus religiosa. Pharmacogn. Rev. 2010, 4, 195. [Google Scholar] [CrossRef] [PubMed]
- Kmail, A.; Rahman, R.; Nisar, S.; Jilani, M.I. Banyan tree-the sacred medicinal tree with potential health and pharmacological benefits. Int. J. Chem. Biochem. Sci. 2018, 13, 52–57. [Google Scholar]
- Shah, N. Herbal folk medicines in Northern India. J. Ethnopharmacol. 1982, 6, 293–301. [Google Scholar] [CrossRef]
- Gregory, M.; Divya, B.; Mary, R.A.; Viji, M.H.; Kalaichelvan, V.; Palanivel, V. Anti–ulcer activity of Ficus religiosa leaf ethanolic extract. Asian Pac. J. Trop. Biomed. 2013, 3, 554–556. [Google Scholar] [CrossRef]
- Kulshreshtha, M.; Goswami, M.; Rao, C.; Ashwlayan, V.; Yadav, S. Estimation of antioxidant potential of aqueous extract of Ficus bengalensis leaf on gastric ulcer. Int. J. Pharm. Sci. Rev. Res. 2011, 9, 122–126. [Google Scholar]
- Haneef, J.; Thankayyan, R.S.K.; Sithul, H.; Sreeharshan, S. Bax translocation mediated mitochondrial apoptosis and caspase dependent photosensitizing effect of Ficus religiosa on cancer cells. PLoS ONE 2012, 7, e40055. [Google Scholar] [CrossRef]
- Saloni, J.; Sakthivel, J. Evaluation of antioxidant and anticancer potential of flavonoids from aerial roots of Ficus benghalensis Linn. Int. J. Pharm. Res. 2019, 11, 11–20. [Google Scholar]
- Pandit, R.; Phadke, A.; Jagtap, A. Antidiabetic effect of Ficus religiosa extract in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2010, 128, 462–466. [Google Scholar] [CrossRef]
- Gulecha, V.; Sivakumar, T.; Upaganlawar, A.; Mahajan, M.; Upasani, C. Screening of Ficus religiosa leaves fractions for analgesic and anti-inflammatory activities. Indian J. Pharmacol. 2011, 43, 662. [Google Scholar]
- Patil, V.; Pimprikar, R.; Patil, V. Pharmacognostical studies and evaluation of anti-inflammatory activity of Ficus bengalensis Linn. J. Young Pharm. 2009, 1, 49. [Google Scholar] [CrossRef]
- Daniel, R.S.; Devi, K.; Augusti, K.; Nair, C. Mechanism of action of antiatherogenic and related effects of Ficus bengalensis Linn. flavonoids in experimental animals. Indian J. Exp. Biol. 2003, 41, 296–303. [Google Scholar] [PubMed]
- Rathi, P.; Nath, R.; Pant, K.; Dixit, R.; Pal, R.; Kumar, R. Evaluation of Hypolipidemic and TNF-Î ± Lowering Effect of Ficus religiosa in Dyslipidemic Wistar Rats. Curr. Res. Diabetes Obes. J. 2019, 10, 104–112. [Google Scholar]
- Aswar, M.; Aswar, U.; Watkar, B.; Vyas, M.; Wagh, A.; Gujar, K.N. Anthelmintic activity of Ficus benghalensis. Int. J. Green Pharm. (IJGP) 2008, 2, 170–172. [Google Scholar] [CrossRef]
- Hyo, W.; Hye, Y.; Chau, V.; Young, H.; Young, K. Methnol extract of Ficus leaf inhibits the production of nitric oxide and Proinflammatory cytokines in LPS stimulated microglia via the MAPK pathway. Phytother. Res. 2008, 22, 1064–1069. [Google Scholar]
- Gabhe, S.; Tatke, P.; Khan, T. Evaluation of the immunomodulatory activity of the methanol extract of Ficus benghalensis roots in rats. Indian J. Pharmacol. 2006, 38, 271. [Google Scholar] [CrossRef]
- Mallurwar, V.; Pathak, A. Studies on immunomodulatory activity of Ficus religiosa. Indian J. Pharm. Educ. Res. 2008, 42, 341–343. [Google Scholar]
- Mukherjee, P.K.; Saha, K.; Murugesan, T.; Mandal, S.; Pal, M.; Saha, B. Screening of anti-diarrhoeal profile of some plant extracts of a specific region of West Bengal, India. J. Ethnopharmacol. 1998, 60, 85–89. [Google Scholar] [CrossRef]
- Taur, D.; Nirmal, S.; Patil, R.; Kharya, M. Antistress and antiallergic effects of Ficus bengalensis bark in asthma. Nat. Prod. Res. 2007, 21, 1266–1270. [Google Scholar] [CrossRef]
- Kapoor, M.; Jasani, N.; Acharya, N.; Acharya, S.; Kumar, V. Phytopharmacological evaluation and anti–asthmatic activity of Ficus religiosa leaves. Asian Pac. J. Trop. Med. 2011, 4, 642–644. [Google Scholar] [CrossRef]
- Singh, D.; Goel, R.K. Anticonvulsant effect of Ficus religiosa: Role of serotonergic pathways. J. Ethnopharmacol. 2009, 123, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, K.; Bastakoti, R.R. Ethnomedicinal knowledge and healthcare practices among the Tharus of Nawalparasi district in central Nepal. For. Ecol. Manag. 2009, 257, 2066–2072. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Pharmacology of Ficus religiosa—A review. IOSR J. Pharm. 2017, 7, 49–60. [Google Scholar] [CrossRef]
- Rajiv, P.; Sivaraj, R. Screening for phytochemicals and antimicrobial activity of aqueous extract of Ficus religiosa Linn. Int. J. Pharm. Pharm. Sci. 2012, 4, 207–209. [Google Scholar]
- Manorenjitha, M.; Norita, A.; Adillah, A.; Asmawi, M. Chemical profile of Ficus religiosa (Linn.) stem. Int. J. Life Sci. Med. Res. 2014, 4, 32. [Google Scholar]
- Aqil, F.; Ahmad, I. Broad-spectrum antibacterial and antifungal properties of certain traditionally used Indian medicinal plants. World J. Microbiol. Biotechnol. 2003, 19, 653–657. [Google Scholar] [CrossRef]
- Poudel, A.; Satyal, P.; Setzer, W.N. Composition and bioactivities of the leaf Essential oil of Ficus religiosa Linn. Am. J. Essent. Oils Nat. Prod. 2015, 2, 16–17. [Google Scholar]
- Naira, N.; Rohini, R.; Syed, M.; Amit, K. Wound healing activity of the hydro alcoholic extract of Ficus religiosa leaves in rats. Internet J. Altern. Med. 2009, 6, 2–7. [Google Scholar]
- Grison-Pigé, L.; Hossaert-McKey, M.; Greeff, J.M. Fig volatile compounds—A first comparative study. Phytochemistry 2002, 61, 61–71. [Google Scholar] [CrossRef]
- Makhija, I.K.; Sharma, I.P.; Khamar, D. Phytochemistry and Pharmacological properties of Ficus religiosa: An overview. Ann. Biol. Res. 2010, 1, 171–180. [Google Scholar]
- Murugesu, S.; Selamat, J.; Perumal, V. Phytochemistry, pharmacological properties, and recent applications of Ficus benghalensis and Ficus religiosa. Plants 2021, 10, 2749. [Google Scholar] [CrossRef] [PubMed]
- Rathee, D.; Rathee, S.; Rathee, P.; Deep, A.; Anandjiwala, S.; Rathee, D. HPTLC densitometric quantification of stigmasterol and lupeol from Ficus religiosa. Arab. J. Chem. 2015, 8, 366–371. [Google Scholar] [CrossRef]
- Warrier, P.K. Indian Medicinal Plants: A Compendium of 500 Species; Orient Blackswan: Andhra Pradesh, India, 1993; Volume 5. [Google Scholar]
- Ali, M.; Qadry, J. Amino-acid-composition of fruits and seeds of medicinal-plants. J. Indian Chem. Soc. 1987, 64, 230–231. [Google Scholar]
- Patel, R.; Gautam, P. Medicinal potency of Ficus benghalensis: A review. Int. J. Med. Chem. Anal. 2014, 4, 53–58. [Google Scholar]
- Shukla, R.; Gupta, S.; Gambhir, J.; Prabhu, K.; Murthy, P. Antioxidant effect of aqueous extract of the bark of Ficus bengalensis in hypercholesterolaemic rabbits. J. Ethnopharmacol. 2004, 92, 47–51. [Google Scholar] [CrossRef]
- Joseph, B.; Raj, S.J. Phytopharmacological and phytochemical properties of three Ficus species—An overview. Int. J. Pharma Bio Sci. 2010, 1, 246–253. [Google Scholar]
- Gill, N.; Rashmi, A.; Anmol, K.; Manpreet, K. Free radical scavenging activity and phytochemical investigation of Ficus benjamina fruit. Int. J. Univ. Pharm. Bio. Sci. 2016, 5, 14–26. [Google Scholar]
- Govindan, V.; Francis, G. Qualitative and quantitative determination of secondary metabolites and antioxidant potential of Ficus benghalensis linn seed. Int. J. Pharm. Pharm. Sci. 2015, 7, 118–124. [Google Scholar]
- Bhaskara Rao, K.; Ojha, V.; Preeti; Kumar, G.; Karthik, L. Phytochemical composition and antioxidant activity of Ficus benghalensis (Moraceae) leaf extract. J. Biol. Act. Prod. Nat. 2014, 4, 236–248. [Google Scholar]
- Malik, H.; Javaid, S.; Fawad Rasool, M.; Samad, N.; Rizwan Ahamad, S.; Alqahtani, F.; Imran, I. Amelioration of scopolamine-induced amnesic, anxiolytic and antidepressant effects of Ficus benghalensis in behavioral experimental models. Medicina 2020, 56, 144. [Google Scholar] [CrossRef]
- Naquvi, K.J.; Ali, M.; Ahamad, J. Two new phytosterols from the stem bark of Ficus bengalensis L. J. Saudi Chem. Soc. 2015, 19, 650–654. [Google Scholar] [CrossRef]
- Roskams, A.J.; Connor, J.R. Aluminum access to the brain: A role for transferrin and its receptor. Proc. Natl. Acad. Sci. USA 1990, 87, 9024–9027. [Google Scholar] [CrossRef] [PubMed]
- Oğuz, E.O.; Enli, Y.; Şahin, B.; Gönen, C.; Turgut, G. Aluminium sulphate exposure increases oxidative stress and suppresses brain development in Ross broiler chicks. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2012, 18, BR103. [Google Scholar] [CrossRef] [PubMed]
- Cherubini, E.; Miles, R.M. The CA3 region of the hippocampus: How is it? What is it for? How does it do it? Front. Cell. Neurosci. 2015, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Massand, A.; Rai, R.; Rai, A.R.; Joy, T.; Murlimanju, B.; Marathe, A. Effect of Methanolic Leaf Extract of Ficus religiosa on Neuronal Degeneration: A Pilot Study in Male Albino Wistar Rats. Indian J. Public Health Res. Dev. 2020, 11, 685. [Google Scholar] [CrossRef]
- Bhangale, J.O.; Acharya, N.S.; Acharya, S.R. Protective effect of Ficus religiosa (L.) against 3-nitropropionic acid induced Huntington disease. Orient. Pharm. Exp. Med. 2016, 16, 165–174. [Google Scholar] [CrossRef]
- Schulz, J.; Matthews, R.; Henshaw, D.; Beal, M. Neuroprotective strategies for treatment of lesions produced by mitochondrial toxins: Implications for neurodegenerative diseases. Neuroscience 1996, 71, 1043–1048. [Google Scholar] [CrossRef]
- Devi, W.; Sengottuvelu, S.; Haja, S.; Lalitha, V.; Sivakumar, T. Memory enhancing activities of Ficus religiosa leaves in rodents. Int. J. Res. Ayurveda Pharm. (IJRAP) 2011, 2, 834–838. [Google Scholar]
