Abstract
The goal of this work is to explore if the changes induced by d-fagomine in the gut microbiota are compatible with its effect on body weight and inflammation markers in rats. Methods: Sprague Dawley rats were fed a standard diet supplemented with d-fagomine (or not, for comparison) for 6 months. The variables measured were body weight, plasma mediators of inflammation (hydroxyeicosatetraenoic acids, leukotriene B4, and IL-6), and the concentration of acetic acid in feces and plasma. The composition and diversities of microbiota in cecal content and feces were estimated using 16S rRNA metabarcoding and high-throughput sequencing. We found that after just 6 weeks of intake d-fagomine significantly reduced body weight gain, increased the plasma acetate concentration, and reduced the plasma concentration of the pro-inflammatory biomarkers’ leukotriene B4, interleukin 6 and 12 hydroxyeicosatetraenoic acids. These changes were associated with a significantly increased prevalence of Bacteroides and Prevotella feces and increased Bacteroides, Prevotella, Clostridium, and Dysgonomonas while reducing Anaerofilum, Blautia, and Oribacterium in cecal content. In conclusion, d-fagomine induced changes in the composition and diversity of gut microbiota similar to those elicited by dietary fiber and compatible with its anti-inflammatory and body-weight-reducing effects.
1. Introduction
d-Fagomine, ((2R, 3R, 4R)-2-hydroxymethylpiperidine-3,4-diol; 1,2-dideoxynojirimycin), is a polyhydroxylated piperidine (a saturated six-atom ring formed of five carbon atoms and a nitrogen atom). The spatial configurations of the hydroxyl groups in d-fagomine coincide with those of simple sugars such as d-glucose and d-mannose.
d-Fagomine was first isolated from seeds of buckwheat (Fagopyrum esculentum Moench, Polygonaceae) [1] and later found in other plant sources such as the leaves of Morus bombycis (Moraceae); root bark, fruit, and leaves of mulberry (Morus alba, Moraceae); roots of Lycium chinense (Solanaceae); roots and leaves of Xanthocercis zambesiaca (Fabaceae); pods of Angylocalyx pynaertii (Fabaceae); and leaves of Baphia nitida (Fabaceae) [2,3]. As d-fagomine is chemically and biologically stable, it remains unaltered during boiling, baking, frying, and fermentation [4]. d-fagomine has been part of the diet in European, Asian, and American countries, mainly as a component of buckwheat-based foodstuffs, such as noodles, groats, pancakes, boiled flour, fried dough, beer, cookies, and bread. The estimated total intake of d-fagomine resulting from a diet that includes such foodstuffs as the only source of carbohydrates is between 3 and 17 mg per day [4].
As an intestinal glycosidase inhibitor, d-fagomine reduces the postprandial blood glucose concentration after oral administration of either sucrose or starch, as evidenced in both rats [5] and humans (results accessible at https://clinicaltrials.gov/ct2/show/NCT01811303). Other observations have revealed a second possible action of d-fagomine and maybe also of other iminocyclitols: the selective inhibition of bacterial adhesion to the intestinal mucosa. In vitro studies have shown that d-fagomine selectively agglutinates fimbriated Enterobacteriales, such as Escherichia coli and Salmonella enterica serovar Typhimurium, and consequently, it inhibits the adhesion of these bacteria to pig intestinal mucosa [5]. These results suggest that d-fagomine may modify the composition of the gut microbiota. The first evidence supporting this hypothesis was the observation that d-fagomine counteracted diet-induced increases in the populations of gut Enterobacteriales while reducing weight gain [6]. We later suggested that the action of d-fagomine on some microbial populations might explain, at least in part, its functional effect against fat-induced impaired glucose tolerance and hepatic inflammation [7]. d-Fagomine has the capacity to stabilize levels of putatively beneficial gut bacteria (e.g., the Bacteroidetes phylum, Bifidobacteriales order, and Prevotella genus) in normal rats fed a standard diet for an extended period of time [8,9]. When combined with fish omega-3 polyunsaturated fatty acids (PUFAs), d-fagomine has an early effect compatible with fast changes in bacterial composition, while the protective anti-inflammatory action of PUFAs appears to be less related to changes in gut microbiota [9].
As evidence of the iminocyclitol d-fagomine having a decisive influence on the composition of gut microbiota is accumulating, we have embarked on a thorough characterization of these changes. We present here a study of the microbial composition in the gut (feces and cecal content) of Sprague Dawley rats administered d-fagomine for a period of six months and compare it with gut microbial composition in control animals. We also examine the possible association of the observed changes in microbial populations with the effects of d-fagomine on body weight and inflammation markers.
2. Materials and Methods
2.1. Experimental Design and Sample Collection
A total of 18 male Sprague Dawley rats from Envigo (Indianapolis, IN, USA), aged 8–9 weeks, were used. All animal handling was conducted in the morning to minimize the effects of circadian rhythms. The rats were housed (n = 3 per cage) under controlled conditions of humidity (60%), and temperature (22 ± 2 °C) with a 12 h light-12 h dark cycle. They were randomly divided into 2 groups (n = 9/group): the control group was fed a standard diet of 2014 Teklad Global 14% Protein chow from Envigo (STD); and the group was fed the same standard diet supplemented with 0.96 g d-fagomine/kg feed (FG). The dose of d-fagomine was established based on the results of previous studies of bacterial adhesion in vitro [5] and of its in vivo effects on gut Enterobacteriales [6]. The animals were fed ad libitum with free access to feed and water (Ribes, Barcelona, Spain).
Feed consumption and body weight were monitored three times per week throughout the experiment. Energy intake was calculated as an estimate of metabolizable energy based on the Atwater factors, assigning 4 kcal/g protein, 9 kcal/g fat, and 4 kcal/g available carbohydrates. Fecal samples were collected by abdominal massage after week 23.
An oral glucose tolerance test (OGTT) was performed on fasted animals. A solution of glucose (1 g/kg body weight) was administered by oral gavage before the test, and blood glucose concentration was measured 15, 30, 45, 60, 90, and 120 min after glucose intake. Blood glucose concentration was measured by the enzyme electrode method, using an Ascensia ELITE XL blood glucose meter (Bayer Consumer Care, Basel, Switzerland).
After 6 months of supplementation, the rats were fasted overnight and anesthetized intraperitoneally with ketamine from Merial Laboratorios (Barcelona, Spain) and xylacine from Quimica Farmaceutica (Barcelona, Spain) (80 and 10 mg/kg body weight, respectively). Blood samples were collected intracardiac puncture, and plasma was separated by centrifugation. Cecal contents were extracted, weighed, and immediately frozen in liquid N2. All the samples were stored at −80 °C until analysis.
2.2. Plasma Aminotransferases, Insulin, and Cholesterol
Plasma aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities were measured by means of spectrophotometry using the corresponding commercial kits (Spinreact, Girona, Spain) in a COBAS MIRA autoanalyzer (Roche Diagnostics System, Madrid, Spain).
Plasma insulin levels were measured in fasted animals using the rat/mouse insulin enzyme-linked immunosorbent assay (ELISA) kit from Millipore Corporation (Billerica, MA, USA).
Plasma total cholesterol, high-density lipoprotein (HDL) cholesterol, and low-density lipoprotein (LDL) cholesterol were measured using a spectrophotometric method and the corresponding kits from Spinreact (Girona, Spain).
2.3. DNA Extraction and Sequencing
Total DNA was extracted individually using a QIAampTM DNA Stool Mini Kit from QIAGEN (Hilden, Germany) from both feces and cecal content and quantified using a Nanodrop 8000 Spectrophotometer (ThermoScientific, Waltham, MA, USA). All DNA samples were diluted to 5 ng/µL and used to amplify the V3–V4 regions of the 16S ribosomal RNA (rRNA) gene, using the following universal primers in a limited cycle PCR:
- forward primer, 5′ TCG GCA GCG TCA GAT GTG TAT AAG AGA CAG CCT ACG GGN GGC WGC AG;
- reverse primer, 5′ GTC TCG TGG GCT CGG AGA TGT GTA TAA GAG ACA GGA CTA CHV GGG TAT CTA ATC C.
The V3–V4 region of the 16S rRNA gene was sequenced on an Illumina MiSeq platform at the Servei de Genòmica i Bioinformàtica of the Universitat Autònoma de Barcelona (Bellaterra, Cerdanyola del Vallés, Spain) following the manufacturer’s guidelines and instructions.
