Neuroprotective Effects of Bifidobacterium breve CCFM1067 in MPTP-Induced Mouse Models of Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
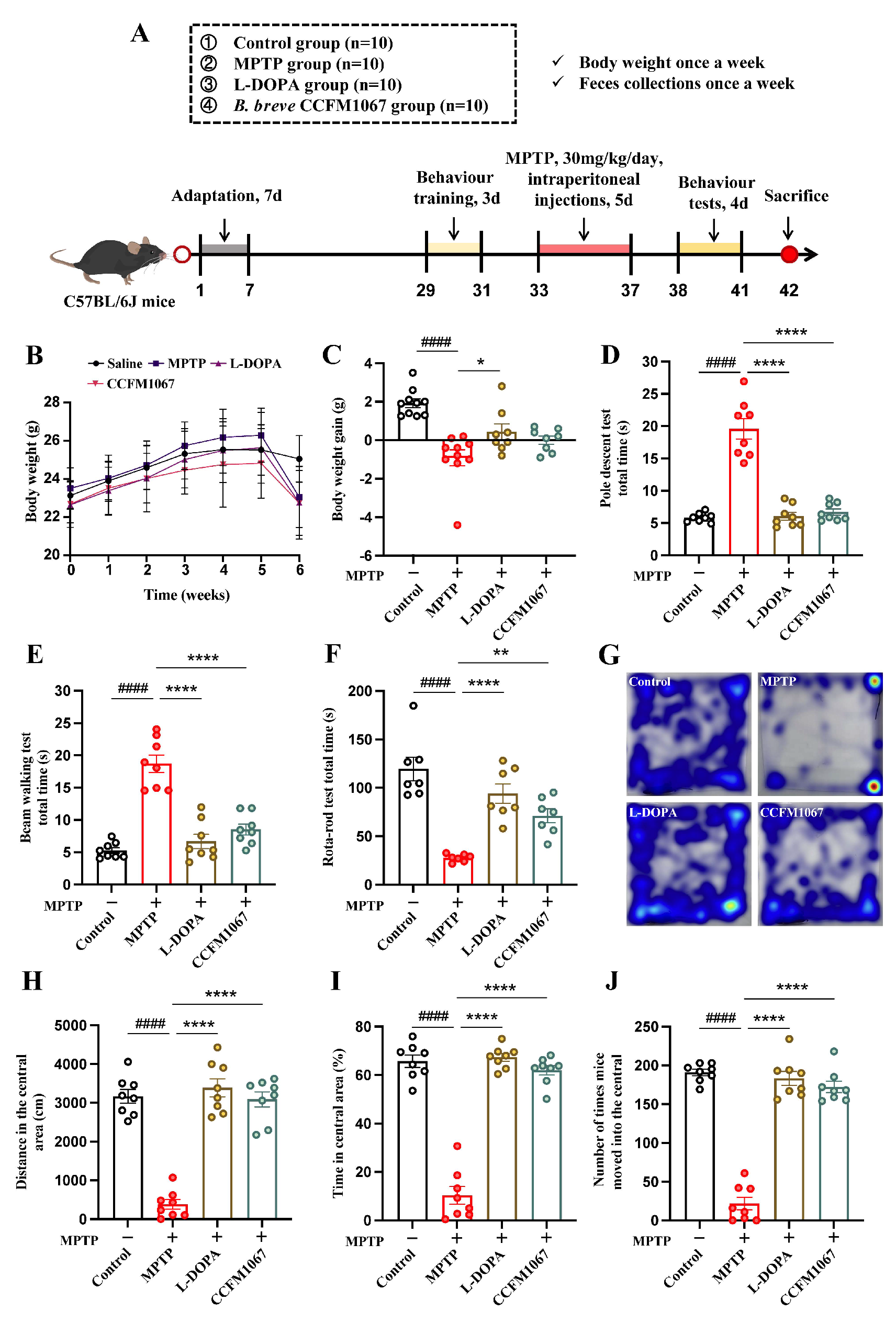
2.1. Animals and Experimental Design
2.2. Bifidobacterium breve CCFM1067 Preparation
2.3. Behavioral Tests for Motor Functions
2.4. Neurochemical and Biochemicall Analyses
2.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.6. Immunohistochemistry
2.7. Gut Microbial and Bioinformatics Analysis
2.8. Statistical Analysis
3. Results
3.1. B. breve CCFM1067 Improves MPTP-Induced Motor Impairments
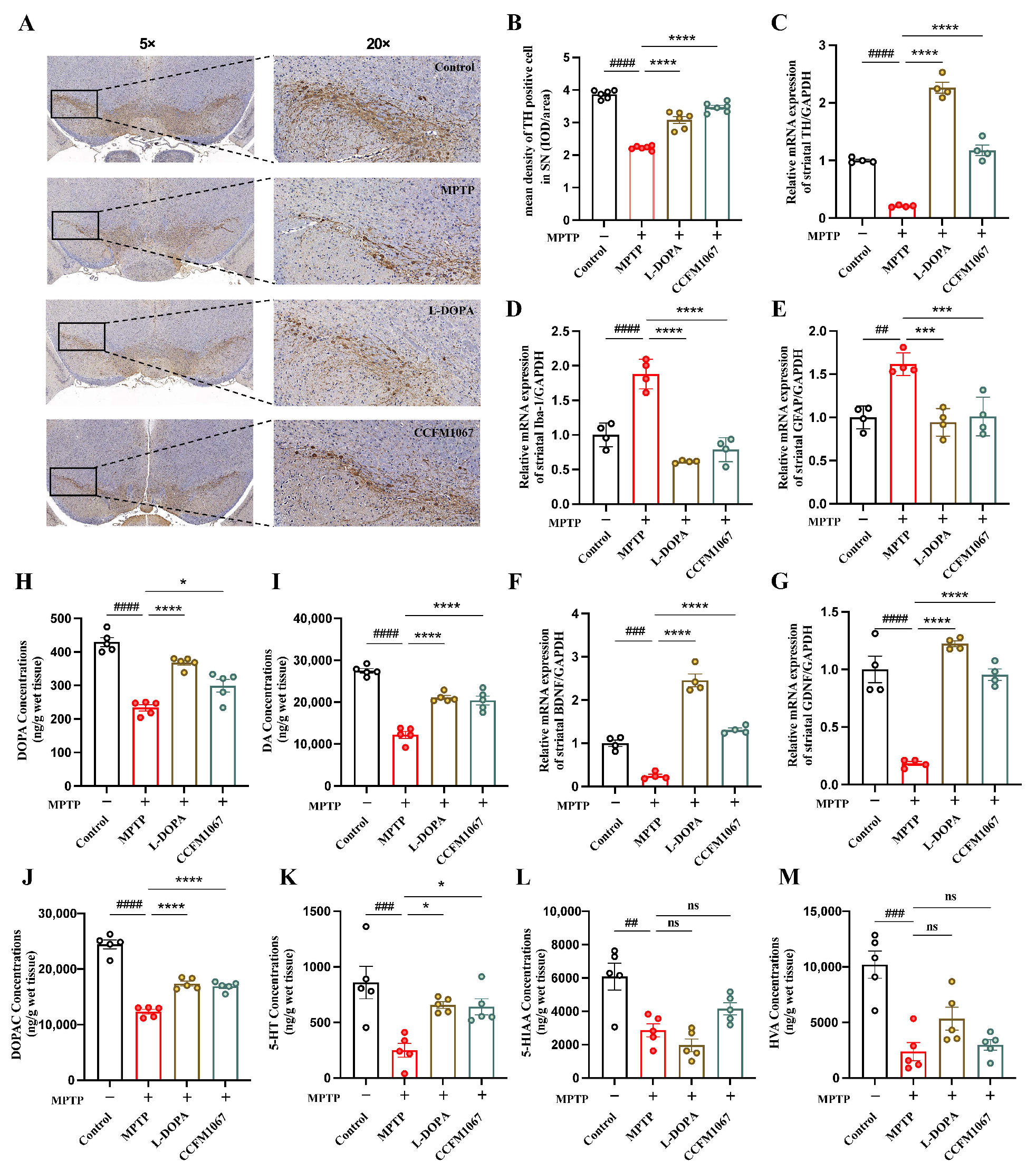
3.2. B. breve CCFM1067 Alleviates MPTP-Induced Neuropathological Alterations
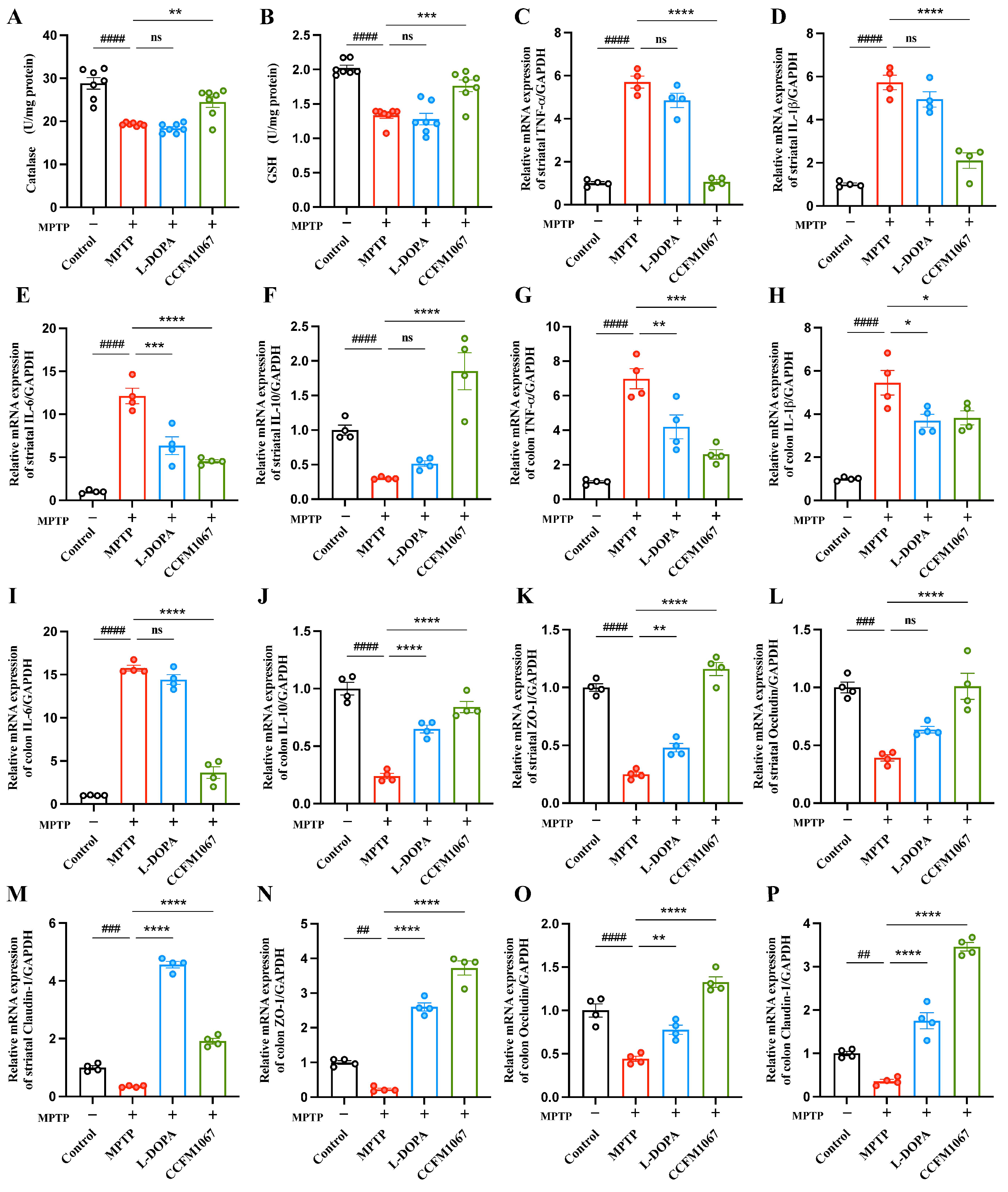
3.3. B. breve CCFM1067 Increases Antioxidant Levels and Reduces MPTP-Induced Neuroinflammation
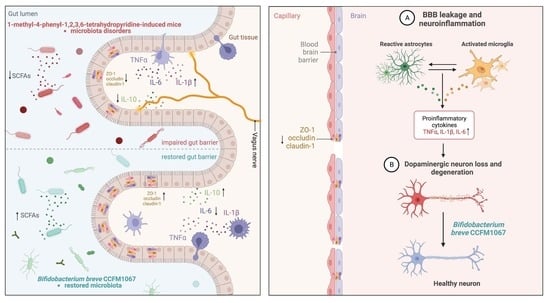
3.4. B. breve CCFM1067 Treatment Helps Improve Blood–Brain and Intestinal Barrier Damage
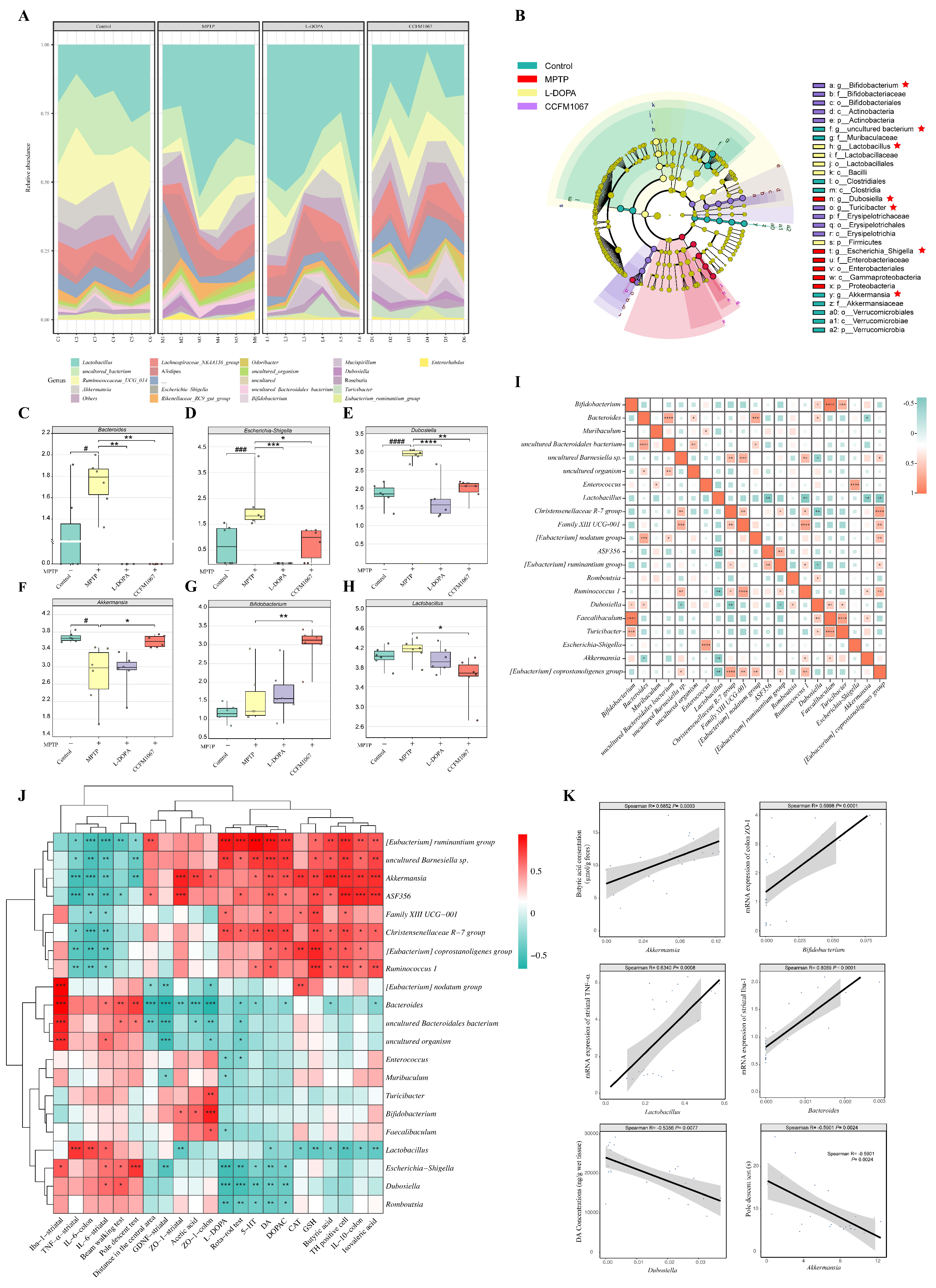
3.5. B. breve CCFM1067 Ameliorated the Dysbiosis of the Mouse Gut Microbiota of MPTP-Induced
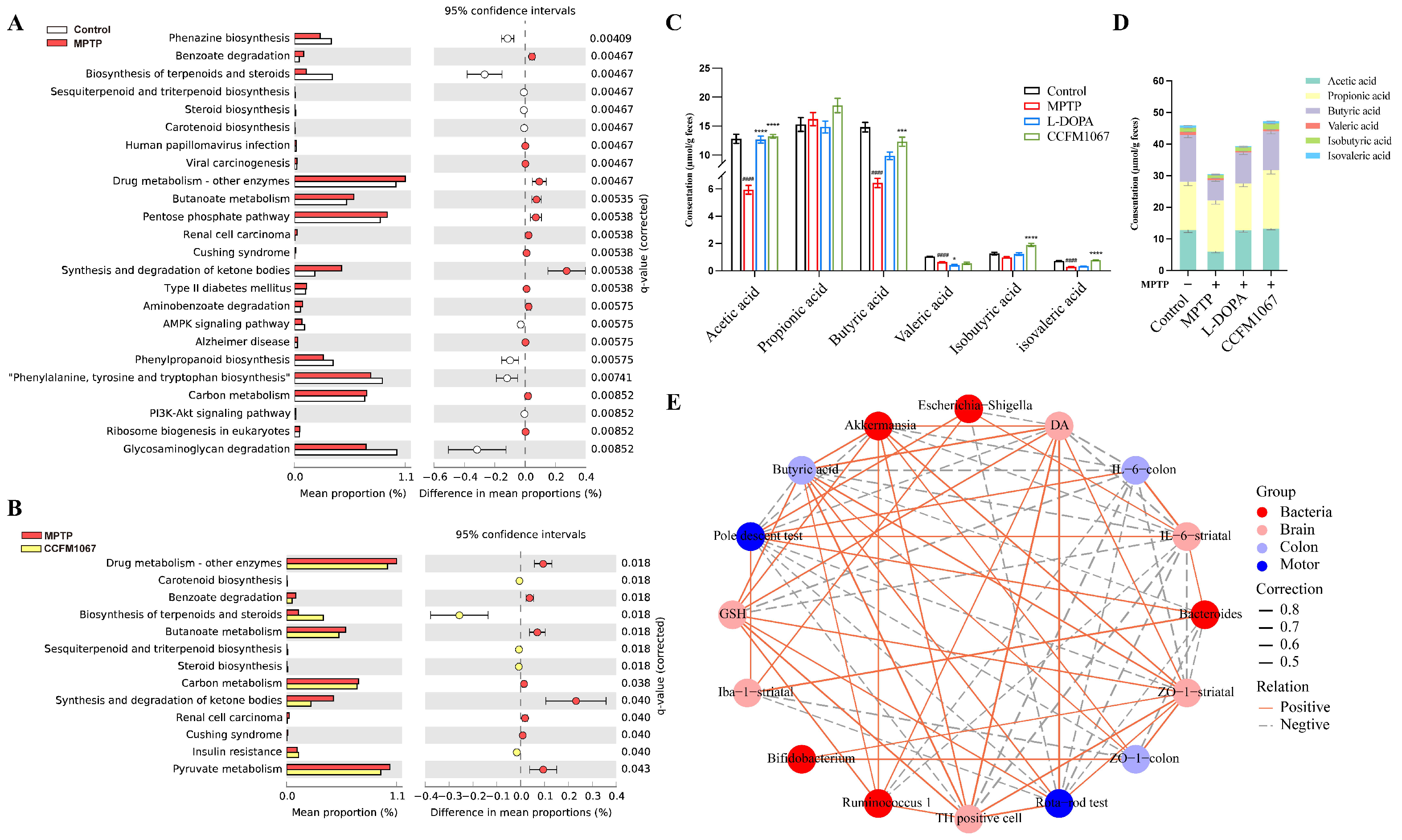
3.6. Functional Predictions Suggested That B. breve CCFM1067 May Modify Functional Modules of the Gut Microbiota
3.7. Correlations Support the Involvement of the MGBA in the MPTP-Treated Mouse Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| α-SYN | α-synuclein |
| BBB | Blood–brain barrier |
| BDNF | Brain-derived neurotrophic factor |
| CAT | Catalase |
| CNS | Central nervous system |
| DA | Dopamine |
| DAB | 3,3′-diaminobenzidine |
| DOPAC | 3,4-dihydroxyphenylacetic acid |
| ELISA | Enzyme-linked immunosorbent assay |
| ENS | Enteric nervous system |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GDNF | Glial cell line-derived neurotrophic factor |
| GFAP | Glial fibrillary acidic protein |
| GSH | Glutathione |
| HPLC | High Performance Liquid Chromatography |
| HVA | Homovanillic acid |
| Iba1 | Ionized calcium binding adapter molecule 1 |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| IHC | Immunohistochemistry |
| LDA | Linear discriminant analysis |
| L-DOPA | Levodopa |
| LPS | Lipopolysaccharide |
| MGBA | Microbiota–gut–brain axis |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| MRS | De Man Rogosa Sharpe |
| NBT | Narrow-beam test |
| OUT | Operational taxonomic unit |
| OFT | Open field test |
| PCA | Principal component analysis |
| PCoA | Principal coordinate analysis |
| PD | Parkinson’s disease |
| perMANOVA | Permutational multivariate analysis of variance |
| PICRUSt | Phylogenetic Investigation of Communities by Reconstruction of Unobserved States |
| PT | Pole test |
| QIIME | Quantitative Insights into Microbial Ecology |
| qRT-PCR | Quantitative real-time polymerase chain reaction assay |
| ROS | Reactive oxygen species |
| RTR | Rotarod test |
| SCFAs | Short-chain fatty acids |
| SEM | Standard error of mean |
| SN | Substantia nigra |
| SOD | Superoxide dismutase |
| TNF-α | Tumor necrosis factor-α |
| TH | Tyrosine hydroxylase |
| TrkB | Tyrosine Kinase receptor B |