- Ghafoor, A.; Tahir, M.; Lone, K.P.; Faisal, B.; Latif, W. The effect of Ficus carica L.(Anjir) leaf extract on gentamicin induced nephrotoxicity in adult male albino mice. J. Ayub. Med. Coll. Abbottabad 2015, 27, 398–401. [Google Scholar]
- Vyawahare, N.; Khandelwal, A.; Batra, V.; Nikam, A. Herbal anticonvulsants. J. Herb. Med. Toxicol. 2007, 1, 9–14. [Google Scholar]
- Singh, D.; Singh, B.; Goel, R.K. Hydroethanolic leaf extract of Ficus religiosa lacks anticonvulsant activity in acute electro and chemo convulsion mice models. J. Pharm. Negat. Results 2011, 2, 58. [Google Scholar]
- Singh, D.; Singh, B.; Goel, R.K. Role of saponins for the anticonvulsant effect of adventitious roots of Ficus religiosa. Pharm. Biol. 2012, 50, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Mishra, A.; Goel, R.K. Effect of saponin fraction from Ficus religiosa on memory deficit, and behavioral and biochemical impairments in pentylenetetrazol kindled mice. Epilepsy. Behav. 2013, 27, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.S.; Patil, C.; Patil, S.; Jadhav, R. Anticonvulsant activity of aqueous root extract of Ficus religiosa. J. Ethnopharmacol. 2011, 133, 92–96. [Google Scholar] [CrossRef]
- Ahuja, D.; Bijjem, K.R.V.; Kalia, A.N. Bronchospasm potentiating effect of methanolic extract of Ficus religiosa fruits in guinea pigs. J. Ethnopharmacol. 2011, 133, 324–328. [Google Scholar] [CrossRef]
- Bagdy, G.; Kecskemeti, V.; Riba, P.; Jakus, R. Serotonin and epilepsy. J. Neurochem. 2007, 100, 857–873. [Google Scholar] [CrossRef]
- Statnick, M.A.; Dailey, J.W.; Jobe, P.C.; Browning, R.A. Abnormalities in brain serotonin concentration, high-affinity uptake, and tryptophan hydroxylase activity in severe-seizure genetically epilepsy-prone rats. Epilepsia 1996, 37, 311–321. [Google Scholar] [CrossRef]
- Kaur, H.; Singh, D.; Singh, B.; Goel, R.K. Anti-amnesic effect of Ficus religiosa in scopolamine-induced anterograde and retrograde amnesia. Pharm. Biol. 2010, 48, 234–240. [Google Scholar] [CrossRef]
- Vinutha, B.; Prashanth, D.; Salma, K.; Sreeja, S.; Pratiti, D.; Padmaja, R.; Radhika, S.; Amit, A.; Venkateshwarlu, K.; Deepak, M. Screening of selected Indian medicinal plants for acetylcholinesterase inhibitory activity. J. Ethnopharmacol. 2007, 109, 359–363. [Google Scholar] [CrossRef]
- Stalin, C.; Gunasekaran, V.; Jayabalan, G. Evaluation of Neuroprotective Effect of Ficus benghalensis against Alloxan Induced Diabetic Neuropathy in Rats. Int. J. Pharmacol. Phytochem. Ethnomed. 2016, 4, 52–60. [Google Scholar] [CrossRef]
- Chandra, P.; Sachan, N.; Chaudhary, A.; Yadav, M.; Kishore, K.; Ghosh, A.K. Acute & Sub Chronic Toxicity Studies and Pharmacological Evaluation of Ficus bengalensis L. (Family: Moraceae) on Scopolamine-Induced Memory Impairmentin Experimental Animals. Indian J. Drugs 2013, 1, 6–16. [Google Scholar]
- Panday, D.R.; Rauniar, G. Effect of root-extracts of Ficus benghalensis (Banyan) in memory, anxiety, muscle co-ordination and seizure in animal models. BMC Complement. Altern. Med. 2016, 16, 429. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, N.M. β-Amyrin rich Bombax ceiba leaf extract with potential neuroprotective activity against scopolamine-induced memory impairment in rats. Rec. Nat. Prod. 2018, 12, 480. [Google Scholar] [CrossRef]
- Park, S.J.; Ahn, Y.J.; Oh, S.R.; Lee, Y.; Kwon, G.; Woo, H.; Lee, H.E.; Jang, D.S.; Jung, J.W.; Ryu, J.H. Amyrin attenuates scopolamine-induced cognitive impairment in mice. Biol. Pharm. Bull. 2014, 37, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-J.; Koh, S.-H. The role of PI3K/AKT pathway and its therapeutic possibility in Alzheimer’s disease. Hanyang Med. Rev. 2017, 37, 18–24. [Google Scholar] [CrossRef]
- Zhu, X.; Castellani, R.J.; Takeda, A.; Nunomura, A.; Atwood, C.S.; Perry, G.; Smith, M.A. Differential activation of neuronal ERK, JNK/SAPK and p38 in Alzheimer disease: The ‘two hit’ hypothesis. Mech. Ageing Dev. 2001, 123, 39–46. [Google Scholar] [CrossRef]
- Giovannini, M.G. The role of the extracellular signal-regulated kinase pathway in memory encoding. Rev. Neurosci. 2006, 17, 619–634. [Google Scholar] [CrossRef]
- Peineau, S.; Taghibiglou, C.; Bradley, C.; Wong, T.P.; Liu, L.; Lu, J.; Lo, E.; Wu, D.; Saule, E.; Bouschet, T. LTP inhibits LTD in the hippocampus via regulation of GSK3β. Neuron 2007, 53, 703–717. [Google Scholar] [CrossRef]
- Aragão, G.F.; Carneiro, L.M.V.; Rota-Junior, A.P.; Bandeira, P.N.; Lemos, T.L.G.d.; Viana, G.S.d.B. Alterations in brain amino acid metabolism and inhibitory effects on PKC are possibly correlated with anticonvulsant effects of the isomeric mixture of α-and β-amyrin from Protium heptaphyllum. Pharm. Biol. 2015, 53, 407–413. [Google Scholar] [CrossRef]
- Wu, G.; Yu, J.; Wang, L.; Ren, S.; Zhang, Y. PKC/CREB pathway mediates the expressions of GABAA receptor subunits in cultured hippocampal neurons after low-Mg2+ solution treatment. Epilepsy Res. 2018, 140, 155–161. [Google Scholar] [CrossRef]
- Kun, X.; Zuhua, G. Amyrin exerts potent anxiolytic and antidepressant effects via mechanisms involving monoamine oxidase and γ-aminobutyric acid in mouse hippocampus. Trop. J. Pharm. Res. 2019, 18, 1673–1681. [Google Scholar]
- Riederer, P.; Laux, G. MAO-inhibitors in Parkinson’s Disease. Exp. Neurobiol. 2011, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Sharmaa, N.; Khuranaa, N.; Muthuramanb, A.; Utrejac, P. Azelaic acid attenuatesrotenone-induced behavioural alterations in parkinson’s disease rat model. Plant Arch. 2021, 21, 2333–2337. [Google Scholar] [CrossRef]
- Castor, K.; Shenoi, S.; Edminster, S.; Tran, T.; King, K.; Chui, H.; Pogoda, J.; Fonteh, A.; Harrington, M. Urine dicarboxylic acids change in pre-symptomatic Alzheimer’s disease and reflect loss of energy capacity and hippocampal volume. PLoS ONE 2020, 15, e0231765. [Google Scholar] [CrossRef] [PubMed]
- Budzynska, B.; Skalicka-Wozniak, K.; Kruk-Slomka, M.; Wydrzynska-Kuzma, M.; Biala, G. In Vivo modulation of the behavioral effects of nicotine by the coumarins xanthotoxin, bergapten, and umbelliferone. Psychopharmacology 2016, 233, 2289–2300. [Google Scholar] [CrossRef]
- Liang, Y.; Xie, L.; Liu, K.; Cao, Y.; Dai, X.; Wang, X.; Lu, J.; Zhang, X.; Li, X. Bergapten: A review of its pharmacology, pharmacokinetics, and toxicity. Phytother. Res. 2021, 35, 6131–6147. [Google Scholar] [CrossRef]
- Dincel, D.; Hatipoğlu, S.D.; Gören, A.C.; Topcu, G. Anticholinesterase furocoumarins from Heracleum platytaenium, a species endemic to the Ida Mountains. Turk. J. Chem. 2013, 37, 675–683. [Google Scholar]
- Orhan, I.; Tosun, F.; Şener, B. Coumarin, anthroquinone and stilbene derivatives with anticholinesterase activity. Z. Nat. C 2008, 63, 366–370. [Google Scholar] [CrossRef]
- Senol, F.S.; Woźniak, K.S.; Khan, M.T.H.; Orhan, I.E.; Sener, B.; Głowniak, K. An in vitro and in silico approach to cholinesterase inhibitory and antioxidant effects of the methanol extract, furanocoumarin fraction, and major coumarins of Angelica officinalis L. fruits. Phytochem. Lett. 2011, 4, 462–467. [Google Scholar] [CrossRef]
- Kowalczyk, J.; Kurach, Ł.; Boguszewska-Czubara, A.; Skalicka-Woźniak, K.; Kruk-Słomka, M.; Kurzepa, J.; Wydrzynska-Kuźma, M.; Biała, G.; Skiba, A.; Budzyńska, B. Bergapten improves scopolamine-induced memory impairment in mice via cholinergic and antioxidative mechanisms. Front. Neurosci. 2020, 14, 730. [Google Scholar] [CrossRef]
- Huong, D.T.L.; Choi, H.C.; Rho, T.C.; Lee, H.S.; Lee, M.K.; Kim, Y.H. Inhibitory activity of monoamine oxidase by coumarins from Peucedanum japonicum. Arch. Pharmacal Res. 1999, 22, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.-U.; Ijaz, M.U.; Shah, F.A.; Khan, A.W.; Li, S. Neuroprotective Effects of Berbamine, Bergepten, and Carveol on Paclitaxel-Induced Peripheral Neuropathy. Res. Sq. 2022, 1–23. [Google Scholar] [CrossRef]
- Yang, Y.-F.; Xu, W.; Song, W.; Ye, M.; Yang, X.-W. Transport of twelve coumarins from Angelicae Pubescentis Radix across a MDCK-pHaMDR cell monolayer—An in vitro model for blood-brain barrier permeability. Molecules 2015, 20, 11719–11732. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-F.; Zhang, L.; Yang, X.-W. Distribution assessments of coumarins from Angelicae Pubescentis Radix in rat cerebrospinal fluid and brain by Liquid Chromatography Tandem Mass Spectrometry analysis. Molecules 2018, 23, 225. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.L.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef]
- Cowley, S.; Paterson, H.; Kemp, P.; Marshall, C.J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell 1994, 77, 841–852. [Google Scholar] [CrossRef]
- Obara, Y.; Aoki, T.; Kusano, M.; Ohizumi, Y. β-Eudesmol induces neurite outgrowth in rat pheochromocytoma cells accompanied by an activation of mitogen-activated protein kinase. J. Pharmacol. Exp. Ther. 2002, 301, 803–811. [Google Scholar] [CrossRef]
- Kim, K.Y. Anti-inflammatory and ECM gene expression modulations of β-eudesmol via NF-κB signaling pathway in normal human dermal fibroblasts. Biomed. Dermatol. 2018, 2, 3. [Google Scholar] [CrossRef]
- Barot, J.; Saxena, B. Therapeutic effects of eugenol in a rat model of traumatic brain injury: A behavioral, biochemical, and histological study. J. Tradit. Complement. Med. 2021, 11, 318–327. [Google Scholar] [CrossRef]
