Vitamin A Nutritional Status Is a Key Determinant of Bone Mass in Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Measurement of Bone Mineral Content, Bone Mineral Density, and Body Fat Percentage
2.3. Measurement of Plasma Retinol Concentration and Dietary Vitamin A Nutritional Status
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Children
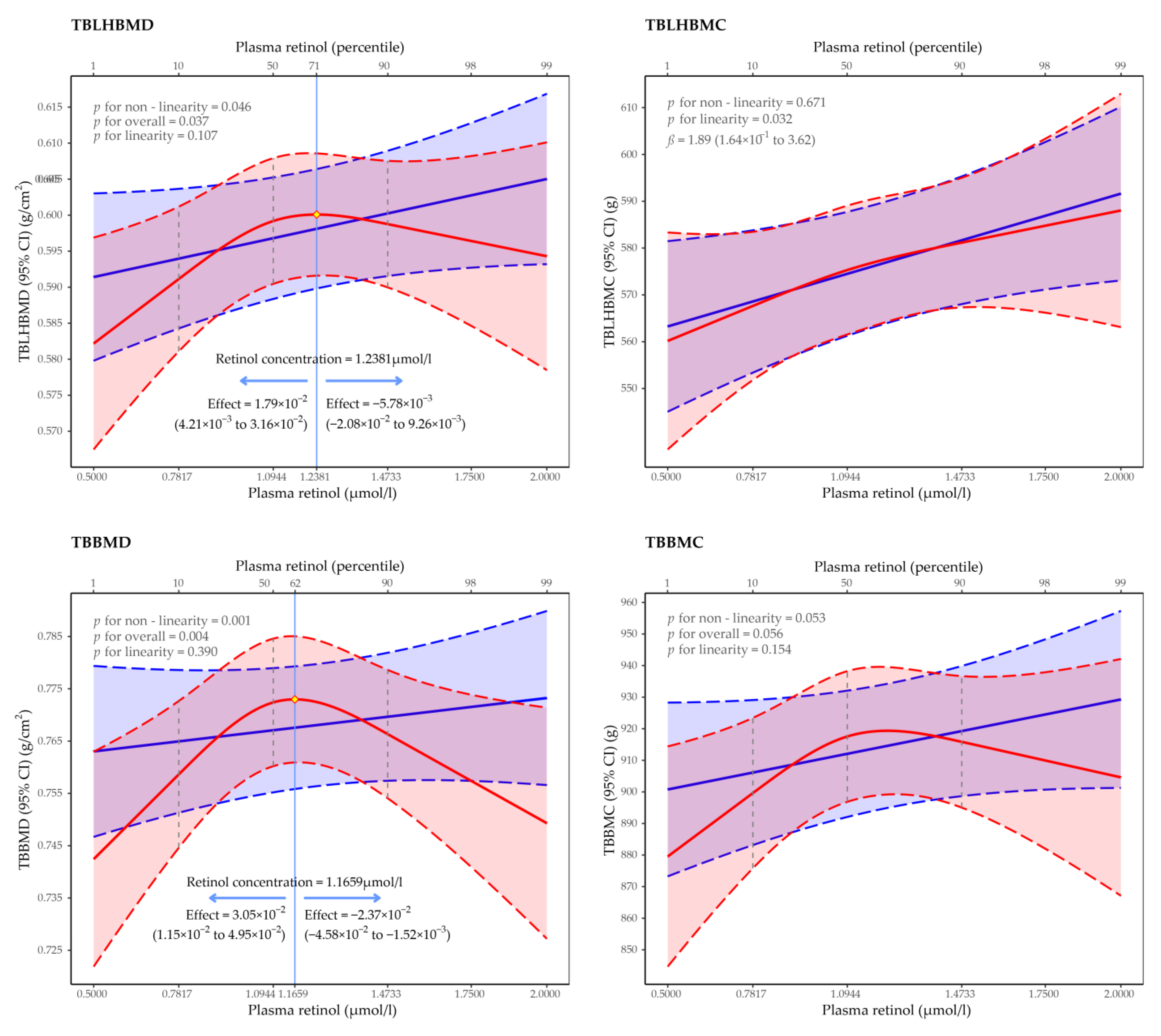
3.2. Regression of Restricted Cubic Spline of the Association between Plasma Retinol Concentration and BMD and BMC
3.3. Multiple Linear Regression Analysis of the Association between VA and BMD and BMC
4. Discussion
4.1. Associations of Plasma Retinol Concentration with Bone Mineral Status
4.2. Dietary VA Intake
4.3. Mechanisms
4.4. Strength and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Consensus development conference: Diagnosis, prophylaxis, and treatment of osteoporosis. Am. J. Med. 1993, 94, 646–650. [CrossRef]
- Xia, W.; Zhang, Z.; Lin, H.; Jin, X.; Yu, W.; Fu, Q. Guidelines for the diagnosis and management of primary osteoporosis (2017). Chin. Soc. Osteoporos. Bone Miner. Res. 2019, 25, 281–309. [Google Scholar]
- Si, L.; Winzenberg, T.M.; Jiang, Q.; Chen, M.; Palmer, A.J. Projection of osteoporosis-related fractures and costs in China: 2010–2050. Osteoporos. Int. 2015, 26, 1929–1937. [Google Scholar] [CrossRef]
- Bonjour, J.P.; Theintz, G.; Buchs, B.; Slosman, D.; Rizzoli, R. Critical years and stages of puberty for spinal and femoral bone mass accumulation during adolescence. J. Clin. Endocrinol. Metab. 1991, 73, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Ciosek, Ż.; Kot, K.; Kosik-Bogacka, D.; Łanocha-Arendarczyk, N.; Rotter, I. The Effects of Calcium, Magnesium, Phosphorus, Fluoride, and Lead on Bone Tissue. Biomolecules 2021, 11, 506. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.S.; Corey, P.N.; Cheung, A.M. Effects of vitamin E on bone turnover markers among US postmenopausal women. J. Bone Min. Res. 2012, 27, 1368–1380. [Google Scholar] [CrossRef]
- Lips, P.; van Schoor, N.M. The effect of vitamin D on bone and osteoporosis. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 585–591. [Google Scholar] [CrossRef]
- Ramesh, P.; Jagadeesan, R.; Sekaran, S.; Dhanasekaran, A.; Vimalraj, S. Flavonoids: Classification, Function, and Molecular Mechanisms Involved in Bone Remodelling. Front. Endocrinol. 2021, 12, 779638. [Google Scholar] [CrossRef]
