The Fitter I Am, the Larger I Feel—The Vicious Circle of Physical Exercise in Anorexia Nervosa
Abstract
1. Introduction
2. Methods
2.1. Study Design and Ethics
2.2. Population
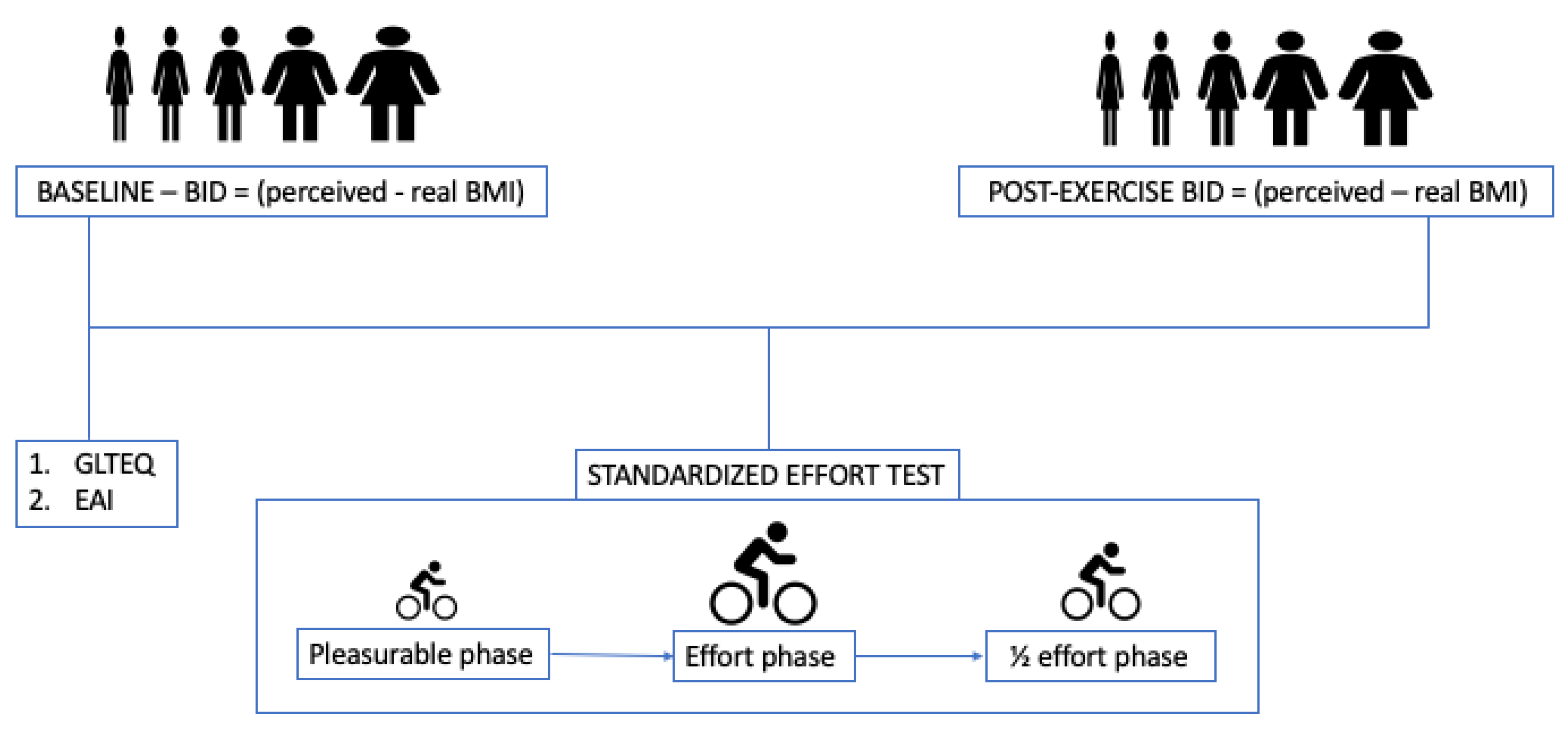
2.3. Variables
2.4. Procedure
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zipfel, S.; Giel, K.E.; Bulik, C.M.; Hay, P.; Schmidt, U. Anorexia nervosa: Aetiology, assessment, and treatment. Lancet Psychiatry 2015, 2, 1099–1111. [Google Scholar] [CrossRef]
- Favaro, A.; Santonastaso, P.; Manara, R.; Bosello, R.; Bommarito, G.; Tenconi, E.; Di Salle, F. Disruption of Visuospatial and Somatosensory Functional Connectivity in Anorexia Nervosa. Biol. Psychiatry 2012, 72, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Mitchison, D.; Hay, P.; Griffiths, S.; Murray, S.B.; Bentley, C.; Gratwick-Sarll, K.; Harrison, C.; Mond, J. Disentangling body image: The relative associations of overvaluation, dissatisfaction, and preoccupation with psychological distress and eating disorder behaviors in male and female adolescents. Int. J. Eat. Disord. 2016, 50, 118–126. [Google Scholar] [CrossRef]
- Phillipou, A.; Gurvich, C.; Castle, D.J.; Abel, L.A.; Rossell, S.L. Comprehensive neurocognitive assessment of patients with anorexia nervosa. World J. Psychiatry 2015, 5, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Cholet, J.; Lambert, S.; Vénisse, J.-L.; Grall-Bronnec, M. 1426—Anorexia nervosa restrictive type and visuospatial and visuoconstructive abilities. From a clinical study of hospitalized patients. Eur. Psychiatry 2013, 28, 1. [Google Scholar] [CrossRef]
- Phillipou, A.; Castle, D.J.; Rossell, S.L. Direct comparisons of anorexia nervosa and body dysmorphic disorder: A systematic review. Psychiatry Res. 2019, 274, 129–137. [Google Scholar] [CrossRef]
- Li, W.; Lai, T.M.; Bohon, C.; Loo, S.K.; McCurdy, D.; Strober, M.; Bookheimer, S.; Feusner, J. Anorexia nervosa and body dysmorphic disorder are associated with abnormalities in processing visual information. Psychol. Med. 2015, 45, 2111–2122. [Google Scholar] [CrossRef] [PubMed]
- Fassino, S.; Pieró, A.; Daga, G.A.; Leombruni, P.; Mortara, P.; Rovera, G.G. Attentional biases and frontal functioning in anorexia nervosa. Int. J. Eat. Disord. 2002, 31, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Walsh, B.T. The Enigmatic Persistence of Anorexia Nervosa. Am. J. Psychiatry 2013, 170, 477–484. [Google Scholar] [CrossRef]
- Tabri, N.; Murray, H.B.; Thomas, J.J.; Franko, D.L.; Herzog, D.B.; Eddy, K.T. Overvaluation of body shape/weight and engagement in non-compensatory weight-control behaviors in eating disorders: Is there a reciprocal relationship? Psychol. Med. 2015, 45, 2951–2958. [Google Scholar] [CrossRef]