- Sun, X.; Wang, D.; Zhang, T.; Lu, X.; Duan, F.; Ju, L.; Zhuang, X.; Jiang, X. Eugenol attenuates cerebral ischemia-reperfusion injury by enhancing autophagy via AMPK-mTOR-P70S6K pathway. Front. Pharmacol. 2020, 11, 84. [Google Scholar] [CrossRef]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Mu, Y.; Jiang, Y.; Song, R.; Yi, J.; Zhou, J.; Sun, J.; Jiao, X.; Prinz, R.A.; Li, Y. Inhibition of p70 S6 kinase activity by A77,1726 induces autophagy and enhances the degradation of superoxide dismutase 1 (SOD1) protein aggregates. Cell Death Dis. 2018, 9, 407. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.N. Neuroprotective efficacy of eugenol and isoeugenol in acrylamide-induced neuropathy in rats: Behavioral and biochemical evidence. Neurochem. Res. 2013, 38, 330–345. [Google Scholar] [CrossRef] [PubMed]
- Irie, Y.; Itokazu, N.; Anjiki, N.; Ishige, A.; Watanabe, K.; Keung, W.M. Eugenol exhibits antidepressant-like activity in mice and induces expression of metallothionein-III in the hippocampus. Brain Res. 2004, 1011, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Said, M.M.; Abd Rabo, M.M. Neuroprotective effects of eugenol against aluminiuminduced toxicity in the rat brain. Arh. Z. Hig. Rada I Toksikol. 2017, 68, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, A.H.; Merrill, D.A.; Coppola, G.; Tsukada, S.; Schroeder, B.E.; Shaked, G.M.; Wang, L.; Blesch, A.; Kim, A.; Conner, J.M. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nat. Med. 2009, 15, 331–337. [Google Scholar] [CrossRef]
- Pandini, G.; Satriano, C.; Pietropaolo, A.; Gianì, F.; Travaglia, A.; La Mendola, D.; Nicoletti, V.G.; Rizzarelli, E. The inorganic side of NGF: Copper (II) and Zinc (II) affect the NGF mimicking signaling of the N-terminus peptides encompassing the recognition domain of TrkA receptor. Front. Neurosci. 2016, 10, 569. [Google Scholar] [CrossRef]
- Moreira Vasconcelos, C.F.; da Cunha Ferreira, N.M.; Hardy Lima Pontes, N.; de Sousa dos Reis, T.D.; Basto Souza, R.; Aragao Catunda Junior, F.E.; Vasconcelos Aguiar, L.M.; Maranguape Silva da Cunha, R. Eugenol and its association with levodopa in 6-hydroxydopamine-induced hemiparkinsonian rats: Behavioural and neurochemical alterations. Basic Clin. Pharmacol. Toxicol. 2020, 127, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Dubey, K.; Anand, B.G.; Shekhawat, D.S.; Kar, K. Eugenol prevents amyloid formation of proteins and inhibits amyloid-induced hemolysis. Sci. Rep. 2017, 7, 40744. [Google Scholar] [CrossRef]
- Nagy, I.; Jancsó, G.; Urbán, L. The role of the vanilloid (capsaicin) receptor (TRPV1) in physiology and pathology. Eur. J. Pharmacol. 2004, 500, 351–369. [Google Scholar] [CrossRef]
- Silva dos Santos, J.; Goncalves Cirino, J.P.; de Oliveira Carvalho, P.; Ortega, M.M. The pharmacological action of kaempferol in central nervous system diseases: A review. Front. Pharmacol. 2021, 11, 565700. [Google Scholar] [CrossRef] [PubMed]
- Beg, T.; Jyoti, S.; Naz, F.; Ali, F.; Ali, S.K.; Reyad, A.M.; Siddique, Y.H. Protective effect of kaempferol on the transgenic Drosophila model of Alzheimer’s disease. CNS Neurol. Disord.-Drug Targets (Former. Curr. Drug Targets-CNS Neurol. Disord.) 2018, 17, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Mohammadi, S.; Jabbari, S.; Shahidi, S. Intracerebroventricular microinjection of kaempferol on memory retention of passive avoidance learning in rats: Involvement of cholinergic mechanism (s). Int. J. Neurosci. 2019, 129, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Hanaki, M.; Murakami, K.; Akagi, K.-I.; Irie, K. Structural insights into mechanisms for inhibiting amyloid β42 aggregation by non-catechol-type flavonoids. Bioorganic Med. Chem. 2016, 24, 304–313. [Google Scholar] [CrossRef]
- Pan, X.; Liu, X.; Zhao, H.; Wu, B.; Liu, G. Antioxidant, anti-inflammatory and neuroprotective effect of kaempferol on rotenone-induced Parkinson’s disease model of rats and SH-S5Y5 cells by preventing loss of tyrosine hydroxylase. J. Funct. Foods 2020, 74, 104140. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Cheng, X.; Li, W.-H.; Liu, M.; Wang, Y.-H.; Du, G.-H. Kaempferol attenuates LPS-induced striatum injury in mice involving anti-neuroinflammation, maintaining BBB integrity, and down-regulating the HMGB1/TLR4 pathway. Int. J. Mol. Sci. 2019, 20, 491. [Google Scholar] [CrossRef]
- Filomeni, G.; Graziani, I.; De Zio, D.; Dini, L.; Centonze, D.; Rotilio, G.; Ciriolo, M.R. Neuroprotection of kaempferol by autophagy in models of rotenone-mediated acute toxicity: Possible implications for Parkinson’s disease. Neurobiol. Aging 2012, 33, 767–785. [Google Scholar] [CrossRef]
- Li, S.; Pu, X.-P. Neuroprotective effect of kaempferol against a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse model of Parkinson’s disease. Biol. Pharm. Bull. 2011, 34, 1291–1296. [Google Scholar] [CrossRef]
- Yang, Y.; Bai, L.; Li, X.; Xiong, J.; Xu, P.; Guo, C.; Xue, M. Transport of active flavonoids, based on cytotoxicity and lipophilicity: An evaluation using the blood–brain barrier cell and Caco-2 cell models. Toxicol. Vitr. 2014, 28, 388–396. [Google Scholar] [CrossRef]
- Hu, L.-D.; Wang, J.; Chen, X.-J.; Yan, Y.-B. Lanosterol modulates proteostasis via dissolving cytosolic sequestosomes/aggresome-like induced structures. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2020, 1867, 118617. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, Z.; Tian, X.; Chen, L.; Lee, S.; Huynh, T.; Ge, C.; Zhou, R. Lanosterol disrupts the aggregation of amyloid-β peptides. ACS Chem. Neurosci. 2019, 10, 4051–4060. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Amanullah, A.; Mishra, R.; Kumar, A.; Mishra, A. Lanosterol suppresses the aggregation and cytotoxicity of misfolded proteins linked with neurodegenerative diseases. Mol. Neurobiol. 2018, 55, 1169–1182. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.; Jackson-Lewis, V.; Wong, L.; Shui, G.; Goh, A.; Kesavapany, S.; Jenner, A.; Fivaz, M.; Przedborski, S.; Wenk, M. Lanosterol induces mitochondrial uncoupling and protects dopaminergic neurons from cell death in a model for Parkinson’s disease. Cell Death Differ. 2012, 19, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Johnson, S.L.; Liu, W.; DaSilva, N.A.; Meschwitz, S.; Dain, J.A.; Seeram, N.P. Evaluation of polyphenol anthocyanin-enriched extracts of blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry for free radical scavenging, reactive carbonyl species trapping, anti-glycation, anti-β-amyloid aggregation, and microglial neuroprotective effects. Int. J. Mol. Sci. 2018, 19, 461. [Google Scholar]
- Suresh, S.; Begum, R.F.; Singh, A.; Chitra, V. Anthocyanin as a Therapeutic in Alzheimer’s Disease: A Systematic Review of Preclinical Evidences. Ageing Res. Rev. 2022, 76, 101595. [Google Scholar] [CrossRef]
- Ullah, R.; Khan, M.; Shah, S.A.; Saeed, K.; Kim, M.O. Natural antioxidant anthocyanins—A hidden therapeutic candidate in metabolic disorders with major focus in neurodegeneration. Nutrients 2019, 11, 1195. [Google Scholar] [CrossRef]
- Winter, A.N.; Bickford, P.C. Anthocyanins and their metabolites as therapeutic agents for neurodegenerative disease. Antioxidants 2019, 8, 333. [Google Scholar] [CrossRef]
- Khan, M.S.; Ikram, M.; Park, T.J.; Kim, M.O. Pathology, risk factors, and oxidative damage related to type 2 diabetes-mediated Alzheimer’s disease and the rescuing effects of the potent antioxidant anthocyanin. Oxidative Med. Cell. Longev. 2021, 2021, 4051207. [Google Scholar] [CrossRef]
- Miguel, M.G. Anthocyanins: Antioxidant and/or anti-inflammatory activities. J. Appl. Pharm. Sci. 2011, 1, 7–15. [Google Scholar]
- El-Shiekh, R.A.; Ashour, R.M.; Abd El-Haleim, E.A.; Ahmed, K.A.; Abdel-Sattar, E. Hibiscus sabdariffa L.: A potent natural neuroprotective agent for the prevention of streptozotocin-induced Alzheimer’s disease in mice. Biomed. Pharmacother. 2020, 128, 110303. [Google Scholar] [CrossRef]
- Kerr, F.; Sofola-Adesakin, O.; Ivanov, D.K.; Gatliff, J.; Gomez Perez-Nievas, B.; Bertrand, H.C.; Martinez, P.; Callard, R.; Snoeren, I.; Cocheme, H.M. Direct Keap1-Nrf2 disruption as a potential therapeutic target for Alzheimer’s disease. PLoS Genet. 2017, 13, e1006593. [Google Scholar] [CrossRef] [PubMed]
- Reichard, J.F.; Motz, G.T.; Puga, A. Heme oxygenase-1 induction by NRF2 requires inactivation of the transcriptional repressor BACH1. Nucleic Acids Res. 2007, 35, 7074–7086. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Ou, S.; Wu, T.; Zhou, L.; Tang, H.; Jiang, M.; Xu, J.; Guo, K. Lycopene alleviates oxidative stress via the PI3K/Akt/Nrf2pathway in a cell model of Alzheimer’s disease. PeerJ 2020, 8, e9308. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, R.; Zhao, H.; Chen, G.; Jiang, Y.; Lyu, X.; Wu, T. Reduction of aging-induced oxidative stress and activation of autophagy by bilberry anthocyanin supplementation via the AMPK–mTOR signaling pathway in aged female rats. J. Agric. Food Chem. 2019, 67, 7832–7843. [Google Scholar] [CrossRef] [PubMed]
- Youdim, K.A.; Dobbie, M.S.; Kuhnle, G.; Proteggente, A.R.; Abbott, N.J.; Rice-Evans, C. Interaction between flavonoids and the blood–brain barrier: In Vitro studies. J. Neurochem. 2003, 85, 180–192. [Google Scholar] [CrossRef]
- Piccialli, I.; Tedeschi, V.; Caputo, L.; Amato, G.; De Martino, L.; De Feo, V.; Secondo, A.; Pannaccione, A. The Antioxidant Activity of Limonene Counteracts Neurotoxicity Triggered byAβ1-42 Oligomers in Primary Cortical Neurons. Antioxidants 2021, 10, 937. [Google Scholar] [CrossRef]
