Abstract
Docosahexaenoic acid-containing lysophosphatidylcholine (DHA-LysoPC) is presented as the main transporter of DHA from blood plasma to the brain. This is related to the major facilitator superfamily domain-containing protein 2A (Mfsd2a) symporter expression in the blood–brain barrier that recognizes the various lyso-phospholipids that have choline in their polar head. In order to stabilize the DHA moiety at the sn-2 position of LysoPC, the sn-1 position was esterified by the shortest acetyl chain, creating the structural phospholipid 1-acetyl,2-docosahexaenoyl-glycerophosphocholine (AceDoPC). This small structure modification allows the maintaining of the preferential brain uptake of DHA over non-esterified DHA. Additional properties were found for AceDoPC, such as antioxidant properties, especially due to the aspirin-like acetyl moiety, as well as the capacity to generate acetylcholine in response to the phospholipase D cleavage of the polar head. Esterification of DHA within DHA-LysoPC or AceDoPC could elicit more potent neuroprotective effects against neurological diseases.
1. Introduction
Lipids are major constituents of living cells, as they are important structural components of cell membranes. Polyunsaturated fatty acids (PUFAs) are long-chain fatty acids (18 carbons or more) that contain two or more double bonds. Depending on the location of the last double bond, PUFAs are classified into families such as omega-3 (last double bond on the third carbon starting from the methyl group) and omega-6 (last double bond on the sixth carbon from the methyl group). Contrary to fatty acids that can be synthesized in the human body, some of them cannot be produced de novo and must be incorporated through diet [1]. The latter are called essential fatty acids, and they include omega-6 family precursor linoleic acid (18:2n-6) and omega-3 family precursor α-linolenic acid (LNA, 18:3n-3). By a cascade of alternating desaturase and elongase enzymatic reactions, which are common to both families, longer PUFAs are biosynthesized from their respective precursors [2]. PUFAs are mainly found esterified within glycerophospholipids present in cell membranes at the sn-1 and sn-2 positions. Glycerophospholipids are grouped by the structure of their polar head group on the sn-3 position. Their amphiphilic nature (one hydrophilic head group and two hydrophobic fatty acids) confers fluidity and selective permeability to the membranes they constitute [3,4]. They are also precursors for signaling metabolites, including eicosanoids, growth hormones, and regulators, and they participate in important physiological processes, such as anti-inflammatory or pro-inflammatory responses [5,6,7,8].
There is a specific enrichment of essential fatty acids in human tissues, notably in the retina, brain, and heart. Contrary to arachidonic acid (ArA, 20:4n-6), which is the major PUFA of human tissues, docosahexaenoic acid (DHA, 22:6n-3) is the most prominent fatty acid in the brain, where it is considered functionally essential [9,10]. DHA concentration is especially high in neurons where it facilitates development and synaptic functions [11], with a special interest in human brain evolution [12]. A proper balance between omega-6 and omega-3 supplementation during pregnancy and infancy is required for correct neural development [13,14,15]. Along with aging, a decrease in long-chain PUFA levels in the brain has been observed, especially for DHA levels [16,17,18]. These deficiencies are correlated to a cognitive decline in normal aging but might be even more detrimental in pathological aging. In Alzheimer’s disease, decreases in PUFAs, particularly in essential fatty acids such as DHA, have been observed [19,20,21]. These results hint at a possible correlation between neurodegenerative diseases and cerebral DHA deficiency.
DHA has many beneficial properties, especially for cerebral diseases such as Alzheimer’s disease, that were covered in numerous reviews [22,23,24,25,26]. These include pro-neurogenic, anti-oxidative, anti-inflammatory, and anti-apoptotic properties. These potent neuroprotective effects might be partly due to the conversion of DHA into active secondary metabolites such as protectins, including protectin DX [27], resolvins, and maresins [28]. DHA can also be transformed into N-Docosahexaenoylethanolamide, an endocannabinoid-like lipid mediator that has been named synaptamide due to its capacity to induce synaptogenesis, neurogenesis, and neurite outgrowth [29,30,31]. Due to its enrichment in double bonds, DHA can also provide fluidity to cell membranes [3,32,33]. As increasing its accretion into the brain through esterification into structured phospholipids improves cognitive functions in healthy brains [34], it might also heighten its neuroprotection against neuronal death.
Since DHA biosynthesis from the essential n-3 precursor α-linolenic acid is very low in humans, except during pregnancy [35], the accretion of dietary DHA from blood is quite crucial. An adequate brain DHA content then depends on both food intake and blood availability [36]. Since blood DHA is present in different chemical forms, especially esterified in glycerolipids [37], its transport to the brain through the highly selective blood–brain barrier is a required step. The involvement of DHA-containing lysophosphatidylcholine (DHA-LysoPC) as an efficient transporter of DHA to the brain, as well as its metabolism and potent neuroprotective effects, is discussed in this review. This review also introduces some studies focusing on the effects of DHA-containing phospholipids on models of neurological diseases.
2. Transport of DHA, Esterified in Phospholipids, to the Brain
2.1. LysoPC as a Preferential Transporter of DHA to the Brain
Early studies have shown that plasma unsaturated LysoPC bound to plasma albumin could mainly result from liver phospholipase A1 activity [38,39]. It was then hypothesized that unsaturated fatty acids (UFAs) could be available to the brain from those LysoPC and non-esterified UFAs, both being bound to plasma albumin [40]. The results clearly showed that intravenously injected albumin-bound UFAs (18:1n-9, 18:2n-6 and 20:4n-6) were 10-fold less incorporated into rat brains than UFAs esterified at the sn-2 position of LysoPC [40]. Interestingly, this preferential uptake from LysoPC was not observed for the saturated fatty acid 16:0 [40]. DHA, being an abundant and crucial PUFA in the brain, has been studied in both forms (non-esterified and esterified at the sn-2 position of LysoPC), and its uptake by the brain was also compared with other organs. The preferential uptake of DHA esterified in LysoPC was confirmed in the brain (with at least 10-fold ratio) but not in other organs such as the heart, kidney, and liver, with an even preferential uptake of non-esterified DHA in the heart and liver [41].
The preferential brain uptake of UFAs esterified in LysoPC was later confirmed and explained through the expression of the symporter major facilitator superfamily domain-containing protein 2A (Mfsd2a), almost exclusively expressed on endothelial cells of the blood–brain barrier [42]. Mfsd2a was further studied for its 3D structure and for its interactions with choline phospholipids having a long hydrophobic chain (at least 16:0), including LysoPC, Lyso-Platelet-Activating Factor (LysoPAF), and even PAF, suggesting that the short acetyl chain in PAF does not alter the transport ability of Mfsd2a [43].
