Clinical Correlates of Measured and Predicted Resting Energy Expenditure in Patients with Anorexia Nervosa: A Retrospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
- Harris–Benedict equation: REEHB (Kcal/day) = 655.1 + (9.56 × weight in kg) + (1.84 × height in cm) − (4.67 × age in years) [10]
- Schebendach equation: REESch (Kcal/day) = (1.84 × Harris-Benedict predicted REE) − 1435 [12]
- Muller 1 equation: REEMuller1 (Kcal/day) = 239 × [0.07122 × weight in kg − 0.02149 × age in years + 0.731] [11]
- Muller 2 equation: REEMuller2 (Kcal/day) = 239 [0.08961 × FFM in kg +0.05662 × FM in kg + 0.667] [11].
2.3. Statistical Analyses
3. Results
3.1. Sample Description
3.2. Analysis of Factors Associated with REEIC
3.3. Level of Agreement between Estimated REE and REEIC
3.4. Analysis of Factors Associated with Diff(Sch-IC) Variability
3.5. Adjustment of the Schebendach Equation to Estimate REESch Using Factors Associated with Diff(Sch-IC) Variability
3.6. Comparison between the Adjusted REESch and REEIC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dobrescu, S.R.; Dinkler, L.; Gillberg, C.; Råstam, M.; Gillberg, C.; Wentz, E. Anorexia nervosa: 30-year outcome. Br. J. Psychiatry 2020, 216, 97–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attia, E. Anorexia Nervosa: Current Status and Future Directions. Annu. Rev. Med. 2010, 61, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Smink, F.R.E.; van Hoeken, D.; Hoek, H.W. Epidemiology of Eating Disorders: Incidence, Prevalence and Mortality Rates. Curr. Psychiatry Rep. 2012, 14, 406–414. [Google Scholar] [CrossRef] [Green Version]
- Moskowitz, L.; Weiselberg, E. Anorexia Nervosa/Atypical Anorexia Nervosa. Curr. Probl. Pediatr. Adolesc. Health Care 2017, 47, 70–84. [Google Scholar] [CrossRef]
- van Hoeken, D.; Hoek, H.W. Review of the burden of eating disorders: Mortality, disability, costs, quality of life, and family burden. Curr. Opin. Psychiatry 2020, 33, 521–527. [Google Scholar] [CrossRef]
- Krahn, D.D.; Rock, C.; Dechert, R.E.; Nairin, K.K.; Hasse, S.A. Changes in resting energy expenditure and body composition in anorexia nervosa patients during refeeding. J. Am. Diet. Assoc. 1993, 93, 434–438. [Google Scholar] [CrossRef]
- Levine, J.A. Measurement of energy expenditure. Public Health Nutr. 2005, 8, 1123–1132. [Google Scholar] [CrossRef]
- Shephard, R.J. Open-circuit respirometry: A brief historical review of the use of Douglas bags and chemical analyzers. Eur. J. Appl. Physiol. 2017, 117, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Psota, T.; Chen, K.Y. Measuring energy expenditure in clinical populations: Rewards and challenges. Eur. J. Clin. Nutr. 2013, 67, 436–442. [Google Scholar] [CrossRef] [Green Version]
- Harris, J.A.; Benedict, F.G. A Biometric Study of Basal Metabolism in Man; Carnegie Institute: Washington, DC, USA, 1919. [Google Scholar]