The 16S rRNA OTU counts were analyzed using the R package of Phyloseq (version 1.30.0) [10]. Statistical significance of the relative abundances of taxa was evaluated using the Wald test in the DESeq2 package (version 1.30.1) [11].
2.4. Acetic Acid Concentration
Acetic acid (the major short-chain fatty acid, SCFA) was analyzed in plasma and fecal samples after 6 months of supplementation by gas chromatography using a previously described method [12] with some modifications. Briefly, the freeze-dried feces (~50 mg dry matter) or plasma (~50 µL) and a solution (1.5 mL) containing the internal standard 2-ethylbutyric acid (6.67 mg/L) and oxalic acid (2.97 g/L) in acetonitrile/water (3:7 for feces and 7:3 for plasma) was added. Then, SCFAs were extracted for 10 min using a rotating mixer. The suspension was centrifuged, and the supernatant was filtered through a 0.45 µm nylon filter. Next, an aliquot of the supernatant (0.7 mL) was diluted with acetonitrile/water 3:7 to a final volume of 1 mL. After this, the SCFAs were analyzed using a Trace2000 gas chromatograph coupled to a flame ionization detector (ThermoFinnigan, Waltham, MA, USA) equipped with an INNOWax capillary column, 30 m × 530 µm, with a 1 µm film unit (Agilent, Santa Clara, CA, USA). The Chrom-Card software ver. 2.4.1. was used for data processing. Helium was used as the carrier gas with a linear velocity of 5 mL/min. The GC oven temperature was programmed as follows: 80 °C (hold for 1 min) to 120 °C at 15 °C/min (hold for 4 min) to 130 °C at 5 °C/min (hold for 4 min) to 235 °C at 8 °C/min (hold for 4 min). A flame ionization detector (FID) was used at a base temperature of 240 °C.
2.5. Plasma Mediators of Inflammation
Several hydroxyeicosatetraenoic acids (HETEs) and leukotriene B4 (LTB4), lipid mediators derived from the metabolism of arachidonic acid (ARA), were determined in plasma by liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS), using a method modified from Dasilva et al. [13]. Erythrocyte-free plasma samples (90 µL) were thawed, diluted in the presence of butylated hydroxytoluene (BHT), and spiked with the internal standard (12HETE-d8; Cayman Chemicals, Ann Arbor, MI, USA). Then, the samples were centrifuged (800× g, 10 min), and the lipids in the supernatants were purified by solid-phase extraction (SPE). The LC-MS/MS analyzer consisted of a Dionex UltiMate 3000 Series chromatograph (Thermo Fisher, Rockford, IL, USA) coupled to a dual-pressure linear ion-trap mass spectrometer, LTQ Velos Pro (Thermo Fisher, Rockford, IL, USA) operating in negative electrospray ionization (ESI) mode. A Symmetry C18 Column 3.5 µm, 150 mm × 2.1 mm inner diameter (Waters, Milford, MA, USA) with a C18 4 × 2 mm guard cartridge (Phenomenex, Torrance, CA, USA) was used in the separation step. Samples (10 µL) were eluted with a binary system consisting of 0.02% aqueous formic acid [A] and 0.02% formic acid in methanol [B] under gradient conditions of: 0 min, 60% B; 2 min, 60% B; 12 min, 80% B; 13 min, 80% B; 23 min, 100% B; 25 min, 100% B; and 30 min, 60% B, at a flow rate of 0.2 mL/min.
Concentrations of plasma IL-6 were determined using Milliplex xMAP multiplex technology on a Luminex xMAP instrument (Millipore, Austin, TX, USA). The MILLIPLEX Analyst 5.1 (VigeneTech; Carlisle, PA, USA) software was used for data analysis.
2.6. Statistical Analysis
All data manipulation and statistical analyses were performed using GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA). Statistical significance was determined by two-way ANOVA for repeated measures of body weight or the Wilcoxon or Student’s t test to compare the groups studied. PERMANOVA was applied for each β-diversity determination. The results are expressed as means with their standard errors (SEM). Differences were considered significant when p < 0.05.
3. Results
3.1. Body and Cecum Weights, Feed Intake, and OGTT
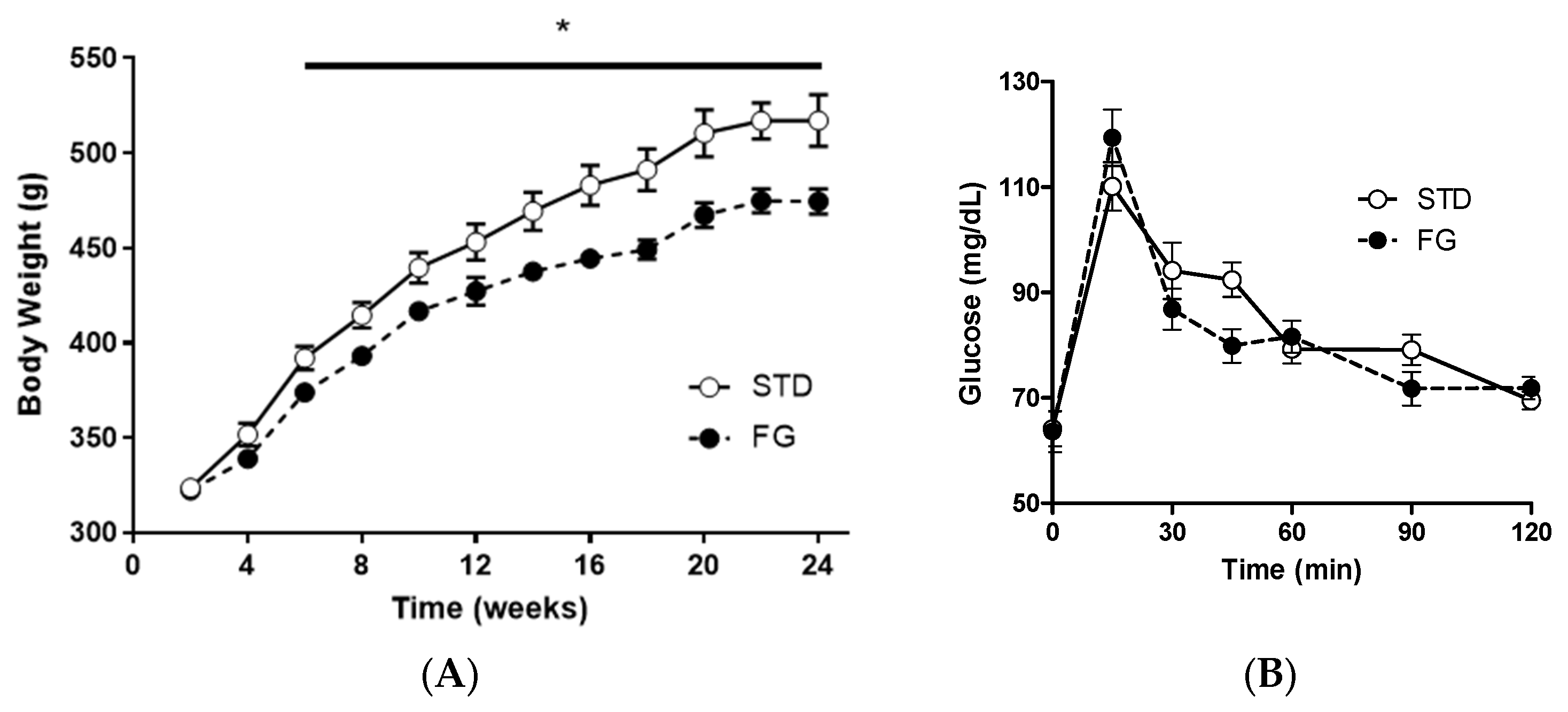
The animals in the FG group gained less weight than those in the STD group from week 6 until the end of the experiment (Figure 1A), while there were no significant differences in the OGTT (Figure 1B).
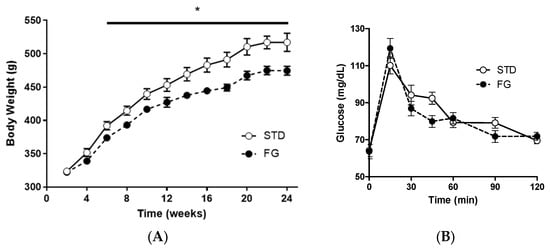
Figure 1.