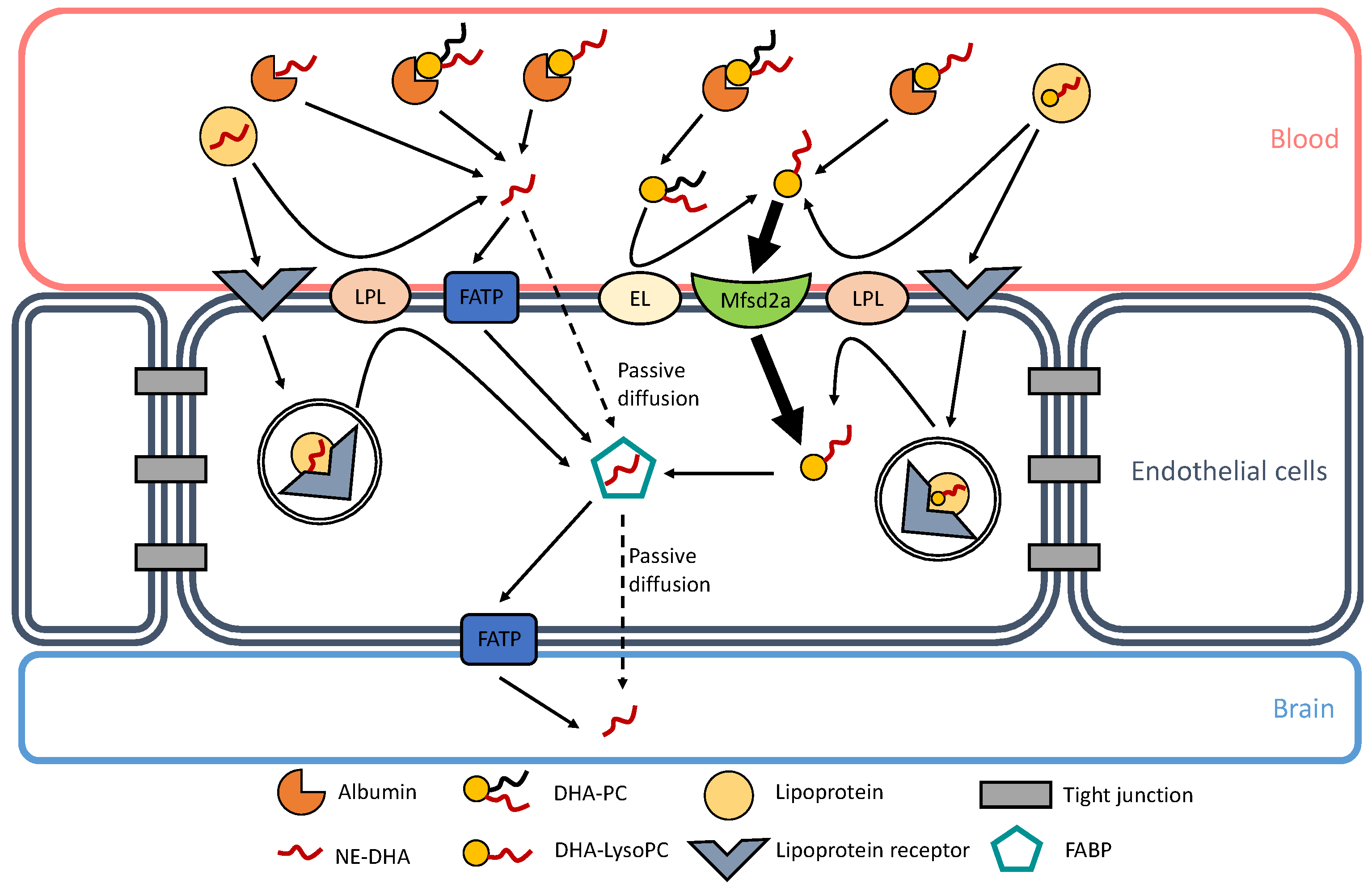
The preferential brain uptake of DHA, and other PUFAs of functional interest such as ArA, when esterified in LysoPC at the most observed physiologically sn-2 position is relevant because of a substantial amount of this lysolipid in plasma. Indeed, similar amounts of non-esterified ArA and DHA, and that esterified in LysoPC, are associated with rat plasma albumin [41]. This is also valid for human plasma, with about equal amounts of ArA-containing LysoPC and DHA-LysoPC associated with high-density and low-density lipoproteins as well [44]. Though higher brain accretion was observed with DHA esterified in phosphatidylcholines (PC) and phosphatidylserines (PS) compared to DHA-containing triacylglycerols [45,46], the highest brain uptake was shown with DHA-LysoPC [47]. Multiple mechanisms and factors can affect DHA esterification [48], such as hypercapnia/ischemia [49] and maternal obesity [50]. Currently known mechanisms of DHA transport through the BBB are represented in Figure 1.
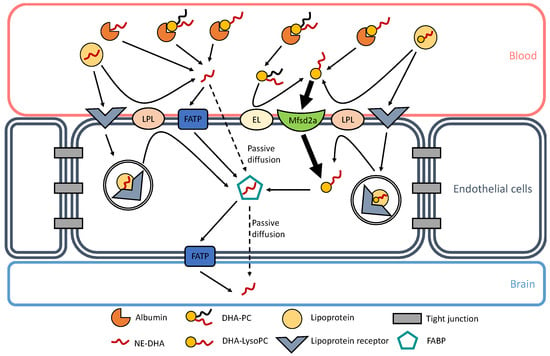
Figure 1.
Docosahexaenoic acid (DHA) transport through the blood–brain barrier (BBB). Currently known mechanisms of DHA transport are represented in this figure. In blood, albumin can bind non-esterified DHA (NE-DHA), DHA-containing lysophosphatidylcholine (DHA-LysoPC), and DHA-containing phosphatidylcholine (DHA-PC). NE-DHA is released from albumin in the vicinity of endothelial cell membranes and is incorporated into the endothelium by passive diffusion or transportation through fatty acid transport proteins (FATP). DHA-LysoPC is also released from albumin and can be actively transported into the endothelium through the symport major facilitator superfamily domain-containing protein 2A (Mfsd2a). DHA-PC can also be released from albumin and can generate DHA-LysoP C through the action of an endothelial lipase (EL), as shown by Chen and Subbaiah [51]. Lipoproteins are other carriers of NE-DHA and DHA-LysoPC. They can release NE-DHA and DHA-LysoPC through the action of lipoprotein lipases (LPL). Lipoproteins can also bind to their receptors and go through transcystosis. Inside endothelial cells, lipoproteins can be hydrolyzed and can release either NE-DHA or DHA-LysoPC. In the endothelium, NE-DHA is bound to fatty acid binding proteins (FABP) for it to cross the intercellular space to reach brain cells. NE-DHA can either diffuse passively through the endothelial-brain barrier or be transported through FATP.