| ZO-1 | Zonula occludens-1 |
| 5-HT | 5-hydroxytryptamine |
| 5-HIAA | 5-hydroxyindoleacetic acid |
References
- Hayes, M.T. Parkinson’s Disease and Parkinsonism. Am. J. Med. 2019, 132, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Vélez, G.E.; Zoghbi, H.Y. Parkinson’s Disease Genetics and Pathophysiology. Annu. Rev. Neurosci. 2021, 44, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Ozansoy, M.; Başak, A.N. The Central Theme of Parkinson’s Disease: α-Synuclein. Mol. Neurobiol. 2013, 47, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.B.; O’Callaghan, J.P. Biomarkers of Parkinson’s Disease: Present and Future. Metabolism 2015, 64, S40–S46. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Visanji, N.P.; Liu, L.W.C.; Lang, A.E.; Pfeiffer, R.F. Gastrointestinal Dysfunction in Parkinson’s Disease. Lancet Neurol. 2015, 14, 625–639. [Google Scholar] [CrossRef]
- Liddle, R.A. Parkinson’s Disease from the Gut. Brain Res. 2018, 1693, 201–206. [Google Scholar] [CrossRef]
- Liao, J.-F.; Cheng, Y.-F.; You, S.-T.; Kuo, W.-C.; Huang, C.-W.; Chiou, J.-J.; Hsu, C.-C.; Hsieh-Li, H.-M.; Wang, S.; Tsai, Y.-C. Lactobacillus Plantarum PS128 Alleviates Neurodegenerative Progression in 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Mouse Models of Parkinson’s Disease. Brain Behav. Immun. 2020, 90, 26–46. [Google Scholar] [CrossRef]
- Lei, Q.; Wu, T.; Wu, J.; Hu, X.; Guan, Y.; Wang, Y.; Yan, J.; Shi, G. Roles of A-synuclein in Gastrointestinal Microbiome Dysbiosis-related Parkinson’s Disease Progression (Review). Mol. Med. Rep. 2021, 24, 734. [Google Scholar] [CrossRef]
- Unger, M.M.; Spiegel, J.; Dillmann, K.-U.; Grundmann, D.; Philippeit, H.; Bürmann, J.; Faßbender, K.; Schwiertz, A.; Schäfer, K.-H. Short Chain Fatty Acids and Gut Microbiota Differ between Patients with Parkinson’s Disease and Age-Matched Controls. Parkinsonism Relat. Disord. 2016, 32, 66–72. [Google Scholar] [CrossRef]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, F.; Liu, Y.; Ma, T.; Jin, H.; Quan, K.; Leng, B.; Zhao, J.; Yuan, X.; Li, Z.; et al. Probiotics Synergized with Conventional Regimen in Managing Parkinson’s Disease. NPJ Parkinsons. Dis. 2022, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.-H.; Kuo, C.-W.; Hsieh, K.-H.; Shieh, M.-J.; Peng, C.-W.; Chen, Y.-C.; Chang, Y.-L.; Huang, Y.-Z.; Chen, C.-C.; Chang, P.-K.; et al. Probiotics Alleviate the Progressive Deterioration of Motor Functions in a Mouse Model of Parkinson’s Disease. Brain Sci. 2020, 10, 206. [Google Scholar] [CrossRef] [PubMed]
- Tamtaji, O.R.; Taghizadeh, M.; Daneshvar Kakhaki, R.; Kouchaki, E.; Bahmani, F.; Borzabadi, S.; Oryan, S.; Mafi, A.; Asemi, Z. Clinical and Metabolic Response to Probiotic Administration in People with Parkinson’s Disease: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Nutr. 2019, 38, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.H.; Lim, S.-Y.; Chong, K.K.; Manap, M.A.A.A.; Hor, J.W.; Lim, J.L.; Low, S.C.; Chong, C.W.; Mahadeva, S.; Lang, A.E. Probiotics for Constipation in Parkinson Disease: A Randomized Placebo-Controlled Study. Neurology 2021, 96, e772–e782. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, D.; Ancusa, O.E.; Georgescu, L.A.; Ionita, I.; Reisz, D. Nonmotor Gastrointestinal Disorders in Older Patients with Parkinson’s Disease: Is There Hope? Clin. Interv. Aging 2016, 11, 1601–1608. [Google Scholar] [CrossRef]