- Yee, M.M.F.; Chin, K.Y.; Ima-Nirwana, S.; Wong, S.K. Vitamin A and Bone Health: A Review on Current Evidence. Molecules 2021, 26, 1757. [Google Scholar] [CrossRef]
- Fairfield, K.M.; Fletcher, R.H. Vitamins for chronic disease prevention in adults: Scientific review. Jama 2002, 287, 3116–3126. [Google Scholar] [CrossRef]
- Gerster, H. Vitamin A—Functions, dietary requirements and safety in humans. Int. J. Vitam. Nutr. Res. 1997, 67, 71–90. [Google Scholar] [PubMed]
- Sinopoli, A.; Caminada, S.; Isonne, C.; Santoro, M.M.; Baccolini, V. What Are the Effects of Vitamin A Oral Supplementation in the Prevention and Management of Viral Infections? A Systematic Review of Randomized Clinical Trials. Nutrients 2022, 14, 4081. [Google Scholar] [CrossRef] [PubMed]
- Stephensen, C.B. Vitamin A, infection, and immune function. Annu. Rev. Nutr. 2001, 21, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Tanumihardjo, S.A. Carotenoids and Bone Health. In Nutrition and Health; Chapter 14; Humana Press: Totowa, NJ, USA, 2013; pp. 237–245. [Google Scholar]
- Villamor, E.; Fawzi, W.W. Effects of vitamin a supplementation on immune responses and correlation with clinical outcomes. Clin. Microbiol. Rev. 2005, 18, 446–464. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.T.; Zeng, F.F.; Li, B.L.; Lin, J.S.; Liang, Y.Y.; Chen, Y.M. Higher dietary carotenoid intake associated with lower risk of hip fracture in middle-aged and elderly Chinese: A matched case-control study. Bone 2018, 111, 116–122. [Google Scholar] [CrossRef]
- de Jonge, E.A.; Jong, J.; Ki, C.; Campos-Obando, N.; Booij, L.; Franco, O.H.; Hofman, A.; Uitterlinden, A.G.; Rivadeneira, F.; Zillikens, M.C. Dietary vitamin A intake and bone health in the elderly: The Rotterdam Study. Eur. J. Clin. Nutr. 2015, 69, 1360–1368. [Google Scholar] [CrossRef]
- Li, X.; Liu, X. Associations of serum vitamins levels with bone mineral density in the different race-ethnicities US adults. BMC Musculoskelet. Disord. 2021, 22, 137. [Google Scholar] [CrossRef]
- Maggio, D.; Polidori, M.C.; Barabani, M.; Tufi, A.; Ruggiero, C.; Cecchetti, R.; Aisa, M.C.; Stahl, W.; Cherubini, A. Low levels of carotenoids and retinol in involutional osteoporosis. Bone 2006, 38, 244–248. [Google Scholar] [CrossRef]