- Müller, M.J.; Bosy-Westphal, A.; Klaus, S.; Kreymann, G.; Lührmann, P.M.; Neuhäuser-Berthold, M.; Noack, R.; Pirke, K.M.; Platte, P.; Selberg, O.; et al. World Health Organization equations have shortcomings for predicting resting energy expenditure in persons from a modern, affluent population: Generation of a new reference standard from a retrospective analysis of a German database of resting energy expenditure. Am. J. Clin. Nutr. 2004, 80, 1379–1390. [Google Scholar]
- Schebendach, M.J.; Golden, N.H.; Jacobson, M.S.; Arden, M.; Pettei, M.; Hardoff, D.; Bauman, N.; Reichert, P.; Copperman, N.; Hertz, S.; et al. Indirect calorimetry in the nutritional management of eating disorders. Int. J. Eat. Disord. 1995, 17, 59–66. [Google Scholar] [CrossRef]
- Marra, M.; Polito, A.; De Filippo, E.; Cuzzolaro, M.; Ciarapica, D.; Contaldo, F.; Scalfi, L. Are the general equations to predict BMR applicable to patients with anorexia nervosa? Eat. Weight Disord. 2002, 7, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Cuerda, C.; Ruiz, A.; Velasco, C.; Bretón, I.; Camblor, M.; García-Peris, P. How accurate are predictive formulas calculating energy expenditure in adolescent patients with anorexia nervosa? Clin. Nutr. 2007, 26, 100–106. [Google Scholar] [CrossRef] [PubMed]
- El Ghoch, M.; Alberti, M.; Capelli, C.; Calugi, S.; Grave, R.D. Resting Energy Expenditure in Anorexia Nervosa: Measured versus Estimated. J. Nutr. Metab. 2012, 2012, 652932. [Google Scholar] [CrossRef] [PubMed]
- Aliasgharzadeh, S.; Mahdavi, R.; Jafarabadi, M.A.; Namazi, N. Comparison of Indirect Calorimetry and Predictive Equations in Estimating Resting Metabolic Rate in Underweight Females. Iran. J. Public Health 2015, 44, 822–829. [Google Scholar]
- da Costa, R.F.; Silva, A.M.; Masset, K.V.D.S.B.; Cesário, T.D.M.; Cabral, B.G.D.A.T.; Ferrari, G.; Dantas, P.M.S. Development and Cross-Validation of a Predictive Equation for Fat-Free Mass in Brazilian Adolescents by Bioelectrical Impedance. Front. Nutr. 2022, 9, 820736. [Google Scholar] [CrossRef]
- Campa, F.; Matias, C.; Gatterer, H.; Toselli, S.; Koury, J.C.; Andreoli, A.; Melchiorri, G.; Sardinha, L.B.; Silva, A.M. Classic Bioelectrical Impedance Vector Reference Values for Assessing Body Composition in Male and Female Athletes. Int. J. Environ. Res. Public Health 2019, 16, 5066. [Google Scholar] [CrossRef] [Green Version]
- Nana, A.; Slater, G.; Stewart, A.; Burke, L.M. Methodology Review: Using Dual-Energy X-ray Absorptiometry (DXA) for the Assessment of Body Composition in Athletes and Active People. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 198–215. [Google Scholar] [CrossRef]
- Correa, C.H.G.; Mejía, F.M.; González, P.A.C.; Claros, J.A.V.; Arroyabe, H.D.C. Bioelectrical impedance analysis and dual x-ray absorptiometry agreement for skeletal muscle mass index evaluation in sarcopenia diagnosis. Physiol. Meas. 2020, 41, 064005. [Google Scholar] [CrossRef]
- Bolanowski, M.; E Nilsson, B. Assessment of human body composition using dual-energy x-ray absorptiometry and bioelectrical impedance analysis. Med. Sci. Monit. 2001, 7, 1029–1033. [Google Scholar]