- Barichella, M.; Pacchetti, C.; Bolliri, C.; Cassani, E.; Iorio, L.; Pusani, C.; Pinelli, G.; Privitera, G.; Cesari, I.; Faierman, S.A.; et al. Probiotics and Prebiotic Fiber for Constipation Associated with Parkinson Disease: An RCT. Neurology 2016, 87, 1274–1280. [Google Scholar] [CrossRef]
- Cassani, E.; Privitera, G.; Pezzoli, G.; Pusani, C.; Madio, C.; Iorio, L.; Barichella, M. Use of Probiotics for the Treatment of Constipation in Parkinson’s Disease Patients. Minerva Gastroenterol. Dietol. 2011, 57, 117–121. [Google Scholar]
- Krüger, J.F.; Hillesheim, E.; Pereira, A.C.S.N.; Camargo, C.Q.; Rabito, E.I. Probiotics for Dementia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr. Rev. 2021, 79, 160–170. [Google Scholar] [CrossRef]
- Ilie, O.-D.; Paduraru, E.; Robea, M.-A.; Balmus, I.-M.; Jijie, R.; Nicoara, M.; Ciobica, A.; Nita, I.-B.; Dobrin, R.; Doroftei, B. The Possible Role of Bifidobacterium Longum BB536 and Lactobacillus Rhamnosus HN001 on Locomotor Activity and Oxidative Stress in a Rotenone-Induced Zebrafish Model of Parkinson’s Disease. Oxidative Med. Cell. Longev. 2021, 2021, 9629102. [Google Scholar] [CrossRef]
- Abdelhamid, M.; Zhou, C.; Jung, C.-G.; Michikawa, M. Probiotic Bifidobacterium Breve MCC1274 Mitigates Alzheimer’s Disease-Related Pathologies in Wild-Type Mice. Nutrients 2022, 14, 2543. [Google Scholar] [CrossRef]
- Cao, J.; Amakye, W.K.; Qi, C.; Liu, X.; Ma, J.; Ren, J. Bifidobacterium Lactis Probio-M8 Regulates Gut Microbiota to Alleviate Alzheimer’s Disease in the APP/PS1 Mouse Model. Eur. J. Nutr. 2021, 60, 3757–3769. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. Administration of Bifidobacterium Breve Improves the Brain Function of Aβ(1-42)-Treated Mice via the Modulation of the Gut Microbiome. Nutrients 2021, 13, 1602. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Sugahara, H.; Shimada, K.; Mitsuyama, E.; Kuhara, T.; Yasuoka, A.; Kondo, T.; Abe, K.; Xiao, J.-Z. Therapeutic Potential of Bifidobacterium Breve Strain A1 for Preventing Cognitive Impairment in Alzheimer’s Disease. Sci. Rep. 2017, 7, 13510. [Google Scholar] [CrossRef]
- Ishii, T.; Furuoka, H.; Kaya, M.; Kuhara, T. Oral Administration of Probiotic Bifidobacterium Breve Improves Facilitation of Hippocampal Memory Extinction via Restoration of Aberrant Higher Induction of Neuropsin in an MPTP-Induced Mouse Model of Parkinson’s Disease. Biomedicines 2021, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Tian, P.; Zhu, H.; Zou, R.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Lactobacillus Paracasei CCFM1229 and Lactobacillus Rhamnosus CCFM1228 Alleviated Depression- and Anxiety-Related Symptoms of Chronic Stress-Induced Depression in Mice by Regulating Xanthine Oxidase Activity in the Brain. Nutrients 2022, 14, 1294. [Google Scholar] [CrossRef]