(A) Body weight evolution and (B) OGTT of rats (n = 8–9) supplemented or not with d-fagomine for 6 months. Comparisons were conducted using ANOVA for repeated measures. * p < 0.05.
Feed intake (STD: 4.6 g/day/100 g body weight, SEM 0.5; FG: 4.8 g/day/100 g body weight, SEM 0.5) and energy intake (STD: 13.3 kcal/day/100 g body weight, SEM 1.4; FG: 13.8 kcal/day/100 g body weight, SEM 1.2) were similar for both groups throughout the experiment.
At the end of the study, there were no differences in cecum weight between the groups (STD: 5.1 g, SEM 0.4; FG: 5.2 g, SEM 0.2).
3.2. Plasma Aminotransferases, Insulin, and Cholesterol
The animals in the FG group did not show any difference in plasma aminotransferases, fasting insulin, or cholesterol levels at the end of the study (Table 1).

Table 1.
Plasma variables in the STD and FG groups of rats (n = 7–9/group) at the end of the study (week 23).
3.3. Relative Composition of Microbial Communities
There were no differences between the groups at the phylum taxonomical level. In feces, the class Bacteroidia was significantly (p < 0.05) increased by d-fagomine supplementation, while the class Flavobacteriia was reduced. Similar changes were detected in the orders Bacteroidales and Flavobacteriales. There were no changes in cecal content at these taxonomical levels.
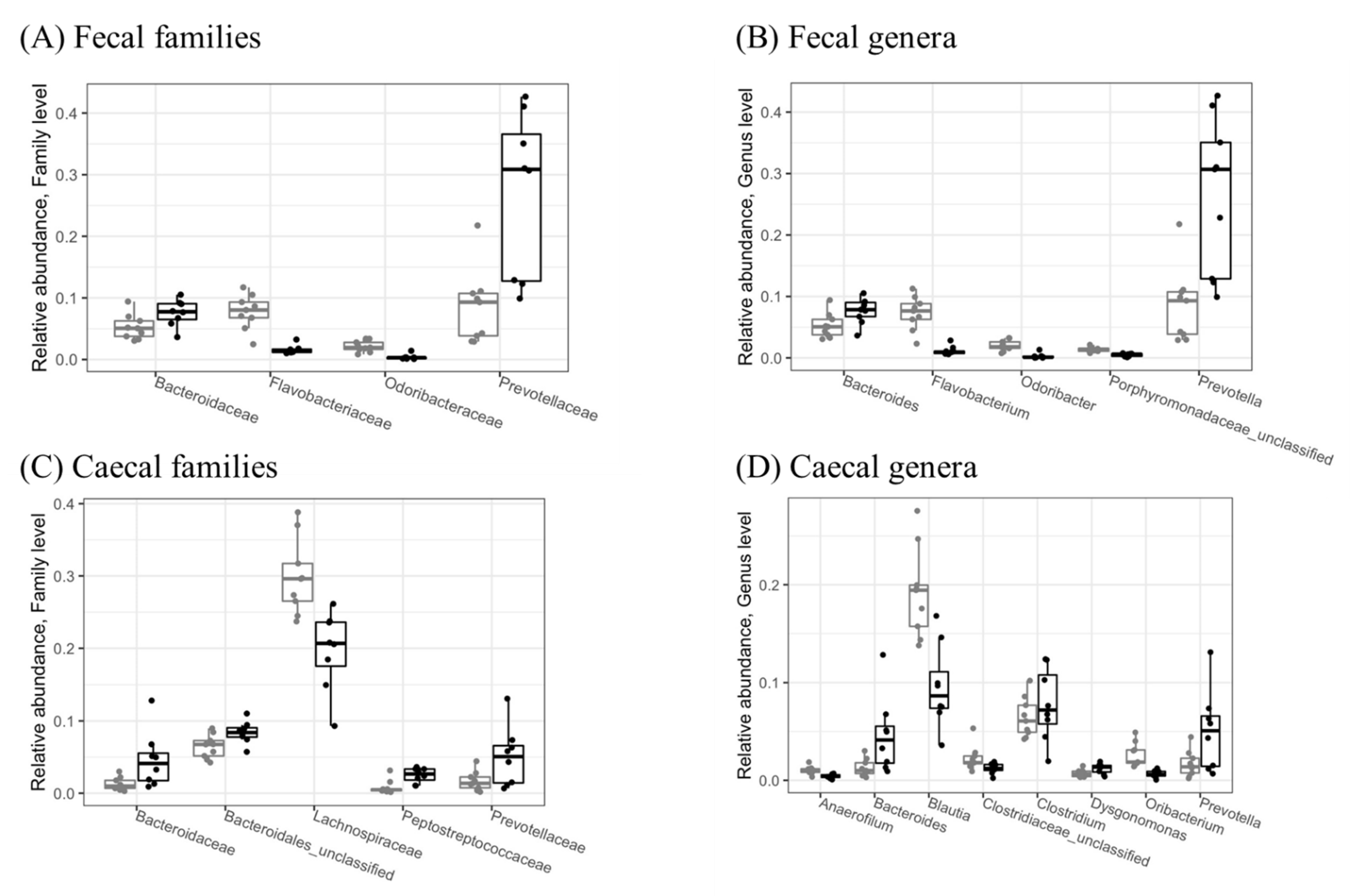
At the family level, d-fagomine intake significantly reduced Flavobacteriaceae and Odoribacteraceae while increasing Bacteroidaceae and Prevotellaceae in feces (Figure 2A). These last two families, as well as Peptostreptococcaceae, were also increased in cecal content (Figure 2C), while Lachnospiraceae was significantly (p < 0.05) reduced.
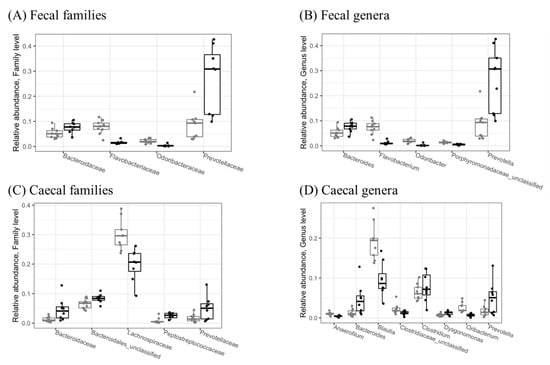
Figure 2.
Relative abundances of significantly different (p < 0.05) microbial families (A,C) and genera (B,D) in fecal (A,B) and cecal (C,D) samples collected from the STD (grey) and FG (black) groups of rats (n = 8–9) at the end of the study (week 23). Only taxa with relative abundance > 1% are shown. Statistical significance was evaluated using the Wald test in DESeq2, version 1.30.1.
At the genus level, d-fagomine supplementation significantly (p < 0.05) increased Bacteroides and Prevotella while reducing Flavobacterium and Odoribacter in feces (Figure 2B). The increased genera in cecal content were also Bacteroides and Prevotella, together with Clostridium and Dysgonomonas (Figure 2D), while the genera Anaerofilum, Blautia and Oribacterium were significantly reduced by d-fagomine intake.
