Slow Drinking of Beer Attenuates Subjective Sedative Feeling in Healthy Volunteers: A Randomized Crossover Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Volunteers
2.2. Study Design
2.3. Blood Analyses and Subjective Sensation Assessments
2.4. Statistical Analysis
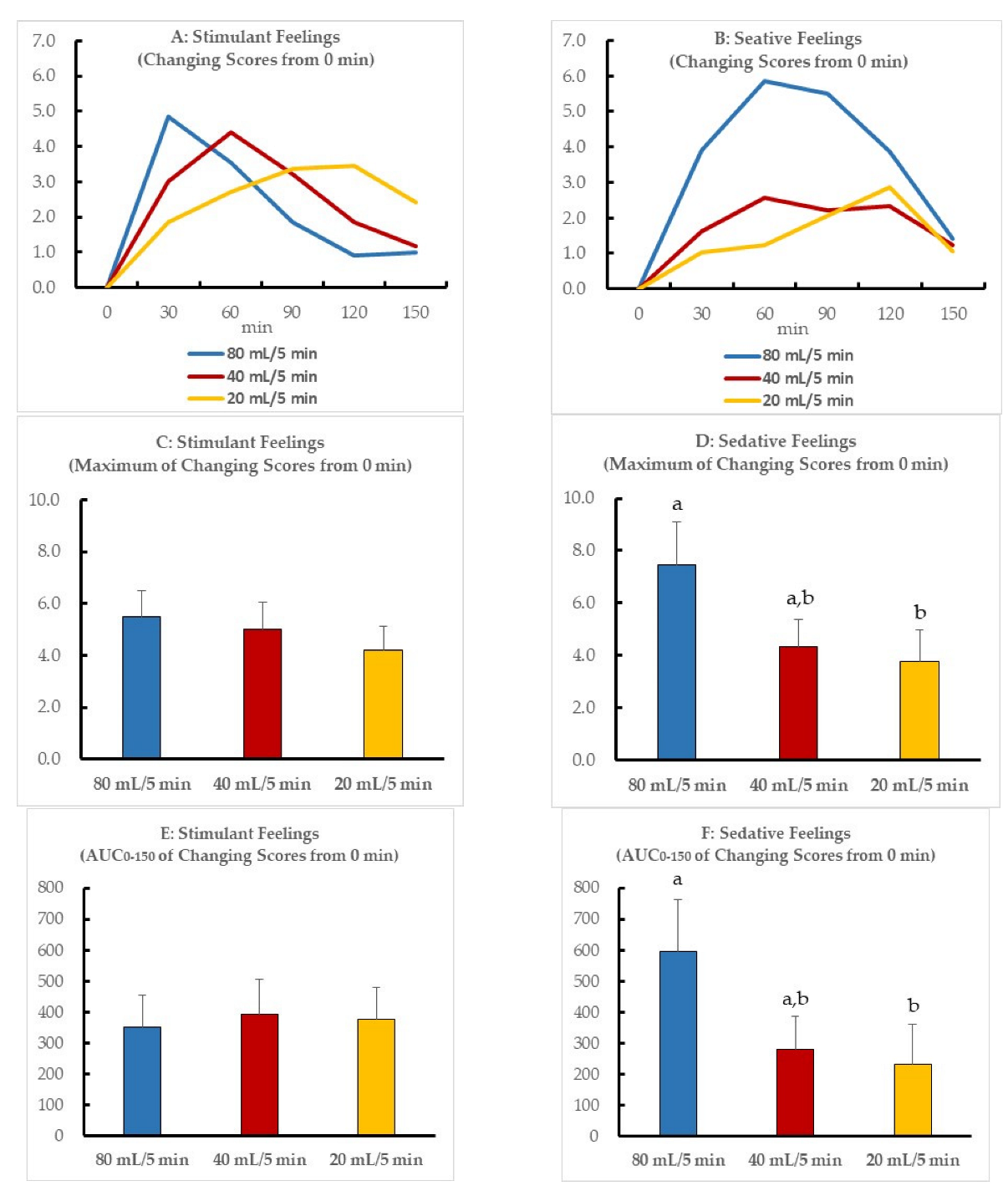
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iranpour, A.; Nakhaee, N. A review of alcohol-related harms: A recent update. Addict. Health 2019, 11, 129–137. [Google Scholar]
- Shield, K.; Manthey, J.; Rylett, M.; Probst, C.; Wettlaufer, A.; Parry, C.D.H.; Rehm, J. National, regional, and global burdens of disease from 2000 to 2016 attributable to alcohol use: A comparative risk assessment study. Lancet Public Health 2020, 5, e51–e61. [Google Scholar] [CrossRef]
- Chikritzhs, T.; Livingston, M. Alcohol and the risk of injury. Nutrients 2021, 13, 2777. [Google Scholar] [CrossRef]
- Morean, M.E.; Corbin, W.R. Subjective response to alcohol: A critical review of the literature. Alcohol. Clin. Exp. Res. 2010, 34, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Quinn, P.D.; Fromme, K. Subjective response to alcohol challenge: A quantitative review. Alcohol. Clin. Exp. Res. 2011, 35, 1759–1770. [Google Scholar] [CrossRef] [PubMed]
- Bujarski, S.; Hutchison, K.E.; Roche, D.J.; Ray, L.A. Factor structure of subjective responses to alcohol in light and heavy drinkers. Alcohol. Clin. Exp. Res. 2015, 39, 1193–1202. [Google Scholar] [CrossRef]
- Kalant, H. Pharmacokinetics of ethanol: Absorption, distribution, and elimination. In The Pharmacology of Alcohol and Alcohol Dependence; Beglieter, H., Kissin, B., Eds.; Oxford University Press: New York, NY, USA, 1996; pp. 15–58. [Google Scholar]
- Mitchell, M.C., Jr.; Teigen, E.L.; Ramchandani, V.A. Absorption and peak blood alcohol concentration after drinking beer, wine, or spirits. Alcohol. Clin. Exp. Res. 2014, 38, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
- Shultz, J.; Weiner, H.; Westcott, J. Retardation of ethanol absorption by food in the stomach. J. Stud. Alcohol 1980, 41, 861–870. [Google Scholar] [CrossRef]