- Tian, P.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium with the Role of 5-Hydroxytryptophan Synthesis Regulation Alleviates the Symptom of Depression and Related Microbiota Dysbiosis. J. Nutr. Biochem. 2019, 66, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Guatteo, E.; Rizzo, F.R.; Federici, M.; Cordella, A.; Ledonne, A.; Latini, L.; Nobili, A.; Viscomi, M.T.; Biamonte, F.; Landrock, K.K.; et al. Functional Alterations of the Dopaminergic and Glutamatergic Systems in Spontaneous α-Synuclein Overexpressing Rats. Exp. Neurol. 2017, 287, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Haavik, J.; Toska, K. Tyrosine Hydroxylase and Parkinson’s Disease. Mol. Neurobiol. 1998, 16, 285–309. [Google Scholar] [CrossRef] [PubMed]
- Kawahata, I.; Fukunaga, K. Degradation of Tyrosine Hydroxylase by the Ubiquitin-Proteasome System in the Pathogenesis of Parkinson’s Disease and Dopa-Responsive Dystonia. Int. J. Mol. Sci. 2020, 21, 3779. [Google Scholar] [CrossRef]
- Nicholson, S.L.; Brotchie, J.M. 5-Hydroxytryptamine (5-HT, Serotonin) and Parkinson’s Disease—Opportunities for Novel Therapeutics to Reduce the Problems of Levodopa Therapy. Eur. J. Neurol. 2002, 9 (Suppl. S3), 1–6. [Google Scholar] [CrossRef]
- Yan, T.; Mao, Q.; Zhang, X.; Wu, B.; Bi, K.; He, B.; Jia, Y. Schisandra Chinensis Protects against Dopaminergic Neuronal Oxidative Stress, Neuroinflammation and Apoptosis via the BDNF/Nrf2/NF-ΚB Pathway in 6-OHDA-Induced Parkinson’s Disease Mice. Food Funct. 2021, 12, 4079–4091. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.J.; Watson, J.J.; Shoemark, D.K.; Barua, N.U.; Patel, N.K. GDNF, NGF and BDNF as Therapeutic Options for Neurodegeneration. Pharmacol. Ther. 2013, 138, 155–175. [Google Scholar] [CrossRef] [PubMed]
- Palasz, E.; Wysocka, A.; Gasiorowska, A.; Chalimoniuk, M.; Niewiadomski, W.; Niewiadomska, G. BDNF as a Promising Therapeutic Agent in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 1170. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ali, T.; Zheng, C.; He, K.; Liu, Z.; Shah, F.A.; Li, N.; Yu, Z.-J.; Li, S. Anti-Depressive-like Behaviors of APN KO Mice Involve Trkb/BDNF Signaling Related Neuroinflammatory Changes. Mol. Psychiatry 2022, 27, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakopoulou, V.; Botsakis, K.; Delis, F.; Mourtzi, T.; Tzatzarakis, M.N.; Dimopoulou, A.; Poulia, N.; Antoniou, K.; Stathopoulos, G.T.; Matsokis, N.; et al. Anti-Neuroinflammatory, Protective Effects of the Synthetic Microneurotrophin BNN-20 in the Advanced Dopaminergic Neurodegeneration of “Weaver” Mice. Neuropharmacology 2020, 165, 107919. [Google Scholar] [CrossRef] [PubMed]
- Patel, M. Targeting Oxidative Stress in Central Nervous System Disorders. Trends Pharmacol. Sci. 2016, 37, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.H.; Wang, X.; Zhu, X. Mitochondrial Defects and Oxidative Stress in Alzheimer Disease and Parkinson Disease. Free Radic. Biol. Med. 2013, 62, 90–101. [Google Scholar] [CrossRef]