- Scalfi, L.; Marra, M.; De Filippo, E.; Caso, G.; Pasanisi, F.; Contaldo, F. The prediction of basal metabolic rate in female patients with anorexia nervosa. Int. J. Obes. 2001, 25, 359–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tresley, J.; Sheean, P.M. Refeeding syndrome: Recognition is the key to prevention and management. J. Am. Diet. Assoc. 2008, 108, 2105–2108. [Google Scholar] [CrossRef] [PubMed]
- de Zwaan, M.; Aslam, Z.; Mitchell, J.E. Research on energy expenditure in individuals with eating disorders: A review. Int. J. Eat. Disord. 2002, 32, 127–134. [Google Scholar] [CrossRef]
- Zipfel, S.; Mack, I.; Baur, L.A.; Hebebrand, J.; Touyz, S.; Herzog, W.; Abraham, S.; Davies, P.S.; Russell, J. Impact of exercise on energy metabolism in anorexia nervosa. J. Eat. Disord. 2013, 1, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manuelli, M.; Blundell, J.E.; Biino, G.; Cena, H. Body composition and resting energy expenditure in women with anorexia nervosa: Is hyperactivity a protecting factor? Clin. Nutr. Espen. 2019, 29, 160–164. [Google Scholar] [CrossRef]
- Van Wymelbeke, V.; Brondel, L.; Marcel Brun, J.; Rigaud, D. Factors associated with the increase in resting energy expenditure during refeeding in malnourished anorexia nervosa patients. Am. J. Clin. Nutr. 2004, 80, 1469–1477. [Google Scholar] [CrossRef]
- Karczewska-Kupczewska, M.; Adamska, A.; Nikołajuk, A.; Otziomek, E.; Górska, M.; Kowalska, I.; Strączkowski, M. Circulating interleukin 6 and soluble forms of its receptors in relation to resting energy expenditure in women with anorexia nervosa. Clin. Endocrinol. 2013, 79, 812–816. [Google Scholar] [CrossRef]
- Onur, S.; Haas, V.; Bosy-Westphal, A.; Hauer, M.; Paul, T.; Nutzinger, D.; Klein, H.; Müller, M.J. L-tri-iodothyronine is a major determinant of resting energy expenditure in underweight patients with anorexia nervosa and during weight gain. Eur. J. Endocrinol. 2005, 152, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Castellini, G.; Castellani, W.; Lelli, L.; Sauro, C.L.; Dini, C.; Lazzeretti, L.; Bencini, L.; Mannucci, E.; Ricca, V. Association between resting energy expenditure, psychopathology and HPA-axis in eating disorders. World J. Clin. Cases 2014, 2, 257–264. [Google Scholar] [CrossRef]
- Maguire, S.; Surgenor, L.J.; Le Grange, D.; Lacey, H.; Crosby, R.D.; Engel, S.G.; Fromholtz, K.M.; Bamford, B.; Touyz, S. Examining a staging model for anorexia nervosa: Empirical exploration of a four stage model of severity. J. Eat. Disord. 2017, 5, 41. [Google Scholar] [CrossRef] [Green Version]
- Treasure, J.; Stein, D.; Maguire, S. Has the time come for a staging model to map the course of eating disorders from high risk to severe enduring illness? An examination of the evidence. Early Interv. Psychiatry 2015, 9, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Strober, M.; Freeman, R.; Morrell, W. The long-term course of severe anorexia nervosa in adolescents: Survival analysis of recovery, relapse, and outcome predictors over 10–15 years in a prospective study. Int. J. Eat. Disord. 1997, 22, 339–360. [Google Scholar] [CrossRef]