- Cattarin, J.A.; Thompson, J.K. A Three-Year Longitudinal Study of Body Image, Eating Disturbance, and General Psychological Functioning in Adolescent Females. Eat. Disord. 1994, 2, 114–125. [Google Scholar] [CrossRef]
- Holtkamp, K.; Hebebrand, J.; Herpertz-Dahlmann, B. The contribution of anxiety and food restriction on physical activity levels in acute anorexia nervosa. Int. J. Eat. Disord. 2004, 36, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Vocks, S.; Legenbauer, T.; Heil, A. Food intake affects state body image: Impact of restrained eating patterns and concerns about eating, weight and shape. Appetite 2007, 49, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Grave, R.D.; Calugi, S.; Marchesini, G. Compulsive exercise to control shape or weight in eating disorders: Prevalence, associated features, and treatment outcome. Compr. Psychiatry 2008, 49, 346–352. [Google Scholar] [CrossRef]
- Davis, C.; Katzman, D.K.; Kirsh, C. Compulsive physical activity in adolescents with anorexia nervosa: A psychobehavioral spiral of pathology. J. Nerv. Ment. Dis. 1999, 187, 336–342. [Google Scholar] [CrossRef]
- Pietrowsky, R.; Straub, K.; Hachl, P. Body dissatisfaction in female restrained eaters depends on food deprivation. Appetite 2003, 40, 285–290. [Google Scholar] [CrossRef]
- Di Lodovico, L.; Hatteea, H.; Couton, C.; Duriez, P.; Treasure, J.; Gorwood, P. Physical exercise-related endophenotypes in anorexia nervosa. Int. J. Eat. Disord. 2021, 54, 1181–1188. [Google Scholar] [CrossRef]
- Gianini, L.M.; Klein, D.A.; Call, C.; Mayer, L.; Foltin, R.W.; Walsh, B.T.; Wang, Y.; Wu, P.; Attia, E. The reinforcing effect of exercise in anorexia nervosa: Clinical correlates and relationship to outcome. Eat. Disord. 2016, 24, 412–423. [Google Scholar] [CrossRef]
- Hale, B.D.; Roth, A.D.; DeLong, R.E.; Briggs, M.S. Exercise dependence and the drive for muscularity in male bodybuilders, power lifters, and fitness lifters. Body Image 2010, 7, 234–239. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar] [CrossRef]
- French, C.C.; Beaumont, J.G. A clinical study of the automated assessment of intelligence by the Mill Hill vocabulary test and the standard progressive matrices test. J. Clin. Psychol. 1990, 46, 129–140. [Google Scholar] [CrossRef]
- Garner, D.M. Eating Disorder Inventory-2: Professional Manual; Psychological Assessment Resources: Odessa, FL, USA, 1991. [Google Scholar]
- Criquillon-Doublet, S.; Divac, S.; Gaillac, V.; Dardennes, R.; Guelfi, J.D. Le ‘eating disorder inventory’ (EDI). In Psychopathologie Quantitative; Masson: Paris, France, 1995; pp. 249–260. [Google Scholar]
- Garner, D.; Olmstead, M.P.; Polivy, J. Development and validation of a multidimensional eating disorder inventory for anorexia nervosa and bulimia. Int. J. Eat. Disord. 1983, 2, 15–34. [Google Scholar] [CrossRef]