- Picca, A.; Guerra, F.; Calvani, R.; Romano, R.; Coelho-Júnior, H.J.; Bucci, C.; Marzetti, E. Mitochondrial Dysfunction, Protein Misfolding and Neuroinflammation in Parkinson’s Disease: Roads to Biomarker Discovery. Biomolecules 2021, 11, 1508. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Z.; Zhao, L.; Zhao, Y.; Yang, G.; Wang, C.; Gao, L.; Niu, C.; Li, S. Lactobacillus Plantarum DP189 Reduces α-SYN Aggravation in MPTP-Induced Parkinson’s Disease Mice via Regulating Oxidative Damage, Inflammation, and Gut Microbiota Disorder. J. Agric. Food Chem. 2022, 70, 1163–1173. [Google Scholar] [CrossRef]
- Smeyne, M.; Smeyne, R.J. Glutathione Metabolism and Parkinson’s Disease. Free Radic. Biol. Med. 2013, 62, 13–25. [Google Scholar] [CrossRef]
- Wei, Y.; Lu, M.; Mei, M.; Wang, H.; Han, Z.; Chen, M.; Yao, H.; Song, N.; Ding, X.; Ding, J.; et al. Pyridoxine Induces Glutathione Synthesis via PKM2-Mediated Nrf2 Transactivation and Confers Neuroprotection. Nat. Commun. 2020, 11, 941. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Peana, M.; Maes, M.; Dadar, M.; Severin, B. The Glutathione System in Parkinson’s Disease and Its Progression. Neurosci. Biobehav. Rev. 2021, 120, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Jenner, P.; Olanow, C.W. Oxidative Stress and the Pathogenesis of Parkinson’s Disease. Neurology 1996, 47, S161–S170. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Ban, Y.; Khan, S. Antioxidant Activity of Calycosin against α-Synuclein Amyloid Fibrils-Induced Oxidative Stress in Neural-like Cells as a Model of Preventive Care Studies in Parkinson’s Disease. Int. J. Biol. Macromol. 2021, 182, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Ortíz de Zárate, A.; Pérez-Torralba, M.; Bonet Isidro, I.; López, C.; Claramunt, R.M.; Martínez-Casanova, D.; Sánchez-Vera, I.; Jiménez-González, J.; Lavandera, J.L. 1,5-Benzodiazepin-2(3H)-Ones: In Vitro Evaluation as Antiparkinsonian Agents. Antioxidants 2021, 10, 1584. [Google Scholar] [CrossRef]
- Cheng, J.; Liao, Y.; Dong, Y.; Hu, H.; Yang, N.; Kong, X.; Li, S.; Li, X.; Guo, J.; Qin, L.; et al. Microglial Autophagy Defect Causes Parkinson Disease-like Symptoms by Accelerating Inflammasome Activation in Mice. Autophagy 2020, 16, 2193–2205. [Google Scholar] [CrossRef]
- Sun, M.-F.; Zhu, Y.-L.; Zhou, Z.-L.; Jia, X.-B.; Xu, Y.-D.; Yang, Q.; Cui, C.; Shen, Y.-Q. Neuroprotective Effects of Fecal Microbiota Transplantation on MPTP-Induced Parkinson’s Disease Mice: Gut Microbiota, Glial Reaction and TLR4/TNF-α Signaling Pathway. Brain Behav. Immun. 2018, 70, 48–60. [Google Scholar] [CrossRef]
- Han, X.; Sun, S.; Sun, Y.; Song, Q.; Zhu, J.; Song, N.; Chen, M.; Sun, T.; Xia, M.; Ding, J.; et al. Small Molecule-Driven NLRP3 Inflammation Inhibition via Interplay between Ubiquitination and Autophagy: Implications for Parkinson Disease. Autophagy 2019, 15, 1860–1881. [Google Scholar] [CrossRef]
- Lahooti, B.; Chhibber, T.; Bagchi, S.; Varahachalam, S.P.; Jayant, R.D. Therapeutic Role of Inflammasome Inhibitors in Neurodegenerative Disorders. Brain Behav. Immun. 2021, 91, 771–783. [Google Scholar] [CrossRef]