3.4. α- and β-Diversities of the Fecal and Cecal Microbiota
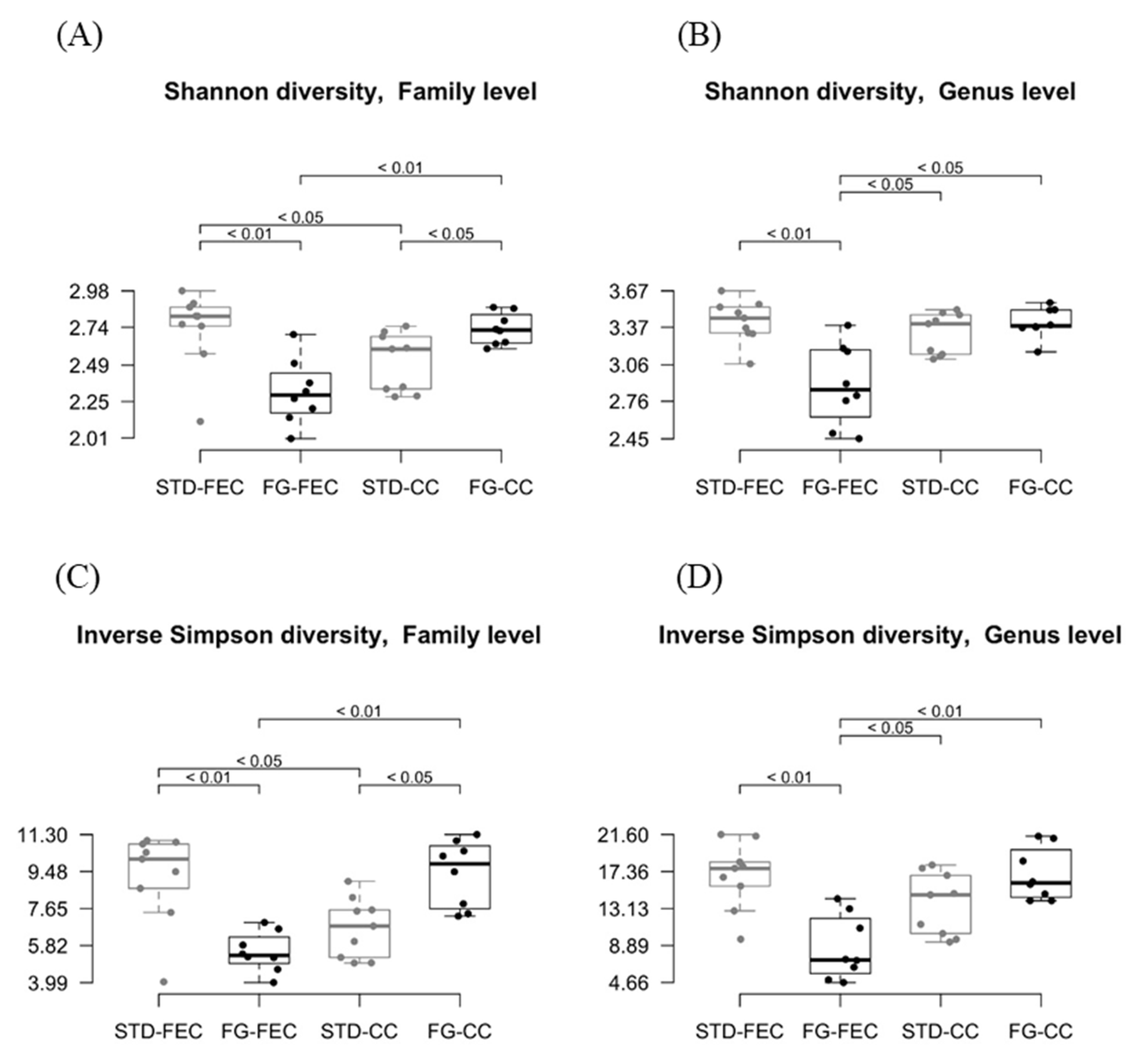
The α-diversity was similar in the two groups at the phylum level. The Shannon index indicated a reduction (p < 0.05 at the class and order levels, p < 0.01 at the family and genus levels; Figure 3A,B) of the α-diversity in fecal samples from the FG group, while α-diversity was increased in cecal samples (p < 0.05 at the family level; Figure 3A). Similar results were obtained using the inverse Simpson index (Figure 3C,D).
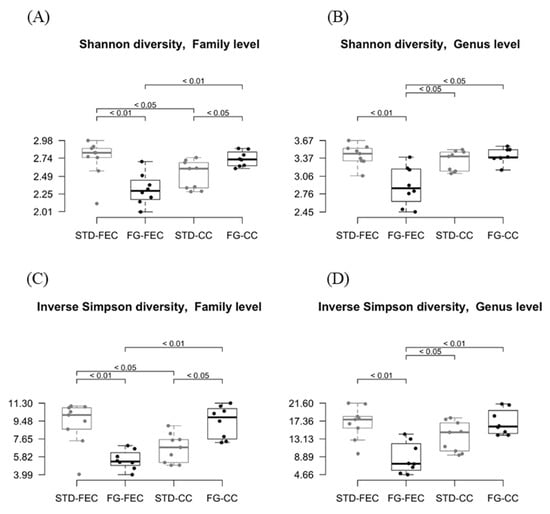
Figure 3.
Estimates of Shannon (A,B) and inverse Simpson (C,D) α-diversities of fecal and cecal microbiota at family (A,C) and genus (B,D) levels in the STD (grey) and FG (black) groups of rats (n = 8–9/group) at the end of the study (week 23). Statistical significance was assessed using the Wilcox test.
In fecal samples, β-diversity was similar at the phylum, class, and order levels (Table 2). The weighted UniFrac distance was significantly (p < 0.05) different at the family, genus, and species levels between controls and animals supplemented with d-fagomine. In cecal content, the weighted UniFrac distance was significantly (p < 0.05) different between the STD and FG groups at all the taxonomical levels.

Table 2.
β-diversity of the fecal and cecal microbiota in the STD and FG groups of rats (n = 8–9/group) at the end of the study (week 23).
3.5. Plasma and Fecal Acetic Acid
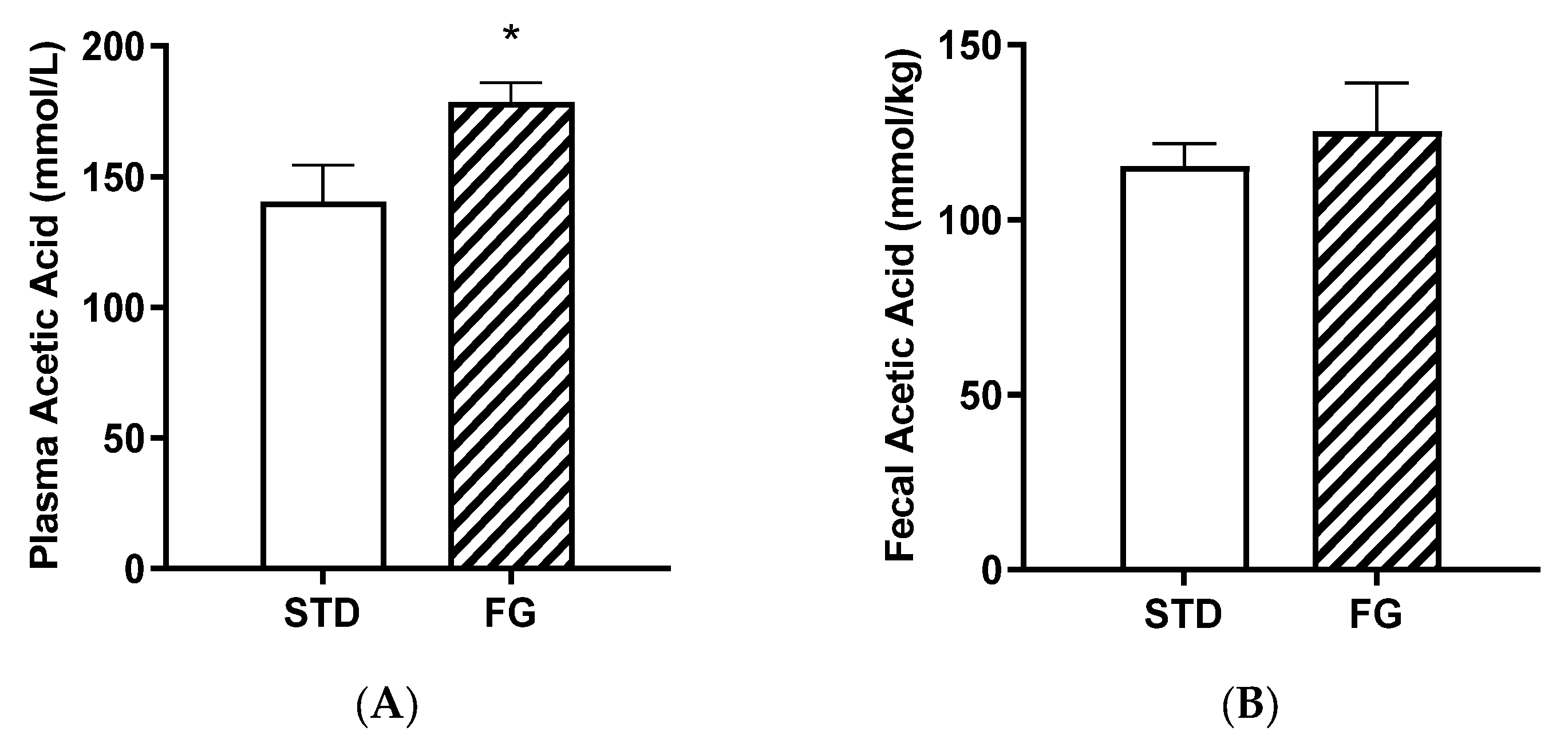
d-Fagomine supplementation (FG group) increased the plasma acetate content with respect to the STD group (p < 0.05, Figure 4A), while the differences were not significant in feces (Figure 4B).