- Pannaccione, A.; Boscia, F.; Scorziello, A.; Adornetto, A.; Castaldo, P.; Sirabella, R.; Taglialatela, M.; Di Renzo, G.; Annunziato, L. Up-regulation and increased activity of KV3. 4 channels and their accessory subunit MinK-related peptide 2 induced by amyloid peptide are involved in apoptotic neuronal death. Mol. Pharmacol. 2007, 72, 665–673. [Google Scholar] [CrossRef]
- Zhou, W.; Yoshioka, M.; Yokogoshi, H. Sub-chronic effects of s-limonene on brain neurotransmitter levels and behavior of rats. J. Nutr. Sci. Vitaminol. 2009, 55, 367–373. [Google Scholar] [CrossRef]
- Boiangiu, R.S.; Brinza, I.; Hancianu, M.; Erdogan Orhan, I.; Eren, G.; Gündüz, E.; Ertas, H.; Hritcu, L.; Cioanca, O. Cognitive facilitation and antioxidant effects of an essential oil mix on scopolamine-induced amnesia in rats: Molecular modeling of in vitro and in vivo approaches. Molecules 2020, 25, 1519. [Google Scholar] [CrossRef]
- Abuhamdah, S.; Abuhamdah, R.; Howes, M.-J.R.; Al-Olimat, S.; Ennaceur, A.; Chazot, P.L. Pharmacological and neuroprotective profile of an essential oil derived from leaves of A loysia citrodora Palau. J. Pharm. Pharmacol. 2015, 67, 1306–1315. [Google Scholar] [CrossRef]
- Lomarat, P.; Sripha, K.; Phanthong, P.; Kitphati, W.; Thirapanmethee, K.; Bunyapraphatsara, N. In Vitro biological activities of black pepper essential oil and its major components relevant to the prevention of Alzheimer’s disease. Thai J. Pharm. Sci. (TJPS) 2015, 39, 94–101. [Google Scholar]
- Sadiki, F.Z.; El Idrissi, M.; Cioanca, O.; Trifan, A.; Hancianu, M.; Hritcu, L.; Postu, P.A. Tetraclinis articulata essential oil mitigates cognitive deficits and brain oxidative stress in an Alzheimer’s disease amyloidosis model. Phytomedicine 2019, 56, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.-H.; Sheen, L.-Y.; Chang, S.-T. Evaluation of anxiolytic potency of essential oil and S-(+)-linalool from Cinnamomum osmophloeum ct. linalool leaves in mice. J. Tradit. Complement. Med. 2015, 5, 27–34. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Caputo, L.; Piccialli, I.; Ciccone, R.; de Caprariis, P.; Massa, A.; De Feo, V.; Pannaccione, A. Lavender and coriander essential oils and their main component linalool exert a protective effect against amyloid-β neurotoxicity. Phytother. Res. 2021, 35, 486–493. [Google Scholar] [CrossRef]
- Yuan, C.; Shin, M.; Park, Y.; Choi, B.; Jang, S.; Lim, C.; Yun, H.S.; Lee, I.-S.; Won, S.-Y.; Cho, K.S. Linalool Alleviates Aβ42-Induced Neurodegeneration via Suppressing ROS Production and Inflammation in Fly and Rat Models of Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2021, 2021, 8887716. [Google Scholar] [CrossRef]
- Sabogal-Guáqueta, A.M.; Posada-Duque, R.; Cortes, N.C.; Arias-Londoño, J.D.; Cardona-Gómez, G.P. Changes in the hippocampal and peripheral phospholipid profiles are associated with neurodegeneration hallmarks in a long-term global cerebral ischemia model: Attenuation by Linalool. Neuropharmacology 2018, 135, 555–571. [Google Scholar] [CrossRef]
- Sabogal-Guáqueta, A.M.; Osorio, E.; Cardona-Gómez, G.P. Linalool reverses neuropathological and behavioral impairments in old triple transgenic Alzheimer’s mice. Neuropharmacology 2016, 102, 111–120. [Google Scholar] [CrossRef]
- Silva Brum, L.; Emanuelli, T.; Souza, D.; Elisabetsky, E. Effects of linalool on glutamate release and uptake in mouse cortical synaptosomes. Neurochem. Res. 2001, 26, 191–194. [Google Scholar] [CrossRef]
- Sabogal-Guáqueta, A.M.; Hobbie, F.; Keerthi, A.; Oun, A.; Kortholt, A.; Boddeke, E.; Dolga, A. Linalool attenuates oxidative stress and mitochondrial dysfunction mediated by glutamate and NMDA toxicity. Biomed. Pharmacother. 2019, 118, 109295. [Google Scholar] [CrossRef]
- Li, Y.; Lv, O.; Zhou, F.; Li, Q.; Wu, Z.; Zheng, Y. Linalool inhibits LPS-induced inflammation in BV2 microglia cells by activating Nrf2. Neurochem. Res. 2015, 40, 1520–1525. [Google Scholar] [CrossRef]
- Xu, X.; Lv, H.; Xia, Z.; Fan, R.; Zhang, C.; Wang, Y.; Wang, D. Rhein exhibits antioxidative effects similar to Rhubarb in a rat model of traumatic brain injury. BMC Complement. Altern. Med. 2017, 17, 140. [Google Scholar] [CrossRef] [PubMed]
- Badshah, H.; Ali, T.; Rehman, S.-U.; Amin, F.-U.; Ullah, F.; Kim, T.H.; Kim, M.O. Protective effect of lupeol against lipopolysaccharide-induced neuroinflammation via the p38/c-Jun N-terminal kinase pathway in the adult mouse brain. J. Neuroimmune Pharmacol. 2016, 11, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Koirala, P.; Seong, S.H.; Jung, H.A.; Choi, J.S. Comparative molecular docking studies of lupeol and lupenone isolated from Pueraria lobata that inhibits BACE1: Probable remedies for Alzheimer’s disease. Asian Pac. J. Trop. Med. 2017, 10, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Kaundal, M.; Akhtar, M.; Deshmukh, R. Lupeol Isolated from Betula alnoides Ameliorates Amyloid Beta Induced Neuronal Damage via Targeting Various Pathological Events and Alteration in Neurotransmitter Levels in Rat’s Brain. J. Neurol. Neurosci. 2017, 8, 195. [Google Scholar] [CrossRef]
- Ahmad, R.; Khan, A.; Lee, H.J.; Ur Rehman, I.; Khan, I.; Alam, S.I.; Kim, M.O. Lupeol, a plant-derived triterpenoid, protects mice brains against Aβ-induced oxidative stress and neurodegeneration. Biomedicines 2020, 8, 380. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Khan, A.; Rehman, I.U.; Lee, H.J.; Khan, I.; Kim, M.O. Lupeol Treatment Attenuates Activation of Glial Cells and Oxidative-Stress-Mediated Neuropathology in Mouse Model of Traumatic Brain Injury. Int. J. Mol. Sci. 2022, 23, 6086. [Google Scholar] [CrossRef]
- Oliveira-Junior, M.S.; Pereira, E.P.; de Amorim, V.C.M.; Reis, L.T.C.; do Nascimento, R.P.; da Silva, V.D.A.; Costa, S.L. Lupeol inhibits LPS-induced neuroinflammation in cerebellar cultures and induces neuroprotection associated to the modulation of astrocyte response and expression of neurotrophic and inflammatory factors. Int. Immunopharmacol. 2019, 70, 302–312. [Google Scholar] [CrossRef]
- Malik, A.; Jamil, U.; Butt, T.T.; Waquar, S.; Gan, S.H.; Shafique, H.; Jafar, T.H. In Silico and in vitro studies of lupeol and iso-orientin as potential antidiabetic agents in a rat model. Drug Des. Dev. Ther. 2019, 13, 1501. [Google Scholar] [CrossRef] [PubMed]
- Villalba, H.; Shah, K.; Albekairi, T.H.; Sifat, A.E.; Vaidya, B.; Abbruscato, T.J. Potential role of myo-inositol to improve ischemic stroke outcome in diabetic mouse. Brain Res. 2018, 1699, 166–176. [Google Scholar] [CrossRef]
- Villalba, H.; Vaidya, B.; Cucullo, L.; Abbruscato, T.J. Myo-Inositol Improves Ischemic Stroke Outcome after both Nicotine Containing Electronic Cigarette and Tobacco Smoke Exposure. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Kandashvili, M.; Gamkrelidze, G.; Tsverava, L.; Lordkipanidze, T.; Lepsveridze, E.; Lagani, V.; Burjanadze, M.; Dashniani, M.; Kokaia, M.; Solomonia, R. Myo-Inositol Limits Kainic Acid-Induced Epileptogenesis in Rats. Int. J. Mol. Sci. 2022, 23, 1198. [Google Scholar] [CrossRef] [PubMed]
- Kalacheva, A.; Gromova, O.; Grishina, T.; Gogoleva, I.; Demidov, V.; Torshin, I. An experimental study of anticonvulsant effects of myo-inositol and folic acid. Zhurnal Nevrol. Psikhiatrii Im. SS Korsakova 2016, 116, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Voevodskaya, O.; Sundgren, P.C.; Strandberg, O.; Zetterberg, H.; Minthon, L.; Blennow, K.; Wahlund, L.-O.; Westman, E.; Hansson, O.; Group, S.B.S. Myo-inositol changes precede amyloid pathology and relate to APOE genotype in Alzheimer disease. Neurology 2016, 86, 1754–1761. [Google Scholar] [CrossRef] [PubMed]
- Voevodskaya, O.; Poulakis, K.; Sundgren, P.; Van Westen, D.; Palmqvist, S.; Wahlund, L.-O.; Stomrud, E.; Hansson, O.; Westman, E.; Group, S.B.S. Brain myoinositol as a potential marker of amyloid-related pathology: A longitudinal study. Neurology 2019, 92, e395–e405. [Google Scholar] [CrossRef] [PubMed]
- Spector, R. Myo-inositol transport through the blood-brain barrier. Neurochem. Res. 1988, 13, 785–787. [Google Scholar] [CrossRef]
- Yao, Y.; Lin, G.; Xie, Y.; Ma, P.; Li, G.; Meng, Q.; Wu, T. Preformulation studies of myricetin: A natural antioxidant flavonoid. Die Pharm.-Int. J. Pharm. Sci. 2014, 69, 19–26. [Google Scholar]
- Liu, M.; Guo, H.; Li, Z.; Zhang, C.; Zhang, X.; Cui, Q.; Tian, J. Molecular level insight into the benefit of myricetin and dihydromyricetin uptake in patients with Alzheimer’s diseases. Front. Aging Neurosci. 2020, 12, 601603. [Google Scholar] [CrossRef]
- Kimura, A.M.; Tsuji, M.; Yasumoto, T.; Mori, Y.; Oguchi, T.; Tsuji, Y.; Umino, M.; Umino, A.; Nishikawa, T.; Nakamura, S. Myricetin prevents high molecular weight Aβ1-42 oligomer-induced neurotoxicity through antioxidant effects in cell membranes and mitochondria. Free Radic. Biol. Med. 2021, 171, 232–244. [Google Scholar] [CrossRef]
- Tamagno, E.; Parola, M.; Bardini, P.; Piccini, A.; Borghi, R.; Guglielmotto, M.; Santoro, G.; Davit, A.; Danni, O.; Smith, M. β-Site APP cleaving enzyme up-regulation induced by 4-hydroxynonenal is mediated by stress-activated protein kinases pathways. J. Neurochem. 2005, 92, 628–636. [Google Scholar] [CrossRef]
- Su, B.; Wang, X.; Lee, H.-g.; Tabaton, M.; Perry, G.; Smith, M.A.; Zhu, X. Chronic oxidative stress causes increased tau phosphorylation in M17 neuroblastoma cells. Neurosci. Lett. 2010, 468, 267–271. [Google Scholar] [CrossRef]