- Chen, X.; Hu, Y.; Cao, Z.; Liu, Q.; Cheng, Y. Cerebrospinal Fluid Inflammatory Cytokine Aberrations in Alzheimer’s Disease, Parkinson’s Disease and Amyotrophic Lateral Sclerosis: A Systematic Review and Meta-Analysis. Front. Immunol. 2018, 9, 2122. [Google Scholar] [CrossRef]
- Alrafiah, A.; Al-Ofi, E.; Obaid, M.T.; Alsomali, N. Assessment of the Levels of Level of Biomarkers of Bone Matrix Glycoproteins and Inflammatory Cytokines from Saudi Parkinson Patients. BioMed Res. Int. 2019, 2019, 2690205. [Google Scholar] [CrossRef] [PubMed]
- Belarbi, K.; Cuvelier, E.; Bonte, M.-A.; Desplanque, M.; Gressier, B.; Devos, D.; Chartier-Harlin, M.-C. Glycosphingolipids and Neuroinflammation in Parkinson’s Disease. Mol. Neurodegener. 2020, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Matheoud, D.; Cannon, T.; Voisin, A.; Penttinen, A.-M.; Ramet, L.; Fahmy, A.M.; Ducrot, C.; Laplante, A.; Bourque, M.-J.; Zhu, L.; et al. Intestinal Infection Triggers Parkinson’s Disease-like Symptoms in Pink1(-/-) Mice. Nature 2019, 571, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ning, J.; Bao, X.Q.; Shang, M.; Ma, J.; Li, G.; Zhang, D. Fecal microbiota transplantation protects rotenone-induced Parkinson’s disease mice via suppressing inflammation mediated by the lipopolysaccharide-TLR4 signaling pathway through the microbiota-gut-brain axis. Microbiome 2021, 9, 226. [Google Scholar] [CrossRef]
- Romano, S.; Savva, G.M.; Bedarf, J.R.; Charles, I.G.; Hildebrand, F.; Narbad, A. Meta-Analysis of the Parkinson’s Disease Gut Microbiome Suggests Alterations Linked to Intestinal Inflammation. NPJ Parkinsons. Dis. 2021, 7, 27. [Google Scholar] [CrossRef]
- Lubomski, M.; Tan, A.H.; Lim, S.-Y.; Holmes, A.J.; Davis, R.L.; Sue, C.M. Parkinson’s Disease and the Gastrointestinal Microbiome. J. Neurol. 2020, 267, 2507–2523. [Google Scholar] [CrossRef]
- Houser, M.C.; Tansey, M.G. The Gut-Brain Axis: Is Intestinal Inflammation a Silent Driver of Parkinson’s Disease Pathogenesis? NPJ Parkinsons Dis. 2017, 3, 3. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, Y.; Ray Chaudhuri, K.; Reynolds, R.; Tan, E.-K.; Pettersson, S. The Role of Gut Dysbiosis in Parkinson’s Disease: Mechanistic Insights and Therapeutic Options. Brain 2021, 144, 2571–2593. [Google Scholar] [CrossRef]
- Caputi, V.; Giron, M.C. Microbiome-Gut-Brain Axis and Toll-Like Receptors in Parkinson’s Disease. Int. J. Mol. Sci. 2018, 19, 1689. [Google Scholar] [CrossRef]
- Varatharaj, A.; Galea, I. The Blood-Brain Barrier in Systemic Inflammation. Brain Behav. Immun. 2017, 60, 1–12. [Google Scholar] [CrossRef]
- Haruwaka, K.; Ikegami, A.; Tachibana, Y.; Ohno, N.; Konishi, H.; Hashimoto, A.; Matsumoto, M.; Kato, D.; Ono, R.; Kiyama, H.; et al. Dual Microglia Effects on Blood Brain Barrier Permeability Induced by Systemic Inflammation. Nat. Commun. 2019, 10, 5816. [Google Scholar] [CrossRef]
- Więckowska-Gacek, A.; Mietelska-Porowska, A.; Wydrych, M.; Wojda, U. Western Diet as a Trigger of Alzheimer’s Disease: From Metabolic Syndrome and Systemic Inflammation to Neuroinflammation and Neurodegeneration. Ageing Res. Rev. 2021, 70, 101397. [Google Scholar] [CrossRef] [PubMed]