- Eberenz, K.P.; Gleaves, D. An examination of the internal consistency and factor structure of the eating disorder inventory-2 in a clinical sample. Int. J. Eat. Disord. 1994, 16, 371–379. [Google Scholar] [CrossRef]
- Godin, G. The Godin-Shephard Leisure-Time Physical Activity Questionnaire. Health Fit J. Can. 2011, 4, 18–22. [Google Scholar]
- Terry, A.; Szabo, A.; Griffiths, M. The exercise addiction inventory: A new brief screening tool. Addict. Res. Theory 2004, 12, 489–499. [Google Scholar] [CrossRef]
- Griffiths, M.D.; Szabo, A.; Terry, A. The exercise addiction inventory: A quick and easy screening tool for health practitioners. BJSM 2005, 39, e30. [Google Scholar] [CrossRef] [PubMed]
- Moussally, J.M.; Rochat, L.; Posada, A.; Van Der Linden, M. A database of body-only computer-generated pictures of women for body-image studies: Development and preliminary validation. Behav. Res. Methods 2017, 49, 172–183. [Google Scholar] [CrossRef]
- George, D.; Mallery, P. SPSS for Windows Step by Step: A Simple Guide and Reference 17.0 Update, 10th ed.; Pearson: Boston, MA, USA, 2010. [Google Scholar]
- Hair, J. Multivariate Data Analysis: A Global Perspective, 7th ed.; Prenctice Hall: Upper Saddle River, NJ, USA, 2009. [Google Scholar]
- Di Lodovico, L.; Gorwood, P. The relationship between moderate to vigorous physical activity and cognitive rigidity in anorexia nervosa. Psychiatry Res. 2020, 284, 112703. [Google Scholar] [CrossRef]
- Rizk, M.; Lalanne, C.; Berthoz, S.; Kern, L.; Godart, N.; EVHAN Group. Problematic Exercise in Anorexia Nervosa: Testing Potential Risk Factors against Different Definitions. PLoS ONE 2015, 10, e0143352. [Google Scholar] [CrossRef]
- Klein, D.A.; Bennett, A.S.; Schebendach, J.; Foltin, R.W.; Devlin, M.J.; Walsh, B.T. Exercise ‘addiction’ in anorexia nervosa: Model development and pilot data. CNS Spectr. 2004, 9, 531–537. [Google Scholar] [CrossRef]
- Monteleone, A.M.; Castellini, G.; Volpe, U.; Ricca, V.; Lelli, L.; Monteleone, P.; Maj, M. Neuroendocrinology and brain imaging of reward in eating disorders: A possible key to the treatment of anorexia nervosa and bulimia nervosa. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 80, 132–142. [Google Scholar] [CrossRef]
- Giel, K.E.; Kullmann, S.; Preißl, H.; Bischoff, S.C.; Thiel, A.; Schmidt, U.; Zipfel, S.; Teufel, M. Understanding the reward system functioning in anorexia nervosa: Crucial role of physical activity. Biol. Psychol. 2013, 94, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Tchanturia, K.; Anderluh, M.B.; Morris, R.G.; Rabe-Hesketh, S.; Collier, D.A.; Sanchez, P.; Treasure, J. Cognitive flexibility in anorexia nervosa and bulimia nervosa. J. Int. Neuropsychol. Soc. 2004, 10, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Taranis, L.; Goodwin, H.; Haycraft, E. Compulsive exercise and eating disorders. Eur. Eat. Disord. Rev. 2011, 19, 174–189. [Google Scholar] [CrossRef] [PubMed]