- Lasagna-Reeves, C.A.; Castillo-Carranza, D.L.; Sengupta, U.; Clos, A.L.; Jackson, G.R.; Kayed, R. Tau oligomers impair memory and induce synaptic and mitochondrial dysfunction in wild-type mice. Mol. Neurodegener. 2011, 6, 39. [Google Scholar] [CrossRef]
- Shimmyo, Y.; Kihara, T.; Akaike, A.; Niidome, T.; Sugimoto, H. Multifunction of myricetin on Aβ: Neuroprotection via a conformational change of Aβ and reduction of Aβ via the interference of secretases. J. Neurosci. Res. 2008, 86, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Ono, K.; Takamura, Y.; Mizuguchi, M.; Ikeda, T.; Nishijo, H.; Yamada, M. Phenolic compounds prevent the oligomerization of α-synuclein and reduce synaptic toxicity. J. Neurochem. 2015, 134, 943–955. [Google Scholar] [CrossRef]
- Jing, N.; Li, X. Dihydromyricetin attenuates inflammation through TLR4/NF-kappaB pathway. Open Med. 2019, 14, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Chen, H.; Lu, W.; Wu, Y.; Wu, X.; Xia, D.; Zhu, J. Myricetin induces protective autophagy by inhibiting the phosphorylation of mTOR in HepG2 cells. Anat. Rec. 2018, 301, 786–795. [Google Scholar] [CrossRef]
- DeToma, A.S.; Choi, J.S.; Braymer, J.J.; Lim, M.H. Myricetin: A naturally occurring regulator of metal-induced amyloid-β aggregation and neurotoxicity. ChemBioChem 2011, 12, 1198–1201. [Google Scholar] [CrossRef]
- Kou, X.; Liu, X.; Chen, X.; Li, J.; Yang, X.; Fan, J.; Yang, Y.; Chen, N. Ampelopsin attenuates brain aging of D-gal-induced rats through miR-34a-mediated SIRT1/mTOR signal pathway. Oncotarget 2016, 7, 74484. [Google Scholar] [CrossRef] [PubMed]
- Shadfar, S.; Hwang, C.J.; Lim, M.-S.; Choi, D.-Y.; Hong, J.T. Involvement of inflammation in Alzheimer’s disease pathogenesis and therapeutic potential of anti-inflammatory agents. Arch. Pharmacal Res. 2015, 38, 2106–2119. [Google Scholar] [CrossRef]
- Weston-Green, K.; Clunas, H.; Naranjo, C.J. A review of the potential use of pinene and linalool as terpene-based medicines for brain health: Discovering novel therapeutics in the flavours and fragrances of cannabis. Front. Psychiatry 2021, 12, 583211. [Google Scholar] [CrossRef]
- Porres-Martínez, M.; González-Burgos, E.; Carretero, M.E.; Gómez-Serranillos, M.P. In Vitro neuroprotective potential of the monoterpenes α-pinene and 1,8-cineole against H2O2-induced oxidative stress in PC12 cells. Z. Nat. C 2016, 71, 191–199. [Google Scholar] [CrossRef]
- Khan-Mohammadi-Khorrami, M.K.; Asle-Rousta, M.; Rahnema, M.; Amini, R. Neuroprotective effect of alpha-pinene is mediated by suppression of the TNF-α/NF-κB pathway in Alzheimer’s disease rat model. J. Biochem. Mol. Toxicol. 2022, 36, e23006. [Google Scholar] [CrossRef] [PubMed]
- Khoshnazar, M.; Bigdeli, M.R.; Parvardeh, S.; Pouriran, R. Attenuating effect of α-pinene on neurobehavioural deficit, oxidative damage and inflammatory response following focal ischaemic stroke in rat. J. Pharm. Pharmacol. 2019, 71, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Khoshnazar, M.; Parvardeh, S.; Bigdeli, M.R. Alpha-pinene exerts neuroprotective effects via anti-inflammatory and anti-apoptotic mechanisms in a rat model of focal cerebral ischemia-reperfusion. J. Stroke Cerebrovasc. Dis. 2020, 29, 104977. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-Y.; Lee, C.; Park, G.H.; Jang, J.-H. Amelioration of Scopolamine-Induced Learning and Memory Impairment by?-Pinene in C57BL/6 Mice. Mult. Bioactivities Tradit. Med. Herbs Treat. Neurodegener. Dis. 2017, 2017, 4926815. [Google Scholar] [CrossRef] [PubMed]
- Somani, G.; Kulkarni, C.; Shinde, P.; Shelke, R.; Laddha, K.; Sathaye, S. In Vitro acetylcholinesterase inhibition by psoralen using molecular docking and enzymatic studies. J. Pharm. Bioallied Sci. 2015, 7, 32. [Google Scholar]
- Kong, L.D.; Tan, R.X.; Woo, A.Y.H.; Cheng, C.H.K. Inhibition of rat brain monoamine oxidase activities by psoralen and isopsoralen: Implications for the treatment of affective disorders. Pharmacol. Toxicol. 2001, 88, 75–80. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, D.; Sehgal, A.; Singh, S.; Sharma, N.; Zengin, G.; Andronie-Cioara, F.L.; Toma, M.M.; Bungau, S.; Bumbu, A.G. Role of monoamine oxidase activity in Alzheimer’s disease: An insight into the therapeutic potential of inhibitors. Molecules 2021, 26, 3724. [Google Scholar] [CrossRef]
- Wu, C.-R.; Chang, C.-L.; Hsieh, P.-Y.; Lin, L.-W.; Ching, H. Psoralen and isopsoralen, two coumarins of Psoraleae Fructus, can alleviate scopolamine-induced amnesia in rats. Planta Med. 2007, 73, 275–278. [Google Scholar] [CrossRef]
- Shimmyo, Y.; Kihara, T.; Akaike, A.; Niidome, T.; Sugimoto, H. Flavonols and flavones as BACE-1 inhibitors: Structure–activity relationship in cell-free, cell-based and in silico studies reveal novel pharmacophore features. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2008, 1780, 819–825. [Google Scholar] [CrossRef]
- Sriraksa, N.; Wattanathorn, J.; Muchimapura, S.; Tiamkao, S.; Brown, K.; Chaisiwamongkol, K. Cognitive-enhancing effect of quercetin in a rat model of Parkinson’s disease induced by 6-hydroxydopamine. Evid. -Based Complement. Altern. Med. 2012, 2012, 823206. [Google Scholar] [CrossRef]
- Ansari, M.A.; Abdul, H.M.; Joshi, G.; Opii, W.O.; Butterfield, D.A. Protective effect of quercetin in primary neurons against Aβ (1–42): Relevance to Alzheimer’s disease. J. Nutr. Biochem. 2009, 20, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Ay, M.; Luo, J.; Langley, M.; Jin, H.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A.G. Molecular mechanisms underlying protective effects of quercetin against mitochondrial dysfunction and progressive dopaminergic neurodegeneration in cell culture and MitoPark transgenic mouse models of Parkinson’s Disease. J. Neurochem. 2017, 141, 766–782. [Google Scholar] [CrossRef] [PubMed]
- Sandhir, R.; Mehrotra, A. Quercetin supplementation is effective in improving mitochondrial dysfunctions induced by 3-nitropropionic acid: Implications in Huntington’s disease. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2013, 1832, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Kuhad, A.; Singla, S.; Arora, V.; Chopra, K. Neuroprotective effect of sesamol and quercetin against QA induced neurotoxicity: An experimental paradigm of Huntington’s disease. J. Neurol. Sci. 2013, 333, e149–e150. [Google Scholar] [CrossRef]
- Grewal, A.K.; Singh, T.G.; Sharma, D.; Sharma, V.; Singh, M.; Rahman, M.H.; Najda, A.; Walasek-Janusz, M.; Kamel, M.; Albadrani, G.M. Mechanistic insights and perspectives involved in neuroprotective action of quercetin. Biomed. Pharmacother. 2021, 140, 111729. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G.; Cole, T.B.; Furlong, C.E.; Costa, L.G. Paraoxonase 2 (PON2) in the mouse central nervous system: A neuroprotective role? Toxicol. Appl. Pharmacol. 2011, 256, 369–378. [Google Scholar] [CrossRef]
- Saw, C.L.L.; Guo, Y.; Yang, A.Y.; Paredes-Gonzalez, X.; Ramirez, C.; Pung, D.; Kong, A.-N.T. The berry constituents quercetin, kaempferol, and pterostilbene synergistically attenuate reactive oxygen species: Involvement of the Nrf2-ARE signaling pathway. Food Chem. Toxicol. 2014, 72, 303–311. [Google Scholar] [CrossRef]
- Chen, L.; Sun, L.; Liu, Z.; Wang, H.; Xu, C. Protection afforded by quercetin against H2O2-induced apoptosis on PC12 cells via activating PI3K/Akt signal pathway. J. Recept. Signal Transduct. 2016, 36, 98–102. [Google Scholar] [CrossRef]
- Park, J.-Y.; Lim, M.-S.; Kim, S.-I.; Lee, H.J.; Kim, S.-S.; Kwon, Y.-S.; Chun, W. Quercetin-3-O-β-D-glucuronide suppresses lipopolysaccharide-induced JNK and ERK phosphorylation in LPS-challenged RAW264. 7 cells. Biomol. Ther. 2016, 24, 610. [Google Scholar] [CrossRef]
- Lavoie, S.; Chen, Y.; Dalton, T.P.; Gysin, R.; Cuénod, M.; Steullet, P.; Do, K.Q. Curcumin, quercetin, and tBHQ modulate glutathione levels in astrocytes and neurons: Importance of the glutamate cysteine ligase modifier subunit. J. Neurochem. 2009, 108, 1410–1422. [Google Scholar] [CrossRef]
- de Boer, V.C.; de Goffau, M.C.; Arts, I.C.; Hollman, P.C.; Keijer, J. SIRT1 stimulation by polyphenols is affected by their stability and metabolism. Mech. Ageing Dev. 2006, 127, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.; Rice-Evans, C.; Williams, R.J. Modulation of pro-survival Akt/protein kinase B and ERK1/2 signaling cascades by quercetin and its in vivo metabolites underlie their action on neuronal viability. J. Biol. Chem. 2003, 278, 34783–34793. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.Y.; Rice-Evans, C.; Williams, R.J. Quercetin attenuates inflammatory responses in BV-2 microglial cells: Role of MAPKs on the Nrf2 pathway and induction of heme oxygenase-1. PLoS ONE 2015, 10, e0141509. [Google Scholar] [CrossRef] [PubMed]
- Sabogal-Guáqueta, A.M.; Munoz-Manco, J.I.; Ramírez-Pineda, J.R.; Lamprea-Rodriguez, M.; Osorio, E.; Cardona-Gómez, G.P. The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology 2015, 93, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fan, X.; Tang, T.; Fan, R.; Zhang, C.; Huang, Z.; Peng, W.; Gan, P.; Xiong, X.; Huang, W. Rhein and rhubarb similarly protect the blood-brain barrier after experimental traumatic brain injury via gp91phox subunit of NADPH oxidase/ROS/ERK/MMP-9 signaling pathway. Sci. Rep. 2016, 6, 37098. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, X.; Chen, A.; Cheng, X.; Zhang, G.; Sun, J.; Zhao, Y.; Huang, Y.; Zhu, Y. Rhein protects against cerebral ischemic-/reperfusion-induced oxidative stress and apoptosis in rats. Int. J. Mol. Med. 2018, 41, 2802–2812. [Google Scholar] [CrossRef]