- Dodiya, H.B.; Forsyth, C.B.; Voigt, R.M.; Engen, P.A.; Patel, J.; Shaikh, M.; Green, S.J.; Naqib, A.; Roy, A.; Kordower, J.H.; et al. Chronic Stress-Induced Gut Dysfunction Exacerbates Parkinson’s Disease Phenotype and Pathology in a Rotenone-Induced Mouse Model of Parkinson’s Disease. Neurobiol. Dis. 2020, 135, 104352. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms Regulating Intestinal Barrier Integrity and Its Pathological Implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Du, Z.R.; Wang, X.; Sun, X.R.; Zhao, Q.; Zhao, F.; Wong, W.T.; Wong, K.H.; Dong, X.-L. Polymannuronic Acid Prebiotic plus Lacticaseibacillus Rhamnosus GG Probiotic as a Novel Synbiotic Promoted Their Separate Neuroprotection against Parkinson’s Disease. Food Res. Int. 2022, 155, 111067. [Google Scholar] [CrossRef]
- Xie, W.; Gao, J.; Jiang, R.; Liu, X.; Lai, F.; Tang, Y.; Xiao, H.; Jia, Y.; Bai, Q. Twice Subacute MPTP Administrations Induced Time-Dependent Dopaminergic Neurodegeneration and Inflammation in Midbrain and Ileum, as Well as Gut Microbiota Disorders in PD Mice. Neurotoxicology 2020, 76, 200–212. [Google Scholar] [CrossRef]
- Sun, J.; Li, H.; Jin, Y.; Yu, J.; Mao, S.; Su, K.-P.; Ling, Z.; Liu, J. Probiotic Clostridium Butyricum Ameliorated Motor Deficits in a Mouse Model of Parkinson’s Disease via Gut Microbiota-GLP-1 Pathway. Brain Behav. Immun. 2021, 91, 703–715. [Google Scholar] [CrossRef]
- Liu, P.; Wu, L.; Peng, G.; Han, Y.; Tang, R.; Ge, J.; Zhang, L.; Jia, L.; Yue, S.; Zhou, K.; et al. Altered Microbiomes Distinguish Alzheimer’s Disease from Amnestic Mild Cognitive Impairment and Health in a Chinese Cohort. Brain Behav. Immun. 2019, 80, 633–643. [Google Scholar] [CrossRef]
- Qian, Y.; Yang, X.; Xu, S.; Wu, C.; Song, Y.; Qin, N.; Chen, S.-D.; Xiao, Q. Alteration of the Fecal Microbiota in Chinese Patients with Parkinson’s Disease. Brain Behav. Immun. 2018, 70, 194–202. [Google Scholar] [CrossRef]
- Chen, T.-J.; Feng, Y.; Liu, T.; Wu, T.-T.; Chen, Y.-J.; Li, X.; Li, Q.; Wu, Y.-C. Fisetin Regulates Gut Microbiota and Exerts Neuroprotective Effect on Mouse Model of Parkinson’s Disease. Front. Neurosci. 2020, 14, 549037. [Google Scholar] [CrossRef]
- Zhou, X.; Lu, J.; Wei, K.; Wei, J.; Tian, P.; Yue, M.; Wang, Y.; Hong, D.; Li, F.; Wang, B.; et al. Neuroprotective Effect of Ceftriaxone on MPTP-Induced Parkinson’s Disease Mouse Model by Regulating Inflammation and Intestinal Microbiota. Oxidative Med. Cell. Longev. 2021, 2021, 9424582. [Google Scholar] [CrossRef]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A Purified Membrane Protein from Akkermansia Muciniphila or the Pasteurized Bacterium Improves Metabolism in Obese and Diabetic Mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef]