In early studies comparing non-esterified PUFAs and PUFA-containing LysoPCs, the latter were sn-2-acyl-LysoPCs, to mimic the physiological situations in which LysoPCs are produced by the cleavage of 1-palmitoyl/stearoyl,2-arachidonoyl/docosahexaenoyl-GPC by phospholipase A1 or triacylglycerol lipase having a phospholipase A1 activity [38]. However, studies showing the involvement of Mfsd2a in LysoPC uptake did not consider the position of the acyl, or alkyl in the case of LysoPAF [43]. This suggests that the position isomers of the acyl moieties might not be crucial for the LysoPC uptake. Indeed, the incubation of sn-2-acyl-LysoPCs in physiological conditions leads to the migration of acyl groups from the sn-2 to the sn-1 position of LysoPCs [44,47]. This does not indicate whether one specific position isomer is required for its brain uptake, as one isomer may be converted or retro-converted into the other before being taken up. However, to maintain DHA at the supposed physiological sn-2 position of LysoPC, it was decided to stabilize it by esterifying the sn-1 position with the shortest acetyl moiety [52]. The resulting structured phospholipid, 1-acetyl,2-docosahexaenoyl-glycerophosphocholine, was named AceDoPC.
2.2. Stabilized Form of DHA-Containing LysoPC: AceDoPC
When 14C-labeled DHA in AceDoPC or 14C-labeled non-esterified DHA were intravenously injected into rats, there was no different 14C-DHA uptake by the heart and liver, but there was a significantly higher uptake of DHA in the brain from AceDoPC compared to non-esterified DHA, while both forms equally decreased from plasma [53]. Analysis of 14C-DHA-containing AceDoPC, PC, and phosphatidylethanolamine (PE) within the brain 1, 24, and 48 h after AceDoPC injection showed a progressively decreased AceDoPC level and increased labeled PC and PE levels [53]. Interestingly, 14C-LysoPC was present in small similar amounts for the three times considered, suggesting that AceDoPC was de-acetylated within the brain, and LysoPC was quickly metabolized for DHA redistribution within PC and PE [53].
An in vitro reconstituted blood–brain barrier (BBB) showed the passage of DHA esterified in AceDoPC or PC, or non-esterified DHA, across the BBB [54]. DHA crossing from AceDoPC was significantly higher than from PC, the latter being less efficient than non-esterified DHA [53]. These results are in agreement with a preferential crossing of the reconstituted BBB model by DHA-LysoPC compared to non-esterified DHA [55]. The molecular modeling of AceDoPC and DHA-LysoPC showed a very similar 3D structure [53], differing only by the acetyl moiety at the sn-1 position, and AceDoPC could be recognized by Mfsd2a as well, explaining similar preferential BBB crossing.
A recent study in human volunteers who ingested 50 mg of 13C-labeled DHA, esterified in AceDoPC or a triacylglycerol (TAG), showed that the blood bioavailability of 13C-DHA was higher from AceDoPC than from TAG [56]. In brief, around twice as much DHA accumulated in red cells from AceDoPC, and this preference was especially clear in PE after 6 days with a transient accumulation in PC after 3 days, which fits well with a long-term accumulation in brain PE [57] if extrapolated from red cells, which could be considered as biomarkers of DHA accumulation in the brain [58].
3. Neuroprotective Properties of DHA-Containing Phospholipids
3.1. DHA-Containing Phospholipid for the Treatment of Alzheimer’s Disease
Amyloid beta (Aβ) induced neurotoxicity can lead to the elevation of oxidative stress in the brain. In an in vitro model of Aβ1-42 neurotoxicity, primary neurons treated with PC from eggs showed less neuronal death with a reduced lactate dehydrogenase release [59]. In a rat model injected with Aβ1-40, diets enriched with DHA-containing PC (DHA-PC) or PS (DHA-PS) could increase the antioxidative enzyme superoxide dismutase (SOD) level and could reduce lipid peroxidation, inflammatory, and apoptotic levels, alongside improving spatial learning cognitive functions [60]. An increase of glutathione peroxidase (GSH-Px) and SOD activities with the improvement of cognitive deficits have also been shown in Aβ25-35-induced Alzheimer’s disease rat models treated for 30 days with DHA-PC [61]. In humans, a prospective follow-up study showed that subjects with baseline plasma DHA-PC levels in the upper quartile had 39% and 47% lower risks of developing Alzheimer disease and all-cause dementia, respectively, compared with participants with levels in the lower 3 quartiles [62].
In a study of senescence-accelerated prone 8 (SAMP8), mice were fed with a high-fat diet, as a model of Alzheimer’s disease, or with a diet enriched with DHA-PC or DHA-PS, which both increased the activity of antioxidative enzymes GSH-Px and SOD while decreasing malondialdehyde, a marker of lipid peroxidation [63]. The mice also showed enhanced cognitive performances, improved neuroprotection through decreased neuroinflammation and apoptosis, and amelioration in Aβ pathology.
The observed improvement of brain health and cognitive functions in the pathology of Alzheimer’s disease could be due not only to the neuroprotective effects of DHA (anti-inflammatory, anti-oxidative, and anti-apoptotic) but also to the beneficial transport of DHA through the BBB, increasing its bioavailability in neural cells. Another working hypothesis is that DHA can also prevent the accumulation of Aβ peptides [22,64,65,66] and the formation of fibrils [67,68,69], thus decreasing the apoptotic effects of oligomers. DHA is suggested to act on multiple pleiotropic mechanisms, leading to beneficial effects on the pathology of Alzheimer’s disease [21,23]. An additional hypothesis is that the choline moiety in the polar head of PC is crucial for neuroprotection, as Ko M. et al. reported that PC but not PS was able to protect against Aβ-induced cell toxicity [59]. It may be speculated that this choline moiety might contribute to acetylcholine production, resulting from PC hydrolysis by phospholipase D, with the resulting choline being further converted by endogenous acetyl-CoA.
3.2. Potential Therapy to Other Neurological Diseases
Mice treated with 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine (MPTP) to mimic oxidative damage induced by the pathology of Parkinson’s disease were fed with DHA- and eicosapentaenoic acid (EPA)-containing phospholipids, which were extracted from squid roe and contained mainly DHA-PC, DHA-containing PE (DHA-PE), and DHA-LysoPC [70]. Compared to the control group (only treated with MPTP), mice fed with DHA/EPA-PC had increased levels of antioxidative enzymes (GSH-Px and SOD) along with a reduction of motor impairments and a decrease of pro-apoptotic markers. Further study on the same model showed that a DHA-PC enriched diet could elevate activities of glutathione and SOD, alleviate the loss of dopaminergic neurons following MPTP treatment (notably through the reduction of pro-apoptotic markers), and dampen cognitive impairments in locomotor activity [71]. Parkinson’s disease is mainly characterized by the abnormal aggregation of α-synuclein protein forming Lewy bodies, an imbalance in the levels of reactive oxygen species, and the loss of dopaminergic neurons. Omega-3 fatty acids, and particularly DHA, can interact with α-synuclein to prevent its detrimental oligomerization [72,73,74]. Another pathway of action of DHA is the modulation of dopamine-induced neurodegeneration [75,76] and the enhancement of anti-oxidative pathways [76].
In a model of dementia induced by short-term memory and learning impairment by treatment with scopolamine, mice fed with squid PC (enriched in DHA) performed better in a spatial-learning memory test and had increased antioxidative activity and a lower lipid peroxidation level compared to the control group [77]. Interestingly, elevated levels of acetylcholinesterase activity induced by scopolamine injection were reduced with squid PC treatment. In a following study by the same research group, it was shown that a DHA-deficient diet could lead to further damage due to scopolamine treatment through oxidative stress, apoptosis, inflammation, and delayed neurodevelopment [78], hinting at possible preventive therapy through a balanced omega-3 diet.