- Monteleone, P.; Maj, M. Dysfunctions of leptin, ghrelin, BDNF and endocannabinoids in eating disorders: Beyond the homeostatic control of food intake. Psychoneuroendocrinology 2013, 38, 312–330. [Google Scholar] [CrossRef] [PubMed]
- Schorr, M.; Miller, K.K. The endocrine manifestations of anorexia nervosa: Mechanisms and management. Nat. Rev. Endocrinol. 2017, 13, 174–186. [Google Scholar] [CrossRef]
- Kosmiski, L.; Schmiege, S.J.; Mascolo, M.; Gaudiani, J.; Mehler, P.S. Chronic starvation secondary to anorexia nervosa is associated with an adaptive suppression of resting energy expenditure. J. Clin. Endocrinol. Metab. 2014, 99, 908–914. [Google Scholar] [CrossRef] [Green Version]
- Speciani, M.; Barak, Y.; Damanhuri, H.; De Ronchi, D.; Panariello, F.; Atti, A.R. A Perspective on Chronic and Long-Lasting Anorexia Nervosa. Front. Psychiatry. 2021, 12, 756669. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- Fairburn, C.G.; Beglin, S.J. Assessment of eating disorders: Interview or self-report questionnaire? Int. J. Eat. Disord. 1994, 16, 363–370. [Google Scholar] [CrossRef]
- Meule, A. Reconsidering the use of cut-off scores for the Eating Disorder Examination-Questionnaire. Eat. Disord. 2021, 29, 480–484. [Google Scholar] [CrossRef]
- Steinborn, M.B.; Langner, R.; Flehmig, H.C.; Huestegge, L. Methodology of performance scoring in the d2 sustained-attention test: Cumulative-reliability functions and practical guidelines. Psychol. Assess. 2018, 30, 339–357. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Stubbs, R.J.; Hopkins, M.; Finlayson, G.S.; Duarte, C.; Gibbons, C.; Blundell, J.E. Potential effects of fat mass and fat-free mass on energy intake in different states of energy balance. Eur. J. Clin. Nutr. 2018, 72, 698–709. [Google Scholar] [CrossRef] [PubMed]
- Egan, A.M.; Collins, A.L. Dynamic changes in energy expenditure in response to underfeeding: A review. Proc. Nutr. Soc. 2021, 4, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bailly, M.; Boscaro, A.; Pereira, B.; Féasson, L.; Boirie, Y.; Germain, N.; Galusca, B.; Courteix, D.; Thivel, D.; Verney, J. Is constitutional thinness really different from anorexia nervosa? A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2021, 22, 913–971. [Google Scholar] [CrossRef] [PubMed]
- McDuffie, J.R.; Adler-Wailes, D.C.; Elberg, J.; Steinberg, E.N.; Fallon, E.M.; Tershakovec, A.M.; Arslanian, S.A.; Delany, J.P.; Bray, G.A.; Yanovski, J.A. Prediction equations for resting energy expenditure in overweight and normal-weight black and white children. Am. J. Clin. Nutr. 2004, 80, 365–373. [Google Scholar] [CrossRef]
- Chima, L.; Mulrooney, H.M.; Warren, J.; Madden, A.M. A systematic review and quantitative analysis of resting energy expenditure prediction equations in healthy overweight and obese children and adolescents. J. Hum. Nutr. Diet. 2020, 33, 373–385. [Google Scholar] [CrossRef]
- Amaro-Gahete, F.J.; Jurado-Fasoli, L.; De-la-O, A.; Gutierrez, Á.; Castillo, M.J.; Ruiz, J.R. Accuracy and Validity of Resting Energy Expenditure Predictive Equations in Middle-Aged Adults. Nutrients 2018, 10, 1635. [Google Scholar] [CrossRef] [Green Version]