- Promislow, J.H.; Goodman-Gruen, D.; Slymen, D.J.; Barrett-Connor, E. Retinol intake and bone mineral density in the elderly: The Rancho Bernardo Study. J. Bone Min. Res. 2002, 17, 1349–1358. [Google Scholar] [CrossRef]
- Regu, G.M.; Kim, H.; Kim, Y.J.; Paek, J.E.; Lee, G.; Chang, N.; Kwon, O. Association between Dietary Carotenoid Intake and Bone Mineral Density in Korean Adults Aged 30-75 Years Using Data from the Fourth and Fifth Korean National Health and Nutrition Examination Surveys (2008–2011). Nutrients 2017, 9, 1025. [Google Scholar] [CrossRef]
- Sun, L.L.; Li, B.L.; Xie, H.L.; Fan, F.; Yu, W.Z.; Wu, B.H.; Xue, W.Q.; Chen, Y.M. Associations between the dietary intake of antioxidant nutrients and the risk of hip fracture in elderly Chinese: A case-control study. Br. J. Nutr. 2014, 112, 1706–1714. [Google Scholar] [CrossRef] [PubMed]
- Mata-Granados, J.M.; Cuenca-Acevedo, J.R.; de Castro, M.D.L.; Holick, M.F.; Quesada-Gómez, J.M. Vitamin D insufficiency together with high serum levels of vitamin A increases the risk for osteoporosis in postmenopausal women. Arch. Osteoporos. 2013, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Michaëlsson, K.; Lithell, H.; Vessby, B.; Melhus, H. Serum retinol levels and the risk of fracture. N. Engl. J. Med. 2003, 348, 287–294. [Google Scholar] [CrossRef]
- Chen, G.; Yan, H.; Hao, Y.; Shrestha, S.; Wang, J.; Li, Y.; Wei, Y.; Pan, J.; Zhang, Z. Comparison of various anthropometric indices in predicting abdominal obesity in Chinese children: A cross-sectional study. BMC Pediatr. 2019, 19, 127. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, G.; Pan, X. China Food Composition; Peking University Medical Press: Beijing, China, 2009; Volume 42, pp. 795–799. [Google Scholar]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sport Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef]
- Paik, J.M.; Farwell, W.R.; Taylor, E.N. Demographic, dietary, and serum factors and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporos. Int. 2012, 23, 1727–1736. [Google Scholar] [CrossRef]
- Pluijm, S.M.; Visser, M.; Smit, J.H.; Popp-Snijders, C.; Roos, J.C.; Lips, P. Determinants of bone mineral density in older men and women: Body composition as mediator. J. Bone Min. Res. 2001, 16, 2142–2151. [Google Scholar] [CrossRef]
- Djakoure, C.; Guibourdenche, J.; Porquet, D.; Pagesy, P.; Peillon, F.; Li, J.Y.; Evain-Brion, D. Vitamin A and retinoic acid stimulate within minutes cAMP release and growth hormone secretion in human pituitary cells. J. Clin. Endocrinol. Metab. 1996, 81, 3123–3126. [Google Scholar]