The potential use of DHA’s beneficial properties on neuropsychiatric disorders is also currently under study [79]. Through its potency to reduce anti-inflammation and to promote neurogenesis, DHA was shown to reduce inflammatory markers in both in vitro and clinical studies [80]. The authors found correlations between higher levels of anti-inflammatory markers linked to DHA and lower levels of depressive symptoms. Similarly, in an in vivo study of forced swimming tests on rats, a decrease of inflammatory cytokines and an increase in serotonin levels were observed with omega-3 supplementation, suggesting anti-depressant effects of DHA [81]. Interestingly, dietary supplementation of DHA-containing phospholipids in a mice model of depression rescued depression-like behavior and inhibited neuroinflammation, suggesting increased effects on depression through DHA esterification in phospholipids [82].
3.3. AceDoPC as a Potential Antioxidant and Neurogenesis Inducer
In the case of AceDoPC initially, sn2-DHA-LysoPC was acetylated at the sn-1 position to prevent the migration of DHA from the sn-2 position as discussed above [52], but it appears that such an acetylation also confers some antioxidant activities to AceDoPC compared to non-esterified DHA. This was observed in an experimental stroke with a more significant lower size of post-stroke lesions and decreased oxidative stress after AceDoPC intravenous injection [83]. In an in vitro model of stroke on adult neural stem cells, strong antioxidant actions of AceDoPC could be seen on prostanoids and lipoxygenase product formation, with lipoxygenase products from ArA (leukotriene B4, LTB4, and 15-Hydroxyeicosatetraenoic acid, 15-HETE) being surprisingly more affected than prostanoids [84].
The inhibition of prostanoid formation could be explained by the inhibition of cyclooxygenases (COX), as shown in using purified COX-1 and COX-2 [85], suggesting an aspirin-like effect of the acetyl-containing AceDoPC. Beyond these effects on lipid metabolism, the treatment of AceDoPC by phospholipase D (PLD) leads to acetylcholine formation, likely by the combination of the acetyl group of the molecule with the released choline moiety due to PLD cleavage process [85]. AceDoPC also acts as an inhibitor of lipopolysaccharide-induced neuroinflammation, both in vitro and in vivo, with some specificities compared to DHA-PC [86].
In adult neural stem cells, nanomolar concentrations of AceDoPC increased neurogenesis by 2.5 fold (compared to the control) in the presence of AceDoPC, while 1.5 fold increase with non-esterified DHA was observed [84]. Enhanced neurogenesis by AceDoPC was even higher under pathological conditions (under hypoxia/ischemia-like conditions) while no effect was observed on gliogenesis. Another phospholipid that is structurally similar to AceDoPC but contains protectin DX, a metabolite of DHA, at the sn-2 position, was also produced [87]. This phospholipid, labeled AceDxPC, might enhance the beneficial effects of AceDoPC.
An additional interest of AceDoPC is due to the quick loss of its acetyl moiety [53] then releasing sn-2-DHA- LysoPC (with DHA at the sn-2 position), which is quickly isomerized into sn-1-DHA-LysoPC (with DHA at the sn-1 position) [44], a substrate for producing synaptamide [88].
4. Conclusions
Essential omega-3 fatty acids are major constituents of cell membranes. DHA is especially prominent in neuronal cells and is necessary for the healthy neurodevelopment and healthy aging of the human brain. Since DHA shares many common signaling pathways with omega-6 ArA, the balance between omega-3 and omega-6 species can determine whether tissues will be inflamed, oxidized, or apoptotic under pathological damage. This is of particular importance in the case of neurodegenerative diseases, where proper homeostasis is required for maintaining neuron survival and cognitive functions.
PUFAs are scarcely synthesized de novo from their precursors and must be largely incorporated through a balanced diet. Since BBB is a selective barrier of the nutrients passing from blood to the brain, there is a need for new strategies to efficiently transport omega-3 PUFAs to neural cells. In summary, this review points out the role of LysoPC as a preferential transporter of DHA to the brain, particularly by crossing the BBB, likely through the Mfsd2a symporter. DHA being acylated at the sn-2 position of LysoPC after the cleavage of DHA-containing PC by triacylglycerol lipase/phospholipase A1, it mainly migrates to the sn-1 position with time. Preventing such a migration by acetylating the sn-1 position led to further studies. The resulting 1-acetyl,2-docosahexaenoyl-glycerophosphocholine, AceDoPC, was then studied as a transporter of DHA to the brain. In addition to its efficiency for DHA brain uptake, AceDoPC appeared as a potential antioxidant and an acetylcholine source through its cleavage by phospholipase D.
This review also highlights the potential beneficial effects of DHA being esterified within structured phospholipids compared to its non-esterified form in the pathology of neurodegenerative diseases. Increasing the bioavailability of DHA might enhance its anti-inflammatory, anti-oxidative, and anti-apoptotic effects, but the conformation of the DHA-containing phospholipid could also exert supplementary beneficial effects on the degeneration of neuronal cells.