- Jacquemot, A.M.M.C.; Park, R. The Role of Interoception in the Pathogenesis and Treatment of Anorexia Nervosa: A Narrative Review. Front. Psychiatry 2020, 11, 281. [Google Scholar] [CrossRef]
- Lopez, C.; Tchanturia, K.; Stahl, D.; Booth, R.; Holliday, J.; Treasure, J. An examination of the concept of central coherence in women with anorexia nervosa. Int. J. Eat. Disord. 2008, 41, 143–152. [Google Scholar] [CrossRef]
- Lopez, C.; Tchanturia, K.; Stahl, D.; Treasure, J. Weak central coherence in eating disorders: A step towards looking for an endophenotype of eating disorders. J. Clin. Exp. Neuropsychol. 2009, 31, 117–125. [Google Scholar] [CrossRef]
- Holliday, J.; Tchanturia, K.; Landau, S.; Collier, D.; Treasure, J. Is Impaired Set-Shifting an Endophenotype of Anorexia Nervosa? Am. J. Psychiatry 2005, 162, 2269–2275. [Google Scholar] [CrossRef]
- Eshkevari, E.; Rieger, E.; Longo, M.R.; Haggard, P.; Treasure, J. Persistent body image disturbance following recovery from eating disorders. Int. J. Eat. Disord. 2014, 47, 400–409. [Google Scholar] [CrossRef]
- Tchanturia, K.; Serpell, L.; Troop, N.; Treasure, J. Perceptual illusions in eating disorders: Rigid and fluctuating styles. J. Behav. Ther. Exp. Psychiatry 2001, 32, 107–115. [Google Scholar] [CrossRef]
- Sauchelli, S.; Arcelus, J.; Granero, R.; Jiménez-Murcia, S.; Agüera, Z.; Del Pino-Gutiérrez, A.; Fernández-Aranda, F. Dimensions of Compulsive Exercise across Eating Disorder Diagnostic Subtypes and the Validation of the Spanish Version of the Compulsive Exercise Test. Front. Psychol. 2016, 7, 1852. [Google Scholar] [CrossRef]
- Cassin, S.; von Ranson, K. Personality and eating disorders: A decade in review. Clin. Psychol. Rev. 2005, 25, 895–916. [Google Scholar] [CrossRef] [PubMed]
- Lampard, A.M.; Byrne, S.M.; McLean, N.; Fursland, A. The Eating Disorder Inventory-2 Perfectionism scale: Factor structure and associations with dietary restraint and weight and shape concern in eating disorders. Eat. Behav. 2012, 13, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Coniglio, K.A.; Becker, K.R.; Franko, D.L.; Zayas, L.V.; Plessow, F.; Eddy, K.T.; Thomas, J.J. Won’t stop or can’t stop? Food restriction as a habitual behavior among individuals with anorexia nervosa or atypical anorexia nervosa. Eat. Behav. 2017, 26, 144–147. [Google Scholar] [CrossRef]