- Kochavi, B.; Mendelowitsch, S.; Enoch-Levy, A.; Yaroslavsky, A.; Toledano, A.; Modan-Moses, D.; Stein, D. Resting energy expenditure in acutely ill and stabilized patients with anorexia nervosa and bulimia nervosa. Int. J. Eat. Disord. 2020, 53, 1460–1468. [Google Scholar] [CrossRef]
- Weijs, P.J.; Kruizenga, H.M.; van Dijk, A.E.; van der Meij, B.S.; Langius, J.A.; Knol, D.L.; van Schijndel, R.J.M.S.; van Bokhorst-de van der Schueren, M.A.E. Validation of predictive equations for resting energy expenditure in adult outpatients and inpatients. Clin. Nutr. 2008, 27, 150–157. [Google Scholar] [CrossRef]
- Casper, R.C. Might Starvation-Induced Adaptations in Muscle Mass, Muscle Morphology and Muscle Function Contribute to the Increased Urge for Movement and to Spontaneous Physical Activity in Anorexia Nervosa? Nutrients 2020, 12, 2060. [Google Scholar] [CrossRef]
- Mueller, S.M.; Immoos, M.; Anliker, E.; Drobnjak, S.; Boutellier, U.; Toigo, M. Reduced Bone Strength and Muscle Force in Women 27 Years After Anorexia Nervosa. J. Clin. Endocrinol. Metab. 2015, 100, 2927–2933. [Google Scholar] [CrossRef] [Green Version]
- Pettersson, C.; Svedlund, A.; Wallengren, O.; Swolin-Eide, D.; Paulson Karlsson, G.; Ellegård, L. Dietary intake and nutritional status in adolescents and young adults with anorexia nervosa: A 3-year follow-up study. Clin. Nutr. 2021, 40, 5391–5398. [Google Scholar] [CrossRef] [PubMed]
- Misra, M. Long-term skeletal effects of eating disorders with onset in adolescence. Ann. N. Y. Acad. Sci. 2008, 1135, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Agüera, Z.; Romero, X.; Arcelus, J.; Sánchez, I.; Riesco, N.; Jiménez-Murcia, S.; González-Gómez, J.; Granero, R.; Custal, N.; Fernández-Aranda, F. Changes in Body Composition in Anorexia Nervosa: Predictors of Recovery and Treatment Outcome. PLoS ONE 2015, 10, e0143012. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Variable | Mean +/− SD * [or Median (Q1–Q3)] | Category: N (%) |
|---|---|---|
| Age (years) | 22 (19–28.75) | |
| Current BMI (Kg/m2) | 17.68 (16.05–19.46) | |
| Current weight (Kg) | 47 (43.1–52.95) | |
| Current height (cm) | 163.57 +/− 5.96 | |
| FFM (Kg) | 37.43 +/− 4.3 | |
| FM (Kg) | 10.14 +/− 5.41 | |
| AN duration (years) | 5 (2–11) | |
| AN subtype | Restrictive: 66 (47.82%) Non-restrictive: 69 (50%) | |
| Food selectivity | Yes: 100 (72.46%) No: 36 (26.08%) | |
| Meal skipping | Yes: 56 (40.58%) No: 79 (57.24%) | |
| Bulimic episodes | Yes: 46 (33.33%) No: 90 (65.21%) | |
| Vomiting | Yes: 55 (39.85%) No: 81 (58.69%) | |
| Physical hyperactivity | Yes: 66 (47.82%) No: 70 (50.72%) | |
| Snack eating | Yes: 19 (13.76%) No: 117 (84.78%) | |
| Compulsory eating | Yes: 31 (22.46%) No: 103 (74.63%) | |
| Polydipsia | Yes: 10 (7.24%) No: 126 (91.3%) | |
| Fasting practices | Yes: 49 (35.5%) No: 87 (63.04%) | |
| Laxative use | Yes: 16 (11.59%) No: 120 (86.95%) | |