Author Contributions
Conceptualization, A.L.V., M.L. and N.B.-H.; Writing—original draft preparation, A.L.V., M.L. and N.B.-H.; writing—review and editing, A.L.V., M.L. and N.B.-H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the financial support from INSA Lyon, CNRS, LaMCoS, UMR5259 and I@L Carnot institute, and the Functional Lipidomics Platform (IMBL, LaMCoS).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Spector, A.A.; Kim, H.-Y. Discovery of essential fatty acids. J. Lipid Res. 2015, 56, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Leonard, A.; Pereira, S.L.; Sprecher, H.; Huang, Y.-S. Elongation of long-chain fatty acids. Prog. Lipid Res. 2004, 43, 36–54. [Google Scholar] [CrossRef]
- Stillwell, W.; Wassall, S.R. Docosahexaenoic acid: Membrane properties of a unique fatty acid. Chem. Phys. Lipids 2003, 126, 1–27. [Google Scholar] [CrossRef]
- Yang, X.; Sheng, W.; Sun, G.Y.; Lee, J.C.-M. Effects of fatty acid unsaturation numbers on membrane fluidity and α-secretase-dependent amyloid precursor protein processing. Neurochem. Int. 2011, 58, 321–329. [Google Scholar] [CrossRef]
- Lagarde, M.; Bernoud-Hubac, N.; Calzada, C.; Véricel, E.; Guichardant, M. Lipidomics of essential fatty acids and oxygenated metabolites. Mol. Nutr. Food Res. 2013, 57, 1347–1358. [Google Scholar] [CrossRef] [PubMed]
- Kuda, O. Bioactive metabolites of docosahexaenoic acid. Biochimie 2017, 136, 12–20. [Google Scholar] [CrossRef]
- Christie, W.W.; Harwood, J.L. Oxidation of polyunsaturated fatty acids to produce lipid mediators. Essays Biochem. 2020, 64, 401–421. [Google Scholar] [CrossRef]
- Das, U.N. Essential Fatty Acids and Their Metabolites in the Pathobiology of Inflammation and Its Resolution. Biomolecules 2021, 11, 1873. [Google Scholar] [CrossRef]
- Crawford, M.; Doyle, W.; Leaf, A.; Leighfield, M.; Ghebremeskel, K.; Phylactos, A. Nutrition and Neurodevelopmental Disorders. Nutr. Health 1993, 9, 81–97. [Google Scholar] [CrossRef]
- Salem, N.; Litman, B.; Kim, H.-Y.; Gawrisch, K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids 2001, 36, 945–959. [Google Scholar] [CrossRef]
- Cao, D.; Kevala, K.; Kim, J.; Moon, H.-S.; Jun, S.B.; Lovinger, D.; Kim, H.-Y. Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function. J. Neurochem. 2009, 111, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M.A.; Bloom, M.; Broadhurst, C.L.; Schmidt, W.F.; Cunnane, S.C.; Galli, C.; Gehbremeskel, K.; Linseisen, F.; Lloyd-Smith, J.; Parkington, J. Evidence for the unique function of docosahexaenoic acid during the evolution of the modern hominid brain. Lipids 1999, 34, S39–S47. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.S.; Astwood, J.D.; Gautier, S.; Kuratko, C.N.; Nelson, E.B.; Salem, N. Effects of long-chain polyunsaturated fatty acid supplementation on neurodevelopment in childhood: A review of human studies. Prostaglandins Leukot. Essent. Fat. Acids 2010, 82, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Guesnet, P.; Alessandri, J.-M. Docosahexaenoic acid (DHA) and the developing central nervous system (CNS)–Implications for dietary recommendations. Biochimie 2011, 93, 7–12. [Google Scholar] [CrossRef]
- Sakayori, N.; Kikkawa, T.; Tokuda, H.; Kiryu, E.; Yoshizaki, K.; Kawashima, H.; Yamada, T.; Arai, H.; Kang, J.X.; Katagiri, H.; et al. Maternal dietary imbalance between omega-6 and omega-3 polyunsaturated fatty acids impairs neocortical development via epoxy metabolites. Stem Cells 2016, 34, 470–482. [Google Scholar] [CrossRef]
- López, G.; de Boschero, M.I.; Castagnet, P.; Giusto, N. Age-associated changes in the content and fatty acid composition of brain glycerophospholipids. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1995, 112, 331–343. [Google Scholar] [CrossRef]
- Giusto, N.M.; Salvador, G.A.; Castagnet, P.I.; Pasquaré, S.J.; De Boschero, M.G.I. Age-associated changes in central nervous system glycerolipid composition and metabolism. Neurochem. Res. 2002, 27, 1513–1523. [Google Scholar] [CrossRef]
- Bourre, J.M. Roles of unsaturated fatty acids (especially omega-3 fatty acids) in the brain at various ages and during ageing. J. Nutr. Health Aging 2004, 8, 163–174. Available online: http://www.ncbi.nlm.nih.gov/pubmed/15129302 (accessed on 21 November 2016).
- Conquer, J.A.; Tierney, M.C.; Zecevic, J.; Bettger, W.J.; Fisher, R.H. Fatty acid analysis of blood plasma of patients with alzheimer’s disease, other types of dementia, and cognitive impairment. Lipids 2000, 35, 1305–1312. [Google Scholar] [CrossRef]
- Fabelo, N.; Martín, V.; Marín, R.; Moreno, D.; Ferrer, I.; Díaz, M. Altered lipid composition in cortical lipid rafts occurs at early stages of sporadic Alzheimer’s disease and facilitates APP/BACE1 interactions. Neurobiol. Aging 2014, 35, 1801–1812. [Google Scholar] [CrossRef]
- Mesa-Herrera, F.; Taoro-González, L.; Valdés-Baizabal, C.; Diaz, M.; Marín, R. Lipid and Lipid Raft Alteration in Aging and Neurodegenerative Diseases: A Window for the Development of New Biomarkers. Int. J. Mol. Sci. 2019, 20, 3810. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.O.; Kuchenbecker, J.; Grösgen, S.; Burg, V.K.; Hundsdörfer, B.; Rothhaar, T.L.; Friess, P.; de Wilde, M.C.; Broersen, L.M.; Penke, B.; et al. Docosahexaenoic Acid Reduces Amyloid β Production via Multiple Pleiotropic Mechanisms. J. Biol. Chem. 2011, 286, 14028–14039. [Google Scholar] [CrossRef] [PubMed]
- Belkouch, M.; Hachem, M.; Elgot, A.; Lo Van, A.; Picq, M.; Guichardant, M.; Lagarde, M.; Bernoud-Hubac, N. The pleiotropic effects of omega-3 docosahexaenoic acid on the hallmarks of Alzheimer’s disease. J. Nutr. Biochem. 2016, 38, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lo Van, A.; Sakayori, N.; Hachem, M.; Belkouch, M.; Picq, M.; Lagarde, M.; Osumi, N.; Bernoud-Hubac, N. Mechanisms of DHA transport to the brain and potential therapy to neurodegenerative diseases. Biochimie 2016, 130, 163–167. [Google Scholar] [CrossRef]