- Hänninen, A.; Toivonen, R.; Pöysti, S.; Belzer, C.; Plovier, H.; Ouwerkerk, J.P.; Emani, R.; Cani, P.D.; De Vos, W.M. Akkermansia Muciniphila Induces Gut Microbiota Remodelling and Controls Islet Autoimmunity in NOD Mice. Gut 2018, 67, 1445–1453. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Q.; Cheng, L.; Buch, H.; Zhang, F. Akkermansia Muciniphila Is a Promising Probiotic. Microb. Biotechnol. 2019, 12, 1109–1125. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, Y.; Chen, S.; Zeng, Y.; Fu, X.; Chen, T.; Luo, S.; Zhang, X. The Role of the Probiotic Akkermansia Muciniphila in Brain Functions: Insights Underpinning Therapeutic Potential. Crit. Rev. Microbiol. 2022, 11, 1–26. [Google Scholar] [CrossRef]
- Pascale, A.; Marchesi, N.; Govoni, S.; Barbieri, A. Targeting the Microbiota in Pharmacology of Psychiatric Disorders. Pharmacol. Res. 2020, 157, 104856. [Google Scholar] [CrossRef]
- Scheperjans, F.; Aho, V.; Pereira, P.A.B.; Koskinen, K.; Paulin, L.; Pekkonen, E.; Haapaniemi, E.; Kaakkola, S.; Eerola-Rautio, J.; Pohja, M.; et al. Gut Microbiota Are Related to Parkinson’s Disease and Clinical Phenotype. Mov. Disord. 2015, 30, 350–358. [Google Scholar] [CrossRef]
- Hung, T.V.; Suzuki, T. Short-Chain Fatty Acids Suppress Inflammatory Reactions in Caco-2 Cells and Mouse Colons. J. Agric. Food Chem. 2018, 66, 108–117. [Google Scholar] [CrossRef]
- Burokas, A.; Arboleya, S.; Moloney, R.D.; Peterson, V.L.; Murphy, K.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Targeting the Microbiota-Gut-Brain Axis: Prebiotics Have Anxiolytic and Antidepressant-like Effects and Reverse the Impact of Chronic Stress in Mice. Biol. Psychiatry 2017, 82, 472–487. [Google Scholar] [CrossRef]
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F., 3rd; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut Microbes Promote Colonic Serotonin Production through an Effect of Short-Chain Fatty Acids on Enterochromaffin Cells. FASEB J. 2015, 29, 1395–1403. [Google Scholar] [CrossRef]
- Wenzel, T.J.; Gates, E.J.; Ranger, A.L.; Klegeris, A. Short-Chain Fatty Acids (SCFAs) Alone or in Combination Regulate Select Immune Functions of Microglia-like Cells. Mol. Cell. Neurosci. 2020, 105, 103493. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Chu, C.; Yu, L.; Zhai, Q.; Wang, S.; Zhao, J.; Zhang, H.; Chen, W.; Tian, F. Neuroprotective Effects of Bifidobacterium breve CCFM1067 in MPTP-Induced Mouse Models of Parkinson’s Disease. Nutrients 2022, 14, 4678. https://doi.org/10.3390/nu14214678
Li T, Chu C, Yu L, Zhai Q, Wang S, Zhao J, Zhang H, Chen W, Tian F. Neuroprotective Effects of Bifidobacterium breve CCFM1067 in MPTP-Induced Mouse Models of Parkinson’s Disease. Nutrients. 2022; 14(21):4678. https://doi.org/10.3390/nu14214678
Chicago/Turabian StyleLi, Tiantian, Chuanqi Chu, Leilei Yu, Qixiao Zhai, Shunhe Wang, Jianxin Zhao, Hao Zhang, Wei Chen, and Fengwei Tian. 2022. "Neuroprotective Effects of Bifidobacterium breve CCFM1067 in MPTP-Induced Mouse Models of Parkinson’s Disease" Nutrients 14, no. 21: 4678. https://doi.org/10.3390/nu14214678
APA StyleLi, T., Chu, C., Yu, L., Zhai, Q., Wang, S., Zhao, J., Zhang, H., Chen, W., & Tian, F. (2022). Neuroprotective Effects of Bifidobacterium breve CCFM1067 in MPTP-Induced Mouse Models of Parkinson’s Disease. Nutrients, 14(21), 4678. https://doi.org/10.3390/nu14214678