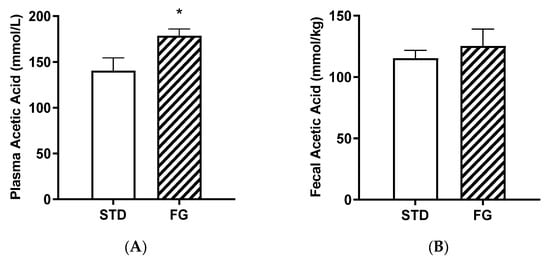
Figure 4.
Acetic acid concentrations in plasma (A) and feces (B) from rats (n = 8–9) supplemented or not with d-fagomine for 6 months. Comparisons were conducted using Student’s t-test. * p < 0.05.
3.6. Biomarkers of Inflammation
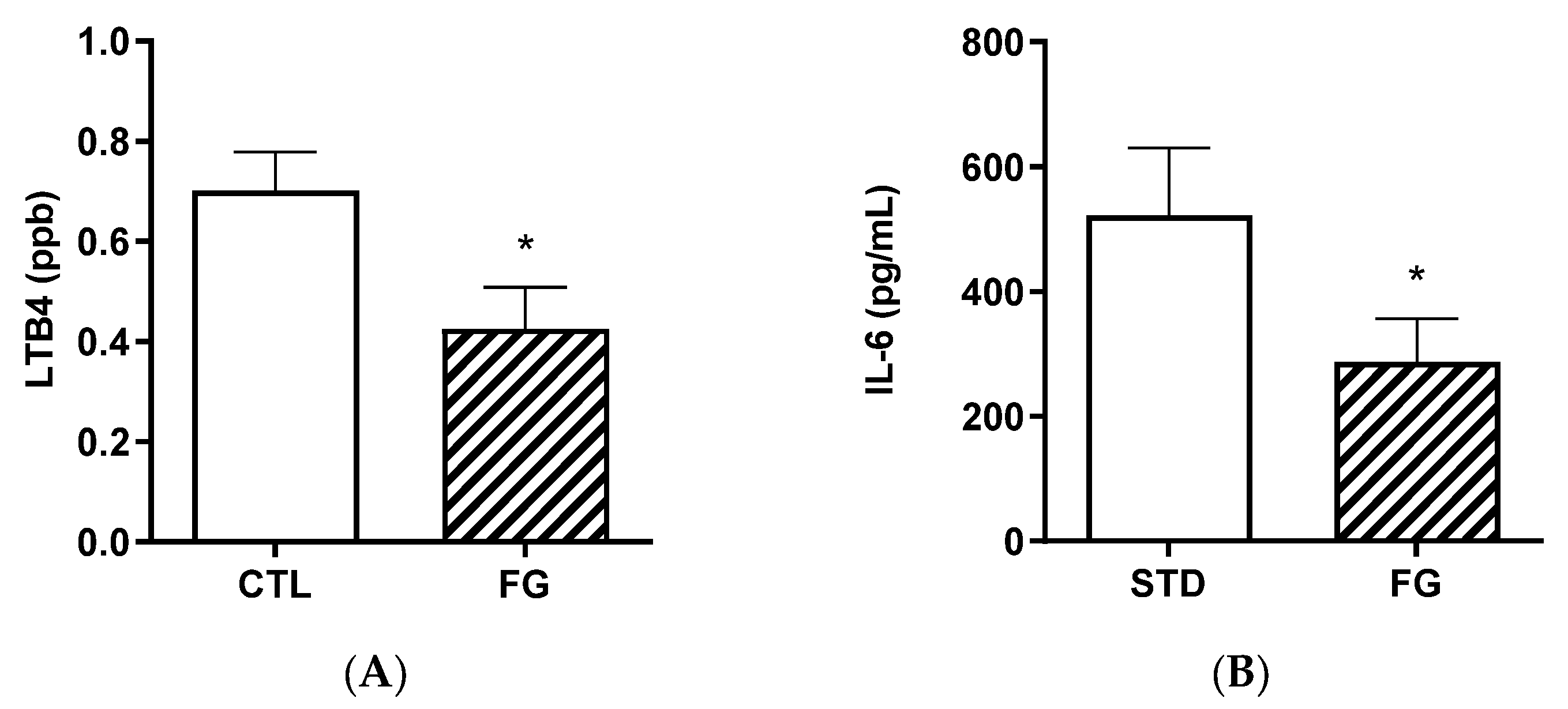
Plasma LTB4 and IL-6 were significantly (p < 0.05) lower in animals after 6 months of supplementation with d-fagomine with respect to those not supplemented (Figure 5).
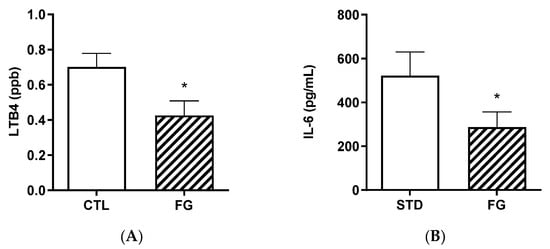
Figure 5.
Biomarkers of inflammation, LTB4 (A) and IL-6 (B) in plasma from rats (n = 8–9) supplemented or not with d-fagomine for 6 months. Comparisons were conducted using Student’s t-test. * p < 0.05.
Furthermore, the plasma concentration of 12HETE was significantly (p < 0.05) reduced in supplemented animals (FG group), while the other ARA-derived pro-inflammatory hydroxyeicosatetraenoic acids were not modified by d-fagomine supplementation (Table 3).

Table 3.
Lipid mediators derived from arachidonic acid from rats (n = 8–9) supplemented or not with d-fagomine for 6 months.
4. Discussion
In agreement with our previous observations [9], d-fagomine supplementation had a reducing effect on body weight gain in healthy rats after just 1.5 months (Figure 1A). Previous studies also showed that d-fagomine reduced body weight gain in rats fed energy-dense diets [6,7,14]. Therefore, body weight control is a consistent effect of d-fagomine in both normoweight and overweight animals. To explain the reduction in body weight, we first focused on gut Enterobacteriales, as this group had previously been associated with weight gain and inflammation [6,15]. We later found that d-fagomine had a more pronounced influence on other microbial orders, such as Prevotellaceae, Lactobacilliaceae, and Bifidobacteriaceae [8,9]. So, to better assess the changes induced by d-fagomine at various taxonomical levels, here we address the study of the gut genome by 16S rRNA gene sequencing techniques.