- Bi, F.; Ma, H.; Ji, C.; Chang, C.; Liu, W.; Xie, K. Rhein protects against neurological deficits after traumatic brain injury in mice via inhibiting neuronal pyroptosis. Front. Pharmacol. 2020, 11, 564367. [Google Scholar] [CrossRef]
- Yin, Z.; Gao, D.; Du, K.; Han, C.; Liu, Y.; Wang, Y.; Gao, X. Rhein Ameliorates Cognitive Impairment in an APP/PS1 Transgenic Mouse Model of Alzheimer’s Disease by Relieving Oxidative Stress through Activating the SIRT1/PGC-1α Pathway. Oxidative Med. Cell. Longev. 2022, 2022, 2524832. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, R.; Luo, J.; Tang, T.; Xing, Z.; Xia, Z.; Peng, W.; Wang, W.; Lv, H.; Huang, W. An ultra high performance liquid chromatography with tandem mass spectrometry method for plasma and cerebrospinal fluid pharmacokinetics of rhein in patients with traumatic brain injury after administration of rhubarb decoction. J. Sep. Sci. 2015, 38, 1100–1108. [Google Scholar] [CrossRef]
- Budzynska, B.; Faggio, C.; Kruk-Slomka, M.; Samec, D.; Nabavi, S.F.; Sureda, A.; Devi, K.P.; Nabavi, S.M. Rutin as neuroprotective agent: From bench to bedside. Curr. Med. Chem. 2019, 26, 5152–5164. [Google Scholar] [CrossRef]
- Habtemariam, S. Rutin as a natural therapy for Alzheimer’s disease: Insights into its mechanisms of action. Curr. Med. Chem. 2016, 23, 860–873. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.-Y.; Ma, J.; Kong, X.-X.; Wang, X.-F.; Li, S.-S.; Qi, X.-L.; Yan, Y.-H.; Cheng, J.; Liu, Q.; Jin, W. Sodium rutin ameliorates Alzheimer’s disease–like pathology by enhancing microglial amyloid-β clearance. Sci. Adv. 2019, 5, eaau6328. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-W.; Wang, Y.-J.; Su, Y.-J.; Zhou, W.-W.; Yang, S.-G.; Zhang, R.; Zhao, M.; Li, Y.-N.; Zhang, Z.-P.; Zhan, D.-W. Rutin inhibits β-amyloid aggregation and cytotoxicity, attenuates oxidative stress, and decreases the production of nitric oxide and proinflammatory cytokines. Neurotoxicology 2012, 33, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.-X.; Wang, S.-W.; Yu, X.-L.; Su, Y.-J.; Wang, T.; Zhou, W.-W.; Zhang, H.; Wang, Y.-J.; Liu, R.-T. Rutin improves spatial memory in Alzheimer’s disease transgenic mice by reducing Aβ oligomer level and attenuating oxidative stress and neuroinflammation. Behav. Brain Res. 2014, 264, 173–180. [Google Scholar] [CrossRef]
- Yu, X.-L.; Li, Y.-N.; Zhang, H.; Su, Y.-J.; Zhou, W.-W.; Zhang, Z.-P.; Wang, S.-W.; Xu, P.-X.; Wang, Y.-J.; Liu, R.-T. Rutin inhibits amylin-induced neurocytotoxicity and oxidative stress. Food Funct. 2015, 6, 3296–3306. [Google Scholar] [CrossRef]
- Sun, X.-Y.; Li, L.-J.; Dong, Q.-X.; Zhu, J.; Huang, Y.-R.; Hou, S.-J.; Yu, X.-L.; Liu, R.-T. Rutin prevents tau pathology and neuroinflammation in a mouse model of Alzheimer’s disease. J. Neuroinflamm. 2021, 18, 131. [Google Scholar] [CrossRef]
- Baluchnejadmojarad, T.; Jamali-Raeufy, N.; Zabihnejad, S.; Rabiee, N.; Roghani, M. Troxerutin exerts neuroprotection in 6-hydroxydopamine lesion rat model of Parkinson’s disease: Possible involvement of PI3K/ERβ signaling. Eur. J. Pharmacol. 2017, 801, 72–78. [Google Scholar] [CrossRef]
- Khan, M.; Raza, S.S.; Javed, H.; Ahmad, A.; Khan, A.; Islam, F.; Safhi, M.M.; Islam, F. Rutin protects dopaminergic neurons from oxidative stress in an animal model of Parkinson’s disease. Neurotox. Res. 2012, 22, 1–15. [Google Scholar] [CrossRef]
- Cordeiro, L.M.; Machado, M.L.; da Silva, A.F.; Baptista, F.B.O.; da Silveira, T.L.; Soares, F.A.A.; Arantes, L.P. Rutin protects Huntington’s disease through the insulin/IGF1 (IIS) signaling pathway and autophagy activity: Study in Caenorhabditis elegans model. Food Chem. Toxicol. 2020, 141, 111323. [Google Scholar] [CrossRef]
- Pandini, G.; Pace, V.; Copani, A.; Squatrito, S.; Milardi, D.; Vigneri, R. Insulin has multiple antiamyloidogenic effects on human neuronal cells. Endocrinology 2013, 154, 375–387. [Google Scholar] [CrossRef]
- Kim, B.; Feldman, E.L. Insulin resistance as a key link for the increased risk of cognitive impairment in the metabolic syndrome. Exp. Mol. Med. 2015, 47, e149. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Estévez, V.; Defterali, Ç.; Vicario-Abejón, C. IGF-I: A key growth factor that regulates neurogenesis and synaptogenesis from embryonic to adult stages of the brain. Front. Neurosci. 2016, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.M.; Torres-Alemán, I. The many faces of insulin-like peptide signalling in the brain. Nat. Rev. Neurosci. 2012, 13, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Vanmierlo, T.; Bogie, J.F.; Mailleux, J.; Vanmol, J.; Lütjohann, D.; Mulder, M.; Hendriks, J.J. Plant sterols: Friend or foe in CNS disorders? Prog. Lipid Res. 2015, 58, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Jie, F.; Yang, X.; Wu, L.; Wang, M.; Lu, B. Linking phytosterols and oxyphytosterols from food to brain health: Origins, effects, and underlying mechanisms. Crit. Rev. Food Sci. Nutr. 2022, 62, 3613–3630. [Google Scholar] [CrossRef]
- Burg, V.K.; Grimm, H.S.; Rothhaar, T.L.; Grösgen, S.; Hundsdörfer, B.; Haupenthal, V.J.; Zimmer, V.C.; Mett, J.; Weingärtner, O.; Laufs, U. Plant sterols the better cholesterol in Alzheimer’s disease? A mechanistical study. J. Neurosci. 2013, 33, 16072–16087. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N.; Khalid, A. Phytochemical and enzyme inhibitory studies on indigenous medicinal plant Rhazya stricta. Nat. Prod. Res. 2010, 24, 305–314. [Google Scholar] [CrossRef]
- Lee, J.; Weon, J.B.; Ma, C.J. Neuroprotective activity of phytosterols isolated from Artemisia apiacea. Korean J. Pharmacogn. 2014, 45, 214–219. [Google Scholar]
- Pratiwi, R.; Nantasenamat, C.; Ruankham, W.; Suwanjang, W.; Prachayasittikul, V.; Prachayasittikul, S.; Phopin, K. Mechanisms and neuroprotective activities of stigmasterol against oxidative stress-induced neuronal cell death via sirtuin family. Front. Nutr. 2021, 8, 648995. [Google Scholar] [CrossRef]
- Gabay, O.; Sanchez, C.; Salvat, C.; Chevy, F.; Breton, M.; Nourissat, G.; Wolf, C.; Jacques, C.; Berenbaum, F. Stigmasterol: A phytosterol with potential anti-osteoarthritic properties. Osteoarthr. Cartil. 2010, 18, 106–116. [Google Scholar] [CrossRef]
- Jie, F.; Yang, X.; Yang, B.; Liu, Y.; Wu, L.; Lu, B. Stigmasterol attenuates inflammatory response of microglia via NF-κB and NLRP3 signaling by AMPK activation. Biomed. Pharmacother. 2022, 153, 113317. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kim, D.H.; Jung, J.M.; Kim, J.M.; Cai, M.; Liu, X.; Hong, J.G.; Lee, C.H.; Lee, K.R.; Ryu, J.H. The ameliorating effects of stigmasterol on scopolamine-induced memory impairments in mice. Eur. J. Pharmacol. 2012, 676, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, S. Molecular diversity of glutamate receptors and implications for brain function. Science 1992, 258, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Al Rahim, M.; Nakajima, A.; Saigusa, D.; Tetsu, N.; Maruyama, Y.; Shibuya, M.; Yamakoshi, H.; Tomioka, Y.; Iwabuchi, Y.; Ohizumi, Y. 4′-Demethylnobiletin, a bioactive metabolite of nobiletin enhancing PKA/ERK/CREB signaling, rescues learning impairment associated with NMDA receptor antagonism via stimulation of the ERK cascade. Biochemistry 2009, 48, 7713–7721. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J.; Preuss, H.G.; Shara, M. A review of the human clinical studies involving Citrus aurantium (bitter orange) extract and its primary protoalkaloid p-synephrine. Int. J. Med. Sci. 2012, 9, 527. [Google Scholar] [CrossRef]
- Jung, Y.P.; Earnest, C.P.; Koozehchian, M.; Galvan, E.; Dalton, R.; Walker, D.; Rasmussen, C.; Murano, P.S.; Greenwood, M.; Kreider, R.B. Effects of acute ingestion of a pre-workout dietary supplement with and without p-synephrine on resting energy expenditure, cognitive function and exercise performance. J. Int. Soc. Sports Nutr. 2017, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Taslimi, P.; Akıncıoglu, H.; Gülçin, İ. Synephrine and phenylephrine act as α-amylase, α-glycosidase, acetylcholinesterase, butyrylcholinesterase, and carbonic anhydrase enzymes inhibitors. J. Biochem. Mol. Toxicol. 2017, 31, e21973. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-J.; Kim, H.J.; Chun, H.S. Quantitative structure—Activity relationship (QSAR) for neuroprotective activity of terpenoids. Life Sci. 2007, 80, 835–841. [Google Scholar] [CrossRef]
- Cheng, Y.; Dong, Z.; Liu, S. β-Caryophyllene ameliorates the Alzheimer-like phenotype in APP/PS1 Mice through CB2 receptor activation and the PPARγ pathway. Pharmacology 2014, 94, 1–12. [Google Scholar] [CrossRef]
- Guo, K.; Mou, X.; Huang, J.; Xiong, N.; Li, H. Trans-caryophyllene suppresses hypoxia-induced neuroinflammatory responses by inhibiting NF-κB activation in microglia. J. Mol. Neurosci. 2014, 54, 41–48. [Google Scholar] [CrossRef]
- Rao, J.-Y.; Wang, Q.; Wang, Y.-C.; Xiang, F.; Tian, X.-C.; Liu, D.-H.; Dong, Z. β-caryophyllene alleviates cerebral ischemia/reperfusion injury in mice by activating autophagy. Zhongguo Zhong Yao Za Zhi Zhongguo Zhongyao Zazhi China J. Chin. Mater. Med. 2020, 45, 932–936. [Google Scholar]
- NIH. β-Caryophyllene. Summary of Data for Chemical Selection. 1997. Available online: https://ntp.niehs.nih.gov/ntp/htdocs/chem_background/exsumpdf/betacaryophyllene_508.pdf (accessed on 1 October 2022).