- Bratland-Sanda, S.; Sundgot-Borgen, J.; Rø, Ø.; Rosenvinge, J.H.; Hoffart, A.; Martinsen, E.W. I’m not physically active—I only go for walks”: Physical activity in patients with longstanding eating disorders. Int. J. Eat. Disord. 2010, 43, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Strigo, I.A.; Matthews, S.C.; Simmons, A.N.; Oberndorfer, T.; Klabunde, M.; Bsc, L.E.R.; Kaye, W.H. Altered insula activation during pain anticipation in individuals recovered from anorexia nervosa: Evidence of interoceptive dysregulation. Int. J. Eat. Disord. 2013, 46, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Gueguen, J.; Piot, M.-A.; Orri, M.; Gutierre, A.; Le Moan, J.; Berthoz, S.; Falissard, B.; Godart, N. Group Qigong for Adolescent Inpatients with Anorexia Nervosa: Incentives and Barriers. PLoS ONE 2017, 12, e0170885. [Google Scholar] [CrossRef]
| AN | Healthy Controls | Student’s t | DF | p-Value | |
|---|---|---|---|---|---|
| Clinical and socio-demographic variables | |||||
| Age (years) | 23.42 (5.59) | 25.50 (3.16) | −1.89 | 61 | 0.06 |
| BMI (kg/m2) | 16.78 (2.31) | 21.82 (3.08) | −7.45 | 62 | <0.01 |
| EDI-2 total score | 97.29 (46.52) | 28.08 (22.71) | 8.03 | 61 | <0.01 |
| Illness duration (years) | 7.32 (2.34) | - | - | - | - |
| Physical exercise | |||||
| GLTEQ score (MET equivalents) | 63.74 (60.21) | 57.33 (29.54) | 0.49 | 64 | 0.63 |
| EAI score | 17.56 (7.16) | 13.58 (4.55) | 2.74 | 62 | 0.01 |
| AN | Healthy Controls | Student’s t | DF | p-Value | |
|---|---|---|---|---|---|
| Body image distortion (BID) | |||||
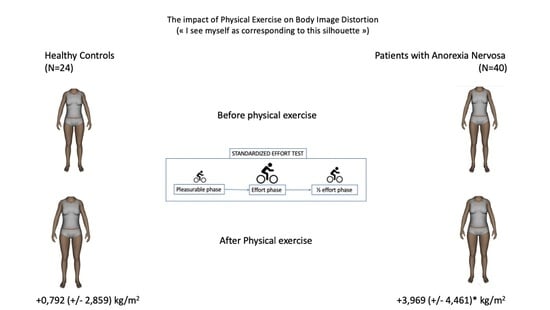
| Self-rated BMI before exercise | 19.016 (4.73) | 21.957 (3.91) | 2.57 | 63 | <0.01 |
| Baseline BID (BMI points: kg/m2) | 3.031 (4.84) | 0.136 (1.91) | 3.43 | 58 | <0.01 |
| Self-rated BMI after physical exercise | 22.985 (5.95) | 22.749 (4.83) | 1.21 | 52 | 0.23 |
| Physical exercise induced-BID (BMI points) | 3.969 (4.46) | 0.792 (2.86) | 3.24 | 52 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Lodovico, L.; Hanachi, M.; Duriez, P.; Gorwood, P. The Fitter I Am, the Larger I Feel—The Vicious Circle of Physical Exercise in Anorexia Nervosa. Nutrients 2022, 14, 4507. https://doi.org/10.3390/nu14214507
Di Lodovico L, Hanachi M, Duriez P, Gorwood P. The Fitter I Am, the Larger I Feel—The Vicious Circle of Physical Exercise in Anorexia Nervosa. Nutrients. 2022; 14(21):4507. https://doi.org/10.3390/nu14214507
Chicago/Turabian StyleDi Lodovico, Laura, Mouna Hanachi, Philibert Duriez, and Philip Gorwood. 2022. "The Fitter I Am, the Larger I Feel—The Vicious Circle of Physical Exercise in Anorexia Nervosa" Nutrients 14, no. 21: 4507. https://doi.org/10.3390/nu14214507
APA StyleDi Lodovico, L., Hanachi, M., Duriez, P., & Gorwood, P. (2022). The Fitter I Am, the Larger I Feel—The Vicious Circle of Physical Exercise in Anorexia Nervosa. Nutrients, 14(21), 4507. https://doi.org/10.3390/nu14214507