- Locatelli, V.; Bianchi, V.E. Effect of GH/IGF-1 on Bone Metabolism and Osteoporsosis. Int. J. Endocrinol. 2014, 2014, 235060. [Google Scholar] [CrossRef]
- Raifen, R.; Altman, Y.; Zadik, Z. Vitamin A levels and growth hormone axis. Horm. Res. 1996, 46, 279–281. [Google Scholar] [CrossRef]
- Dawson, M.I. The importance of vitamin A in nutrition. Curr. Pharm. Des. 2000, 6, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.M. The vitamin A spectrum: From deficiency to toxicity. Am. J. Clin. Nutr. 2000, 71, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Lionikaite, V.; Henning, P.; Drevinge, C.; Shah, F.A.; Palmquist, A.; Wikström, P.; Windahl, S.H.; Lerner, U.H. Vitamin A decreases the anabolic bone response to mechanical loading by suppressing bone formation. Faseb J. 2019, 33, 5237–5247. [Google Scholar] [CrossRef] [PubMed]
- Johansson, S.; Melhus, H. Vitamin A antagonizes calcium response to vitamin D in man. J. Bone Min. Res. 2001, 16, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, A.M.; Ruiz-Ramos, M.; Correa-Muñoz, E.; Mendoza-Núñez, V.M. Oxidative stress as a risk factor for osteoporosis in elderly Mexicans as characterized by antioxidant enzymes. BMC Musculoskelet. Disord. 2007, 8, 124. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Asp. Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- El-Agamey, A.; Lowe, G.M.; McGarvey, D.J.; Mortensen, A.; Phillip, D.M.; Truscott, T.G.; Young, A.J. Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch. Biochem. Biophys. 2004, 430, 37–48. [Google Scholar] [CrossRef]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Res. Int. 2015, 76 Pt 3, 735–750. [Google Scholar] [CrossRef]
- Wang, F.; Wang, N.; Gao, Y.; Zhou, Z.; Liu, W.; Pan, C.; Yin, P.; Yu, X.; Tang, M. β-Carotene suppresses osteoclastogenesis and bone resorption by suppressing NF-κB signaling pathway. Life Sci. 2017, 174, 15–20. [Google Scholar] [CrossRef]
| Variables | Group 1 (n = 42) | Group 2 (n = 171) | Group 3 (n = 85) | Group 4 (n = 86) | Group 5 (n = 42) | p | Total (n = 426) |
|---|---|---|---|---|---|---|---|
| Age (years) | 7.93 (0.80) | 7.94 (1.00) | 8.07 (1.00) | 8.19 (0.96) | 8.17 (1.10) | 0.291 | 8.0 (1.0) |
| Gender (n, %) | 0.514 | ||||||
| Girls | 17 (40.5) | 68 (39.8) | 37 (43.5) | 38 (44.2) | 23 (54.8) | 183 (43.0) | |