Our results from the present study show that d-fagomine induces changes in gut microbiota at different taxonomical levels. Some changes are consistent at the levels of class (increased populations of Bacteroidia, reduced populations of Flavobacteriia), order (increased Bacteroidales, reduced Flavobacteriales), family (increased Bacteroidaceae and Prevotellaceae, reduced Flavobacteriaceae) and genus (increased Bacteroides and Prevotella, reduced Flavobacterium and Odoribacter) in feces. At the genus level, Bacteroides and Prevotella also increased in cecal content (Figure 2D). This result is particularly interesting because cecal content might be a more faithful indicator than feces of effective interactions with the intestinal mucosa. As collecting feces is much easier than obtaining samples from intestinal mucus or epithelial tissue, most of the relations between microbiota and health in humans have been established by studying fecal populations and physiologically relevant variables [16]. While the examination of fecal microbiota has evident diagnostic and clinical significance, cecal content might provide more physiologically relevant information. Significant differences in microbial populations within the mucosal and luminal niches have been reported in healthy humans [17]. In mice, fecal and cecal microbiota profiles are also clustered in different ways, so the fecal microbiome is not necessarily representative of the population actually in contact with the mucosa along the whole of the intestinal tract [18,19]. In our study, the fact that increased populations in both feces and cecal content were only observed for Bacteroides and Prevotella indicates that the most dramatic effect of d-fagomine within the rat intestine may involve these two genera (Figure 2B,D). Bacteroides spp. is microorganisms that are specialized in degrading dietary saccharides such as short-chain fructooligosaccharides (FOS), arabinoxylans, galactoligosacharides, and xylooligosaccharides, as well as resistant starch [20,21]. Prevotella spp., particularly the Prevotella copri complex, also exhibits saccharolytic activity, particularly against xyloglucan [22]. The genus Prevotella is increasingly appearing as a key mediator of the relationship between nutrition, human metabolism, and health [23]. Intriguingly, as increased Prevotella populations have been observed in individuals under conditions of both health and disease, their role in human metabolism is still controversial [23]. Whatever that might be, there is overwhelming evidence that Prevotella spp. in oral and intestinal microbiomes is more abundant in high-fiber-consuming rural populations than in high-protein and high-fat-consuming westernized populations [24,25]. Even at the species level, westernization of the diet leads to a loss of diversity, as shown by the underrepresentation of clades of the P. copri complex [26]. Since P. copri has been mechanistically linked to an improvement in glucose tolerance in mice [27], it may participate decisively in the protective effect of dietary fiber on glucose metabolism. In contradistinction, other results, also in mice, assign an effect of triggering insulin resistance to P. copri via the biosynthesis of branched-chain amino acids [28]. Previous results from our group showed that increased populations of Prevotella associated with the intake of d-fagomine did not have any significant effect on the fasting blood levels of either glucose or insulin in healthy rats [9]; indeed, such populations even appeared together with a reduction in the elevation of the Homeostatic Assessment Model for Insulin Resistance (HOMA-IR) driven by a high-fat diet in rats [29]. All these apparently contradictory results, as well as those reported by many other authors, highlight the importance of addressing the study of the whole intestinal ecosystem in relation to the host’s metabolism. A given microorganism is not intrinsically beneficial or detrimental by itself, and it can play different roles depending on its relationship with other microbial populations. So, it is important to look at other minor variations and the overall effects on the diversity of the microbial ecosystem.
Apart from the changes in Prevotellaceae, d-fagomine induced other statistically significant changes in the bacterial populations at both the family and genus levels. At the family level, Flavobacteriaceae and Odoribacteraceae were reduced in feces (Figure 2A), while Peptostreptococcaceae were increased and Lachnospiraceae reduced in cecal content (Figure 2C). These differences in population shifts between feces and cecal content emphasize the need to consider fecal populations as markers but not necessarily as being responsible for biological effects at the intestinal or systemic level. Increased populations of Flavobacteriaceae are consistent with the reduced intracellular levels of reactive oxygen species (ROS) detected in the worm Caenorhabditis elegans when treated with a prebiotic polysaccharide from the microalga Spirulina (Arthrospira) platensis {Chen, 2020 #16}. At the genus level, Clostridium and Dysgonomonas were increased by d-fagomine supplementation, while Anaerofilum, Blautia, and Oribacterium were significantly reduced in the cecal content (Figure 2D). The strongest reduction was detected in Blautia: a genus belonging to the Lachnospiraceae family. Reports on the effects of Blautia are also contradictory. As relative fecal and cecal abundances of Blautia have been related to increased serum concentrations of glucose and insulin in pigs [30], low levels of this genus may be associated with beneficial effects. In support of this possibility, elevated populations of Blautia have been recorded in mice fed a high-fat diet showing impaired glucose tolerance [31]. In humans, fecal Blautia has been inversely associated with visceral fat area [32]. In contrast, high Blautia populations have been associated with disturbances of glucose metabolism and arterial hypertension, and they strongly correlate with low consumption of resistant starch [33]. The influence of soluble prebiotic dietary fiber on the populations of Blautia and the whole Lachnospiraceae family is also controversial. Lachnospiraceae is abundant in the cecum of mice given a prebiotic oligosaccharide from lotus seed [34]. In contrast, inulin (3 g/kg/day) and Lycium barbarum polysaccharides (400 mg/kg) reduced Blautia and Desulfovibrio while increasing Bifidobacterium, Lactobacillus, and Alistipes in diabetic Sprague-Dawley rats fed a high-fat diet and injected with streptozotocin (STZ) [35]. With some exceptions, the overall picture emerging from most of the literature suggests that good dietary habits relate to low levels of Blautia in both human and animal models.
A reduction in the populations of the genus Oribacterium in the animals given d-fagomine contributed to the reduction recorded for the Lachnospiraceae family (Figure 2) in the cecum. Very little is known about the role of Oribacterium in the intestinal ecosystem. Our results suggest that closer attention should be paid to the contribution of this genus to the effects of Lachnospiraceae.
d-Fagomine also triggers a small increase in the genus Dysgonomonas (Figure 2D). Dysgonomonas forms a phylogenetic cluster within the Bacteroides-Prevotella-Porphyromonas group [36]. Again, there is very limited information on the effects of intestinal Dysgonomonas on the host physiology apart from the observation that it may be involved in SCFA generation in rats [37]. In vitro fermentation studies suggest that the proliferation of Dysgonomonas is fostered by prebiotics such as alginate (arabinoxylans) oligosaccharides [38]. Dysgonomonas may reveal itself to be an important player in the maintenance of balanced gut microbiota as it expresses esterases that facilitate the utilization of prebiotics such as xylan polysaccharides by removing acetyl and feruloyl groups [39].
4.1. Systemic Acetate and Inflammatory Markers
The strong effect of d-fagomine on the populations of fiber-processing Prevotella and Bacteroides agrees with the increased blood acetate levels observed in our study (Figure 5). Acetate is the most abundant bacterial metabolite both in the intestinal tract and systemically, in humans and also in animal models [40,41], and it influences body weight and insulin sensitivity in rats [42]. Prevotella is emerging as a key player in the conversion of soluble dietary fiber into SCFAs, particularly acetate and butyrate [43]. A fiber-like effect of d-fagomine is consistent with the increased blood acetate concentration and the effect of lowering body weight in Sprague Dawley rats reported here (Figure 4 and Figure 1A, respectively). This is also consistent with the lowering of the biomarkers of low-grade inflammation (IL-6, leucotriene LTB4, and hydroxyeicosatetraenoic acid 12-HETE) summarized in Figure 5 and Table 3. Reduced blood levels of IL-6 have been associated with the intake of dietary fiber in humans [44] and rats [45]. LTB4 is a chemotactic lipid mediator of inflammation generated from arachidonic acid (ARA) during the early phase of inflammation [46]. HETEs are also early mediators of inflammation derived along the ARA pathway [46]. The differential effect of d-fagomine on hydroxyeicosatetraenoic acids (Table 3) may help to elucidate the physiological role of 12-HETE, which remains unclear [47]. Variations in other genera, such as Blautia and Dysgonomonas, might contribute to these changes. Our results concerning inflammatory mediators show that the action of d-fagomine includes the prevention of age-related low-level systemic inflammation, which is consistent with its fiber-like effect on the intestinal microbiota. The results are also consistent with the effect of d-fagomine on body weight gain, as fat accumulation has been related to the low-level inflammation triggered by diet- and aging-related moderate dysbiosis [48].
4.2. Diversity
d-Fagomine increased α-diversity (Shannon and inverse Simpson indexes, Figure 3) at the family level in the cecum while it reduced diversity in feces. As microbial α-diversity is positively associated with fiber intake and the metabolic health of the host [49], the effect of d-fagomine, which lowers weight gain, may, at least in part, be attributed to the promotion of diversity in the cecum. The differences in cecal contents between treated and non-treated rats were not significant at the genus level (Figure 3B,D). The reduced diversity in feces after d-fagomine intake may reflect the threefold increase in Prevotellaceae and particularly Prevotella (Figure 2A,B). It should be noted that both the Shannon and inverse Simpson indexes measure not only the total number of taxonomic units but also the relative size of their populations (evenness) [50]. In the cecum, the increase in Prevotellaceae is lower and combined with a reduction in Lachnospiraceae and in its constituting genera Blautia and Oribacterium (Figure 2C,D). The overall result is increased α-diversity. Then the reduction in α-diversity in feces may be the direct consequence of the physiological regulation of intestinal eubiosys as a response to an elevated increase in Prevotellaceae, resulting in an elevated excretion of microorganisms pertaining to this taxon.