- Hachem, M.; Nacir, H. Emerging Role of Phospholipids and Lysophospholipids for Improving Brain Docosahexaenoic Acid as Potential Preventive and Therapeutic Strategies for Neurological Diseases. Int. J. Mol. Sci. 2022, 23, 3969. [Google Scholar] [CrossRef]
- Mora, I.; Arola, L.; Caimari, A.; Escoté, X.; Puiggròs, F. Structured Long-Chain Omega-3 Fatty Acids for Improvement of Cognitive Function during Aging. Int. J. Mol. Sci. 2022, 23, 3472. [Google Scholar] [CrossRef]
- Liu, M.; Boussetta, T.; Makni-Maalej, K.; Fay, M.; Driss, F.; El-Benna, J.; Lagarde, M.; Guichardant, M. Protectin DX, a Double Lipoxygenase Product of DHA, Inhibits Both ROS Production in Human Neutrophils and Cyclooxygenase Activities. Lipids 2014, 49, 49–57. [Google Scholar] [CrossRef]
- Serhan, C.N. Novel Chemical Mediators in the Resolution of Inflammation: Resolvins and Protectins. Anesthesiol. Clin. N. Am. 2006, 24, 341–364. [Google Scholar] [CrossRef]
- Kim, H.Y.; Moon, H.S.; Cao, D.; Lee, J.; Kevala, K.; Jun, S.B.; Lovinger, M.D.; Akbar, M.; Huang, B.X. N-Docosahexaenoylethanolamide promotes development of hippocampal neurons. Biochem. J. 2011, 435, 327–336. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Spector, A.A. Synaptamide, endocannabinoid-like derivative of docosahexaenoic acid with cannabinoid-independent function. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 121–125. [Google Scholar] [CrossRef]
- Tyrtyshnaia, A.; Bondar, A.; Konovalova, S.; Manzhulo, I. Synaptamide Improves Cognitive Functions and Neuronal Plasticity in Neuropathic Pain. Int. J. Mol. Sci. 2021, 22, 12779. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Hossain, S.; Shimada, T.; Shido, O. Docosahexaenoic acid-induced protective effect against impaired learning in amyloid β-Infused rats is associated with increased synaptosomal membrane fluidity. Clin. Exp. Pharmacol. Physiol. 2006, 33, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Hossain, S.; Al Mamun, A.; Matsuzaki, K.; Arai, H. Docosahexaenoic acid: One molecule diverse functions. Crit. Rev. Biotechnol. 2016, 37, 579–597. [Google Scholar] [CrossRef] [PubMed]
- Sugasini, D.; Thomas, R.; Yalagala, P.C.R.; Tai, L.M.; Subbaiah, P.V. Dietary docosahexaenoic acid (DHA) as lysophosphatidylcholine, but not as free acid, enriches brain DHA and improves memory in adult mice. Sci. Rep. 2017, 7, 11263. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.A.; Muhlhausler, B.; Makrides, M. Conversion of linoleic acid and alpha-linolenic acid to long-chain polyunsaturated fatty acids (LCPUFAs), with a focus on pregnancy, lactation and the first 2 years of life. Matern. Child Nutr. 2011, 7, 17–26. [Google Scholar] [CrossRef]
- Domenichiello, A.F.; Kitson, A.P.; Bazinet, R.P. Is docosahexaenoic acid synthesis from α-linolenic acid sufficient to supply the adult brain? Prog. Lipid Res. 2015, 59, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Brossard, N.; Croset, M.; Lecerf, J.; Pachiaudi, C.; Normand, S.; Chirouze, V.; Macovschi, O.; Riou, J.P.; Tayot, J.L.; Lagarde, M. Metabolic fate of an oral tracer dose of [13C]docosahexaenoic acid triglycerides in the rat. Am. J. Physiol. Integr. Comp. Physiol. 1996, 270, R846–R854. [Google Scholar] [CrossRef]
- Robinson, B.S.; Baisted, D.J.; Vance, D. Comparison of albumin-mediated release of lysophosphatidylcholine and lysophosphatidylethanolamine from cultured rat hepatocytes. Biochem. J. 1989, 264, 125–131. [Google Scholar] [CrossRef]
- Bentejac, M.; Bugaut, M.; Delachambre, M.C.; Lecerf, J. Utilization of High-Density Lipoprotein Sphingomyelin by the Developing and Mature Brain in the Rat. J. Neurochem. 1989, 52, 1495–1500. [Google Scholar] [CrossRef]
- Thiès, F.; Delachambre, M.C.; Bentejac, M.; Lagarde, M.; Lecerf, J. Unsaturated Fatty Acids Esterified in 2-Acyl-1-Lysophosphatidylcholine Bound to Albumin Are More Efficiently Taken up by the Young Rat Brain than the Unesterified Form. J. Neurochem. 1992, 59, 1110–1116. [Google Scholar] [CrossRef]
- Thies, F.; Pillon, C.; Moliere, P.; Lagarde, M.; Lecerf, J. Preferential incorporation of sn-2 lysoPC DHA over unesterified DHA in the young rat brain. Am. J. Physiol. Integr. Comp. Physiol. 1994, 267, R1273–R1279. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.N.; Ma, D.; Shui, G.; Wong, P.; Cazenave-Gassiot, A.; Zhang, X.; Wenk, M.R.; Goh, E.L.K.; Silver, D.L. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 2014, 509, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Quek, D.Q.; Nguyen, L.N.; Fan, H.; Silver, D.L. Structural Insights into the Transport Mechanism of the Human Sodium-dependent Lysophosphatidylcholine Transporter MFSD2A. J. Biol. Chem. 2016, 291, 9383–9394. [Google Scholar] [CrossRef]
- Croset, M.; Brossard, N.; Polette, A.; Lagarde, M. Characterization of plasma unsaturated lysophosphatidylcholines in human and rat. Biochem. J. 2000, 345, 61–67. [Google Scholar] [CrossRef]
- Chouinard-Watkins, R.; Lacombe, R.S.; Metherel, A.H.; Masoodi, M.; Bazinet, R.P. DHA Esterified to Phosphatidylserine or Phosphatidylcholine is More Efficient at Targeting the Brain than DHA Esterified to Triacylglycerol. Mol. Nutr. Food Res. 2019, 63, e1801224. [Google Scholar] [CrossRef] [PubMed]
- Destaillats, F.; Oliveira, M.; Schmid, V.B.; Masserey-Elmelegy, I.; Giuffrida, F.; Thakkar, S.K.; Dupuis, L.; Gosoniu, M.L.; Cruz-Hernandez, C. Comparison of the Incorporation of DHA in Circulatory and Neural Tissue When Provided as Triacylglycerol (TAG), Monoacylglycerol (MAG) or Phospholipids (PL) Provides New Insight into Fatty Acid Bioavailability. Nutrients 2018, 10, 620. [Google Scholar] [CrossRef] [PubMed]
- Sugasini, D.; Yalagala, P.C.; Goggin, A.; Tai, L.M.; Subbaiah, P.V. Enrichment of brain docosahexaenoic acid (DHA) is highly dependent upon the molecular carrier of dietary DHA: Lysophosphatidylcholine is more efficient than either phosphatidylcholine or triacylglycerol. J. Nutr. Biochem. 2019, 74, 108231. [Google Scholar] [CrossRef]