| Nicotine consumption | Yes: 48 (34.78%) No: 90 (65.21%) | |
| Current depressive episode | Yes: 35 (25.36%) No: 88 (63.76%) | |
| Current anxiety disorder | Yes: 65 (47.1%) No: 59 (42.75%) | |
| Current obsessive compulsive disorder | Yes: 12 (8.69%) No: 111 (80.43%) | |
| Current post-traumatic stress disorder | Yes: 5 (3.62%) No: 118 (85.5%) | |
| Current alcohol and/or substance use disorder | Yes: 8 (5.79%) No: 114 (82.6%) | |
| History of suicide attempt | Yes: 28 (20.29%) No: 109 (78.98%) | |
| EDE-Q restriction | 3.6 (1.6–4.8) | |
| EDE-Q eating concern | 2.8 (1.6–4) | |
| EDE-Q weight concern | 4 (2–4.8) | |
| EDE-Q shape concern | 4.43 (2.68–5.37) | |
| EDE-Q total score | 3.53 (2.45–4.43) | |
| d2 score ratio | 0.032 (0.017–0.056) | |
| REEIC (Kcal) | 1135.65 +/− 202.2 | |
| REEHB (Kcal) | 1296.27 (1242.04–1340.57) | |
| REESch (Kcal) | 943.12 (850.37–1031.65) | |
| REEMuller1 (Kcal) | 851.48 (768.84–938.25) | |
| REEMuller2 (Kcal) | 1087.91 (1010.9–1174.9) |
| Variable | Bivariate Analysis with REEIC as Dependent Variable: Statistical Test (Correlation for Continuous Variables and Comparison of Means for Categorical Variables) Mean ± SD for Normally Distributed Data or Median (Q1–Q3) | p Value | Linear Regression Model (Model 1) with REEIC as Dependent Variable and All Eligible Independent Variables (Adjusted R2 = 0.559; df = 119; p < 0.001) | Final Linear Regression Model (Model 2) with REEIC as Dependent Variable (Adjusted R2 = 0.579; df = 123; p < 0.001) |
|---|---|---|---|---|
| Age (years) | Spearman’s Rho = 0.068 | 0.437 | ||
| Current BMI (Kg/m2) | Spearman’s Rho = 0.657 | <0.001 | ||
| Current weight (Kg) | Spearman’s Rho = 0.689 | <0.001 | ||
| Current height (cm) | Pearson’s correlation; r = 0.253 | 0.004 | ||
| FFM (Kg) | Pearson’s correlation; r = 0.595 | <0.001 | <0.001 | p < 0.001 |
| FM (Kg) | Spearman’s Rho = 0.599 | <0.001 | <0.001 | p < 0.001 |
| AN duration (years) | Spearman’s Rho = 0.203 | 0.021 | 0.007 | p = 0.058 |
| AN subtype | T test: AN restrictive type: 1052.4 ± 177.56 AN non-restrictive type: 1205.4 ± 184.96 | <0.001 | 0.069 | p = 0.005 |
| Food selectivity | T test: Yes: 1119.63 ± 207.18 No: 1184.33 ± 184.92 | 0.115 | 0.221 | p = 0.1 |
| Meal skipping | T test: Yes: 1168.56 ± 212.28 No: 1105.71 ± 187.06 | 0.078 | 0.938 | |
| Bulimic episodes | T test: Yes: 1187 ± 173.59 No: 1110.47 ± 212.51 | 0.041 | 0.586 | |
| Vomiting | T test: Yes: 1174.09 ± 186.8 No: 1110.58 ± 210.58 | 0.081 | 0.907 | |
| Physical hyperactivity | T test: Yes: 1136.71 ± 198.01 No: 1135.7 ± 208.85 | 0.978 | ||
| Snack eating | T test: Yes: 1265.16 ± 197.13 No: 1115.27 ± 196.94 | 0.003 | 0.931 | |
| Compulsory eating | T test: Yes: 1217.89 ± 211.27 No: 1108.92 ± 192.67 | 0.01 | 0.908 | |
| Polydipsia | T test: Yes: 1173 ± 210.89 No: 1113.42 ± 203.04 | 0.575 | ||
| Fasting practices | T test: Yes: 1167.02 ± 214.33 No: 1117.91 ± 195.04 | 0.185 | ||
| Laxative use | T test: Yes: 1174.68 ± 216.24 No: 1130.73 ± 201.48 | 0.42 | ||
| Nicotine consumption | T test: Yes: 1203.8 ± 173.03 No: 1098.77 ± 208.13 | 0.004 | 0.135 | p = 0.115 |