The comparative variation of species between two ecological niches or the variation between two-time points within the same zone is known as β-diversity. In our study, β-diversity, as measured by the weighted UniFrac distance (containing information on both phylogenetic distance and abundance), was different at the family, genus, and species levels between controls and the animals supplemented with d-fagomine in both feces and cecal content (Table 2). This means that the changes induced by d-fagomine supplementation in particular subgroups of microbial populations discussed above translate to significant variations when considering the whole ecosystem. The increase in α- and β-diversities induced in the cecum indicates that d-fagomine may help to reinstate the intestinal microbiota of city-dwelling populations feeding on fiber-poor westernized diets to a more diverse condition associated with rural life. Since it is supplemented in a proportion as low as 0.1%, d-fagomine might help to bolster the benefits of dietary fiber by promoting the proliferation of fiber-processing bacteria. These fiber-like effects of d-fagomine are evidenced at doses around 100-fold lower than those used for soluble dietary fiber (e.g., fructo oligosaccharides, inulin) in rats and mice [51]. This suggests that d-fagomine intake might result in a reduced percentage of ingested dietary fiber, still producing beneficial effects on gut microbiota, particularly an increase in Prevotella and Bacteroides spp. Therefore, d-fagomine might help to make healthy food more palatable by reinforcing the action of dietary fiber.
5. Conclusions
In conclusion, our results show that d-fagomine exerts a fiber-like effect on the gut microbiota of healthy rats by increasing the populations of Prevotellaceae (mainly Prevotella) and Bacteroidaceae (mainly Bacteroides) as well as by reducing Lachnospiraceae (Blautia and Oribacterium). This fiber-like effect is compatible with increased levels of blood acetate, reduced levels of age-related inflammatory markers, and reduced body weight gain.
Author Contributions
S.R.-R. and J.L.T. conceived and designed the research. J.P. analyzed the sequencing data by bioinformatics. S.A. and B.M.-P. performed the gas chromatography experiments. L.M. and I.M. performed the LC-MS/MS experiments. M.H., S.R.-R., and M.R. conducted the in vivo experiments. S.R.-R. and J.L.T. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Spanish Ministry of Economy, Industry, and Competitiveness (grant numbers AGL2017-83599-R, PID2020-117009RB-I00). We acknowledge the support of the CRG by the MEIC to the EMBL partnership, Centro de Excelencia Severo Ochoa, and CERCA Programme/Generalitat de Catalunya.
Institutional Review Board Statement
All the procedures strictly adhered to the European Union guidelines for the care and management of laboratory animals under license from the Catalan regional authorities (reference no. DAAM7921) and approved by the Spanish CSIC Subcommittee of Bioethical Issues.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Koyama, M.; Sakamura, S. Structure of a new piperidine derivative from buckwheat seeds (Fagopyrum esculentum moench). Agric. Biol. Chem. 1974, 38, 1111–1112. [Google Scholar] [CrossRef]
- Asano, N.; Kato, A.; Miyauchi, M.; Kizu, H.; Tomimori, T.; Matsui, K.; Nash, R.J.; Molyneux, R.J. Specific α-galactosidase inhibitors, N-methylcalystegines structure/activity relationships of calystegines from Lycium Chinense. Eur. J. Biochem. 1997, 248, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Asano, N.; Yamashita, T.; Yasuda, K.; Ikeda, K.; Kizu, H.; Kameda, Y.; Kato, A.; Nash, R.J.; Lee, H.S.; Ryu, K.S. Polyhydroxylated alkaloids isolated from mulberry trees (Morus alba L.) and silkworms (Bombyx mori L.). J. Agric. Food Chem. 2001, 49, 4208–4213. [Google Scholar] [CrossRef] [PubMed]
- Amézqueta, S.; Galán, E.; Vila-Fernández, I.; Pumarola, S.; Carrascal, M.; Abian, J.; Ribas-Barba, L.; Serra-Majem, L.; Torres, J.L. The presence of D-fagomine in the human diet from buckwheat-based foodstuffs. Food Chem. 2013, 136, 1316–1321. [Google Scholar] [CrossRef]
- Gómez, L.; Molinar-Toribio, E.; Calvo-Torras, M.Á.; Adelantado, C.; Juan, M.E.; Planas, J.M.; Cañas, X.; Lozano, C.; Pumarola, S.; Clapés, P.; et al. D-Fagomine lowers postprandial blood glucose and modulates bacterial adhesion. Br. J. Nutr. 2012, 107, 1739–1746. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Molinar-Toribio, E.; Gómez, L.; Pérez-Jiménez, J.; Casado, M.; Clapés, P.; Piña, B.; Torres, J.L. Effect of D-fagomine on excreted enterobacteria and weight gain in rats fed a high-fat high-sucrose diet. Obesity 2014, 22, 976–979. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Hereu, M.; Atienza, L.; Casas, J.; Taltavull, N.; Romeu, M.; Amézqueta, S.; Dasilva, G.; Medina, I.; Torres, J.L. Functional effects of the buckwheat iminosugar D-fagomine on rats with diet-induced prediabetes. Mol. Nutr. Food Res. 2018, 62, e1800373. [Google Scholar] [CrossRef]
- Hereu, M.; Ramos-Romero, S.; García-González, N.; Amézqueta, S.; Torres, J.L. Eubiotic effect of buckwheat D-Fagomine in healthy rats. J. Funct. Foods 2018, 50, 120–126. [Google Scholar] [CrossRef]
- Hereu, M.; Ramos-Romero, S.; Marín-Valls, R.; Amézqueta, S.; Miralles-Pérez, B.; Romeu, M.; Méndez, L.; Medina, I.; Torres, J.L. Combined buckwheat D-fagomine and fish omega-3 PUFAs stabilize the populations of gut Prevotella and Bacteroides while reducing weight gain in rats. Nutrients 2019, 11, 2606. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Schwiertz, A.; Taras, D.; Schafer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Dasilva, G.; Pazos, M.; Gallardo, J.M.; Rodríguez, I.; Cela, R.; Medina, I. Lipidomic analysis of polyunsaturated fatty acids and their oxygenated metabolites in plasma by solid-phase extraction followed by LC-MS. Anal. Bioanal. Chem. 2014, 406, 2827–2839. [Google Scholar] [CrossRef] [PubMed]
- Molinar-Toribio, E.; Pérez-Jiménez, J.; Ramos-Romero, S.; Gómez, L.; Taltavull, N.; Nogués, M.R.; Adeva, A.; Jáuregui, O.; Joglar, J.; Clapés, P.; et al. D-Fagomine attenuates metabolic alterations induced by a high-energy-dense diet in rats. Food Funct. 2015, 6, 2614–2619. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Chassaing, B.; Singh, V.; Pellizzon, M.; Ricci, M.; Fythe, M.D.; Kumar, M.V.; Gewirtz, A.T. Fiber-mediated nourishment of gut microbiota protects against diet-induced obesity by restoring IL-22-mediated colonic health. Cell Host Microbe 2018, 23, 41–53.e4. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Tremaroli, V.; Backhed, F. Linking microbiota to human diseases: A systems biology perspective. Trends Endocrinol. Metab. 2015, 26, 758–770. [Google Scholar] [CrossRef]
- Ringel, Y.; Maharshak, N.; Ringel-Kulka, T.; Wolber, E.A.; Sartor, R.B.; Carroll, I.M. High throughput sequencing reveals distinct microbial populations within the mucosal and luminal niches in healthy individuals. Gut Microbes 2015, 6, 173–181. [Google Scholar] [CrossRef]
- Lkhagva, E.; Chung, H.-J.; Hong, J.; Tang, W.H.W.; Lee, S.-I.; Hong, S.-T.; Lee, S. The regional diversity of gut microbiome along the GI tract of male C57BL/6 mice. BMC Microbiol. 2021, 21. [Google Scholar] [CrossRef]