| Boys | 25 (59.5) | 103 (60.2) | 48 (56.5) | 48 (55.8) | 19 (45.2) | 243 (57.0) | |
| Height (cm) | 127.3 (7.7) | 127.9 (7.9) | 128.2 (7.4) | 130.4 (7.8) | 129.6 (10.6) | 0.112 | 128.5 (8.1) |
| Weight (kg) | 24.4 (4.5) | 25.7 (7.1) | 25.9 (5.9) | 27.8 (6.9) | 28.3 (9.8) | 0.022 | 26.3 (7.0) |
| BMI (kg/m2) | 15.0 (1.4) | 15.6 (2.7) | 15.6 (2.4) | 16.2 (2.7) | 16.6 (3.4) | 0.021 | 15.7 (2.7) |
| MET [kcal/(kg·day)] | 39.9 (4.5) | 39.8 (4.3) | 40.1 (4.5) | 40.1 (4.5) | 40.0 (4.4) | 0.988 | 40.0 (4.4) |
| Energy (kcal/day) | 1333 (418) | 1404 (440) | 1404 (377) | 1545 (452) | 1455 (445) | 0.055 | 1430 (432) |
| Protein (g/day) | 59 (22) | 62 (21) | 64 (23) | 72 (26) | 67 (22) | 0.009 | 65 (23) |
| Fat (g/day) | 38 (14) | 43 (21) | 44 (21) | 47 (19) | 44 (17) | 0.255 | 44 (19) |
| Carbohydrate (g/day) | 193 (63) | 196 (58) | 191 (40) | 214 (63) | 203 (60) | 0.062 | 199 (57) |
| Calcium (mg/day) | 456 (182) | 486 (208) | 492 (209) | 583 (219) | 529 (220) | 0.003 | 508 (212) |
| Phosphorus (mg/day) | 917 (321) | 970 (301) | 986 (303) | 1119 (355) | 1034 (312) | 0.002 | 1004 (321) |
| Delivery mode (n, %) | 0.762 | ||||||
| Vaginal | 20 (47.6) | 85 (49.7) | 47 (55.3) | 39 (45.3) | 20 (47.6) | 211 (49.5) | |
| Cesarean | 22 (52.4) | 86 (50.3) | 38 (44.7) | 47 (54.7) | 22 (52.4) | 215 (50.5) | |
| Feeding patterns (n, %) | 0.457 | ||||||
| Breastfeeding | 34 (81.0) | 146 (85.4) | 78 (91.8) | 72 (83.7) | 36 (85.7) | 366 (85.9) | |
| Artificial feeding | 8 (19.0) | 25 (14.6) | 7 (8.2) | 14 (16.3) | 6 (14.3) | 60 (14.1) | |
| Household income (n, %) | 0.346 | ||||||
| <8000 Yuan/month | 8 (19.0) | 35 (20.5) | 13 (15.3) | 15 (17.4) | 5 (11.9) | 76 (17.8) | |
| 8000~15,000 Yuan/month | 13 (31.0) | 55 (32.2) | 25 (29.4) | 24 (27.9) | 13 (31.0) | 130 (30.5) | |
| >15,000 Yuan/month | 12 (28.6) | 50 (29.2) | 26 (30.6) | 36 (41.9) | 20 (47.6) | 144 (33.8) | |
| Unknown | 9 (21.4) | 31 (18.1) | 21 (24.7) | 11 (12.8) | 4 (9.5) | 76 (17.8) | |
| Maternal education level (n, %) | 0.149 | ||||||
| Secondary or less | 20 (47.6) | 76 (44.4) | 29 (34.1) | 23 (26.7) | 13 (31.0) | 161 (37.8) | |
| University | 18 (42.9) | 84 (49.1) | 49 (57.6) | 53 (61.6) | 26 (61.9) | 230 (54.0) | |
| Postgraduate or above | 4 (9.5) | 11 (6.4) | 7 (8.2) | 10 (11.6) | 3 (7.1) | 35 (8.2) | |
| Paternal education level | 0.246 | ||||||
| Secondary or less | 24 (57.1) | 79 (46.2) | 31 (36.5) | 28 (32.6) | 15 (35.7) | 177 (41.5) | |