| Current depressive episode | T test: Yes: 1109.57 ± 218.78 No: 1137.65 ± 204.97 | 0.514 | ||
| Current anxiety disorder | T test: Yes: 1106.79 ± 233.51 No: 1157.6 ± 172.35 | 0.184 | ||
| Current obsessive compulsive disorder | T test: Yes: 1172.83 ± 222.58 No: 1124.91 ± 207.24 | 0.453 | ||
| Current post-traumatic stress disorder | T test: Yes: 1225.2 ± 350.58 No: 1125.56 ± 201.34 | 0.297 | ||
| Current alcohol and/or substance use disorder | T test: Yes: 1223.57 ± 160.8 No: 1124.56 ± 211 | 0.226 | ||
| History of suicide attempt | T test: Yes: 1210.5 ± 218.1 No: 1117.37 ± 193.63 | 0.03 | 0.911 | |
| EDE-Q restriction | Pearson’s correlation; r = −0.013 | 0.884 | ||
| EDE-Q eating concern | Pearson’s correlation; r = −0.07 | 0.451 | ||
| EDE-Q weight concern | Pearson’s correlation; r = 0.132 | 0.156 | ||
| EDE-Q shape concern | Pearson’s correlation; r = 0.134 | 0.152 | ||
| EDE-Q total score | Pearson’s correlation; r = 0.07 | 0.462 | ||
| d2 score ratio | Pearson’s correlation; r = 0.042 | 0.667 |
| Variable | Bivariate Analysis with Diff(Sch-IC) as Dependent Variable: Statistical Test (Correlation for Continuous Variables and Comparison of Means for Categorical Variables) Mean ± SD for Normally Distributed Data or Median (Q1–Q3) | p Value | Linear Regression Model (Model 3) with Diff(Sch-IC) as Dependent Variable and All Eligible Independent Variables Included (Adjusted R2 = 0.349; df = 119; p < 0.001) | Final Linear Regression Model (Model 4) with Diff(Sch-IC) as Dependent Variable (Adjusted R2 = 0.381; df = 121; p < 0.001) |
|---|---|---|---|---|
| Age (years) | Spearman’s Rho = −0.497 | <0.001 | p = 0.001 | p < 0.001 |
| Current BMI (Kg/m2) | Spearman’s Rho = −0.143 | 0.103 | p = 0.555 | |
| Current weight (Kg) | Spearman’s Rho = −0.026 | 0.767 | ||
| Current height (cm) | Pearson’s correlation; r = 0.232 | 0.008 | p = 0.009 | p = 0.007 |
| FFM (Kg) | Pearson’s correlation; r = 0.096 | 0.273 | ||
| FM (Kg) | Spearman’s Rho = −0.121 | 0.168 | ||
| AN duration | T test: ≤3 years: −77.88 ± 146.39 >3 years: −245.3 ± 149.14 | <0.001 | p = 0.025 | p = 0.012 |
| AN subtype | T test: AN restrictive type: −136.06 ± 164.73 AN non-restrictive type: −225.25 ± 157.1 | 0.002 | p = 0.549 | p = 0.23 |
| Food selectivity | T test: Yes: −164.12 ± 163.64 No: −239.71 ± 172.44 | 0.026 | p = 0.124 | p = 0.068 |
| Meal skipping | T test: Yes: −210.59 ± 173.42 No: −160.62 ± 162.56 | 0.097 | p = 0.694 | |
| Bulimic episodes | T test: Yes: −166.8 ± 170 No: −216.77 ± 162.39 | 0.113 | p = 0.957 | |
| Vomiting | T test: Yes: −214.3 ± 158.56 No: −162.36 ± 172.85 | 0.088 | p = 0.952 | |
| Physical hyperactivity | T test: Yes: −165.99 ± 163.03 No: −199.13 ± 173.01 | 0.266 | ||
| Snack eating | T test: Yes: −283.72 ± 162.74 No: −167.2 ± 164.46 | 0.006 | p = 0.821 | |
| Compulsory eating | T test: Yes: −228.31 ± 153.93 No: −167.53 ± 169.29 | 0.088 | p = 0.781 | p = 0.545 |
| Polydipsia | T test: Yes: −237.68 ± 215.52 No: −179.39 ± 164.89 | 0.319 | ||