- Ayaz, M.; Junaid, M.; Ullah, F.; Subhan, F.; Sadiq, A.; Ali, G.; Ovais, M.; Shahid, M.; Ahmad, A.; Wadood, A. Anti-Alzheimer’s studies on β-sitosterol isolated from Polygonum hydropiper L. Front. Pharmacol. 2017, 8, 697. [Google Scholar] [CrossRef] [PubMed]
- Bari, W.U.; Zahoor, M.; Zeb, A.; Khan, I.; Nazir, Y.; Khan, A.; Rehman, N.U.; Ullah, R.; Shahat, A.A.; Mahmood, H.M. Anticholinesterase, antioxidant potentials, and molecular docking studies of isolated bioactive compounds from Grewia optiva. Int. J. Food Prop. 2019, 22, 1386–1396. [Google Scholar] [CrossRef]
- Shi, C.; Wu, F.; Zhu, X.; Xu, J. Incorporation of β-sitosterol into the membrane increases resistance to oxidative stress and lipid peroxidation via estrogen receptor-mediated PI3K/GSK3β signaling. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 2538–2544. [Google Scholar] [CrossRef]
- Schmitz, G.; Grandl, M. Update on lipid membrane microdomains. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Salazar, M.; Rojo, A.I.; Velasco, D.; de Sagarra, R.M.; Cuadrado, A. Glycogen synthase kinase-3β inhibits the xenobiotic and antioxidant cell response by direct phosphorylation and nuclear exclusion of the transcription factor Nrf2. J. Biol. Chem. 2006, 281, 14841–14851. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, L.; Hou, W.; Wu, J. β-Sitosterol alleviates inflammatory response via inhibiting the activation of ERK/p38 and NF-κB pathways in LPS-exposed BV2 cells. BioMed Res. Int. 2020, 2020, 7532306. [Google Scholar] [CrossRef]
- Kim, H.-J.; Fan, X.; Gabbi, C.; Yakimchuk, K.; Parini, P.; Warner, M.; Gustafsson, J.-Å. Liver X receptor β (LXRβ): A link between β-sitosterol and amyotrophic lateral sclerosis–Parkinson’s dementia. Proc. Natl. Acad. Sci. USA 2008, 105, 2094–2099. [Google Scholar] [CrossRef]
- Ye, J.-Y.; Li, L.; Hao, Q.-M.; Qin, Y.; Ma, C.-S. β-Sitosterol treatment attenuates cognitive deficits and prevents amyloid plaque deposition in amyloid protein precursor/presenilin 1 mice. Korean J. Physiol. Pharmacol. 2020, 24, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Hossen, M.A.; Ali Reza, A.; Amin, M.B.; Nasrin, M.S.; Khan, T.A.; Rajib, M.H.R.; Tareq, A.M.; Haque, M.A.; Rahman, M.A.; Haque, M.A. Bioactive metabolites of Blumea lacera attenuate anxiety and depression in rodents and computer-aided model. Food Sci. Nutr. 2021, 9, 3836–3851. [Google Scholar] [CrossRef]
- Baell, J.; Walters, M.A. Chemistry: Chemical con artists foil drug discovery. Nature 2014, 513, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Gilberg, E.; Stumpfe, D.; Bajorath, J. Activity profiles of analog series containing pan assay interference compounds. RSC Adv. 2017, 7, 35638–35647. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The essential medicinal chemistry of curcumin: Miniperspective. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef] [PubMed]
- Baker, M. Deceptive curcumin offers cautionary tale for chemists. Nature 2017, 541, 144–145. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Cock, I. The safe usage of herbal medicines: Counter-indications, cross-reactivity and toxicity. Pharmacogn. Commun. 2015, 5, 2–50. [Google Scholar]
- Mathur, S.; Hoskins, C. Drug development: Lessons from nature. Biomed. Rep. 2017, 6, 612–614. [Google Scholar] [CrossRef]
- Drummond, E.; Wisniewski, T. Alzheimer’s disease: Experimental models and reality. Acta Neuropathol. 2017, 133, 155–175. [Google Scholar] [CrossRef]
- Ricciarelli, R.; Fedele, E. The amyloid cascade hypothesis in Alzheimer’s disease: It’s time to change our mind. Curr. Neuropharmacol. 2017, 15, 926–935. [Google Scholar] [CrossRef]
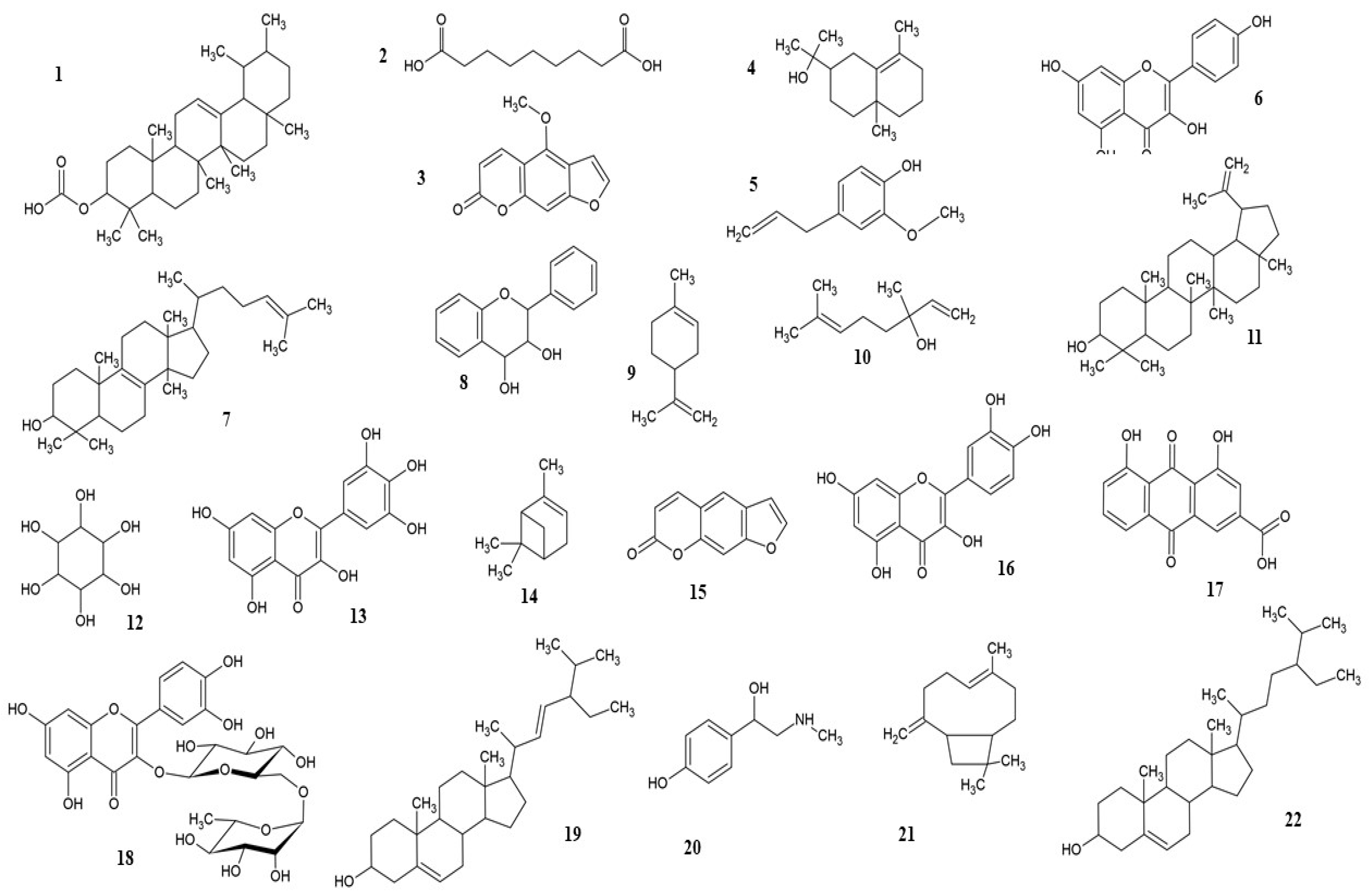
| Name | Plant Part | Extract | Model | Dose (mg/kg) | Action | Ref. |
|---|---|---|---|---|---|---|
| F. religiosa | Leaves | Methanolic | BV2 cell lines | Inhibits proinflammatory cytokine production; downregulates MAPK/ERK/JNK/NF-κB; improves the number and quality of neurons | [36,64,65,66,67,68,69,70,71,72,73] | |
| AlCl3-induced | 200 and 300 | |||||
| Petroleum ether | 3-NP-, 6-OHDA-induced | 200 and 400 | Anti-AChE; reduces oxidative stress | |||
| Ethanolic | Scopolamine-, sodium nitrite- induced | 100 | Anti-amnesic and nootropic | |||
| Root | Hydroethanolic | PTZ-induced | 1, 2, 4 | Anticonvulsant | [74,75,76] | |
| Aqueous | Strychnine-, PTZ-induced | 25, 50, 100 | Anticonvulsant | |||
| Fruit | Methanolic | MES-, picrotoxin, scopolamine-induced | 25, 50, 100 | Antiamnesic, anticonvulsant | [77,78,79,80] | |
| 10, 50, 100 | ||||||
| Ethyl acetate | PTZ-induced | 1, 2, 4 | Reduces oxidative stress, anticonvulsant, anti-AChE | |||
| Bark | Methanolic | In vitro | Anti-AChE | [81] | ||
| F. benghalensis | Leaves | Methanolic | Alloxan-induced | 200 and 400 | Improves motor coordination | [82] |
| Bark | Methanolic | Scopolamine- induced | 100, 200, 300 | Anxiolytic and antidepressant | [62,83] | |
| Aqueous | Scopolamine- induced | 150 and 300 | Cognitive enhancement | |||
| Root | Aqueous | PTZ-, MES-induced | 100 and 200 | Anxiolytic, memory-enhancing, muscle-relaxant, seizure-modifying effect | [84] |
| Name | Class and MW | BBB Permeability | Model | Dose/Concentration | MOA | Pathways Affected | Medicinal Chemistry (PAINS) | Ref. |
|---|---|---|---|---|---|---|---|---|
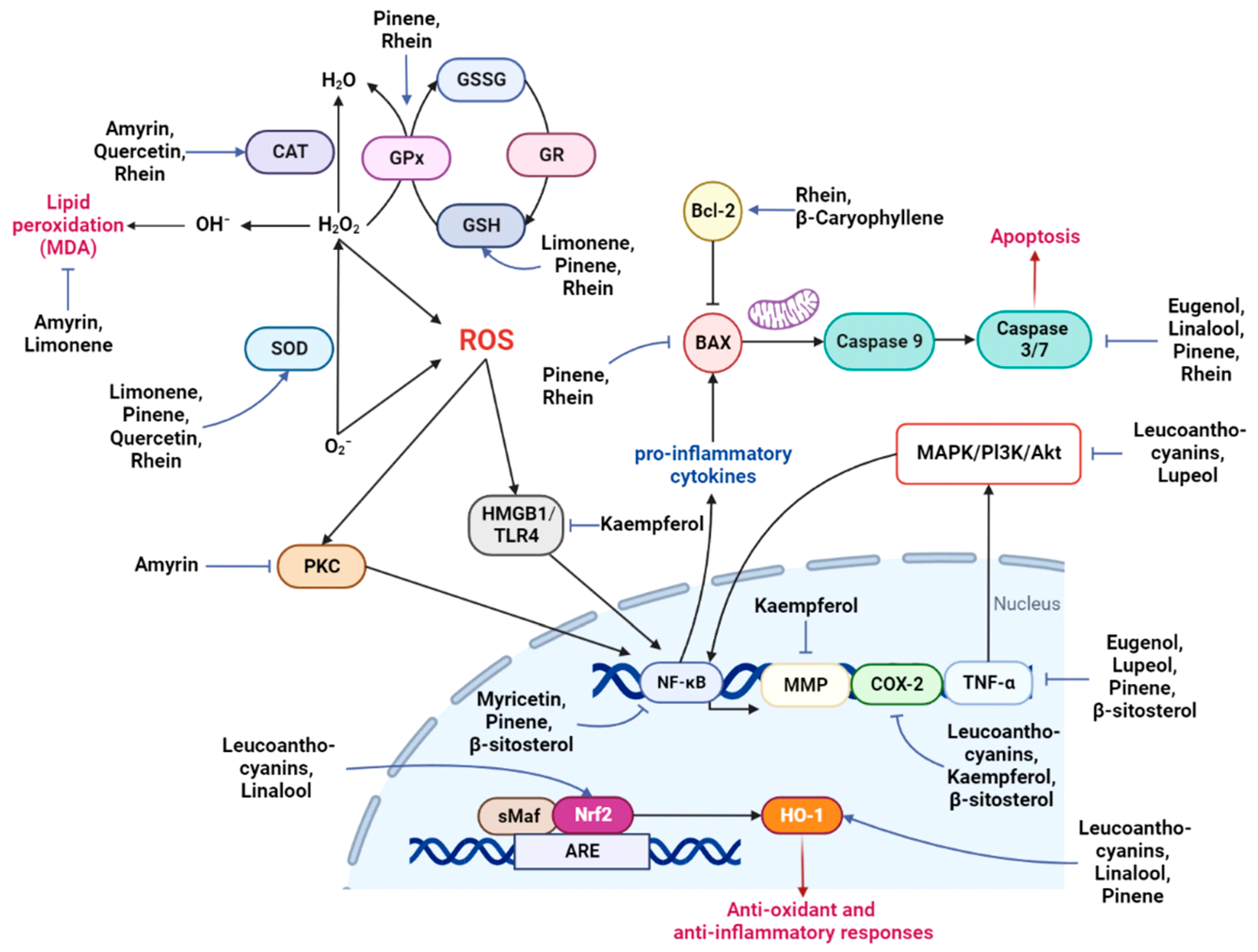
| Amyrin | Phytosterol 426.72 | PTZ-induced seizures | 25 and 50 mg/kg | Antioxidant | ERK activation, GSK inhibition, memory enhancement; MAO inhibition; elevation of GABA; inhibits PKC; increases CAT; decreases MDA; inhibits AChE | 0 alerts | [85,86,87,88,89,90,91,92,93,94] | |
| Azelaic acid | Dicarboxylic acid 188.22 | Rotenone-induced PD model | 80 mg/kg | Improves motor functions | 0 alerts | [95,96] | ||
| Bergapten | Furanocoumarin 216.19 | √ | Scopolamine-induced amnesia; paclitaxel-induced neuropathic pain | 25 and 50 mg/kg; 25, 50, and 100 mg/kg | Enzyme inhibition | Inhibits AChE, BchE, and MAO; memory enhancement; anti-depressant | 0 alerts | [97,98,99,100,101,102,103,104,105,106] |
| Eudesmol | Sesquiterpenoids 222.37 | PC-12 cells | 100 and 150 µM | Neurite extension | Induced neurite extension; MAPK activation; phosphorylation of the CREB | 0 alerts | [107,108,109,110] | |
| Eugenol | Polyphenol 164.2 | √ | TBI rats; I/R damage; acrylamide-induced neuropathic rats; aluminum-induced toxicity; hydroxydopamine-induced PD model | 25, 50, and 100 mg/kg; 50 and 100 mg/kg; 10 mg/kg; 6 mg/kg; 0.1, 1, and 10 mg/kg | Anti-inflammatory, autophagy, antioxidant | Improves memory and motor functions; decreases AChE, TNF-α, and caspase-3; increases BDNF and serotonin; inhibits amyloid formation; increases MT-III, promotes neurogenesis | 0 alerts | [111,112,113,114,115,116,117,118,119,120,121,122] |