| University | 14 (33.3) | 72 (42.1) | 42 (49.4) | 46 (53.5) | 22 (52.4) | 196 (46.0) | |
| Postgraduate or above | 4 (9.5) | 20 (11.7) | 12 (14.1) | 12 (14.0) | 5 (11.9) | 53 (12.4) | |
| Use of calcium supplement (n, %) | 0.900 | ||||||
| No | 25 (59.5) | 102 (59.6) | 56 (65.9) | 53 (61.6) | 25 (59.5) | 261 (61.3) | |
| Yes | 17 (40.5) | 69 (40.4) | 29 (34.1) | 33 (38.4) | 17 (40.5) | 165 (38.7) | |
| Use of multivitamin supplement (n, %) | 0.521 | ||||||
| No | 38 (90.5) | 139 (81.3) | 67 (78.8) | 72 (83.7) | 36 (85.7) | 352 (82.6) | |
| Yes | 4 (9.5) | 32 (18.7) | 18 (21.2) | 14 (16.3) | 6 (14.3) | 74 (17.4) | |
| BMC (g) | |||||||
| Total body | 882 (105) | 919 (128) | 927 (129) | 967 (149) a | 951 (178) | 0.009 | 930 (138) |
| Total body less head | 554 (87) | 572 (103) | 581 (95) | 616 (120) a,b | 615 (154) | 0.004 | 585 (111) |
| BMD (g/cm2) | |||||||
| Total body | 0.757 (0.047) | 0.778 (0.060) | 0.784 (0.063) | 0.797 (0.063) a | 0.783 (0.077) | 0.014 | 0.781 (0.063) |
| Total body less head | 0.590 (0.050) | 0.602 (0.061) | 0.610 (0.058) | 0.627 (0.062) a,b | 0.621 (0.088) | 0.006 | 0.609 (0.063) |
| TBF (kg) | 6.3 (2.0) | 7.3 (3.6) | 7.2 (3.1) | 8.0 (3.9) | 8.7 (4.7) | 0.015 | 7.4 (3.6) |
| Plasma retinol concentration (μmol/L) | 0.67 (0.11) | 0.95 (0.09) | 1.16 (0.04) | 1.34 (0.08) | 1.65 (0.19) | <0.001 | 1.11 (0.28) |
| Vitamin A (μg RE/day) | 550 (280) | 648 (344) | 642 (287) | 768 (424) | 795 (366) | 0.001 | 676 (354) |
| α-carotene (μg /day) | 763 (882) | 823 (879) | 814 (692) | 1004 (1086) | 905 (788) | 0.491 | 860 (883) |
| β-carotene (μg /day) | 2038 (1401) | 2343 (1648) | 2234 (1320) | 2817 (1958) | 2559 (1301) | 0.052 | 2408 (1615) |
| β-cryptoxanthin (μg/day) | 86.5 (75.5) | 91.4 (84.8) | 93.6 (76.0) | 110.3 (113.5) | 116.4 (186.8) | 0.417 | 97.6 (103.0) |
| Variables | Per SD Increase in Dietary VA Intake of Different Forms | |||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | |||||
| β | 95% CI | p | β | 95% CI | p | |
| Dietary VA intake | ||||||
| TB BMC (g) | 13.2 | (2.75, 23.6) | 0.001 | 6.16 | (−1.11, 13.4) | 0.098 |
| TB BMD (g/cm2) | 9.21 × 10−3 | (4.21 × 10−3, 1.42 × 10−2) | <0.001 | 5.86 × 10−3 | (1.56 × 10−3, 1.02 × 10−2) | 0.008 |
| TBLH BMC (g) | 7.119 | (−1.27, 15.5) | 0.097 | 2.53 | (−2.31, 7.37) | 0.306 |