| Fasting practices | T test: Yes: −211.65 ± 177.45 No: −166.75 ± 166.83 | 0.144 | p = 0.384 | p = 0.254 |
| Laxative use | T test: Yes: −246.34 ± 199.8 No: −174.55 ± 162.7 | 0.111 | p = 0.979 | |
| Nicotine consumption | T test: Yes: −230.83 ± 148.5 No: −157.49 ± 172.36 | 0.016 | p = 0.31 | p = 0.208 |
| Current depressive episode | T test: Yes: −158.88 ± 172.69 No: −184.59 ± 170.19 | 0.465 | ||
| Current anxiety disorder | T test: Yes: −180.74 ± 182 No: −174.91 ± 156.85 | 0.853 | ||
| Current obsessive compulsive disorder | T test: Yes: −206.51 ± 192.88 No: −174.11 ± 168.54 | 0.535 | ||
| Current post-traumatic stress disorder | T test: Yes: −142.07 ± 226.39 No: −178.97 ± 168.81 | 0.638 | ||
| Current alcohol and/or substance use disorder | T test: Yes: −188.53 ± 129.52 No: −176.6 ± 174.03 | 0.859 | ||
| History of suicide attempt | T test: Yes: −231.8 ± 164.29 No: −169.16 ± 167.32 | 0.081 | p = 0.593 | |
| EDE-Q restriction | Pearson’s correlation; r = 0.093 | 0.311 | ||
| EDE-Q eating concern | Pearson’s correlation; r = 0.025 | 0.787 | ||
| EDE-Q weight concern | Pearson’s correlation; r= −0.059 | 0.528 | ||
| EDE-Q shape concern | Spearman’s Rho= −0.02 | 0.832 | ||
| EDE-Q total score | Pearson’s correlation; r = 0.018 | 0.85 | ||
| d2 score ratio | Pearson’s correlation; r = −0.091 | 0.344 |
| Mean Difference | SE | t | pbonf | ||
|---|---|---|---|---|---|
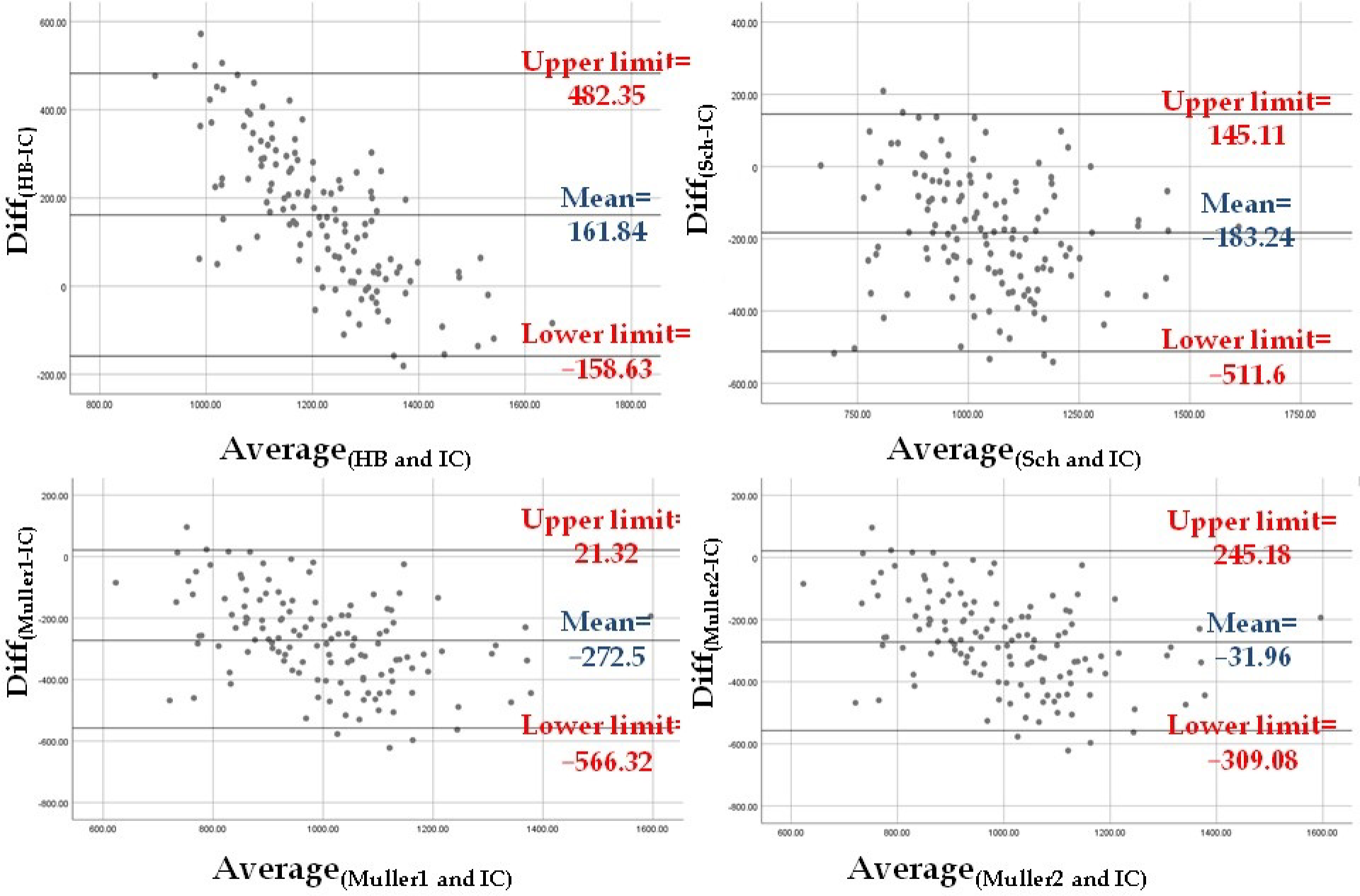
| REEIC | REEHB | −160.511 | 9.500 | −16.896 | <0.001 |
| REESch | 185.138 | 9.500 | 19.489 | <0.001 | |
| REEMuller1 | 274.368 | 9.500 | 28.882 | <0.001 | |
| REEMuller2 | 34.158 | 9.500 | 3.596 | 0.005 | |
| Adjusted REESch | 0.150 | 9.500 | 0.016 | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bou Khalil, R.; Sultan, A.; Seneque, M.; Richa, S.; Lefebvre, P.; Renard, E.; Courtet, P.; Maimoun, L.; Guillaume, S. Clinical Correlates of Measured and Predicted Resting Energy Expenditure in Patients with Anorexia Nervosa: A Retrospective Cohort Study. Nutrients 2022, 14, 2727. https://doi.org/10.3390/nu14132727
Bou Khalil R, Sultan A, Seneque M, Richa S, Lefebvre P, Renard E, Courtet P, Maimoun L, Guillaume S. Clinical Correlates of Measured and Predicted Resting Energy Expenditure in Patients with Anorexia Nervosa: A Retrospective Cohort Study. Nutrients. 2022; 14(13):2727. https://doi.org/10.3390/nu14132727
Chicago/Turabian StyleBou Khalil, Rami, Ariane Sultan, Maude Seneque, Sami Richa, Patrick Lefebvre, Eric Renard, Philippe Courtet, Laurent Maimoun, and Sebastien Guillaume. 2022. "Clinical Correlates of Measured and Predicted Resting Energy Expenditure in Patients with Anorexia Nervosa: A Retrospective Cohort Study" Nutrients 14, no. 13: 2727. https://doi.org/10.3390/nu14132727
APA StyleBou Khalil, R., Sultan, A., Seneque, M., Richa, S., Lefebvre, P., Renard, E., Courtet, P., Maimoun, L., & Guillaume, S. (2022). Clinical Correlates of Measured and Predicted Resting Energy Expenditure in Patients with Anorexia Nervosa: A Retrospective Cohort Study. Nutrients, 14(13), 2727. https://doi.org/10.3390/nu14132727