- Kitson, A.P.; Stark, K.D.; Duncan, R.E. Enzymes in brain phospholipid docosahexaenoic acid accretion: A PL-ethora of potential PL-ayers. Prostaglandins Leukot. Essent. Fat. Acids 2012, 87, 1–10. [Google Scholar] [CrossRef]
- Otoki, Y.; Metherel, A.H.; Pedersen, T.; Yang, J.; Hammock, B.D.; Bazinet, R.P.; Newman, J.W.; Taha, A.Y. Acute Hypercapnia/Ischemia Alters the Esterification of Arachidonic Acid and Docosahexaenoic Acid Epoxide Metabolites in Rat Brain Neutral Lipids. Lipids 2020, 55, 7–22. [Google Scholar] [CrossRef]
- Powell, T.L.; Barner, K.; Madi, L.; Armstrong, M.; Manke, J.; Uhlson, C.; Jansson, T.; Ferchaud-Roucher, V. Sex-specific responses in placental fatty acid oxidation, esterification and transfer capacity to maternal obesity. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158861. [Google Scholar] [CrossRef]
- Chen, S.; Subbaiah, P.V. Phospholipid and fatty acid specificity of endothelial lipase: Potential role of the enzyme in the delivery of docosahexaenoic acid (DHA) to tissues. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2007, 1771, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Polette, A.; Deshayes, C.; Chantegrel, B.; Croset, M.; Armstrong, J.M.; Lagarde, M. Synthesis of acetyl, docosahexaenoyl-glycerophosphocholine and its characterization using nuclear magnetic resonance. Lipids 1999, 34, 1333–1337. [Google Scholar] [CrossRef] [PubMed]
- Hachem, M.; Géloën, A.; Lo Van, A.; Foumaux, B.; Fenart, L.; Gosselet, F.; Da Silva, P.; Breton, G.; Lagarde, M.; Picq, M.; et al. Efficient Docosahexaenoic Acid Uptake by the Brain from a Structured Phospholipid. Mol. Neurobiol. 2016, 53, 3205–3215. [Google Scholar] [CrossRef] [PubMed]
- Culot, M.; Lundquist, S.; Vanuxeem, D.; Nion, S.; Landry, C.; Delplace, Y.; Dehouck, M.-P.; Berezowski, V.; Fenart, L.; Cecchelli, R. An in vitro blood-brain barrier model for high throughput (HTS) toxicological screening. Toxicol. Vitr. 2008, 22, 799–811. [Google Scholar] [CrossRef]
- Bernoud, N.; Fenart, L.; Bénistant, C.; Pageaux, J.; Dehouck, M.; Molière, P.; Lagarde, M.; Cecchelli, R.; Lecerf, J. Astrocytes are mainly responsible for the polyunsaturated fatty acid enrichment in blood–brain barrier endothelial cells in vitro. J. Lipid Res. 1998, 39, 1816–1824. [Google Scholar] [CrossRef]
- Hachem, M.; Nacir, H.; Picq, M.; Belkouch, M.; Bernoud-Hubac, N.; Windust, A.; Meiller, L.; Sauvinet, V.; Feugier, N.; Lambert-Porcheron, S.; et al. Docosahexaenoic Acid (DHA) Bioavailability in Humans after Oral Intake of DHA-Containing Triacylglycerol or the Structured Phospholipid AceDoPC®. Nutrients 2020, 12, 251. [Google Scholar] [CrossRef] [PubMed]
- Connor, W.; Neuringer, M.; Lin, D. Dietary effects on brain fatty acid composition: The reversibility of n-3 fatty acid deficiency and turnover of docosahexaenoic acid in the brain, erythrocytes, and plasma of rhesus monkeys. J. Lipid Res. 1990, 31, 237–247. [Google Scholar] [CrossRef]
- Létondor, A.; Buaud, B.; Vaysse, C.; Fonseca, L.; Herrouin, C.; Servat, B.; Layé, S.; Pallet, V.; Alfos, S. Erythrocyte DHA level as a biomarker of DHA status in specific brain regions of n-3 long-chain PUFA-supplemented aged rats. Br. J. Nutr. 2014, 112, 1805–1818. [Google Scholar] [CrossRef]
- Ko, M.; Hattori, T.; Abdullah, M.; Gong, J.-S.; Yamane, T.; Michikawa, M. Phosphatidylcholine protects neurons from toxic effects of amyloid β-protein in culture. Brain Res. 2016, 1642, 376–383. [Google Scholar] [CrossRef]
- Wen, M.; Ding, L.; Zhang, L.; Zhou, M.; Xu, J.; Wang, J.; Wang, Y.; Xue, C. DHA-PC and DHA-PS improved Aβ1–40 induced cognitive deficiency uncoupled with an increase in brain DHA in rats. J. Funct. Foods 2016, 22, 417–430. [Google Scholar] [CrossRef]
- Qu, M.-H.; Yang, X.; Wang, Y.; Tang, Q.; Han, H.; Wang, J.; Du Wang, G.; Xue, C.; Gao, Z. Docosahexaenoic Acid-Phosphatidylcholine Improves Cognitive Deficits in an Aβ23-35-Induced Alzheimer’s Disease Rat Model. Curr. Top. Med. Chem. 2015, 16, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, E.J.; Bongard, V.; Beiser, A.; Lamon-Fava, S.; Robins, S.J.; Au, R.; Tucker, K.; Kyle, D.J.; Wilson, P.W.F.; Wolf, P.A. Plasma Phosphatidylcholine Docosahexaenoic Acid Content and Risk of Dementia and Alzheimer Disease. Arch. Neurol. 2006, 63, 1545–1550. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.-M.; Ding, L.; Wen, M.; Che, H.-X.; Huang, J.-Q.; Zhang, T.-T.; Xue, C.-H.; Mao, X.-Z.; Wang, Y.-M. Mechanisms of DHA-enriched phospholipids in improving cognitive deficits in aged SAMP8 mice with high-fat diet. J. Nutr. Biochem. 2018, 59, 64–75. [Google Scholar] [CrossRef]
- Green, K.N.; Martínez-Coria, H.; Khashwji, H.; Hall, E.B.; Yurko-Mauro, K.A.; Ellis, L.; LaFerla, F.M. Dietary Docosahexaenoic Acid and Docosapentaenoic Acid Ameliorate Amyloid- and Tau Pathology via a Mechanism Involving Presenilin 1 Levels. J. Neurosci. 2007, 27, 4385–4395. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.P.; Calon, F.; Morihara, T.; Yang, F.; Teter, B.; Ubeda, O.; Salem, N.; Frautschy, S.A.; Cole, G.M. A Diet Enriched with the Omega-3 Fatty Acid Docosahexaenoic Acid Reduces Amyloid Burden in an Aged Alzheimer Mouse Model. J. Neurosci. 2005, 25, 3032–3040. [Google Scholar] [CrossRef]
- Teng, E.; Taylor, K.; Bilousova, T.; Weiland, D.; Pham, T.; Zuo, X.; Yang, F.; Chen, P.-P.; Glabe, C.G.; Takacs, A.; et al. Dietary DHA supplementation in an APP/PS1 transgenic rat model of AD reduces behavioral and Aβ pathology and modulates Aβ oligomerization. Neurobiol. Dis. 2015, 82, 552–560. [Google Scholar] [CrossRef]
- Hashimoto, M.; Shahdat, H.M.; Yamashita, S.; Katakura, M.; Tanabe, Y.; Fujiwara, H.; Gamoh, S.; Miyazawa, T.; Arai, H.; Shimada, T.; et al. Docosahexaenoic acid disrupts in vitro amyloid β1–40 fibrillation and concomitantly inhibits amyloid levels in cerebral cortex of Alzheimer’s disease model rats. J. Neurochem. 2008, 107, 1634–1646. [Google Scholar] [CrossRef]