- Jian, R.; Cortot, A.; Ducrot, F.; Jobin, G.; Chayvialle, J.A.; Modigliani, R. Effect of ethanol ingestion on postprandial gastric emptying and secretion, biliopancreatic secretions, and duodenal absorption in man. Dig. Dis. Sci. 1986, 31, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M.; Maddox, A.; Bochner, M.; Wishart, J.; Bratasiuk, R.; Collins, P.; Shearman, D. Relationships between gastric emptying of solid and caloric liquid meals and alcohol absorption. Am. J. Physiol. 1989, 257, G291–G298. [Google Scholar] [CrossRef] [PubMed]
- Oneta, C.M.; Simanowski, U.A.; Martinez, M.; Allali-Hassani, A.; Parés, X.; Homann, N.; Conradt, C.; Waldherr, R.; Fiehn, W.; Coutelle, C.; et al. First pass metabolism of ethanol is strikingly influenced by the speed of gastric emptying. Gut 1998, 43, 612–619. [Google Scholar] [CrossRef]
- Millar, K.; Hammersley, R.H.; Finnigan, F. Reduction of alcohol-induced performance impairment by prior ingestion of food. Br. J. Psychol. 1992, 83, 261–278. [Google Scholar] [CrossRef]
- Holt, S. Observations on the relation between alcohol absorption and the rate of gastric emptying. Can. Med. Assoc. J. 1981, 124, 267–297. [Google Scholar]
- Kalaria, T.; Ko, Y.L.; Issuree, K.K.J. Literature review: Drug and alcohol-induced hypoglycaemia. J. Lab. Precis. Med. 2021, 6, 1–16. [Google Scholar] [CrossRef]
- Okada, T.; Mizoi, Y. Studies on the problem of blood acetaldehyde determination in man and level after alcohol intake. Jpn. J. Alcohol Drug Depend. 1982, 17, 141–159. [Google Scholar]
- Namihira, T.; Shinzato, N.; Akamine, H.; Maekawa, H.; Matsui, T. Influence of nitrogen fertilization on tropical-grass silage assessed by ensiling process monitoring using chemical and microbial community analyses. J. Appl. Microbiol. 2010, 108, 1954–1965. [Google Scholar] [CrossRef]
- Martin, C.S.; Earleywine, M.; Musty, R.E.; Perrine, M.W.; Swift, R.M. Development and validation of the biphasic alcohol effects scale. Alcohol. Clin. Exp. Res. 1993, 17, 140–146. [Google Scholar] [CrossRef]