| Kaempferol | Flavonoid 286.23 | √ | Anti-inflammatory, autophagy, antioxidant, anti-amyloid | MMP inhibitor; BDNF modulation: antioxidant; reduces inflammatory cytokines, COX-2, HMGB1/TLR4; anti-AChE; increases dopamine; inhibits Abeta accumulation | 0 alerts | [123,124,125,126,127,128,129,130,131] | ||
| Lanosterol | Phytosterol 426.71 | (MPP+)-induced cell death in the PD cellular model | 0.5 mM | Autophagy | Suppresses the buildup of misfolded protein aggregations/sequestosomes; promotes autophagy; mitochondrial depolarization | 0 alerts | [132,133,134,135] | |
| Leucoanthocyanins | Anthocyanins 242.26 | √ | Kainate-induced learning impairment in rats; LPS-treated adult mice; BV-2 cells | 2%; 24 mg/kg; 50 and 100 μg/ml | Anti-inflammatory, autophagy, antioxidant | Modulates the PI3K/Akt/Nrf2/HO-1 pathway; COX-2/mPGES-1; promotes autophagy by upregulating AMPK–mTOR | 0 alerts | [136,137,138,139,140,141,142,143,144,145,146,147] |
| Limonene | Terpene 136.24 | √ | Aβ-induced in vitro model of AD; scopolamine-induced amnesia rat model; subchronic effects in rats | 10 μg/mL; MO: 1% and 3%; 5, 25, and 50 mg/kg | Anti-inflammatory | Improves cognition; decreases MDA, increases SOD, GSH; anti-AChE and BChE; anti-inflammatory; increases GABA | 0 alerts | [148,149,150,151,152,153,154] |
| Lupeol | Phytosterol 426.72 | √ | Acetic acid-induced writhing, formalin test, carrageenan-induced hyperalgesia, and post-operative pain model; Aβ-induced oxidative stress in mice; TBI mouse model; cerebellar cultures | 25, 50, and 100 mg/kg; 50 mg/kg; 50 mg/kg; 0.1 µM | Anti-inflammatory, antioxidant | MAPK/JNK pathway; downregulates BACE-1, upregulates proinflammatory cytokines; downregulates TNF, iNOS, NLRP3; upregulates GDNF and SHH–GLI signaling | 0 alerts | [164,165,166,167,168,169,170] |
| myo-Inositol | Carbocyclic sugar 180.16 | √ | Kainic acid-induced epilepsy rat model; ischemic stroke injury in animals exposed to tobacco smoke; i streptozotocin-induced mice | 0.1 μCi/mL; 30 mg/kg | Improved memory and motor functions; anticonvulsant | 0 alerts | [171,172,173,174,175,176,177] | |
| Myricetin | Flavonoid 318.23 | √ | Aβ-induced in vitro model of AD; primary neuron cultures | 5 μM; 1 and 10 μM | Anti-inflammatory, autophagy, antioxidant, anti-amyloid | Decreases NF-κB and AMPK/SIRT1 signaling; reduces the levels of inflammatory mediators; autophagy; metal ion chelation; reduces A beta, anti-AChE; restores mitochondrial dysfunction | 1 alert | [178,179,180,181,182,183,184,185,186,187,188,189,190] |
| Pinene | Terpene 136.24 | √ | Aβ-induced rat model; PC-12 cells; focal ischemic stroke model of rats; cerebral ischemia–reperfusion in rats | 50 mg/kg; 10 and 25 µM; 100 mg/kg; 100 mg/kg | Anti-inflammatory, autophagy, antioxidant, anti-amyloid | Improves cognition; increases SOD, GSH, GPX, HO-1; suppresses the TNF-α/NF-κB pathway; increases the expression of choline acetyltransferase, Bcl-1, muscarinic receptors, nAChR, BDNF, and antioxidant transcription factors; decreases Bax, caspase-3 | 0 alerts | [191,192,193,194,195,196] |
| Psoralen | Coumarin 186.16 | Ccopolamine-induced amnesia in rats; in vitro, in silico | 0.1 and 0.3 mg/kg | Enzyme inhibition | Anti-AChE; anti-MAO | 0 alerts | [197,198,199,200] | |
| Quercetin | Flavonoid 302.23 | √ | Primary neuron cultures; MitoPark PD model; 3-NP-induced HD model | 20 μM; 20 and 40 μM; 25 and 175 mg/kg; 25 mg/kg | Anti-inflammatory, antioxidant | BACE-1 inhibitor, decreases proinflammatory cytokines, increases ATP, CAT, SOD; affects PON2, Nrf2–ARE, PI3K, JNK/ERK, TNF-α, SIRT1, CREB, MAPK, NF-κB, AMPK, PGC-1α | 1 alert | [201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216] |
| Rhein | Anthraquinone 284.22 | √ | CCI rats; I/R rats; TBI rat model; APP/PS1 mouse model of AD | 12 mg/kg; 50 and 100 mg/kg; 100 mg/kg; 10 mg/kg | Anti-inflammatory, antioxidant | Increases SOD, GSH, CAT, GSH/GSSG, GSH-Px; enhances Bcl-2; decreases Bax, caspase-3, and ROS, proinflammatory cytokines; activation of the SIRT1/PGC-1α pathway; inhibits the NADPH oxidase/ROS/ERK/MMP-9 signaling pathway | 1 alert | [163,217,218,219,220,221] |
| Rutin | Flavonoid-3-o-glycosides 610.51 | √ | Tau-P301S mouse model; 6-OHDA-induced rat model of PD; Caenorhabditis elegans model of HD | 100 μL; 25 mg/kg; 15–120 μM | Anti-inflammatory, anti-amyloid, antioxidant | Improves memory, reduces Aβ oligomerization, oxidative stress, neurotoxicity, and neuroinflammation; reduces tau; protects dopaminergic neurons; insulin/insulin-like growth factor I pathway | 1 alert | [228,229,230,231,232,233,234,235] |
| Stigmasterol | Phytosterol 412.69 | √ | SH-SY5Y cells; BV2 cells; scopolamine-induced memory loss in mice | 1 μM; 10 and 20 μM; 3, 10, and 30 mg/kg; 10 mg/kg | Anti-inflammatory, antioxidant, anti-amyloid | Anti-AChE; reduces amyloid plaques; reduces ROS; modulates the SIRT1–FoxO3a pathway; inhibits proinflammatory cytokines; represses NF-κB and NLRP3 signaling by AMPK activation; NMDA activation; ERK/CREB activation; improves memory and LTP | 0 alerts | [239,240,241,242,243,244,245,246] |
| Synephrine | Biogenic amine 167.21 | Pre-workout; in vitro | 20 mg | Enzyme inhibition | Anti-BChE and anti-AChE activity; improves the cognitive function | 0 alerts | [247,248,249] | |
| β-Caryophyllene | Sesquiterpene 204.36 | √ | APP/PS1 mice; BV2 cells; I/R injury mouse model | 48 mg/kg; 5 μM; 24 and 72 mg/kg | Anti-inflammatory, autophagy, antioxidant | Anti-BACE and anti-AChE activity; increases the expression of Bcl-2, beclin-1, CB2R; decreases p62; decreases ROS and proinflammatory cytokines | 0 alerts | [250,251,252,253,254] |
| β-Sitosterol | Phytosterol 414.71 | √ | In vitro; HT22 cells and primarily cultured hippocampal cells; LPS- induced BV2 cells | 15 µM; 8 and 16 µM | Anti-inflammatory, antioxidant | Antioxidant; anti-AChE and BChE; prevents plaque deposition; modulates the PI3K/GSK-3β pathway; increases ΔΨm and ATP; decreases the expression of IL-6, iNOS, TNF-α, COX-2, IκB, NF-κB, ERK/p38 | 0 alerts | [255,256,257,258,259,260,261,262] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shim, K.H.; Sharma, N.; An, S.S.A. Mechanistic Insights into the Neuroprotective Potential of Sacred Ficus Trees. Nutrients 2022, 14, 4731. https://doi.org/10.3390/nu14224731
Shim KH, Sharma N, An SSA. Mechanistic Insights into the Neuroprotective Potential of Sacred Ficus Trees. Nutrients. 2022; 14(22):4731. https://doi.org/10.3390/nu14224731
Chicago/Turabian StyleShim, Kyu Hwan, Niti Sharma, and Seong Soo A. An. 2022. "Mechanistic Insights into the Neuroprotective Potential of Sacred Ficus Trees" Nutrients 14, no. 22: 4731. https://doi.org/10.3390/nu14224731
APA StyleShim, K. H., Sharma, N., & An, S. S. A. (2022). Mechanistic Insights into the Neuroprotective Potential of Sacred Ficus Trees. Nutrients, 14(22), 4731. https://doi.org/10.3390/nu14224731