- Hashimoto, M.; Shahdat, H.M.; Katakura, M.; Tanabe, Y.; Gamoh, S.; Miwa, K.; Shimada, T.; Shido, O. Effects of docosahexaenoic acid on in vitro amyloid beta peptide 25–35 fibrillation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2009, 1791, 289–296. [Google Scholar] [CrossRef]
- Eto, M.; Hashimoto, T.; Shimizu, T.; Iwatsubo, T. Characterization of the unique In Vitro effects of unsaturated fatty acids on the formation of amyloid β fibrils. PLoS ONE 2019, 14, e0219465. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, L.; Wen, M.; Du, L.; Gao, X.; Xue, C.; Xu, J.; Wang, Y. Enhanced neuroprotective effect of DHA and EPA-enriched phospholipids against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induced oxidative stress in mice brain. J. Funct. Foods 2016, 25, 385–396. [Google Scholar] [CrossRef]
- Wang, C.; Wang, D.; Xu, J.; Yanagita, T.; Xue, C.; Zhang, T.; Wang, Y. DHA enriched phospholipids with different polar groups (PC and PS) had different improvements on MPTP-induced mice with Parkinson’s disease. J. Funct. Foods 2018, 45, 417–426. [Google Scholar] [CrossRef]
- Sharon, R.; Bar-Joseph, I.; Frosch, M.P.; Walsh, D.M.; Hamilton, J.A.; Selkoe, D.J. The Formation of Highly Soluble Oligomers of α-Synuclein Is Regulated by Fatty Acids and Enhanced in Parkinson’s Disease. Neuron 2003, 37, 583–595. [Google Scholar] [CrossRef]
- Broersen, K.; van den Brink, D.; Fraser, G.; Goedert, M.; Davletov, B. α-Synuclein Adopts an α-Helical Conformation in the Presence of Polyunsaturated Fatty Acids To Hinder Micelle Formation. Biochemistry 2006, 45, 15610–15616. [Google Scholar] [CrossRef]
- Fecchio, C.; Palazzi, L.; de Laureto, P.P. α-Synuclein and Polyunsaturated Fatty Acids: Molecular Basis of the Interaction and Implication in Neurodegeneration. Molecules 2018, 23, 1531. [Google Scholar] [CrossRef] [PubMed]
- Chitre, N.M.; Wood, B.J.; Ray, A.; Moniri, N.H.; Murnane, K.S. Docosahexaenoic acid protects motor function and increases dopamine synthesis in a rat model of Parkinson’s disease via mechanisms associated with increased protein kinase activity in the striatum. Neuropharmacology 2020, 167, 107976. [Google Scholar] [CrossRef]
- Lamontagne-Proulx, J.; Coulombe, K.; Dahhani, F.; Côté, M.; Guyaz, C.; Tremblay, C.; Di Marzo, V.; Flamand, N.; Calon, F.; Soulet, D. Effect of Docosahexaenoic Acid (DHA) at the Enteric Level in a Synucleinopathy Mouse Model. Nutrients 2021, 13, 4218. [Google Scholar] [CrossRef]
- Zhou, M.-M.; Xue, Y.; Sun, S.-H.; Wen, M.; Li, Z.-J.; Xu, J.; Wang, J.-F.; Yanagita, T.; Wang, Y.-M.; Xue, C.-H. Effects of different fatty acids composition of phosphatidylcholine on brain function of dementia mice induced by scopolamine. Lipids Health Dis. 2016, 15, 135. [Google Scholar] [CrossRef]
- Wang, D.-D.; Wu, F.; Ding, L.; Shi, H.-H.; Xue, C.-H.; Wang, Y.-M.; Zhang, T.-T. Dietary n–3 PUFA Deficiency Increases Vulnerability to Scopolamine-Induced Cognitive Impairment in Male C57BL/6 Mice. J. Nutr. 2021, 151, 2206–2214. [Google Scholar] [CrossRef]
- Hashimoto, M.; Maekawa, M.; Katakura, M.; Hamazaki, K.; Matsuoka, Y. Possibility of Polyunsaturated Fatty Acids for the Prevention and Treatment of Neuropsychiatric Illnesses. J. Pharmacol. Sci. 2014, 124, 294–300. [Google Scholar] [CrossRef]
- Borsini, A.; Nicolaou, A.; Camacho-Muñoz, D.; Kendall, A.C.; Di Benedetto, M.G.; Giacobbe, J.; Su, K.-P.; Pariante, C.M. Omega-3 polyunsaturated fatty acids protect against inflammation through production of LOX and CYP450 lipid mediators: Relevance for major depression and for human hippocampal neurogenesis. Mol. Psychiatry 2021, 26, 6773–6788. [Google Scholar] [CrossRef]
- Park, Y.; Moon, H.-J.; Kim, S.-H. N-3 polyunsaturated fatty acid consumption produces neurobiological effects associated with prevention of depression in rats after the forced swimming test. J. Nutr. Biochem. 2012, 23, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, J.; Shi, H.; Zhao, Y.; Yang, J.; Wang, Y.; Yanagita, T.; Xue, C.; Zhang, T. DHA-Enriched Phospholipids Exhibit Anti-Depressant Effects by Immune and Neuroendocrine Regulation in Mice: A Study on Dose- and Structure-Activity Relationship. Mol. Nutr. Food Res. 2022, 2200089. [Google Scholar] [CrossRef] [PubMed]
- Chauveau, F.; Cho, T.-H.; Perez, M.; Guichardant, M.; Riou, A.; Aguettaz, P.; Picq, M.; Lagarde, M.; Berthezene, Y.; Nighoghossian, N.; et al. Brain-Targeting Form of Docosahexaenoic Acid for Experimental Stroke Treatment: MRI Evaluation and Anti-Oxidant Impact. Curr. Neurovascular Res. 2011, 8, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Lo Van, A.; Sakayori, N.; Hachem, M.; Belkouch, M.; Picq, M.; Fourmaux, B.; Lagarde, M.; Osumi, N.; Bernoud-Hubac, N. Targeting the Brain with a Neuroprotective Omega-3 Fatty Acid to Enhance Neurogenesis in Hypoxic Condition in Culture. Mol. Neurobiol. 2018, 56, 986–999. [Google Scholar] [CrossRef]
- Lagarde, M.; Vericel, E.; Picq, M.; Guichardant, M.; Bernoud-Hubac, N.; Fourmaux, B. AceFaPC for the Treatment of Acetylcholine-Dependent Diseases. WO Patent WO-2018162617-A1, 8 March 2018. [Google Scholar]
- Fourrier, C.; Remus-Borel, J.; Greenhalgh, A.D.; Guichardant, M.; Bernoud-Hubac, N.; Lagarde, M.; Joffre, C.; Layé, S. Docosahexaenoic acid-containing choline phospholipid modulates LPS-induced neuroinflammation in vivo and in microglia in vitro. J. Neuroinflamm. 2017, 14, 170. [Google Scholar] [CrossRef]
- Lo Van, A.; Fourmaux, B.; Picq, M.; Guichardant, M.; Lagarde, M.; Bernoud-Hubac, N. Synthesis and Identification of AceDoxyPC, a Protectin-Containing Structured Phospholipid, Using Liquid Chromatography/Mass Spectrometry. Lipids 2017, 52, 751–761. [Google Scholar] [CrossRef]
- Kevala, K.; Lagarde, M.; Spector, A.; Kim, H.-Y. Biosynthesis of N-Docosahexanoylethanolamine from Unesterified Docosahexaenoic Acid and Docosahexaenoyl-Lysophosphatidylcholine in Neuronal Cells. Int. J. Mol. Sci. 2020, 21, 8768. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).