- King, A.C.; Houle, T.; Wit, H.; Holdstock, L.; Schuster, A. Biphasic alcohol response differs in heavy versus light drinkers. Alcohol. Clin. Exp. Res. 2002, 26, 827–835. [Google Scholar] [CrossRef]
- Poprawa, R. A subjective assessment of the short-term effects of alcohol consumption. Preliminary studies on the Polish language adaptation of “the biphasic alcohol effects scale”. Alcohol Drug Addic. 2015, 28, 1–21. [Google Scholar] [CrossRef][Green Version]
- Rueger, S.Y.; McNamara, P.J.; King, A.C. Expanding the utility of the biphasic alcohol effects scale (BAES) and initial psychometric support for the Brief-BAES (B-BAES). Alcohol. Clin. Exp. Res. 2009, 33, 916–924. [Google Scholar] [CrossRef]
- Rueger, S.Y.; King, A.C. Validation of the brief biphasic alcohol effects scale (B-BAES). Alcohol. Clin. Exp. Res. 2013, 37, 470–476. [Google Scholar] [CrossRef]
- Smith, T.; DeMaster, E.G.; Furne, J.K.; Springfield, J.; Levitt, M.D. First-pass gastric mucosal metabolism of ethanol is negligible in the rat. J. Clin. Investig. 1992, 89, 1801–1806. [Google Scholar] [CrossRef][Green Version]
- Mascord, D.; Rogers, J.; Smith, J.; Starmer, G.A.; Whitfield, J.B. Effect of diet on [lactate]/[pyruvate] ratios during alcohol metabolism in man. Alcohol Alcohol. 1989, 24, 189–191. [Google Scholar]
- Lieber, C.S.; Jones, D.P.; Losowsky, M.S.; Davidson, C.S. Interrelation of uric acid and ethanol metabolism in man. J. Clin. Investig. 1962, 41, 1863–1870. [Google Scholar] [CrossRef]
- Nieradko-Iwanicka, B. The role of alcohol consumption in pathogenesis of gout. Crit. Rev. Food Sci. Nutr. 2022, 62, 7129–7137. [Google Scholar] [CrossRef]
- Earleywine, M.; Martin, C.S. Anticipated stimulant and sedative effects of alcohol vary with dosage and limb of the blood alcohol curve. Alcohol. Clin. Exp. Res. 1993, 17, 135–139. [Google Scholar] [CrossRef]
- Holdstock, L.; de Wit, H. Individual differences in the biphasic effects of ethanol. Alcohol. Clin. Exp. Res. 1998, 22, 1903–1911. [Google Scholar] [CrossRef]