| TBLH BMD (g/cm2) | 5.79 × 10−3 | (8.13 × 10−4, 1.07 × 10−2) | 0.022 | 3.04 × 10−3 | (−3.72 × 10−5, 6.12 × 10−3) | 0.053 |
| Dietary α-carotene intake | ||||||
| TB BMC (g) | 18.9 | (−5.60, 43.4) | 0.131 | 13.2 | (−3.03, 29.4) | 0.111 |
| TB BMD (g/cm2) | 1.22 × 10−2 | (4.20 × 10−3, 2.40 × 10−2) | 0.043 | 9.10 × 10−3 | (−5.04 × 10−4, 1.87 × 10−2) | 0.064 |
| TBLH BMC (g) | 12.8 | (−6.78, 32.4) | 0.201 | 9.73 | (−1.03, 20.5) | 0.077 |
| TBLH BMD (g/cm2) | 8.96 × 10−3 | (−2.64 × 10−3, 2.06 × 10−2) | 0.131 | 6.80 × 10−3 | (−4.04 × 10−5, 1.36 × 10−2) | 0.052 |
| Dietary β-carotene intake | ||||||
| TB BMC (g) | 17.3 | (6.36, 28.2) | 0.002 | 5.97 | (−1.48, 13.42) | 0.117 |
| TB BMD (g/cm2) | 9.16 × 10−3 | (3.91 × 10−3, 1.41 × 10−2) | <0.001 | 4.47 × 10−3 | (5.47 × 10−5, 8.88 × 10−2) | 0.048 |
| TBLH BMC (g) | 13.1 | (4.36, 21.8) | 0.003 | 3.92 | (−1.02, 8.86) | 0.121 |
| TBLH BMD (g/cm2) | 8.42 × 10−3 | (3.25 × 10−3, 1.36 × 10−2) | 0.001 | 3.03 × 10−3 | (−1.06 × 10−4, 6.17 × 10−3) | 0.059 |
| Dietary β-cryptoxanthin intake | ||||||
| TB BMC (g) | −5.35 | (−15.9, 5.19) | 0.321 | −4.29 | (−11.3, 2.67) | 0.228 |
| TB BMD (g/cm2) | −1.85 × 10−3 | (−6.95 × 10−3, 3.25 × 10−3) | 0.476 | −1.27 × 10−3 | (−5.41 × 10−3, 2.87 × 10−3) | 0.549 |
| TBLH BMC (g) | −5.20 | (−13.63, 3.23) | 0.227 | −4.28 | (−8.89, 0.33) | 0.070 |
| TBLH BMD (g/cm2) | −2.79 ×10−3 | (−7.79 × 10−3, 2.21 × 10−3) | 0.274 | −2.13 × 10−3 | (−5.07 × 10−3, 8.10 × 10−4) | 0.157 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Huang, J.; Zhou, Y.; Hong, Z.; Lin, X.; Chen, S.; Ye, Y.; Zhang, Z. Vitamin A Nutritional Status Is a Key Determinant of Bone Mass in Children. Nutrients 2022, 14, 4694. https://doi.org/10.3390/nu14214694
Zhang X, Huang J, Zhou Y, Hong Z, Lin X, Chen S, Ye Y, Zhang Z. Vitamin A Nutritional Status Is a Key Determinant of Bone Mass in Children. Nutrients. 2022; 14(21):4694. https://doi.org/10.3390/nu14214694
Chicago/Turabian StyleZhang, Xuanrui, Jiapeng Huang, Yingyu Zhou, Zhen Hong, Xiaoping Lin, Shanshan Chen, Yongnong Ye, and Zheqing Zhang. 2022. "Vitamin A Nutritional Status Is a Key Determinant of Bone Mass in Children" Nutrients 14, no. 21: 4694. https://doi.org/10.3390/nu14214694
APA StyleZhang, X., Huang, J., Zhou, Y., Hong, Z., Lin, X., Chen, S., Ye, Y., & Zhang, Z. (2022). Vitamin A Nutritional Status Is a Key Determinant of Bone Mass in Children. Nutrients, 14(21), 4694. https://doi.org/10.3390/nu14214694