- Pang, W.; Vogensen, F.K.; Nielsen, D.S.; Hansen, A.K. Faecal and caecal microbiota profiles of mice do not cluster in the same way. Lab. Anim. 2012, 46, 231–236. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Schwalm, N.D.; Groisman, E.A. Navigating the gut buffet: Control of polysaccharide utilization in Bacteroides spp. Trends Microbiol. 2017, 25, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Fehlner-Peach, H.; Magnabosco, C.; Raghavan, V.; Scher, J.U.; Tett, A.; Cox, L.M.; Gottsegen, C.; Watters, A.; Wiltshire-Gordon, J.D.; Segata, N.; et al. Distinct polysaccharide utilization profiles of human intestinal Prevotella copri isolates. Cell Host Microbe 2019, 26, 680–690.e5. [Google Scholar] [CrossRef] [PubMed]
- Tett, A.; Pasolli, E.; Masetti, G.; Ercolini, D.; Segata, N. Prevotella diversity, niches and interactions with the human host. Nat. Rev. Microbiol. 2021, 19, 585–599. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Tett, A.; Huang, K.D.; Asnicar, F.; Fehlner-Peach, H.; Pasolli, E.; Karcher, N.; Armanini, F.; Manghi, P.; Bonham, K.; Zolfo, M.; et al. The Prevotella copri complex comprises four distinct clades underrepresented in westernized populations. Cell Host Microbe 2019, 26, 666–679.e7. [Google Scholar] [CrossRef]
- Kovatcheva-Datchary, P.; Nilsson, A.; Akrami, R.; Lee, Y.S.; De Vadder, F.; Arora, T.; Hallen, A.; Martens, E.; Björck, I.; Bäckhed, F. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab. 2015, 22, 971–982. [Google Scholar] [CrossRef]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.H.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376. [Google Scholar] [CrossRef]
- Hereu, M.; Ramos-Romero, S.; Busquets, C.; Atienza, L.; Amézqueta, S.; Miralles-Pérez, B.; Nogués, M.R.; Méndez, L.; Medina, I.; Torres, J.L. Effects of combined D-fagomine and omega-3 PUFAs on gut microbiota subpopulations and diabetes risk factors in rats fed a high-fat diet. Sci. Rep. 2019, 9, 16628. [Google Scholar] [CrossRef]
- Panasevich, M.R.; Wankhade, U.D.; Chintapalli, S.V.; Shankar, K.; Rector, R.S. Cecal versus fecal microbiota in Ossabaw swine and implications for obesity. Physiol Genom. 2018, 50, 355–368. [Google Scholar] [CrossRef]
- Yuan, X.; Zheng, J.; Ren, L.; Jiao, S.; Feng, C.; Du, Y.; Liu, H. Glucosamine Ameliorates Symptoms of High-Fat Diet-Fed Mice by Reversing Imbalanced Gut Microbiota. Front. Pharmacol. 2021, 12, 694107. [Google Scholar] [CrossRef] [PubMed]
- Ozato, N.; Saito, S.; Yamaguchi, T.; Katashima, M.; Tokuda, I.; Sawada, K.; Katsuragi, Y.; Kakuta, M.; Imoto, S.; Ihara, K.; et al. Blautia genus associated with visceral fat accumulation in adults 20–76 years of age. NPJ Biofilms Microbiomes 2019, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Kashtanova, D.A.; Tkacheva, O.N.; Doudinskaya, E.N.; Strazhesko, I.D.; Kotovskaya, Y.V.; Popenko, A.S.; Tyakht, A.V.; Alexeev, D.G. Gut microbiota in patients with different metabolic statuses: Moscow study. Microorganisms 2018, 6, 98. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Chen, J.; Miao, S.; Deng, K.; Liu, J.; Zeng, S.; Zheng, B.; Lu, X. Lotus seed oligosaccharides at various dosages with prebiotic activity regulate gut microbiota and relieve constipation in mice. Food Chem. Toxicol. 2019, 134, 110838. [Google Scholar] [CrossRef]
- Lu, H.; Liu, P.; Zhang, X.; Bao, T.; Wang, T.; Guo, L.; Li, Y.; Dong, X.; Li, X.; Dong, Y.; et al. Inulin and Lycium barbarum polysaccharides ameliorate diabetes by enhancing gut barrier via modulating gut microbiota and activating gut mucosal TLR2+ intraepithelial γδ T cells in rats. J. Funct. Foods 2021, 79, 104407. [Google Scholar] [CrossRef]
- Lawson, P.A.; Falsen, E.; Inganas, E.; Weyant, R.S.; Collins, M.D. Dysgonomonas mossii sp nov., from human sources. Syst. Appl. Microbiol. 2002, 25, 194–197. [Google Scholar] [CrossRef]
- Martinez-Oca, P.; Robles-Vera, I.; Sanchez-Roncero, A.; Escriva, F.; Perez-Vizcaino, F.; Duarte, J.; Alvarez, C.; Fernandez-Millan, E. Gut DYSBIOSIS and altered barrier function precedes the appearance of metabolic syndrome in a rat model of nutrient-induced catch-up growth. J. Nutr. Biochem. 2020, 81, 108383. [Google Scholar] [CrossRef]
- Han, Z.-L.; Yang, M.; Fu, X.-D.; Chen, M.; Su, Q.; Zhao, Y.-H.; Mou, H.-J. Evaluation of Prebiotic Potential of Three Marine Algae Oligosaccharides from Enzymatic Hydrolysis. Mar. Drugs 2019, 17, 173. [Google Scholar] [CrossRef]
- Kmezik, C.; Mazurkewich, S.; Meents, T.; McKee, L.S.; Idstrom, A.; Armeni, M.; Savolainen, O.; Branden, G.; Larsbrink, J. A polysaccharide utilization locus from the gut bacterium Dysgonomonas mossii encodes functionally distinct carbohydrate esterases. J. Biol. Chem. 2021, 296, 100500. [Google Scholar] [CrossRef]
- Hernandez, M.A.G.; Canfora, E.E.; Jocken, J.W.E.; Blaak, E.E. The Short-Chain Fatty Acid Acetate in Body Weight Control and Insulin Sensitivity. Nutrients 2019, 11, 1943. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Fujisawa, K.; Ito, E.; Idei, S.; Kawaguchi, N.; Kimoto, M.; Hiemori, M.; Tsuji, H. Improvement of obesity and glucose tolerance by acetate in Type 2 diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Biosci. Biotechnol. Biochem. 2007, 71, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Salamone, D.; Rivellese, A.A.; Vetrani, C. The relationship between gut microbiota, short-chain fatty acids and type 2 diabetes mellitus: The possible role of dietary fibre. Acta Diabetol. 2021, 58, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Hébert, J.R.; Li, W.; Bertone-Johnson, E.R.; Olendzki, B.; Pagoto, S.L.; Tinker, L.; Rosal, M.C.; Ockene, I.S.; Ockene, J.K.; et al. Association between dietary fiber and markers of systemic inflammation in the Women’s Health Initiative Observational Study. Nutrition 2008, 24, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Melekoglu, E.; Cetinkaya, M.A.; Kepekci-Tekkeli, S.E.; Kul, O.; Samur, G. Effects of prebiotic oligofructose-enriched inulin on gut-derived uremic toxins and disease progression in rats with adenine-induced chronic kidney disease. PLoS ONE 2021, 16, e0258145. [Google Scholar] [CrossRef]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal. Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef]
- Porro, B.; Songia, P.; Squellerio, I.; Tremoli, E.; Cavalca, V. Analysis, physiological and clinical significance of 12-HETE: A neglected platelet-derived 12-lipoxygenase product. J. Chromatogr. B 2014, 964, 26–40. [Google Scholar] [CrossRef]
- Calder, P.C.; Bosco, N.; Bourdet-Sicard, R.; Capuron, L.; Delzenne, N.; Dore, J.; Franceschi, C.; Lehtinen, M.J.; Recker, T.; Salvioli, S.; et al. Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res. Rev. 2017, 40, 95–119. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Backhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Kim, B.R.; Shin, J.; Guevarra, R.B.; Lee, J.H.; Kim, D.W.; Seol, K.H.; Lee, J.H.; Kim, H.B.; Isaacson, R.E. Deciphering diversity indices for a better understanding of microbial communities. J. Microbiol. Biotechnol. 2017, 27, 2089–2093. [Google Scholar] [CrossRef]
- Saha, D.C.; Reimer, R.A. Long-term intake of a high prebiotic fiber diet but not high protein reduces metabolic risk after a high fat challenge and uniquely alters gut microbiota and hepatic gene expression. Nutr. Res. 2014, 34, 789–796. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).