- Stafford, L.D.; Salmon, J. Alcohol health warnings can influence the speed of consumption. J. Public Health 2017, 25, 147–154. [Google Scholar] [CrossRef]
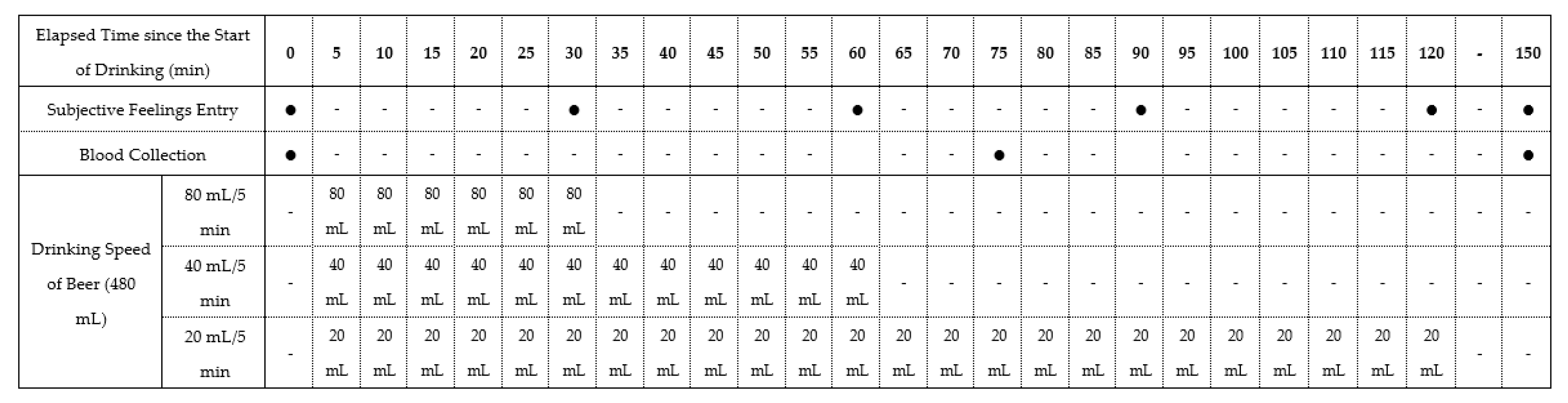
| 1st Experiment | Washout (>1 Week) | 2nd Experiment | Washout (>1 Week) | 3rd Experiment | |
|---|---|---|---|---|---|
| Group 1 (n = 6, ID: 1–6) | 20 mL/5 min | → | 80 mL/5 min | → | 40 mL/5 min |
| Group 2 (n = 6, ID: 7–12) | 40 mL/5 min | → | 20 mL/5 min | → | 80 mL/5 min |
| Group 3 (n = 6, ID: 13–18) | 80 mL/5 min | → | 40 mL/5 min | → | 20 mL/5 min |
| Parameter | Mean (SD) |
|---|---|
| Age (years) | 36.3 (9.9) |
| male, n = 11 | 40.1 (9.9) |
| female, n = 7 | 30.4 (6.3) |
| Height (cm) | 166.7 (7.9) |
| male, n = 11 | 171.7 (4.0) |
| female, n = 7 | 158.9 (6.0) |
| Body weight (kg) | 61.0 (8.6) |
| male, n = 11 | 66.1 (6.1) |
| female, n = 7 | 53.0 (5.1) |
| Body mass index (kg/m2) | 21.9 (2.0) |
| male, n = 11 | 22.4 (2.0) |
| female, n = 7 | 21.0 (1.5) |
| ALT (U/L) | 18.6 (7.6) |
| AST (U/L) | 23.0 (4.0) |
| GGT (U/L) | 28.2 (18.2) |
| LDH (U/L) | 174.6 (25.6) |
| CK (U/L) | 144.1 (63.2) |
| ALP (U/L) | 193.4 (44.6) |
| Total bilirubin (mg/dL) | 0.7 (0.3) |
| Triglyceride (mg/dL) | 80.6 (49.9) |
| Uric acid (mg/dL) | 6.0 (1.2) |
| Total protein (g/dL) | 7.3 (0.5) |
| Albumin (g/dL) | 4.9 (0.4) |
| Glucose (mg/dL) | 80.3 (5.7) |
| Parameter | Drinking Speed (mL/5 min) | 0 min | After 75 min | After 150 min | p c | Maximum |
|---|---|---|---|---|---|---|
| Alcohol (mg/mL) | 80 | 0.0 | 0.34 (0.07) a | 0.17 (0.07) | <0.001 | 0.34 (0.07) a |
| 40 | 0.0 | 0.34 (0.08) a | 0.17 (0.06) | <0.001 | 0.34 (0.08) a | |
| 20 | 0.0 | 0.13 (0.04) b | 0.17 (0.06) | <0.001 | 0.18 (0.05) b | |
| Acetate (μg/mL) | 80 | 0.0 | 18.1 (6.4) | 16.0 (7.8) | <0.001 | 19.6 (7.2) |
| 40 | 0.0 | 20.4 (9.3) | 18.1 (6.4) | <0.001 | 22.1 (8.6) | |
| 20 | 0.0 | 20.3 (9.0) | 15.5 (5.8) | <0.001 | 21.1 (8.5) | |
| Lactate (mg/dL) | 80 | 15.8 (5.0) | 19.7 (4.3) | 17.5 (4.6) | 0.007 | 3.3 (6.7) |
| 40 | 13.5 (6.8) | 19.4 (4.1) | 19.5 (7.1) | <0.001 | 7.5 (8.1) | |
| 20 | 13.9 (5.5) | 18.5 (3.7) | 18.3 (6.5) | 0.027 | 5.6 (7.3) | |
| Pyruvate (mg/dL) | 80 | 1.00 (0.25) | 0.51 (0.12) | 0.47 (0.11) | <0.001 | −0.55 (0.25) |
| 40 | 0.90 (0.34) | 0.48 (0.12) | 0.53 (0.25) | <0.001 | −0.43 (0.44) | |
| 20 | 0.84 (0.25) | 0.48 (0.14) | 0.48 (0.17) | <0.001 | −0.41 (0.26) | |
| Lactate/pyruvate ratio | 80 | 15.6 (3.9) | 38.5 (9.2) | 37.4 (9.4) | <0.001 | 27.1 (9.9) |
| 40 | 14.4 (3.0) | 40.7 (8.3) | 39.0 (10.6) | <0.001 | 30.9 (10.2) | |
| 20 | 16.1 (4.0) | 39.0 (8.9) | 38.8 (9.7) | <0.001 | 28.0 (10.4) | |
| Uric acid (mg/dL) | 80 | 5.8 (1.3) | 6.1 (1.3) | 6.0 (1.3) | <0.001 | 0.3 (0.1) a |
| 40 | 5.7 (1.4) | 6.1 (1.4) | 6.0 (1.5) | <0.001 | 0.4 (0.2) a | |
| 20 | 5.8 (1.2) | 6.0 (1.2) | 6.0 (1.2) | <0.001 | 0.2 (0.2) b |
| Stimulant Feelings | Sedative Feelings | |||||||
|---|---|---|---|---|---|---|---|---|
| Energized | Excited | Up | Stimulant | Sedated | Slow Thoughts | Sluggish | Sedative | |
| BAC-max | 0.010 | −0.096 | −0.023 | −0.037 | 0.394 ** | 0.273 * | 0.014 | 0.278 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oshima, S.; Shiiya, S.; Kato, Y. Slow Drinking of Beer Attenuates Subjective Sedative Feeling in Healthy Volunteers: A Randomized Crossover Pilot Study. Nutrients 2022, 14, 4502. https://doi.org/10.3390/nu14214502
Oshima S, Shiiya S, Kato Y. Slow Drinking of Beer Attenuates Subjective Sedative Feeling in Healthy Volunteers: A Randomized Crossover Pilot Study. Nutrients. 2022; 14(21):4502. https://doi.org/10.3390/nu14214502
Chicago/Turabian StyleOshima, Shunji, Sachie Shiiya, and Yasuhito Kato. 2022. "Slow Drinking of Beer Attenuates Subjective Sedative Feeling in Healthy Volunteers: A Randomized Crossover Pilot Study" Nutrients 14, no. 21: 4502. https://doi.org/10.3390/nu14214502
APA StyleOshima, S., Shiiya, S., & Kato, Y. (2022). Slow Drinking of Beer Attenuates Subjective Sedative Feeling in Healthy Volunteers: A Randomized Crossover Pilot Study. Nutrients, 14(21), 4502. https://doi.org/10.3390/nu14214502






